Summary
Over the past few years it has become clear that over half of the mammalian heart derives from outside the heart field as originally defined. Such a second heart field, however, has not been described in zebrafish, which could explain its smaller, two-chambered heart. Instead, zebrafish have a population of haemangioblasts, which is absent in mammalian embryos, raising the possibility that these cells represent the evolutionary ancestor of the second heart field. Here, we show for the first time that the genetic programmes of these anterior haemangioblasts and the adjacent heart field are co-regulated, by transcription factors previously associated with heart but not blood or endothelial development. We demonstrate that gata4, gata5 and gata6 are essential for anterior haemangioblast specification, and for subsequent myelopoiesis, acting as early as cloche and upstream of scl. The requirement for gata4, gata5 and gata6 in myeloid, endothelial and cardiac specification is in the mesoderm, but these factors also control, from within the endoderm and the yolk syncytial layer, the migration of the cardiac precursors as they differentiate. This genetic link between the blood/endothelial and cardiac programmes supports the notion that this haemangioblast population in zebrafish is an evolutionary antecedent of the second heart field, and has implications for the differentiation of haemangioblasts and cardiomyocytes from pluripotent cells, and for the origins of stem cells in the adult heart.
Keywords: Myelopoiesis, Cardiogenesis, GATA factors, Second heart field, Haemangioblasts, Transcriptional regulation, Evolution, Adult stem cells, Zebrafish
INTRODUCTION
Of the several candidates for stem cells in the adult heart, only one, in addition to having the classical stem cell characteristics of multipotentiality, clonality and self renewal, has also been identified and traced in living embryos (Martin-Puig et al., 2008). This cell is identified on the basis of expression of the homeodomain transcription factor islet 1, and in the embryo these cells represent the second heart field. In mammals and the chick, the four-chambered heart is formed from two distinct regions of the embryo: the primary heart field, which gives rise to the left ventricle and contributes to the atria, and the second or anterior heart field, which gives rise to the right ventricle, the outflow tract and also the atria (reviewed by Laugwitz et al., 2008). Zebrafish have only two-chambered hearts and no second heart field has currently been detected. Instead, anterior to the primary heart field, zebrafish have a population of haemangioblasts not detected in chick or mammalian embryos.
The anterior lateral plate mesoderm (ALM) in zebrafish is a source of haematopoietic, endothelial and cardiogenic cells, with the blood and endothelium coming from the most rostral region and cardiac tissue deriving from the adjacent more posterior population (Fig. 1A). The blood/endothelial precursors in the ALM co-express genes that are later expressed in either blood or endothelium and have therefore been referred to as a putative haemangioblast population (Brown et al., 2000; Gering et al., 1998; Thompson et al., 1998). These ALM haemangioblasts have only a transient existence (between 5 and 10 somites), eventually giving rise to myeloid cells (macrophages and neutrophils), head endothelium and endocardium (Herbomel et al., 1999; Hsu et al., 2001; Roman and Weinstein, 2000), whereas the more posterior cardiac precursors differentiate into the muscle of the two-chambered heart (Stainier and Fishman, 1992; Stainier et al., 1993).
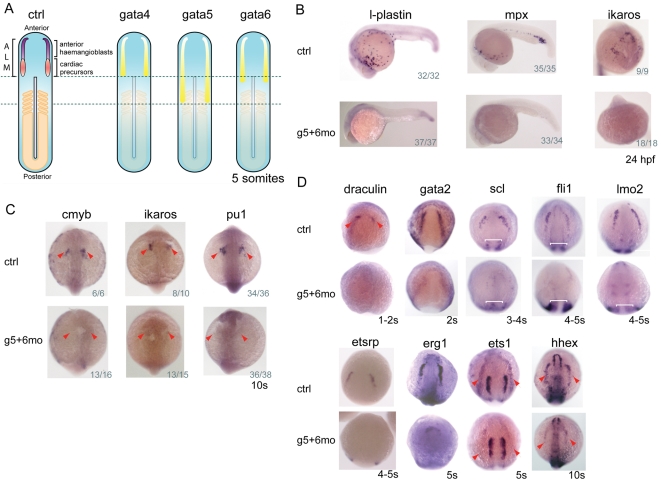
Fig. 1.
gata4, gata5 and gata6 are essential for anterior haemangioblast specification. (A) gata4, gata5 and gata6 are expressed in overlapping yet distinct domains throughout the anterior lateral plate mesoderm (ALM). (B) Expression of l-plastin, mpx and ikaros, genes associated with myelopoiesis, was absent in gata5+gata6 morphants at 24 hpf. (C) Analysis of earlier regulatory myeloid gene expression on knock down of gata5 and gata6 shows ablation of cmyb, ikaros and pu1 at 10 somites (red arrowheads). (B,C) The number of embryos exhibiting this phenotype shown at the bottom right-hand corner of each panel. (D) Expression of haemangioblast-associated genes close to the onset of their expression was downregulated in gata5+gata6 morphants (marked by red arrowheads where necessary). uncx4.1 was used as a somite marker to stage the embryos (white brackets). n=40-100 for each gene analysed, the pictures shown depict >95% of the embryos. Views are: (A) flatmounted, anterior to the top; (B) lateral, anterior to left, except for ikaros, which is an anterior view; (C) dorsal, anterior to the top; (D) anterior-dorsal.
Manipulation of anterior haemangioblast regulators suggests that this programme is antagonistic to the cardiac programme within the ALM (Gering et al., 2003; Schoenebeck et al., 2007). Thus, ectopic expression of blood and endothelial master regulators suppresses the cardiac programme, whereas knocking them down generates ectopic cardiomyocytes in the rostral haemangioblast territory. It is tempting to speculate therefore that this latent cardiac potential found in the anterior haemangioblast population may have been recruited by amniotes during evolution, generating the second heart field and a larger, more complex heart.
Although the anterior haemangioblast and cardiac progenitors express predominantly distinct sets of genes, a few are expressed in both territories; for example, gata4, gata5 and gata6 (Reifers et al., 2000; Reiter et al., 1999; Reiter et al., 2001; Schoenebeck et al., 2007) (this study). Clearly, if the anterior haemangioblast population is the evolutionary precursor of the second heart field, one would expect that they would be under common genetic control prior to the separation of the two programmes. Jointly expressed transcriptional regulators such as gata4, gata5 and gata6 are therefore candidates for such common genetic control. In vertebrates, GATA factors are traditionally described as belonging to two subfamilies: those predominantly expressed and functioning in haematopoiesis and ectodermal patterning (gata1, gata2 and gata3), and those playing a role in cardiac and endodermal formation (gata4, gata5 and gata6) (Molkentin, 2000; Patient and McGhee, 2002). In zebrafish, gata4, gata5 and gata6 have indeed already been shown to be required for normal cardiogenesis and the formation of heart precursors (Holtzinger and Evans, 2007; Peterkin et al., 2003; Peterkin et al., 2007). To determine whether the anterior haemangioblast population is under common genetic control with the heart field, we used morpholinos to knock down gata4, gata5 and gata6, and show for the first time that they are crucial for anterior haemangioblast formation and subsequent myelopoiesis. This requirement is within the mesoderm, although we also show that gata5 and gata6 are required in the yolk syncytial layer (YSL) and the endoderm for the correct migration of cardiac precursors. The ablation of both cardiac and haemangioblast programmes within the ALM suggests that these GATA factors lie at the top of a genetic cascade that is initially common to both of these two lineages. This is confirmed by the continued expression of gata4, gata5 and gata6 in scl morphants and cloche mutants, suggesting that these GATA factors lie upstream of, or parallel to, these well-described blood and endothelial regulatory factors. These data genetically link the anterior haemangioblast and cardiac fields, and are consistent with the former being the evolutionary ancestor of the latter.
MATERIALS AND METHODS
In situ hybridisation of zebrafish embryos
Wild-type and cloche [Clom39] (Stainier, 2001) zebrafish were bred, maintained, and embryos raised and staged using standard conditions (Westerfield, 1993). In situ hybridisations on zebrafish embryos were carried out as previously described (Jowett, 2001). All RNA probes used were labelled with digoxigenin (DIG) and detection of the antibody-alkaline phosphatase was performed using BM purple (Roche). EST clones for ets1 and erg1 were obtained from ImaGenes, clone ID 7051215 and 6966926, respectively. The following forward (5′-ATGATGGATAGCCGGATCCTCG-3′) and reverse (5′-GTCCATGTCTACATCCTCTCC-3′) primers were used to amplify uncx4.1 from cDNA using standard PCR protocols. The PCR fragment was cloned into the pGEM T-Easy vector (Promega) and confirmed by sequencing. To make an in situ hybridisation probe, uncx4.1 was linearised with Spe1 and transcribed with T7. Details of all other probes have been described previously (Patterson et al., 2005; Peterkin et al., 2003; Peterkin et al., 2007).
Morpholino and mRNA injections
The gata5 and gata6 antisense morpholinos (mo) were designed and manufactured by Gene Tools and sequences have been previously described (Peterkin et al., 2003; Peterkin et al., 2007). Combinatorial injections were mixed at a ratio of 1:5 gata6mo:gata5mo and titrated to an appropriate level to avoid non-toxic effects: between 1.25-1.50 ng of gata6mo and 6.25-8.50 ng gata5mo. The sequences of other morpholinos used have been described previously: scl splice mo (Patterson et al., 2005); pu1 mo (Rhodes et al., 2005); and casanova mo (Sakaguchi et al., 2001). scl, pu1 and cas morpholinos were titrated to around amounts previously described; final quantities of 6.5 ng, 15 ng and 10 ng, respectively, were used. YSL injections were performed at the 1000-cell stage using a standard fluorescent control morpholino (Gene Tools) as a lineage tracer. scl and lmo2 mRNAs were synthesised and injected as previously described (Gering et al., 2003), etsrp (etv2 - Zebrafish Information Network) mRNA was synthesised and injected as described (Pham et al., 2007).
RESULTS
gata4, gata5 and gata6 are required for the formation of the anterior haemangioblast
gata4, gata5 and gata6 are expressed in the ALM of zebrafish embryos during early somitogenesis, in the region that gives rise to primitive myeloid cells, head endothelium and endocardium (Herbomel et al., 1999; Reifers et al., 2000; Reiter et al., 1999; Reiter et al., 2001; Schoenebeck et al., 2007) (Fig. 1A, `anterior haemangioblasts'). Initially forming bilateral stripes either side of the embryo, the precursors migrate to a position just anterior to the heart cone as they differentiate, and the myeloid derivatives then disperse throughout the whole embryo, expressing markers such as l-plastin (lymphocyte cytosolic plastin 1 - Zebrafish Information Network) and mpx (myeloperoxidase) in monocytes/macrophages and granulocytes, respectively. To determine whether gata4, gata5 or gata6 play any role in myeloid development, we used morpholinos to combinatorially knock down gata5 and gata6. We have previously shown that these double morphants are essentially a triple knockdown, as gata4 is no longer expressed, which was reconfirmed here (see Fig. S1A in the supplementary material) (Peterkin et al., 2007). In gata5 and gata6 morphants at 24 hours post-fertilization (hpf), both l-plastin and mpx were severely downregulated (Fig. 1B). Loss of the transcription factor ikaros, associated with less-differentiated cells, was also seen in the head region of morphants (Fig. 1B), suggesting that the defect might be prior to terminal differentiation. Probing earlier for cmyb and ikaros expression confirmed this, as expression was absent in morphants close to the onset of their expression at 10 somites (Fig. 1C). Expression of the key myeloid regulator pu1 (spi1 - Zebrafish Information Network) was also ablated at this time (Fig. 1C). These data show that gata4, gata5 and gata6 are essential for the specification of myeloid cells during development.
Recent studies have identified both redundant and non-redundant contributions from gata4, gata5 and gata6 during development (Holtzinger and Evans, 2005; Holtzinger and Evans, 2007; Peterkin et al., 2007). To address this issue in the myeloid population, gata4, gata5 or gata6 morpholinos were injected individually. The expression of both l-plastin and mpx was much less severely downregulated in all three individual morphants than in morphants in which all three were lost together (compare Fig. S1B in the supplementary material with Fig. 1B), which suggests that their activities are additive. Therefore, for the rest of the experiments described here, gata5 and gata6 morpholinos were co-injected, creating a triple knockdown.
The loss of the earliest myeloid regulators at 10 somites led us to explore the extent to which the entire anterior haemangioblast programme is disrupted. draculin and gata2 are the first blood-associated genes to be expressed in the ALM between 1 and 2 somites (Patterson et al., 2005), and expression of both genes was substantially reduced in gata5 and gata6 morphants (Fig. 1D, red arrowheads). Likewise, expression of scl, fli1, lmo2 and etsrp, which are expressed in and required for haemangioblast formation, were severely downregulated (Gering et al., 1998; Liu et al., 2008; Patterson et al., 2007; Patterson et al., 2005; Sumanas and Lin, 2006) (Fig. 1D). Similar downregulation was observed for erg1, ets1 and hhex, whose later expression is in endothelial cells (Liao et al., 2000; Liu and Patient, 2008; Sumanas and Lin, 2006) (Fig. 1D, red arrowheads). By contrast, expression of all of these genes in the posterior lateral mesoderm (PLM), which gives rise to erythroid cells and the major vessels, was unaltered (see Fig. S2 in the supplementary material). Staging of these early embryos was confirmed by counting somite numbers after staining for uncx4.1 expression (Kawakami et al., 2005) (Fig. 1D, white brackets; data not shown). These data demonstrate a very early role for gata4, gata5 and gata6 in the specification of anterior haemangioblasts.
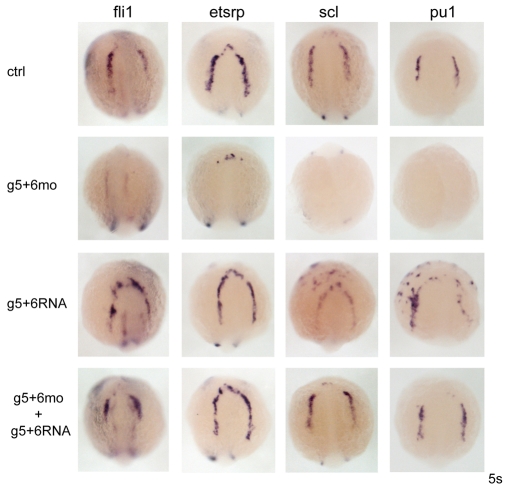
Rescue experiments were carried out to validate morpholino specificity. The severe downregulation of fli1, etsrp, scl and pu1 seen in morphants was rescued by the injection of gata5 and gata6 mRNA (Fig. 2). Overexpression studies of GATA factors have proven to be difficult because of their strong phenotypes (Haworth et al., 2008; Weber et al., 2000); however, low levels of gata5 and gata6 (25 pg) injected into wild-type embryos produced relatively normal embryos morphologically, and the blood-associated genes pu1 and scl were ectopically expressed (Fig. 2). By contrast, very little if any ectopic or increased expression of vascular genes was observed (Fig. 2). A few ectopic patches of cells expressing fli1 were detected, whereas etsrp expression was seen only within the normal bilateral ALM stripes. Thus, although gata5 and gata6 are necessary for all haemangioblast gene expression in the ALM, they are sufficient only for myeloid and not vascular gene expression.
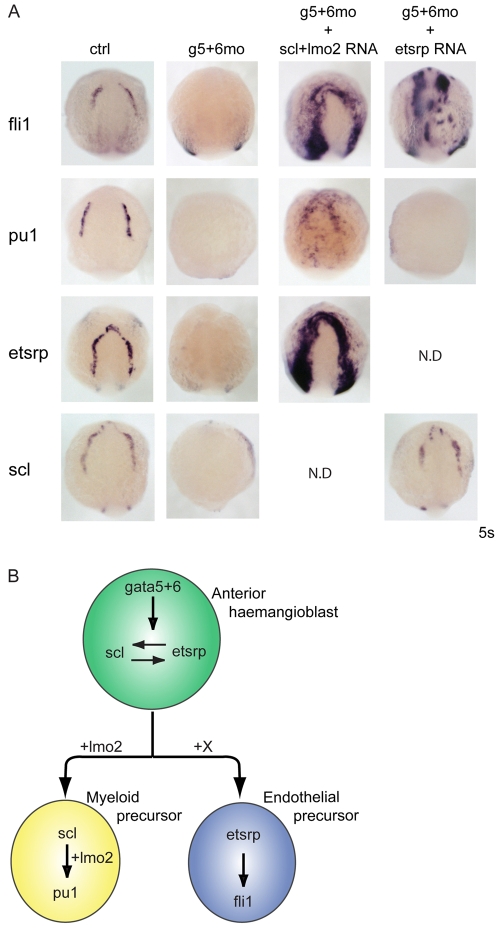
Fig. 2.
gata5 and gata6 rescue and gain-of-function experiments confirm necessity and identify sufficiency for myelopoiesis. Injection of exogenous gata5 and gata6 mRNA (g5+6RNA) into wild-type embryos did not induce fli1 or etsrp, whereas scl and pu1 were ectopically expressed. Rescue of all four genes was seen in gata5 and gata6 morphants co-injected with gata5 and gata6 mRNA (g5+6mo + g5+6RNA) when compared with gata5 and gata6 morphants alone (g5+6mo). Views are anterior-dorsal.
gata5 and gata6 play a migratory role in the YSL
gata5 and gata6 are expressed in the endoderm and the yolk syncytial layer (YSL), as well as in the ALM, in zebrafish embryos (Kikuchi et al., 2001; Reiter et al., 1999; Rodaway et al., 1999) (A.G., T.P. and R.P., unpublished). The YSL appears to be crucial for migration of cardiac precursors to the midline, with YSL defects giving rise to cardia bifida (Sakaguchi et al., 2006). Cardia bifida is also seen in gata5 and gata6 morphants (Peterkin et al., 2007), suggesting that these factors may be acting in the YSL. We therefore wished to determine whether the activity affecting anterior haemangioblast programming is located in the YSL or the ALM. To deplete gata5 and gata6 in the YSL, 1000-cell stage embryos were injected into the YSL with gata5 and gata6 morpholinos, along with a fluorescent morpholino as a tracer. At this stage of development, the YSL gap junctions are complete and no longer open to the embryo itself (Chen and Kimelman, 2000; Kimmel and Law, 1985). Embryos fluorescent only in the YSL were selected at two different stages as embryos lacking gata5 and gata6 in the YSL alone (Fig. 3A, +YSL). Embryos with no fluorescence (-YSL) were collected as injection controls, expressing gata5 and gata6 at wild-type levels. To confirm the efficiency of the morpholinos, a batch of embryos was injected at the one-cell stage, giving rise to fluorescence, and therefore gata5 and gata6 inhibition, throughout the embryo (+embryo). Cardiac and myeloid gene expression was monitored.
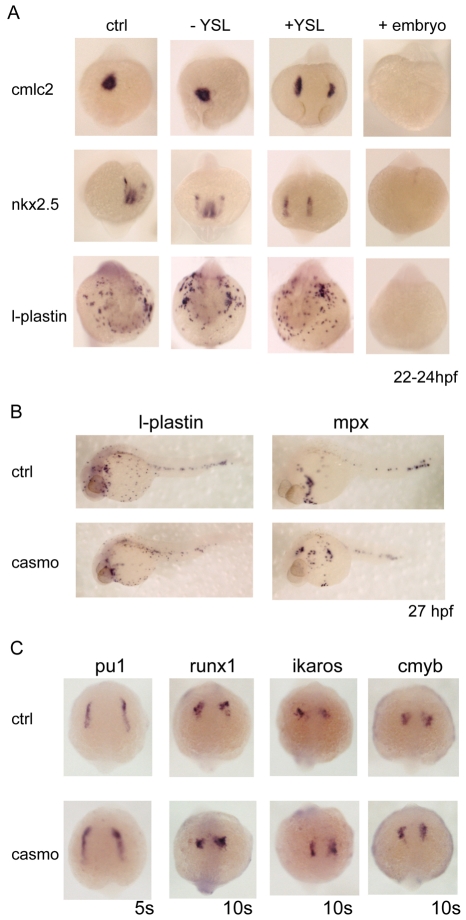
Fig. 3.
Depletion of gata5 and gata6 from the YSL causes cardia bifida but does not affect myelopoiesis. (A) The YSL expression of gata5 and gata6 was depleted by injection of gata5+gata6 morpholinos into the YSL at the 1000-cell stage. To trace the correctly targeted embryos, a fluorescent control morpholino was co-injected. Embryos positive for fluorescence in the YSL alone (+YSL) were selected at several time points and harvested as YSL gata5+gata6-depleted embryos. The embryos showing no fluorescence (-YSL) were collected as negative injection controls and should express wild-type levels of gata5 and gata6. To ensure the efficiency of the morpholinos, positive control embryos were injected with gata5+gata6 morpholinos at the one-cell stage and were fluorescent throughout the embryo (+embryos). To assess heart formation, cmlc2 and nkx2.5 expression was analysed. Depletion of gata5 and gata6 in the YSL (+YSL) showed normal levels of expression of both cmlc2 and nkx2.5, indicating that specification occurs normally in these embryos. However, cardia bifida was observed in the +YSL embryos, demonstrating that gata5 and gata6 are required in the YSL for the correct migration of the cardiac precursors to the midline. By contrast, l-plastin expression was the same as in the wild-type embryos, indicating that gata5 and gata6 expression in the YSL is dispensable for myelopoiesis. Embryos injected at the one-cell stage (+embryos) showed complete absence of cmlc2, nkx2.5 and l-plastin expression, thereby validating morpholino effectiveness. Views are anterior. For each gene and type (±YSL, +embryo) analysed, n=28-38, and the images shown depict the findings for >95% of the embryos. (B,C) Loss of endoderm in casanova morphant embryos but no defects in myelopoiesis. To establish whether endoderm plays a role in myelopoiesis, endodermless embryos were created by injection of casanova morpholinos. Casanova morphants (casmo) were assessed for endoderm formation and myelopoiesis. Myelopoiesis was not affected in cas morphants as l-plastin and mpx remained unaffected (B). Gene expression in the ALM of cas morphants was examined at 5 and 10 somites (C). The formation of myeloid precursors occurred as normal in cas morphants. Expression of pu1 at 5 somites, and runx1, ikaros and cmyb at 10 somites, was unaffected in cas morphants. Views are anterior-dorsal. For each gene analysed n=38-67, and the images shown depict the findings for >95% of the embryos.
As expected, the levels of expression of the cardiac markers cmlc2 and nkx2.5 were normal in the injection controls (-YSL), as were the locations of the expressing cells (Fig. 3A). However, in the embryos in which gata5 and gata6 were absent in the YSL alone (+YSL), 100% of the embryos showed cardia bifida, although the levels of expression remained normal (Fig. 3A). In the embryos used as a control for morpholino efficiency (+embryo), cmlc2 and nkx2.5 expression was absent, as reported previously (Peterkin et al., 2007). Importantly though, the expression of the myeloid marker l-plastin also remained unchanged in + and -YSL embryos, but was ablated when gata5 and gata6 MOs were injected throughout the embryo (+embryo; see Fig. 3A). Thus, gata5 and gata6 do not appear to be required in the YSL for myelopoiesis, but rather in the embryo proper. These experiments also show that, whilst expression of gata5 and gata6 is required in the YSL for cardiac migration, it is not required there for heart specification or differentiation.
gata5 and gata6 play a migratory role in the endoderm
In zebrafish, endoderm is not thought to be required for the specification and differentiation of heart precursors, but it is required for the correct migration of cardiac progenitors to the midline (Alexander et al., 1999; Kikuchi et al., 2001; Schier et al., 1997; Trinh and Stainier, 2004). However, its role in myeloid development has not been assessed, and therefore the role of gata4, gata5 and gata6 in the embryo proper (shown above) could in principle be in the endoderm. In casanova (cas) mutants, which have no endoderm, gata4, gata5 and gata6 expression is still observed in the mesoderm (Alexander et al., 1999; Kikuchi et al., 2001), thus the roles of gata4, gata5 and gata6 in the endoderm and mesoderm should be distinguishable. When wild-type embryos were injected with a cas morpholino, endodermal gata4, gata5 and gata6 expression was lost at 27 hpf, as has been described for cas mutants (Alexander et al., 1999) (see Fig. S3A in the supplementary material, red arrowheads), whereas the expression of these GATA factors in the cardiac mesoderm was maintained, albeit in a pattern similar to that seen in cardia bifida, as is seen for cmlc2 (see Fig. S3A in the supplementary material, black arrowheads). Importantly, expression of l-plastin and mpx at this time was not altered, suggesting that endoderm, and therefore gata4, gata5 and gata6 expression within the endoderm, is not required for myelopoiesis at 27 hpf (Fig. 3B).
Expression of gata4, gata5 and gata6 in the ALM at 5 somites was unaffected in cas morphants (see Fig. S3B in the supplementary material). Furthermore, expression of pu1 at 5 somites and runx1, ikaros and cmyb at 10 somites was also unaffected (Fig. 3C). As a control for morpholino activity (Dickmeis et al., 2001), expression of the endodermal marker sox17 was lost by 50-90% epiboly (data not shown). We therefore conclude that gata4, gata5 and gata6 are required in the mesoderm for anterior haemangioblast, as well as cardiac, specification, whereas gata5 and gata6 are required in the endoderm and the YSL only for the migration of cardiac precursors.
Epistatic relationships between gata4, gata5 and gata6 and haemangioblast genes
Having established that the anterior haemangioblast requirement for gata4, gata5 and gata6 is in the mesoderm itself, we wanted to determine their position in the genetic hierarchy. pu1 expression is lost in gata5 and gata6 morphants but, although this could explain the absence of myelopoiesis, a role for pu1 in haemangioblast formation or endothelial development has not been established (Rhodes et al., 2005; Su et al., 2007). However, analysis of pu1 morphants showed that pu1 is required only for myeloid development and not at all for the endothelial programme (see Fig. S4B,C in the supplementary material). We conclude that the loss of pu1 in gata5 and gata6 morphants (Fig. 1C) and the continued expression of gata4, gata5 and gata6 in pu1 morphants (see Fig. S4B in the supplementary material), together with the more widespread defects observed in haemangioblast formation in gata5 and gata6 morphants, places gata5 and gata6 upstream of pu1. We also note that, even though pu1 was absent in the ALM of gata5 and gata6 morphants, ectopic globin and gata1 was never seen there, in contrast to pu1 morphants (Fig. S4B in the supplementary material; data not shown) (Rhodes et al., 2005). Thus, in the absence of gata4, gata5 and gata6, the ALM is unable to form either myeloid or erythroid blood, which is consistent with a position higher up the hierarchy than pu 1.
scl has been implicated in haemangioblast formation in both the ALM and the PLM of zebrafish embryos (Bussmann et al., 2007; Gering et al., 2003; Patterson et al., 2005). In the ALM, expression of the blood genes pu1, cmyb, runx1 and ikaros is disrupted, along with expression of the endothelial genes flt4 and hhex, when scl is lost, placing it towards the top of the anterior haemangioblast hierarchy (Patterson et al., 2005). To establish the relationship between scl and gata4, gata5 and gata6, the expression of GATA factors was investigated in scl morphants. Embryos were injected with the scl morpholinos previously described (Patterson et al., 2005). Continued expression of gata4, gata5 and gata6 was seen in scl morphants from 7 somites (Fig. 4A) to around 15 somites (data not shown). As a control for morpholino functionality, pu1 was lost in scl morphants, as has been shown previously (Patterson et al., 2005) (Fig. 4A). Thus, as scl is lost in GATA morphants (Fig. 1D), and gata4, gata5 and gata6 expression is maintained in scl morphants (Fig. 4A), gata4, gata5 and gata6 emerge as upstream regulators of scl in the ALM.
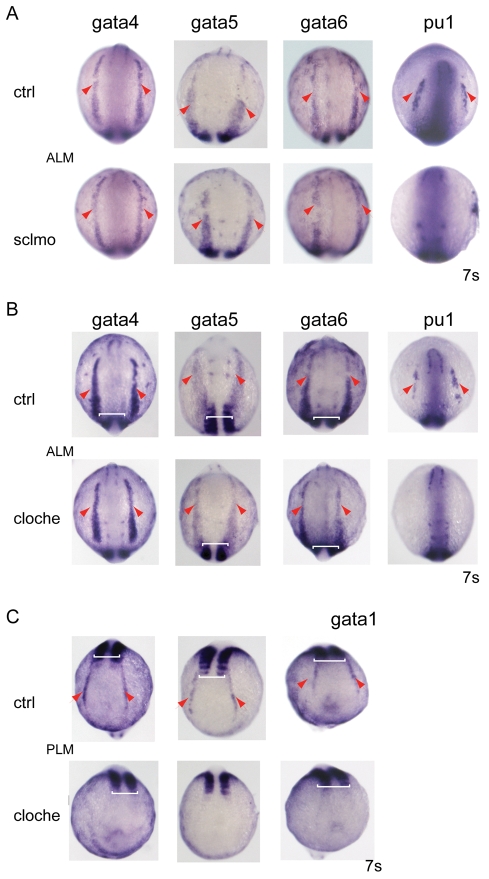
Fig. 4.
Initiation of gata4, gata5 and gata6 expression is independent of scl and cloche. (A) Expression of the GATA factors was analysed in scl morphants. gata4, gata5 and gata6 were expressed as in the wild-type embryos in the ALM (red arrowheads), placing them upstream of scl. Loss of pu1 was used as a control for scl morpholino efficacy. (B) The expression of gata4, gata5 and gata6 in the ALM was unchanged (red arrowheads) in cloche embryos placing the GATA factors parallel to or upstream of cloche. (C) cloche embryos were identified by the absence of gata1 expression (red arrowheads) in the PLM. Pu1 downregulation was an additional control (B). The expression of uncx4.1 was used as a somitic counter to ensure correct staging of the embryos (white brackets). Views are anterior-dorsal. For each gene analysed, n=28-42, and the images shown depict the findings for >95% of the embryos identified as cloche and scl morphant embryos.
cloche mutants lack vascular and haematopoietic tissues, including myeloid cells (Liao et al., 1997; Rhodes et al., 2005; Thompson et al., 1998), and gata4, gata5 and gata6 lie upstream of scl and pu1, both of which can partially rescue myelopoeisis in cloche embryos (Liao et al., 1997; Rhodes et al., 2005). Determining the expression of cloche has not been possible, because of uncertain identification and low expression of the only candidate (Xiong et al., 2008), so we could not monitor cloche expression in gata5 and gata6 morphants. We therefore assessed the expression of gata4, gata5 and gata6 in cloche embryos. To control for both the number of somites and to identify cloche embryos from their wild-type siblings, triple in situ hybridisation was performed (Fig. 4B,C). gata4, gata5 and gata6 expression was monitored in the ALM (red arrowheads), and in the same embryos uncx4.1 staining of early somites was used as a timing control (white brackets), while the loss of gata1 expression in the PLM was used to identify cloche embryos (Stainier et al., 1995). Expression levels of all three GATA factors remained unchanged in cloche embryos (Fig. 4B), even though pu1 expression in the ALM was lost, as shown previously (Lieschke et al., 2002). Thus, gata4, gata5 and gata6 lie upstream or parallel to cloche in the ALM.
To confirm the hierarchical relationships between gata4, gata5 and gata6 and the other haemangioblast-associated genes, we tried to rescue myeloid and vascular gene expression in gata5 and gata6 morphants. Previous studies have shown that co-injection of scl and lmo2 can strongly induce ectopic haemangioblast gene expression, although myeloid gene expression was not expanded and the cells appeared disorganised (Gering et al., 2003). By contrast, etsrp overexpression induced ectopic vasculogenesis and myelopoiesis (Sumanas et al., 2008). Overexpression of scl/lmo2 or estrp mRNAs in wild-type embryos behaved as described above (data not shown). However, injection of scl/lmo2 mRNAs into gata5 and gata6 morphants, while still able to strongly induce fli1 (as in wild-type embryos), was also able to rescue pu1 expression, albeit in a disorganised fashion (Fig. 5A). By contrast, although etsrp was able to induce fli1 expression in the gata5 and gata6 morphants, it could not rescue pu1 expression (Fig. 5A). Together with the gata5 and gata6 overexpression data above, and with recent evidence that etsrp is required for the myeloid programme (Liu and Patient, 2008; Sumanas et al., 2008), these data confirm differential roles for gata5 and gata6 and etsrp in endothelial versus myeloid development (Fig. 5B). gata5 and gata6 are necessary and sufficient for at least pu1 expression, probably via the induction of scl, whereas they are necessary but not sufficient for etsrp and fli1 expression, suggesting that an additional factor is required (X in Fig. 5B). The failure of etsrp to rescue pu1 expression while inducing fli1 expression in gata5 and gata6 morphants, suggests that etsrp is dependent on gata5 and gata6 for its downstream activity in myeloid but not endothelial development. Similarly, the ability of gata5 and gata6 to induce other endothelial markers may depend on etsrp.
Fig. 5.
Interaction of gata5 and gata6 with scl/lmo2 and etsrp. (A) Scl/lmo2 mRNA can rescue both blood (pu1), and endothelium (fli1 and etsrp) development in gata5 and gata6 morphants at the 5 somite stage. By contrast, etsrp overexpression can rescue fli1 but not pu1 expression in gata5 and gata6 morphants. All views are anterior. N.D., not determined. (B) Proposed model for gata5 and gata6 function within the anterior haemangioblast. gata5 and gata6 are required for etsrp and scl expression in the haemangioblast. gata5 and gata6 overexpression can induce scl and pu1 expression, which drives the myeloid lineage, whereas endothelium cannot be induced by gata5 and gata6 mRNA (see Fig. 2). Furthermore, the head vasculature eventually recovers (see Fig. S4A in the supplementary material), suggesting the existence of an unknown GATA-independent signal (X).
The fate of gata4-, gata5- and gata6-depleted mesoderm
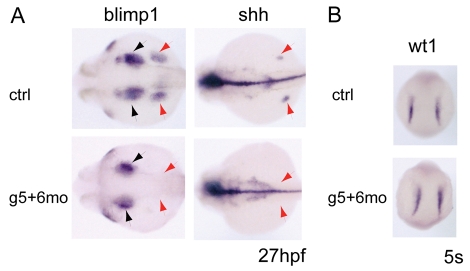
The data presented here and in previous reports show that gata5 and gata6 are required for the specification of the cardiac and haemangioblast programmes in the ALM (Peterkin et al., 2003; Peterkin et al., 2007; Reiter et al., 1999). What happens to these cells in the absence of gata4, gata5 and gata6? We showed previously that expression of nkx2.7 is unaffected in the ALM at 5 somites when haemangioblast- and cardiac-associated genes are already lost in gata5 and gata6 morphants, and that no increase in apoptosis was observed at 10 somites (Peterkin et al., 2007). From these data we can conclude that mesodermal cells are still present and have correctly migrated during gastrulation, but that they are unable to differentiate into either the cardiac or haemangioblast lineages. Additional mesodermal markers expressed in the ALM in the absence of the haemangioblast and cardiac programmes are not currently available, so we monitored adjacent tissues to determine whether they are expanded in the absence of gata5 and gata6. Fin buds are one candidate; however, they are dependent on tbx5 (Ahn et al., 2002; Garrity et al., 2002) and tbx5 is absent in gata5 and gata6 morphants at 10 somites (Peterkin et al., 2007). Consistent with this, a loss of fin buds was evident at 27 hpf, as revealed by the loss of blimp and shh expression (Fig. 6A, red arrows). blimp expression in the pharyngeal endoderm was slightly reduced but still present (Fig. 6A, black arrows). wt1 is expressed in the pronephros just posterior to the heart field (Drummond et al., 1998; Serluca and Fishman, 2001), and this expression was unaffected in gata5 and gata6 morphants (Fig. 6B). Thus, in the absence of gata5 and gata6, the haemangioblast, cardiac and fin bud programmes are not induced, but adjacent tissues are not expanded and mesodermal cells expressing nkx2.7 are still present in the ALM.
Fig. 6.
Fate of ALM cells in gata5 and gata6 morphants. (A) Fin buds are lost in gata5 and gata6 morphants, as seen by the loss of blimp1 and shh expression (red arrows). Pharyngeal endoderm is slightly reduced in morphants (blimp1, black arrows) compared with wild type. (B) Pronephric mesoderm (wt1 expression) remains unchanged in gata5 and gata6 morphants at 5 somites.
What happens to the nkx2.7-expressing cells? Expression of myeloid genes such as runx1, ikaros, draculin, l-plastin and mpx in ALM-derived cells was never seen in gata5 and gata6 morphants (Fig. 1; data not shown), whereas endothelial differentiation appeared to have recovered completely by 22 hpf (see Fig. S4A in the supplementary material). This recovery is consistent with previous data (Holtzinger and Evans, 2007), which have shown that expression of lmo2 appeared normal in gata5 and gata6 morphants around 12 somites, a time at which we saw recovery of expression of several endothelial genes, including lmo2. Thus, it appears that some of the ALM cells in morphants are still able to contribute to head vasculature.
DISCUSSION
gata4, gata5 and gata6 lie at the hierarchical apex of blood and cardiac specification
Data presented here demonstrate for the first time a crucial role for gata4, gata5 and gata6 in haemangioblast as well as cardiac precursor specification. Although Gata4 and Gata6 have been detected in mouse embryos in and around the blood islands and the allantois, another tissue associated with blood and endothelial development, there has hitherto been no evidence for a cell-autonomous role (Bielinska et al., 1996; Caprioli et al., 2001; Dumon et al., 2006; Nemer and Nemer, 2003). Gata5 expression has been observed to increase in embryonic stem (ES) cells differentiated towards a haematopoietic fate, but no functional consequences have been demonstrated (Baird et al., 2001). Here, we show for the first time that expression of these `cardiac' GATA factors is required in the lateral mesoderm for the development of myeloid lineages and the initial programming of their associated endothelial cells.
The presence of gata4, gata5 and gata6 in the endoderm and YSL, as well as in the cardiac and haemangioblastic mesoderm, raised issues concerning the cell autonomy of the role described here. We demonstrate that gata5 and gata6 are indeed crucial in both the YSL and the endoderm for migration of cardiac precursors to the midline, but that they are dispensable there for the specification of both the heart tissue and the haemangioblasts. Thus, the requirement for the GATA factors in specification must reside within the mesoderm. Precedent for inductive interactions between endoderm and blood/endothelial programming comes from experiments performed in mouse and chick (Belaoussoff et al., 1998; Bielinska et al., 1996; Kessel and Fabian, 1987). By contrast, we found that loss of endoderm in casanova morphants, including loss of gata4, gata5 and gata6 expression there, had no effect on haemangioblast specification or myelopoiesis. Differences between zebrafish and mouse/chick may in part reflect the different origins of the endoderm: whereas in chick and mouse, yolk sac haematopoiesis and vasculogenesis occur adjacent to the visceral endoderm, in zebrafish the ALM is adjacent to the definitive endoderm.
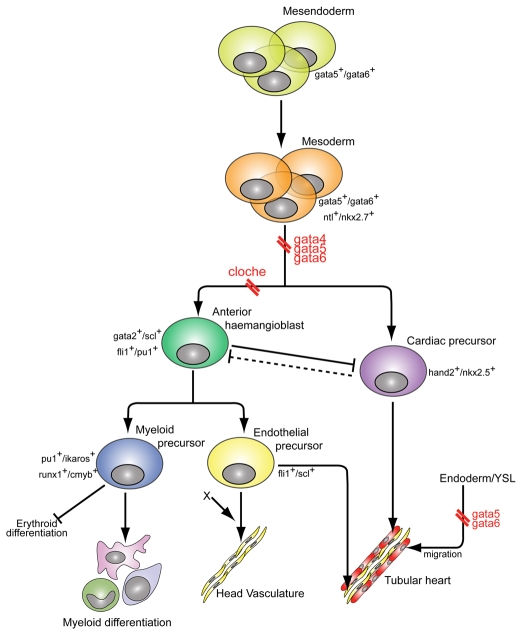
The mutated gene in the zebrafish cloche mutant is thought to be close to the hierarchical apex of blood and endothelial development (Liao et al., 1997; Rhodes et al., 2005; Thompson et al., 1998). Consistent with this notion, the mouse homologue of lycat, a gene cloned from the cloche genetic interval, is essential for blood and endothelial specification in ES cells (Wang et al., 2007). We have now shown that gata4, gata5 and gata6 are required for both haemangioblast and cardiac specification, and that expression of gata4, gata5 and gata6 is unaffected in cloche mutants, placing these GATA genes not only upstream of or parallel to cloche/lycat in the haemangioblast lineage, but also at the apex of the genetic hierarchy common to both the cardiac and haemangioblast programmes (Fig. 7). Based on their early expression patterns (Rodaway and Patient, 2001), we hypothesise that gata5 and gata6 are required very early in the mesendoderm, allowing it to respond to both blood- and cardiac-inducing signals.
Fig. 7.
Cellular hierarchy of anterior haemangioblast and heart formation. gata4, gata5 and gata6 are required for the specification of the anterior haemangioblast and cardiac precursors from the anterior lateral plate mesoderm (double red break). gata4, gata5 and gata6 lie at the apex of the hierarchy, above cloche (double red break) and scl. Previous studies show that loss of the haemangioblast programme causes the expansion of cardiac progenitors (Schoenebeck et al., 2007), suggesting an antagonistic relationship between these two programmes (barred solid and dashed lines). Whether the cardiac precursors can antagonise the anterior haemangioblast population has yet to be demonstrated (dashed line). Data presented here also show a requirement for gata5 and gata6 in the endoderm and the YSL for the correct migration of cardiac precursors towards the midline.
Although the requirement for gata4, gata5 and gata6 for blood development from the anterior haemangioblast is absolute, there appears to be a GATA-independent pathway that is able to rescue endothelial development after GATA expression has ceased. Thus, whereas expression of the myeloid genes in the anterior (pu1, runx1, cmyb, l-plastin and mpx) was never seen in gata5 and gata6 morphants, expression of genes associated with endothelial development (such as fli1, etsrp1, ets1 and hhex), along with haemangioblast genes (scl and lmo2), in their later head endothelial mode, began to recover from around 12 somites and appeared normal by 15 somites, resulting in an apparently normal circulatory system (see Fig. S4A in the supplementary material; data not shown). Thus, it appears that a recovery pathway is available for endothelial development from the ALM but not for blood (Fig. 5B, labelled X). As cloche embryos show a severe downregulation of endothelium throughout development, it is likely that the recovery of head endothelium in GATA morphants is cloche dependent. Thus, even though gata5 and gata6 are required together with cloche for the initiation of the haemangioblast programme, they are apparently not required for maintenance of the endothelial programme in the haemangioblast derivatives.
gata4, gata5 and gata6 and the origins of the second heart field and cardiac stem cells
Attractive candidate stem cells in the adult mouse heart are the islet 1-positive population also found in developing embryos (reviewed by Laugwitz et al., 2008). These cells constitute the second heart field in the mammalian embryo. Although the absence of a second heart field in zebrafish embryos may explain the smaller two-chambered heart produced, the presence of a haemangioblast population anterior to the primary heart field, and its absence in mouse embryos, raises the possibility that this haemangioblast population represents the evolutionary precursor of the mammalian second heart field. Consistent with such a notion, we have found that the two populations do indeed have common genetic control, depending absolutely in both cases on gata4, gata5 and gata6. Interestingly, using the marker islet-1, a second heart field has recently been reported for the amphibian Xenopus laevis (Brade et al., 2007), which has a three-chambered heart and an anterior haemangioblast population (Walmsley et al., 2002), raising the possibility that Amphibia represent an intermediate evolutionary state between fish and amniotes.
Recently, cardiac gene expression has been detected in the anterior haemangioblast population in cloche embryos and in scl/etsrp morphants, suggesting that, normally, the blood/endothelial programme there might be responsible for the suppression of the cardiac programme (Schoenebeck et al., 2007). Consistent with this, overexpression of scl, with either etsrp or lmo2, ablates heart formation (Gering et al., 2003; Schoenebeck et al., 2007). These observations suggest that the blood and cardiac programmes in the ALM are antagonistic. It will be interesting to determine how this antagonism was resolved in favour of the cardiac programme during evolution.
Identifying and characterising stem cells in the adult heart is likely to have implications for treatment of heart disease. Islet 1-positive cells have several of the necessary credentials and are thought to derive from the second heart field. Clearly a better understanding of their genetic programme will facilitate their future manipulation and also their derivation from pluripotent cells. Consistent with the common genetic programme indicated here, cardiomyocytes have recently been obtained from ES cell derivatives expressing the VEGF receptor, flk-1, which is classically associated with haemangioblast development (Kattman et al., 2006). Interestingly, haemangioblasts differentiate from ES cells before cardiomyocytes and in less time than it takes to make haemangioblasts in a mouse embryo (Huber et al., 2004). In zebrafish and more obviously Xenopus embryos, the anterior haemangioblast programme develops earlier than the posterior blood and endothelial programme (Walmsley et al., 2008), raising the intriguing possibility that the haemangioblasts derived from mouse ES cells are expressing the ancestral programme.
Taken together, our work identifies a close genetic relationship between anterior haemangioblasts and cardiac precursors in zebrafish. We reveal a co-dependence of these populations on gata4, gata5 and gata6, placing these genes at the apex of the genetic regulatory hierarchy specifying these two anterior lateral mesoderm derivatives. This common genetic control is consistent with the postulated conversion of one to the other during evolution.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/9/1465/DC1
Supplementary Material
Acknowledgments
We would like to thank Shuo Lin for probes, Didier Stainier for the cloche mutants and Brant Weinstein for the etsrp construct. EST clones were obtained from ImaGenes. We are grateful to the staff of the Biomedical Services Unit for aquatic support. We appreciate the helpful comments from Maggie Walmsley, Filipa Simoes and the reviewers, and thank Philip Pinheiro for his help.
This work was supported by the British Heart Foundation and MRC. Deposited in PMC for release after 6 months.
References
- Ahn, D. G., Kourakis, M. J., Rohde, L. A., Silver, L. M. and Ho, R. K. (2002). T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature 417, 754-758. [DOI] [PubMed] [Google Scholar]
- Alexander, J., Rothenberg, M., Henry, G. L. and Stainier, D. Y. (1999). casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343-357. [DOI] [PubMed] [Google Scholar]
- Baird, J. W., Ryan, K. M., Hayes, I., Hampson, L., Heyworth, C. M., Clark, A., Wootton, M., Ansell, J. D., Menzel, U., Hole, N. et al. (2001). Differentiating embryonal stem cells are a rich source of haemopoietic gene products and suggest erythroid preconditioning of primitive haemopoietic stem cells. J. Biol. Chem. 276, 9189-9198. [DOI] [PubMed] [Google Scholar]
- Belaoussoff, M., Farrington, S. M. and Baron, M. H. (1998). Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development 125, 5009-5018. [DOI] [PubMed] [Google Scholar]
- Bielinska, M., Narita, N., Heikinheimo, M., Porter, S. B. and Wilson, D. B. (1996). Erythropoiesis and vasculogenesis in embryoid bodies lacking visceral yolk sac endoderm. Blood 88, 3720-3730. [PubMed] [Google Scholar]
- Brade, T., Gessert, S., Kuhl, M. and Pandur, P. (2007). The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev. Biol. 311, 297-310. [DOI] [PubMed] [Google Scholar]
- Brown, L. A., Rodaway, A. R., Schilling, T. F., Jowett, T., Ingham, P. W., Patient, R. K. and Sharrocks, A. D. (2000). Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech. Dev. 90, 237-252. [DOI] [PubMed] [Google Scholar]
- Bussmann, J., Bakkers, J. and Schulte-Merker, S. (2007). Early endocardial morphogenesis requires Scl/Tal1. PLoS Genet. 3, e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli, A., Minko, K., Drevon, C., Eichmann, A., Dieterlen-Lievre, F. and Jaffredo, T. (2001). Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev. Biol. 238, 64-78. [DOI] [PubMed] [Google Scholar]
- Chen, S. and Kimelman, D. (2000). The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development 127, 4681-4689. [DOI] [PubMed] [Google Scholar]
- Dickmeis, T., Mourrain, P., Saint-Etienne, L., Fischer, N., Aanstad, P., Clark, M., Strahle, U. and Rosa, F. (2001). A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 15, 1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, I. A., Majumdar, A., Hentschel, H., Elger, M., Solnica-Krezel, L., Schier, A. F., Neuhauss, S. C., Stemple, D. L., Zwartkruis, F., Rangini, Z. et al. (1998). Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125, 4655-4667. [DOI] [PubMed] [Google Scholar]
- Dumon, S., Heath, V. L., Tomlinson, M. G., Gottgens, B. and Frampton, J. (2006). Differentiation of murine committed megakaryocytic progenitors isolated by a novel strategy reveals the complexity of GATA and Ets factor involvement in megakaryocytopoiesis and an unexpected potential role for GATA-6. Exp. Hematol. 34, 654-663. [DOI] [PubMed] [Google Scholar]
- Garrity, D. M., Childs, S. and Fishman, M. C. (2002). The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129, 4635-4645. [DOI] [PubMed] [Google Scholar]
- Gering, M., Rodaway, A. R., Gottgens, B., Patient, R. K. and Green, A. R. (1998). The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 17, 4029-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering, M., Yamada, Y., Rabbitts, T. H. and Patient, R. K. (2003). Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development 130, 6187-6199. [DOI] [PubMed] [Google Scholar]
- Haworth, K. E., Kotecha, S., Mohun, T. J. and Latinkic, B. V. (2008). GATA4 and GATA5 are essential for heart and liver development in Xenopus embryos. BMC Dev. Biol. 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel, P., Thisse, B. and Thisse, C. (1999). Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126, 3735-3745. [DOI] [PubMed] [Google Scholar]
- Holtzinger, A. and Evans, T. (2005). Gata4 regulates the formation of multiple organs. Development 132, 4005-4014. [DOI] [PubMed] [Google Scholar]
- Holtzinger, A. and Evans, T. (2007). Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Dev. Biol. 312, 613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, K., Kanki, J. P. and Look, A. T. (2001). Zebrafish myelopoiesis and blood cell development. Curr. Opin. Hematol. 8, 245-251. [DOI] [PubMed] [Google Scholar]
- Huber, T. L., Kouskoff, V., Fehling, H. J., Palis, J. and Keller, G. (2004). Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432, 625-630. [DOI] [PubMed] [Google Scholar]
- Jowett, T. (2001). Double in situ hybridisation techniques in zebrafish. Methods 23, 345-358. [DOI] [PubMed] [Google Scholar]
- Kattman, S. J., Huber, T. L. and Keller, G. M. (2006). Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell 11, 723-732. [DOI] [PubMed] [Google Scholar]
- Kawakami, Y., Raya, A., Raya, R. M., Rodriguez-Esteban, C. and Belmonte, J. C. (2005). Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature 435, 165-171. [DOI] [PubMed] [Google Scholar]
- Kessel, J. and Fabian, B. (1987). Inhibitory and stimulatory influences on mesodermal erythropoiesis in the early chick blastoderm. Development 101, 45-49. [DOI] [PubMed] [Google Scholar]
- Kikuchi, Y., Agathon, A., Alexander, J., Thisse, C., Waldron, S., Yelon, D., Thisse, B. and Stainier, D. Y. (2001). casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 15, 1493-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel, C. B. and Law, R. D. (1985). Cell lineage of zebrafish blastomeres. II. Formation of the yolk syncytial layer. Dev. Biol. 108, 86-93. [DOI] [PubMed] [Google Scholar]
- Laugwitz, K. L., Moretti, A., Caron, L., Nakano, A. and Chien, K. R. (2008). Islet1 cardiovascular progenitors: a single source for heart lineages? Development 135, 193-205. [DOI] [PubMed] [Google Scholar]
- Liao, W., Bisgrove, B. W., Sawyer, H., Hug, B., Bell, B., Peters, K., Grunwald, D. J. and Stainier, D. Y. (1997). The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development 124, 381-389. [DOI] [PubMed] [Google Scholar]
- Liao, W., Ho, C. Y., Yan, Y. L., Postlethwait, J. and Stainier, D. Y. (2000). Hhex and scl function in parallel to regulate early endothelial and blood differentiation in zebrafish. Development 127, 4303-4313. [DOI] [PubMed] [Google Scholar]
- Lieschke, G. J., Oates, A. C., Paw, B. H., Thompson, M. A., Hall, N. E., Ward, A. C., Ho, R. K., Zon, L. I. and Layton, J. E. (2002). Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Dev. Biol. 246, 274-295. [DOI] [PubMed] [Google Scholar]
- Liu, F. and Patient, R. (2008). Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ. Res. 103, 1147-1154. [DOI] [PubMed] [Google Scholar]
- Liu, F., Walmsley, M., Rodaway, A. and Patient, R. (2008). Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr. Biol. 18, 1234-1240. [DOI] [PubMed] [Google Scholar]
- Martin-Puig, S., Wang, Z. and Chien, K. R. (2008). Lives of a heart cell: tracing the origins of cardiac progenitors. Cell Stem Cell 2, 320-331. [DOI] [PubMed] [Google Scholar]
- Molkentin, J. D. (2000). The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 275, 38949-38952. [DOI] [PubMed] [Google Scholar]
- Nemer, G. and Nemer, M. (2003). Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev. Biol. 254, 131-148. [DOI] [PubMed] [Google Scholar]
- Patient, R. K. and McGhee, J. D. (2002). The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12, 416-422. [DOI] [PubMed] [Google Scholar]
- Patterson, L. J., Gering, M. and Patient, R. (2005). Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood 105, 3502-3511. [DOI] [PubMed] [Google Scholar]
- Patterson, L. J., Gering, M., Eckfeldt, C. E., Green, A. R., Verfaillie, C. M., Ekker, S. C. and Patient, R. (2007). The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109, 2389-2398. [DOI] [PubMed] [Google Scholar]
- Peterkin, T., Gibson, A. and Patient, R. (2003). GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. EMBO J. 22, 4260-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkin, T., Gibson, A. and Patient, R. (2007). Redundancy and evolution of GATA factor requirements in development of the myocardium. Dev. Biol. 311, 623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, V. N., Lawson, N. D., Mugford, J. W., Dye, L., Castranova, D., Lo, B. and Weinstein, B. M. (2007). Combinatorial function of ETS transcription factors in the developing vasculature. Dev. Biol. 303, 772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers, F., Walsh, E. C., Leger, S., Stainier, D. Y. and Brand, M. (2000). Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar). Development 127, 225-235. [DOI] [PubMed] [Google Scholar]
- Reiter, J. F., Alexander, J., Rodaway, A., Yelon, D., Patient, R., Holder, N. and Stainier, D. Y. (1999). Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13, 2983-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter, J. F., Kikuchi, Y. and Stainier, D. Y. (2001). Multiple roles for Gata5 in zebrafish endoderm formation. Development 128, 125-135. [DOI] [PubMed] [Google Scholar]
- Rhodes, J., Hagen, A., Hsu, K., Deng, M., Liu, T. X., Look, A. T. and Kanki, J. P. (2005). Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 8, 97-108. [DOI] [PubMed] [Google Scholar]
- Rodaway, A. R. F. and Patient, R. K. (2001). Mesendoderm, an ancient germ layer? Cell 105, 169-172. [DOI] [PubMed] [Google Scholar]
- Rodaway, A. R. F., Takeda, H., Koshida, S., Price, B. M. J., Smith, J. C., Patient, R. K. and Holder, N. (1999). Induction of the mesendoderm in the zebrafish germ ring by yolk cell derived TGF-β family signals and discrimination of mesoderm and endoderm by FGF. Development 126, 3067-3078. [DOI] [PubMed] [Google Scholar]
- Roman, B. L. and Weinstein, B. M. (2000). Building the vertebrate vasculature: research is going swimmingly. BioEssays 22, 882-893. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, T., Kuroiwa, A. and Takeda, H. (2001). A novel sox gene, 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mech. Dev. 107, 25-38. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, T., Kikuchi, Y., Kuroiwa, A., Takeda, H. and Stainier, D. Y. (2006). The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development 133, 4063-4072. [DOI] [PubMed] [Google Scholar]
- Schier, A. F., Neuhauss, S. C. F., Helde, K. A., Talbot, W. S. and Driever, W. (1997). The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 124, 327-342. [DOI] [PubMed] [Google Scholar]
- Schoenebeck, J. J., Keegan, B. R. and Yelon, D. (2007). Vessel and blood specification override cardiac potential in anterior mesoderm. Dev. Cell 13, 254-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serluca, F. C. and Fishman, M. C. (2001). Pre-pattern in the pronephric kidney field of zebrafish. Development 128, 2233-2241. [DOI] [PubMed] [Google Scholar]
- Stainier, D. Y. (2001). Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2, 39-48. [DOI] [PubMed] [Google Scholar]
- Stainier, D. Y. and Fishman, M. C. (1992). Patterning the zebrafish heart tube: acquisition of anteroposterior polarity. Dev. Biol. 153, 91-101. [DOI] [PubMed] [Google Scholar]
- Stainier, D. Y., Lee, R. K. and Fishman, M. C. (1993). Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 119, 31-40. [DOI] [PubMed] [Google Scholar]
- Stainier, D. Y. R., Weinstein, B. M., Detrich, H. W., Zon, L. I. and Fishman, M. C. (1995). cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121, 3141-3150. [DOI] [PubMed] [Google Scholar]
- Su, F., Juarez, M. A., Cooke, C. L., Lapointe, L., Shavit, J. A., Yamaoka, J. S. and Lyons, S. E. (2007). Differential regulation of primitive myelopoiesis in the zebrafish by Spi-1/Pu.1 and C/ebp1. Zebrafish 4, 187-199. [DOI] [PubMed] [Google Scholar]
- Sumanas, S. and Lin, S. (2006). Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 4, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas, S., Gomez, G., Zhao, Y., Park, C., Choi, K. and Lin, S. (2008). Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111, 4500-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, M. A., Ransom, D. G., Pratt, S. J., MacLennan, H., Kieran, M. W., Detrich, H. W., Vail, B., Huber, T. L., Paw, B., Brownlie, A. J. et al. (1998). The cloche and spadetail genes differentially affect hematopoiesis and vasculogenisis. Dev. Biol. 197, 248-269. [DOI] [PubMed] [Google Scholar]
- Trinh, L. A. and Stainier, D. Y. (2004). Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6, 371-382. [DOI] [PubMed] [Google Scholar]
- Walmsley, M., Ciau-Uitz, A. and Patient, R. (2002). Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development 129, 5683-5695. [DOI] [PubMed] [Google Scholar]
- Walmsley, M., Cleaver, D. and Patient, R. (2008). Fibroblast growth factor controls the timing of Scl, Lmo2, and Runx1 expression during embryonic blood development. Blood 111, 1157-1166. [DOI] [PubMed] [Google Scholar]
- Wang, C., Faloon, P. W., Tan, Z., Lv, Y., Zhang, P., Ge, Y., Deng, H. and Xiong, J. W. (2007). Mouse lysocardiolipin acyltransferase controls the development of hematopoietic and endothelial lineages during in vitro embryonic stem-cell differentiation. Blood 110, 3601-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, H., Symes, C., Walmsley, M. E., Rodaway, A. R. F. and Patient, R. K. (2000). A role for GATA5 in Xenopus endoderm specification. Development 127, 4345-4360. [DOI] [PubMed] [Google Scholar]
- Westerfield, M. (1993). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press.
- Xiong, J. W., Yu, Q., Zhang, J. and Mably, J. D. (2008). An acyltransferase controls the generation of hematopoietic and endothelial lineages in zebrafish. Circ. Res. 102, 1057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.