Abstract
In this report, we reveal that etoposide inhibits the proliferation of SK-N-AS neuroblastoma cancer cells and promotes protein kinase Cδ (PKCδ)- and caspase-dependent apoptosis. Etoposide induces the caspase-3-dependent cleavage of PKCδ to its active p40 fragment, and active PKCδ triggers the processing of caspase-3 by a positive-feedback mechanism. Treatment of cells with the caspase-3-specific inhibitor N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone or caspase-3-specific small interacting RNA (siRNA) prevented the etoposide-induced activation of caspase-8 and inhibited apoptosis. The silencing of the caspase-2 or caspase-8 genes using siRNAs did not affect the etoposide-induced processing of caspase-3, indicating that these caspases lie downstream of caspase-3 in this signaling pathway. Furthermore, the etoposide-induced processing of caspase-2 required the expression of caspase-8, and the etoposide-mediated processing of caspase-8 required the expression of caspase-2, indicating that these two caspases activate each other after etoposide treatment. We also observed that etoposide-mediated apoptosis was decreased by treating the cells with the caspase-6-specific inhibitor benzyloxycarbonyl-Val-Glu(OMe)-Ile-Asp-(OMe)-fluoromethyl ketone and that caspase-6 was activated by a caspase-8-dependent mechanism. Finally, we show that rottlerin blocks etoposide-induced apoptosis by inhibiting the PKCδ-mediated activation of caspase-3 and by degrading caspase-2, which prevents caspase-8 activation. Our results add important insights into how etoposide mediates apoptotic signaling and how targeting these pathways may lead to the development of novel therapeutics for the treatment of neuroblastomas.
Apoptosis is a type of cell death that occurs without the production of inflammation and is essential for human development, tissue homeostasis, and immunity. The dysfunction of the apoptotic signaling process often leads to serious consequences such as cancer (Vermeulen et al., 2005), neurological disorders (Ekshyyan and Aw, 2004), or autoimmune diseases (Mahoney and Rosen, 2005). One of the ways that chemotherapeutic agents kill tumor cells is by inducing apoptosis. The two main apoptotic pathways are the death receptor and mitochondrial pathways (Debatin and Krammer, 2004; Cereghetti and Scorrano, 2006). Apoptotic signaling through these two pathways is dependent on caspases, which comprise a family of cysteine proteases that cleave key protein substrates close to specific aspartic acid residues (Green and Kroemer, 1998). In the death receptor pathway, the cell surface death receptors DR4/DR5 and Fas encounter their specific ligands Apo2L/TRAIL and Fas, respectively, inducing a conformational change that activates the receptors. The activated receptors recruit the adaptor protein FADD, which binds to the initiating caspases-8 and -10 through homophilic death effector domain interactions, forming the death-inducing signaling complex (Ashkenazi and Dixit, 1998; Sprick et al., 2000). The close proximity of the initiating caspases in the death-inducing signaling complex leads to their dimerization and proteolytic activation. The active caspase-8 cleaves the proapoptotic protein Bid as well as the downstream caspases-3, -6, and -7 initiating the apoptotic process (Li et al., 1998). Furthermore, the caspase-8-mediated cleavage of Bid induces the activation of the downstream proapoptotic proteins such as Bak and Bax, which promote mitochondrial cytochrome c release into the cytosol, thereby linking the death receptor and mitochondrial apoptotic pathways (Gogvadze and Orrenius, 2006). Cytosolic cytochrome c associates with caspase-9, dATP, and apoptotic protease activating factor-1, forming the apoptosome complex leading to the activation of caspase-9 (Liu et al., 1996; Jiang and Wang, 2004). The activated caspase-9 cleaves the downstream effector caspases-3, -6, and -7 (Jiang and Wang, 2004). In addition, caspase-2 adds a level of complexity to apoptotic signaling because it has features of both initiator and executioner caspases (Zhivotovsky and Orrenius, 2005). Furthermore, it has been shown that caspase-2 plays a role in mediating genotoxic-induced apoptosis (Tinel and Tschopp, 2004; Panaretakis et al., 2005; Cao et al., 2008).
Protein kinase C (PKC) isozymes comprise a family of at least 10 related serine-threonine kinases that play important roles in the regulation of various cellular processes, including proliferation, differentiation, malignant transformation, and apoptosis (Nishizuka, 1984; Toker, 1998). Based on their structures and cofactor requirements, the PKC isoforms are divided into the classic PKCs (α, β1, β2, and γ), novel (δ, ε, η, and θ), and atypical (ζ and λ/i) groups (Mackay and Twelves, 2007). Members of this family have been shown to be either pro- or antiapoptotic depending on the isoform and cellular context. For instance, PKCα and PKCε have been shown to inhibit apoptosis by phosphorylating or increasing the expression of the antiapoptotic protein Bcl-2 (Gubina et al., 1998; Ruvolo et al., 1998), whereas caspase-3- and caspase-2-dependent activation of PKCδ promotes apoptosis (Reyland et al., 1999; Panaretakis et al., 2005; Lu et al., 2007).
Etoposide is a major antitumor agent that is used to treat a variety of cancers, including neuroblastomas (Simon et al., 2007). It exerts its antineoplastic activity by inhibiting topoisomerase II, which leads to DNA strand breaks, inhibition of DNA replication, and apoptotic cell death (Kaufmann, 1998). However, the detailed mechanism of how etoposide triggers apoptosis has not been clearly defined. The aim of this study was to further the understanding of how the interplay of PKCδ and caspases mediate etoposide-induced apoptosis of cancer cells.
Our results reveal that etoposide inhibits the proliferation of SK-N-AS neuroblastoma cells and promotes PKCδ- and caspase-dependent apoptosis. Furthermore, we show that caspase-3 cleaves PKCδ, active PKCδ processes caspase-3 through a positive-feedback mechanism, and active caspase-3 leads to the activation of caspase-8. The knockdown of caspases-2 or -8 does not affect the etoposide-induced processing of caspase-3, but it does inhibit the caspase-8-dependent activation of caspase-6 and apoptosis. Moreover, we made a novel finding that the etoposide-induced activation of caspase-2 requires caspase-8 expression, and the activation of caspase-8 requires caspase-2 expression, indicating that they directly activate each other after etoposide treatment.
Materials and Methods
Cell Culture, Materials, and Antibodies. The SK-N-AS human neuroblastoma cell line was obtained from American Type Culture Collection (Manassas, VA) and was maintained in Dulbecco's modified Eagle's medium/F-12 medium with 15% fetal calf serum and 100 ng/ml each of penicillin and streptomycin (Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. The caspase-2 inhibitor [benzyloxycarbonyl-Val-Asp(OMe)-Val-Ala-Asp(OMe)-fluoromethylketone], caspase-9 inhibitor (z-LEHD-fmk), caspase-6 inhibitor (z-VEID-fmk), and caspase-3 inhibitor (z-DEVD-fmk) were purchased from R&D Systems (Minneapolis, MN). Etoposide, rottlerin, and Gö6976 were purchased from EMD Biosciences (Gibbstown, NJ). The inhibitors were dissolved in dimethyl sulfoxide and added to the cultured cells so that the final concentration of dimethyl sulfoxide was 0.1%. In this study, the following primary antibodies were used: anti-caspase-8, anti-caspase-6, anti-PKCδ (BD Biosciences, San Jose, CA), anti-caspase-9, anti-caspase-3 (Cell Signaling Technology, Danvers, MA), anti-caspase-2 (Assay Designs, Inc., Ann Arbor, MI), and anti-β-actin clone AC-74 (Sigma-Aldrich, St. Louis, MO).
Western Blotting. SK-N-AS cells were harvested, rinsed in cold phosphate-buffered saline, and lysed in mammalian protein extract reagent (Pierce Biotechnology, Rockford, IL) containing 1% protease inhibitor cocktail (Sigma-Aldrich) followed by centrifugation (10,000g, 15 min). Protein concentration was determined by using the BCA/Cu2SO4 protein (Sigma-Aldrich) assay as described by the manufacturer. One hundred micrograms of lysate was separated on a NuPAGE 4 to 12% or 10% bis-Tris Gel (Invitrogen) and transferred to an Immobilon-P membrane (Thermo Fisher Scientific, Pittsburgh, PA). Membranes were incubated in blocking buffer (phosphate-buffered saline, 0.1% Tween 20, and 5% skim milk) and then incubated first with specific antibodies in blocking buffer followed by the addition of anti-mouse, anti-rabbit (both from GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK), or anti-goat (Santa Cruz Biotechnology, Santa Cruz, CA) IgG antibodies. Immunoreactive proteins were visualized by using SuperSignal West Pico solutions (Pierce Biotechnology).
Small Interfering RNA Preparation and Transfection. The caspase-2 small interfering RNA (siRNA) was synthesized by Invitrogen according to the sequences published in Cao et al. (2008). The ON-TARGET plus SMARTpool PKCδ siRNA, caspase-8 siRNA, caspase-3 siRNA, and siCONTROL NonTargeting siRNA were purchased from Dharmacon RNA Technologies (Lafayette, CO). SK-N-AS cells were seeded at a density of 2 × 105 cells/ml in antibiotic-free medium 1 day before transfection. For transfection, 100 nM siRNA was mixed with DharmaFECT 1 transfection reagent (Dharmacon) according to the manufacturer's instructions. The cells were incubated with the siRNA-DharmaFECT 1 complexes for 72 h and 50 μM etoposide was added for an additional 48 h before further analysis.
Proliferation Assay. SK-N-AS cells (1 × 104) were plated in 100 μl of complete medium in 96-well plates and incubated in the presence or absence of increasing concentrations of etoposide (10, 25, 50, and 100 μM) for 48 h. Twenty microliters of CellTiter 96 Aqueous One solution cell proliferation assay reagent (Promega, Madison, WI) was added and incubated at 37°C for 1 h, and the plate was read in a microplate reader at 490 nM.
Apoptosis Detection Assays. SK-N-AS cells were treated with 50 μM etoposide for 48 h, stained with 10 μM Hoechst 33342 stain (Sigma) for 1 h, and the nuclear morphologies were visualized by fluorescence microscopy using a Nikon Diaphot 200 Inverted Microscope at 40× magnification. The images were captured using a Diagnostic Instruments SPOT color camera (Diagnostic Instruments, Inc., Sterling Heights, MI). For the Annexin V binding assay, the cells were incubated as described above, harvested, and stained with fluorescein isothiocyanate-labeled Annexin V (BD Biosciences) and propidium iodide according to the manufacturer's protocol. The cells were analyzed using a FACScan (BD Biosciences) flow cytometry and CellQuest software (BD Biosciences).
Cytochrome c Release. SK-N-AS cells were treated with 50 μM etoposide for 48 h, and the cytosolic and mitochondrial fractions were generated using a digitonin-based subcellular fractionation technique as described previously (Adrain et al., 2001; Ekert et al., 2001). Equal amounts of cytosolic fractions (100 μg) or mitochondrial fractions (100 μg) were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with an anti-cytochrome c antibody (Santa Cruz Biotechnology).
Results
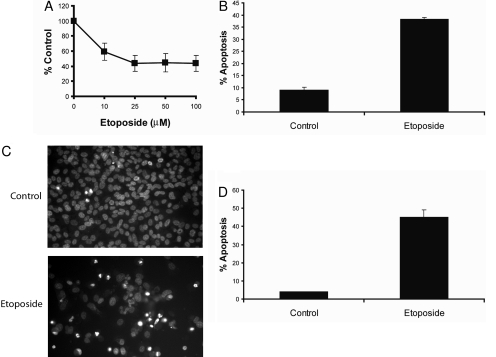
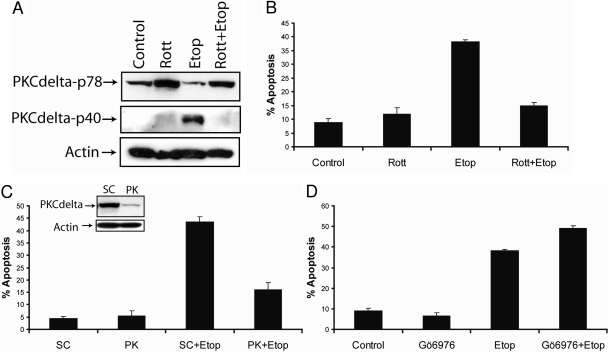
Etoposide Inhibits the Proliferation and Induces PKCδ-Mediated Apoptosis in SK-N-AS Neuroblastoma Cancer Cells. To examine the effect of etoposide on the proliferation of SK-N-AS cells, we treated cells with 10, 25, 50, and 100 μM etoposide for 48 h and performed a cell proliferation assay. As shown in Fig. 1A, etoposide decreased the proliferation of SK-N-AS in a dose-dependent fashion, and the IC50 value was approximately 50 μM. After 48 h, etoposide decreased the proliferation by 50%, and the IC50 value was approximately 50 μM. An early event in apoptosis is the exposure of phosphatidylserine on the outer leaflet of the plasma membrane. Annexin V is a protein that binds to phosphatidylserine and is used to detect apoptotic cells. Furthermore, double staining the cells with annexin V and propidium iodide allowed us to detect apoptotic cells by flow cytometry. We also identified apoptotic cells by fluorescence microscopy of Hoechst 33342-stained SK-N-AS cells, because apoptosis is characterized by changes in nuclear morphology (Willingham, 1999). As shown in Fig. 1, B through D, treatment with 50 μM etoposide for 48 h induced apoptosis in 40% of the cells. Next, we determined whether PKCδ was involved in mediating etoposide's apoptotic signals, because several laboratories have shown that it is involved in mediating chemotherapeutic drug-induced apoptosis in various cell types (Reyland et al., 1999; Basu et al., 2001; Blass et al., 2002; Panaretakis et al., 2005). Immunoblot analysis of whole-cell lysates showed that etoposide induced the cleavage of PKCδ to its constitutive active p40 fragment, suggesting that active PKCδ may be involved in etoposide-mediated apoptosis (Fig. 2A). Therefore, to investigate the function of PKCδ in etoposide-induced apoptosis, we used the PKCδ-specific inhibitor rottlerin (Gschwendt et al., 1994). Rottlerin inhibited the etoposide-induced cleavage and activation of PKCδ and decreased apoptosis from 43 to 15%, revealing that etoposide-triggered apoptosis occurs by a PKCδ-mediated pathway in SK-N-AS cells (Fig. 2, A and B). Furthermore, we silenced PKCδ expression using a PKCδ-specific siRNA and showed that PKCδ gene knockdown reduced etoposide-mediated apoptosis from 45 to 18% (Fig. 2C).
Fig. 1.
Etoposide inhibits the proliferation and triggers apoptosis in SK-N-AS cells. A, cells were treated with 10, 25, 50, or 100 μM etoposide for 48 h, and cell proliferation was determined by using the CellTiter 96 Aqueous One solution cell proliferation assay reagent. Values are representative of three independent experiments, and error bars show S.D. from triplicate counts. B, cells were treated with 50 μM etoposide for 48 h, harvested, and apoptosis was determined by FACScan analysis as described under Materials and Methods. Compensation was executed for each experiment using untreated cells stained with Annexin V and propidium iodide. Error bars show S.D. from triplicate measurements. C, fluorescence microscopy images at 40× magnification of Hoechst 33342-stained nuclei of untreated cells or cells treated with 50 μM etoposide for 48 h. D, quantification of apoptosis was carried out by counting fragmented nuclei stained with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts.
Fig. 2.
Etoposide induces PKCδ-mediated apoptosis in SK-N-AS cells. A, immunoblot analysis of PKCδ and β-actin in cell lysates after the treatment with 2 μM rottlerin, 50 μM etoposide, or 2 μM rottlerin and 50 μM etoposide for 48 h. B, cells were treated as described above, and the percentage of apoptotic cells was determined by staining with annexin V and propidium iodide and analyzed by flow cytometry. All values are representative of three independent experiments, and error bars show S.D. from triplicate measurements. C, immunoblot analysis of PKCδ and β-actin from whole-cell lysates after the transfection with 100 nM nontargeting siRNA (SC) or PKCδ -specific siRNA for 72 h (inset), cells were treated with 50 μM etoposide for an additional 48 h, and apoptosis was determined by counting fragmented nuclei after staining with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts. D, cells were treated with 10 nM Gö6976 for 2 h followed by 50 μM etoposide for 48 h, and the percentage of apoptotic cells was determined by staining with annexin V and propidium iodide and analyzed by flow cytometry. All values are representative of three independent experiments, and error bars show S.D. from triplicate measurements.
The etoposide-triggered apoptosis observed in PKCδ-siRNA-treated cells was most likely due to incomplete silencing of the PKCδ gene or by PKCδ-independent apoptotic signaling (Fig. 2C). To confirm that PKCδ and not other PKC isoforms were involved in etoposide-induced apoptosis, we treated the cells with Gö6976, which is an inhibitor of classic PKCs such as PKCα and -β. The 10 nM Gö6976 concentration that we used has been demonstrated not to inhibit PKCδ or other Ca2+-independent PKC isoforms (Martiny-Baron et al., 1993). Inhibition of the classic PKCs with 10 nM Gö6976 did not decrease apoptosis but instead increased it from 40 to 50%, revealing that the classic PKC isoforms inhibit the etoposide-induced apoptosis in SK-N-AS cells (Fig. 2D). Furthermore, our results show that PKCδ mediates etoposide-induced apoptosis in SK-N-AS cells.
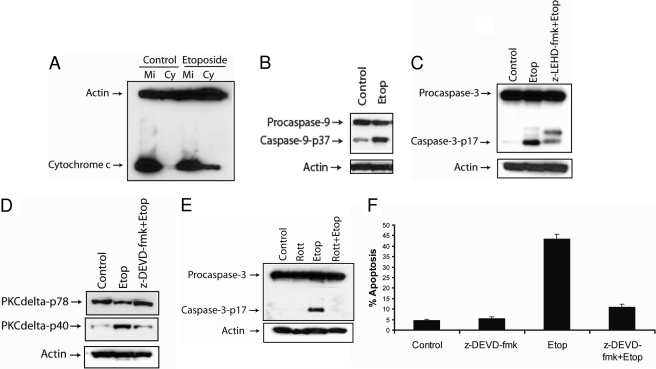
Etoposide Induces Caspase-3-Dependent Apoptosis in SK-N-AS cells. Caspase-9 has been shown to be an apical caspase in DNA damaged-induced apoptosis (Zou et al., 1999; Chen et al., 2000; Yu et al., 2007; Feng et al., 2008). Therefore, we analyzed whether etoposide triggered the activation of caspase-9 in SK-N-AS cells. We observed that etoposide induces mitochondrial cytochrome c release (Fig. 3A) and triggers the activation of caspase-9, as indicated by the production of its p37 band (Fig. 3B). Next, we determined whether the activation of caspase-9 triggered the cleavage of caspase-3 and whether caspase-3 was required for PKCδ activation, because caspase-3 has been shown to cleave PKCδ after etoposide treatment (Reyland et al., 1999; Blass et al., 2002). Treating cells with the caspase-9 inhibitor z-LEHD-fmk decreased the etoposide-induced processing of caspase-3, showing that caspase-9 is involved in cleaving caspase-3 after etoposide treatment (Fig. 3C). Moreover, treating cells with the caspase-3 inhibitor z-DEVD-fmk prevented the etoposide-induced cleavage of PKCδ, indicating that the etoposide-induced activation of PKCδ is mediated by a caspase-3-dependent mechanism (Fig. 3D). Rottlerin treatment inhibited the etoposide-mediated activation of caspase-3, revealing that active PKCδ is required for the processing of caspase-3 (Fig. 3E). These results are in agreement with previous studies showing that PKCδ activates caspase-3 after etoposide treatment (Reyland et al., 1999; Blass et al., 2002). Furthermore, treating cells with the caspase-3 inhibitor decreased apoptosis from 45 to 12%, revealing caspase-3 activation is required for etoposide-induced apoptosis in SK-N-AS cells (Fig. 3F). These results reveal that etoposide induces caspase-3-dependent apoptosis, that caspase-3 is processed by caspase-9 and PKCδ, and that caspase-3 itself can activate PKCδ by a positive-feedback mechanism.
Fig. 3.
Etoposide triggers caspase-3-dependent apoptosis in SK-N-AS cells. A, cells were treated with 50 μM etoposide for 48 h and harvested. The cytosolic fractions were obtained by a digitonin-based subcellular fractionation procedure. One hundred micrograms of cytosolic (Cy) and mitochondrial protein fractions (Mi) was analyzed by SDS-polyacrylamide gel electrophoresis, and cytochrome c and β-actin levels were determined by immunoblotting. B, immunoblot analysis of caspase-9 and β-actin in cell lysates treated with 50 μM etoposide for 48 h. C, immunoblot analysis of caspase-3 and β-actin in cell lysates treated with 50 μM etoposide with or without 20 μM caspase-9 inhibitor (z-LEHD-fmk) for 48 h. D, immunoblot analysis of PKCδ and β-actin in cell lysates treated with 50 μM etoposide with or without 20 μM caspase-3 inhibitor (z-DEVD-fmk) for 48 h. E, immunoblot analysis of caspase-3 and β-actin in cell lysates treated with or without 2 μM rottlerin, 50 μM etoposide, or 2 μM rottlerin and 50 μM etoposide for 48 h. F, cells were treated with 50 μM etoposide with or without 20 μM caspase-3 inhibitor (z-DEVD-fmk) for 48 h, and the percentage of apoptotic cells was quantified by counting fragmented nuclei stained with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts.
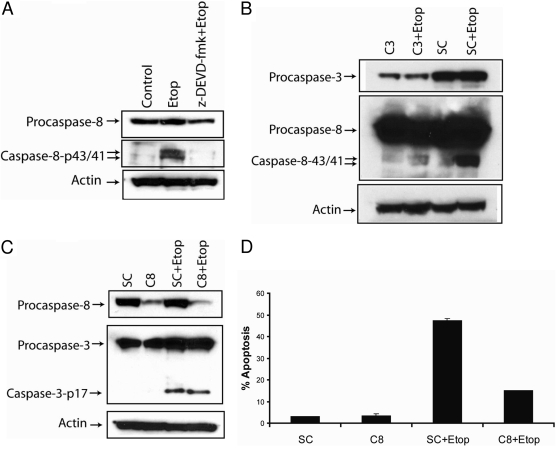
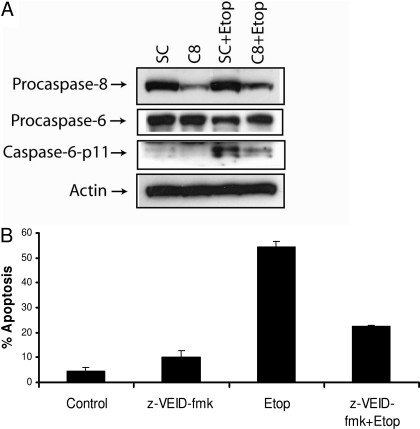
Etoposide Triggers Caspase-8- and Caspase-6-Dependent Apoptosis in SK-N-AS Cells. We next examined the role of caspase-8 in etoposide-induced apoptosis, because it is a central caspase in the death receptor and mitochondrial apoptotic signaling pathways. Etoposide induced the activation of caspase-8 in SK-N-AS cells as indicated by the production of the caspase-8-p43/41-activated bands, and the processing of caspase-8 was decreased by treating the cells with the caspase-3-specific inhibitor z-DEVD-fmk (Fig. 4A) or by silencing the expression of caspase-3 using a caspase-3-specific siRNA (Fig. 4B). These results reveal that the etoposide-induced activation of caspase-8 occurs by a caspase-3-dependent mechanism. Moreover, the knockdown of the caspase-8 using a caspase-8-specific siRNA did not prevent the etoposide-mediated cleavage of caspase-3, indicating that caspase-8 is not required for the processing of caspase-3 in these cells (Fig. 4C). These data are in agreement with previous studies showing that genotoxic stress can trigger caspase-8 activation downstream of caspases-9 and -3 via a mitochondrial amplification loop (Slee et al., 2001; Shamimi-Noori et al., 2008). To delineate the function of caspase-8 in etoposide-induced apoptosis, we silenced the caspase-8 gene using a caspase-8-specific siRNA (Fig. 4C), treated cells with etoposide, and detected apoptotic cells. As shown in Fig. 4D, the knockdown of caspase-8 decreased etoposide-induced apoptosis from 48 to 15%, showing that etoposide-triggered apoptosis occurs by a caspase-8-dependent mechanism. Because the silencing of caspase-8 did not alter the etoposide-induced processing of caspase-3, we analyzed whether caspase-6 was involved in etoposide-induced apoptosis, because caspase-6 can be processed by caspase-8. As shown in Fig. 5A, the knockdown of caspase-8 expression reduced the etoposide-mediated production of the caspase-6-p11 active subunit, indicating that caspase-6 is cleaved by a caspase-8-dependent mechanism. Furthermore, treating the cells with the caspase-6-specific inhibitor z-VEID-fmk decreased etoposide-induced apoptosis from 52 to 23%, revealing that caspase-6 mediates etoposide-induced apoptosis in SK-N-AS cells (Fig. 5B).
Fig. 4.
Etoposide induces caspase-8-dependent apoptosis. A, immunoblot analysis of caspase-8 and β-actin in cell lysates treated with 50 μM etoposide with or without 20 μM caspase-3 inhibitor (z-DEVD-fmk) for 48 h. B, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-3-specific siRNA (C3) for 48 h followed by the addition of 50 μM etoposide for an additional 48 h, and cell lysates were obtained and caspase-3, caspase-8, and β-actin levels were determined by immunoblotting. C, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-8-specific siRNA (C8) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and cell lysates were obtained and caspase-8, caspase-3, and β-actin levels were determined by immunoblotting. D, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-8-specific siRNA (C8) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and the percentage of apoptotic cells were quantified by counting fragmented nuclei after staining with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts.
Fig. 5.
Etoposide induces the caspase-8-dependent activation of caspase-6 and apoptosis. A, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-8-specific siRNA (C8) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and cell lysates were obtained and caspase-8, caspase-6, and β-actin levels were determined by immunoblotting. B, cells were treated with 50 μM etoposide with or without 20 μM caspase-6 inhibitor (z-VEID-fmk) for 48 h, and the percentage of apoptotic cells was quantified by counting fragmented nuclei stained with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts.
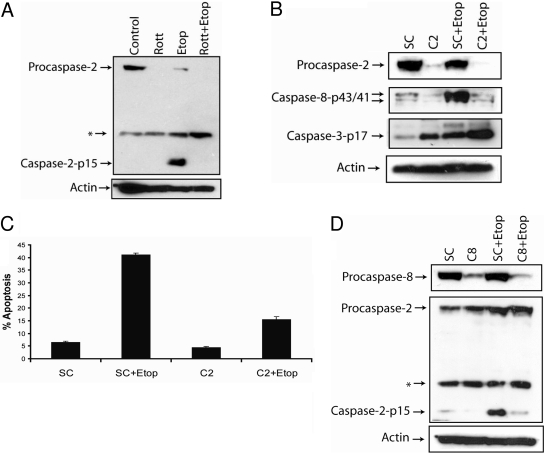
Etoposide Triggers Caspase-2-Dependent Apoptosis in SK-N-AS Cells. Rottlerin has been shown to promote the proteosomal degradation of caspase-2 (Basu et al., 2008). Therefore, we determined whether rottlerin induced the degradation of caspase-2 and whether caspase-2 plays a role in triggering etoposide-mediated apoptosis, because rottlerin inhibits etoposide-induced apoptosis in SK-N-AS cells (Fig. 2B). As shown in Fig. 6A, etoposide triggered caspase-2 activation, and rottlerin induced the degradation of caspase-2, indicating that besides the PKCδ, caspase-2 may also be involved in mediating etoposide's effects in SK-N-AS cells. To directly assess the function of caspase-2 in etoposide-induced apoptosis, we silenced the caspase-2 gene using a caspase-2-specific siRNA (Fig. 6B). The knockdown of caspase-2 expression inhibited the etoposide-induced activation of caspase-8 and decreased apoptosis from 41 to 14%, showing that apoptosis is mediated by a caspase-2-dependent mechanism and that caspase-2 is required for the etoposide-induced activation of caspase-8 in these cells (Fig. 6, B and C). Furthermore, we observed that the knockdown of the caspase-2 gene by siRNA did not inhibit the etoposide-mediated activation of caspase-3, revealing that caspase-2 is not required for the processing of caspase-3 in these cells (Fig. 6B). It is noteworthy that the knockdown of caspase-8 expression, by using a caspase-8-specific siRNA, inhibited the etoposide-induced activation of caspase-2, revealing that caspase-8 and caspase-2 activate each other downstream of caspase-3 in SK-N-AS cells (Fig. 6D).
Fig. 6.
Etoposide induces caspase-2-dependent apoptosis. A, immunoblot analysis of caspase-2 and β-actin in cell lysates treated with or without 2 μM rottlerin, 50 μM etoposide, or 2 μM rottlerin and 50 μM etoposide for 48 h. B, cells were transfected with nontargeting siRNA (SC) or caspase-2-specific siRNA (C2) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and cell lysates were obtained, and caspase-2, caspase-8, caspase-3, and β-actin levels were determined by immunoblotting. C, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-2-specific siRNA (C2) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and the percentage of apoptotic cells was quantified by counting fragmented nuclei after staining with Hoechst 33342 among 200 cells. Shown are representative apoptosis rates from three independent counts. D, cells were transfected with 100 nM nontargeting siRNA (SC) or caspase-8-specific siRNA (C8) for 72 h followed by the addition of 50 μM etoposide for an additional 48 h, and cell lysates were obtained and caspase-8, caspase-2, and β-actin levels were determined by immunoblotting. *, a nonspecific protein that is recognized by the caspase-2-specific antibody.
Discussion
In the present study, we demonstrated that etoposide induces apoptosis in SK-N-AS neuroblastoma cancer cells by a PKCδ- and caspase-dependent mechanism and that caspase-3 serves as the initiating caspase in this process. Moreover, etoposide induces the caspase-3-dependent activation of caspases-8, -2, and -6, which triggers apoptosis. We revealed that the knockdown of the caspase-8 gene or the caspase-2 gene does not affect the etoposide-mediated activation of caspase-3, but the knockdown of caspase-3 expression prevented the activation of caspase-8. In addition, we showed that etoposide induces caspase-3 activation by a caspase-9- and PKCδ-mediated mechanism. For the first time, we showed that caspase-2 and caspase-8 are required for the processing of each other after etoposide treatment and that caspase-6 induces apoptosis downstream of caspase-8. Thus, this work identified novel mechanisms of how etoposide induces apoptosis in cancer cells.
By using the caspase-3 inhibitor z-DEVD-fmk or caspase-3-specific siRNA, we showed that the etoposide-mediated processing of caspase-3 is required for the activation of caspase-8. Furthermore, the knockdown of the caspase-8 or caspase-2 genes using siRNA did not affect the etoposide-mediated processing of caspase-3, revealing that both caspases are activated downstream of caspase-3 in this signaling pathway. Moreover, the silencing of the FADD gene using a FADD-specific siRNA did not inhibit the etoposide-induced processing of caspase-8, indicating that etoposide triggers the activation of caspase-8 by a death receptor-independent pathway (data not shown). These results are in agreement with previous reports showing that genotoxic agents can trigger the caspase-3-dependent activation of caspase-8 by a mitochondrial amplification loop (Slee et al., 2000; von Haefen et al., 2003; Wang et al., 2006; Conrad et al., 2008). It is noteworthy that we showed the expression of caspase-8 to be required for the etoposide-induced activation of caspase-2 in SK-N-AS cells. To our knowledge, this is the first report showing that the expression of caspase-8 is required for the processing of caspase-2 after the treatment with a DNA-damaging agent. We also revealed that the expression of caspase-2 was required for the etoposide-triggered processing of caspase-8. This finding is in agreement with a recent finding by Basu et al. (2008) showing that the knockdown of caspase-2 using a caspase-2-specific siRNA decreased the cisplatin-mediated processing of caspase-8. Furthermore, Shin et al. (2005) showed that caspase-2 expression was required for the processing of caspase-8 after the treatment of human esophageal cancer cells with the protein kinase casein kinase II inhibitor 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole. Current studies are underway to determine whether the formation of the PIDDosome, a protein complex of p53-induced protein with a death domain (PIDD), is required for the etoposide-mediated activation of caspase-2 and whether the expression of caspase-8 is required for the PIDDosome formation, or whether caspase-8 can directly interact with and activate caspase-2. We also found that etoposide induces the caspase-8-dependent activation of caspase-6 and that this effector caspase is required for mediating etoposide's death signals downstream of caspases-3, -2, and -8 in SK-N-AS cells.
Rottlerin was originally identified as a PKCδ-specific inhibitor and has been used to determine the function of PKCδ in genotoxic drug-induced apoptosis (Reyland et al., 1999; Blass et al., 2002; Panaretakis et al., 2005). In this report, we showed that 2 μM rottlerin blocked the etoposide-mediated processing of PKCδ to its 40-kDa active fragment, prevented caspase-3 activation, and inhibited apoptosis. However, recent reports suggest that rottlerin has additional intracellular targets besides PKCδ. For instance, Lim et al. (2008, 2009) reported that 5 to 10 μM rottlerin induced endoplasmic reticulum stress, triggered the CCAAT/enhancer-binding protein homologous protein-mediated up-regulation of DR5, and sensitized HT29 human colon carcinoma cells to tumor necrosis factor-related TRAIL-induced apoptosis by a PKCδ-independent pathway. Moreover, Tillman et al. (2003) reported that rottlerin sensitizes colon carcinoma cells to TRAIL-induced apoptosis by uncoupling the mitochondria via a PKCδ-independent mechanism.
In addition, Kim et al. (2005) showed that rottlerin-sensitized glioma cells to TRAIL-induced apoptosis by decreasing the X-linked inhibitor of apoptosis protein and survivin levels via the inhibition of cdc2 independently of PKCδ. Finally, Basu et al. (2008) showed that rottlerin induces the down-regulation of caspase-2 by a PKCδ-independent pathway. We also observed that 2 μM rottlerin induced the degradation of caspase-2 in SK-N-AS cells, which suggests that besides inhibiting the etoposide-mediated activation of PKCδ, rottlerin also protects cells from etoposide's effects by inhibiting the caspase-2-dependent activation of caspase-8 (Fig. 6, A and B). Moreover, 2 μM rottlerin did not induce apoptosis in SK-N-AS cells (Fig. 2B), but treating cells with 10 μM rottlerin for 24 h triggered a 3-fold increase in apoptosis, increased the expression of CCAAT/enhancer-binding protein homologous protein and DR5, and sensitized SK-N-AS cells to TRAIL (Day et al., unpublished results). These findings reveal that in SK-N-AS cells, 2 μM rottlerin inhibits apoptosis and does not affect DR5 expression (T. W. Day and A. R. SaFa, unpublished results), whereas at a concentration of 10 μM, rottlerin induces DR5 expression and triggered apoptosis. These results indicate that rottlerin has diverse intracellular targets and that the concentration of rottlerin and the type of cancer being studied can dictate whether it functions to inhibit or promote apoptosis.
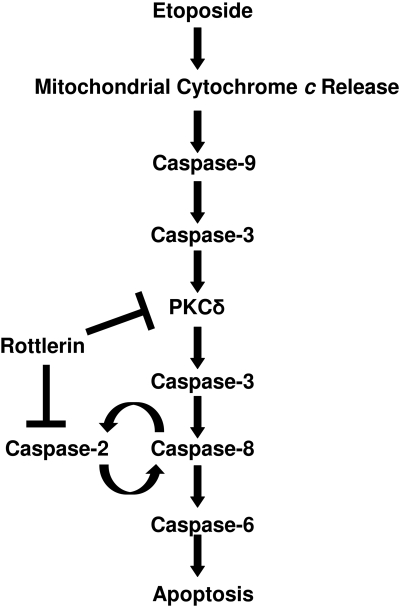
Based on the results from this study, we have proposed a model depicting how etoposide triggers apoptosis in SK-N-AS neuroblastoma cancer cells (Fig. 7). Etoposide induces the release of mitochondrial cytochrome c, leading to the activation of caspase-9. Caspase-9 triggers the activation of caspase-3, which triggers the caspase-3-dependent cleavage of PKCδ to its constitutively active p40 fragment, and active PKCδ triggers the processing of caspase-3 by a positive-feedback mechanism. The activation of caspase-3 leads to the activation of caspase-8. The expression of caspase-2 is required for the processing of caspase-8, and the expression of caspase-8 is required for the processing of caspase-2, indicating that these two caspases form a positive activation loop whereby they activate each other. The activation of caspase-8 triggers the processing of caspase-6 and apoptosis. Rottlerin inhibits etoposide-induced apoptosis by blocking the PKCδ-mediated activation of caspase-3 and by directly down-regulating caspase-2 expression, which prevents caspase-8 activation. The findings from this report have increased our understanding of how etoposide triggers apoptosis and identified key apoptotic proteins that may be targeted to augment etoposide's effectiveness in treating neuroblastomas.
Fig. 7.
Model depicting the etoposide-induced apoptotic signaling pathway in SK-N-AS cells. Etoposide induces the mitochondrial cytochrome c release, leading to the caspase-9-dependent activation of caspase-3. The activation of caspase-3 induces the cleavage of PKCδ, and active PKCδ processes caspase-3 by a positive-feedback mechanism. The activation of caspase-3 leads to the processing of caspase-8, and the expression of caspase-8 and caspase-2 is required for the activation of each other downstream of caspase-3. The etoposide-induced activation of caspase-8 leads to the processing of caspase-6 and apoptosis. Rottlerin inhibits etoposide-induced apoptotic signaling by preventing the PKCδ-mediated activation of caspase-3 and by causing the degradation of caspase-2, which inhibits caspase-8 activation.
This work was supported the National Institutes of Health National Cancer Institute [Grant R01-CA101743]; and the Indiana University Simon Cancer Center.
ABBREVIATIONS: FADD, Fas-associated death domain; PKCδ: protein kinase Cδ; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; PIDD, p53-induced protein with a death domain; siRNA, small interacting RNA; Gö6976, 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole; z-DEVD-fmk, N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone; z-LEHD-fmk, N-benzyloxycarbonyl-Leu-Glu-His-Asp-fluoromethyl ketone; z-VEID-fmk, benzyloxycarbonyl-Val-Glu(OMe)-Ile-Asp(OMe)-fluoromethyl ketone.
References
- Adrain C, Creagh EM, and Martin SJ (2001) Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20 6627-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A and Dixit VM (1998) Death receptors: signaling and modulation. Science 281 1305-1308. [DOI] [PubMed] [Google Scholar]
- Basu A, Adkins B, and Basu C (2008) Down-regulation of caspase-2 by rottlerin via protein kinase C-delta-independent pathway. Cancer Res 68 2795-2802. [DOI] [PubMed] [Google Scholar]
- Basu A, Woolard MD, and Johnson CL (2001) Involvement of protein kinase C-delta in DNA damage-induced apoptosis. Cell Death Differ 8 899-908. [DOI] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, and Brodie C (2002) Tyrosine phosphorylation of protein kinase Cdelta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol 22 182-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Bennett RL, and May WS (2008) c-Myc and caspase-2 are involved in activating Bax during cytotoxic drug-induced apoptosis. J Biol Chem 283 14490-14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM and Scorrano L (2006) The many shapes of mitochondrial death. Oncogene 25 4717-4724. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gong B, and Almasan A (2000) Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ 7 227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DM, Robichaud MR, Mader JS, Boudreau RT, Richardson AM, Giacomantonio CA, and Hoskin DW (2008) 2-Chloro-2′-deoxyadenosine-induced apoptosis in T leukemia cells is mediated via a caspase-3-dependent mitochondrial feedback amplification loop. Int J Oncol 32 1325-1333. [DOI] [PubMed] [Google Scholar]
- Debatin KM and Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23 2950-2966. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Silke J, Hawkins CJ, Verhagen AM, and Vaux DL (2001) DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9. J Cell Biol 152 483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekshyyan O and Aw TY (2004) Apoptosis: a key in neurodegenerative disorders. Curr Neurovasc Res 1 355-371. [DOI] [PubMed] [Google Scholar]
- Feng R, Ma H, Hassig CA, Payne JE, Smith ND, Mapara MY, Hager JH, and Lentzsch S (2008) KD5170, a novel mercaptoketone-based histone deacetylase inhibitor, exerts antimyeloma effects by DNA damage and mitochondrial signaling. Mol Cancer Ther 7 1494-1505. [DOI] [PubMed] [Google Scholar]
- Gogvadze V and Orrenius S (2006) Mitochondrial regulation of apoptotic cell death. Chem Biol Interact 163 4-14. [DOI] [PubMed] [Google Scholar]
- Green D and Kroemer G (1998) The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol 8 267-271. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, and Marks F (1994) Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199 93-98. [DOI] [PubMed] [Google Scholar]
- Gubina E, Rinaudo MS, Szallasi Z, Blumberg PM, and Mufson RA (1998) Overexpression of protein kinase C isoform epsilon but not delta in human interleukin-3-dependent cells suppresses apoptosis and induces bcl-2 expression. Blood 91 823-829. [PubMed] [Google Scholar]
- Jiang X and Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73 87-106. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH (1998) Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim Biophys Acta 1400 195-211. [DOI] [PubMed] [Google Scholar]
- Kim EH, Kim SU, and Choi KS (2005) Rottlerin sensitizes glioma cells to TRAIL-induced apoptosis by inhibition of Cdc2 and the subsequent downregulation of survivin and XIAP. Oncogene 24 838-849. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94 491-501. [DOI] [PubMed] [Google Scholar]
- Lim JH, Park JW, Choi KS, Park YB, and Kwon TK (2009) Rottlerin induces apoptosis via death receptor 5 (DR5) up-regulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis 30 729-736. [DOI] [PubMed] [Google Scholar]
- Lim JH, Park JW, Kim SH, Choi YH, Choi KS, and Kwon TK (2008) Rottlerin induces pro-apoptotic endoplasmic reticulum stress through the protein kinase C-delta-independent pathway in human colon cancer cells. Apoptosis 13 1378-1385. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, and Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86 147-157. [DOI] [PubMed] [Google Scholar]
- Lu W, Lee HK, Xiang C, Finniss S, and Brodie C (2007) The phosphorylation of tyrosine 332 is necessary for the caspase 3-dependent cleavage of PKCdelta and the regulation of cell apoptosis. Cell Signal 19 2165-2173. [DOI] [PubMed] [Google Scholar]
- Mackay HJ and Twelves CJ (2007) Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 7 554-562. [DOI] [PubMed] [Google Scholar]
- Mahoney JA and Rosen A (2005) Apoptosis and autoimmunity. Curr Opin Immunol 17 583-588. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, and Schächtele C (1993) Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem 268 9194-9197. [PubMed] [Google Scholar]
- Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308 693-698. [DOI] [PubMed] [Google Scholar]
- Panaretakis T, Laane E, Pokrovskaja K, Björklund AC, Moustakas A, Zhivotovsky B, Heyman M, Shoshan MC, and Grandér D (2005) Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell 16 3821-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, and Quissell DO (1999) Protein kinase C δ is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem 274 19115-19123. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Deng X, Carr BK, and May WS (1998) A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem 273 25436-25442. [DOI] [PubMed] [Google Scholar]
- Shamimi-Noori S, Yeow WS, Ziauddin MF, Xin H, Tran TL, Xie J, Loehfelm A, Patel P, Yang J, Schrump DS, et al. (2008) Cisplatin enhances the antitumor effect of tumor necrosis factor-related apoptosis-inducing ligand gene therapy via recruitment of the mitochondria-dependent death signaling pathway. Cancer Gene Ther 15 356-370. [DOI] [PubMed] [Google Scholar]
- Shin S, Lee Y, Kim W, Ko H, Choi H, and Kim K (2005) Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J 24 3532-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Längler A, Harnischmacher U, Frühwald MC, Jorch N, Claviez A, Berthold F, and Hero B (2007) Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma. Results of a phase-II trial. J Cancer Res Clin Oncol 133 653-661. [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, and Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276 7320-7326. [DOI] [PubMed] [Google Scholar]
- Slee EA, Keogh SA, and Martin SJ (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 7 556-565. [DOI] [PubMed] [Google Scholar]
- Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, and Walczak H (2000) FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12 599-609. [DOI] [PubMed] [Google Scholar]
- Tillman DM, Izeradjene K, Szucs KS, Douglas L, and Houghton JA (2003) Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res 63 5118-5125. [PubMed] [Google Scholar]
- Tinel A and Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304 843-846. [DOI] [PubMed] [Google Scholar]
- Toker A (1998) Signaling through protein kinase C. Front Biosci 3 D1134-D1147. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, and Berneman ZN (2005) Apoptosis: mechanisms and relevance in cancer. Ann Hematol 84 627-639. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Wieder T, Essmann F, Schulze-Osthoff K, Dörken B, and Daniel PT (2003) Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene 22 2236-2247. [DOI] [PubMed] [Google Scholar]
- Wang P, Song JH, Song DK, Zhang J, and Hao C (2006) Role of death receptor and mitochondrial pathways in conventional chemotherapy drug induction of apoptosis. Cell Signal 18 1528-1535. [DOI] [PubMed] [Google Scholar]
- Willingham MC (1999) Cytochemical methods for the detection of apoptosis. J Histochem Cytochem 47 1101-1110. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang P, Ming L, Wood MA, and Zhang L (2007) SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene 26 4189-4198. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B and Orrenius S (2005) Caspase-2 function in response to DNA damage. Biochem Biophys Res Commun 331 859-867. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, and Wang X (1999) An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274 11549-11556. [DOI] [PubMed] [Google Scholar]