Abstract
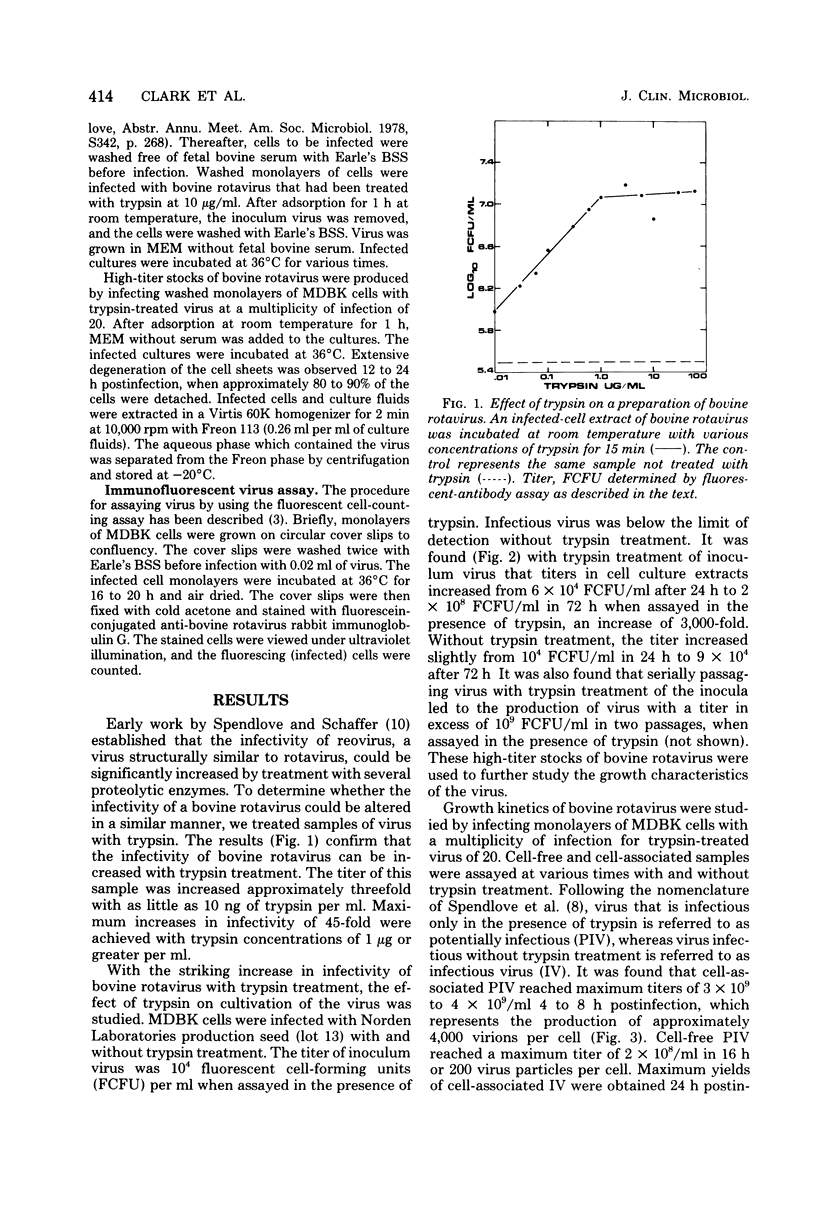
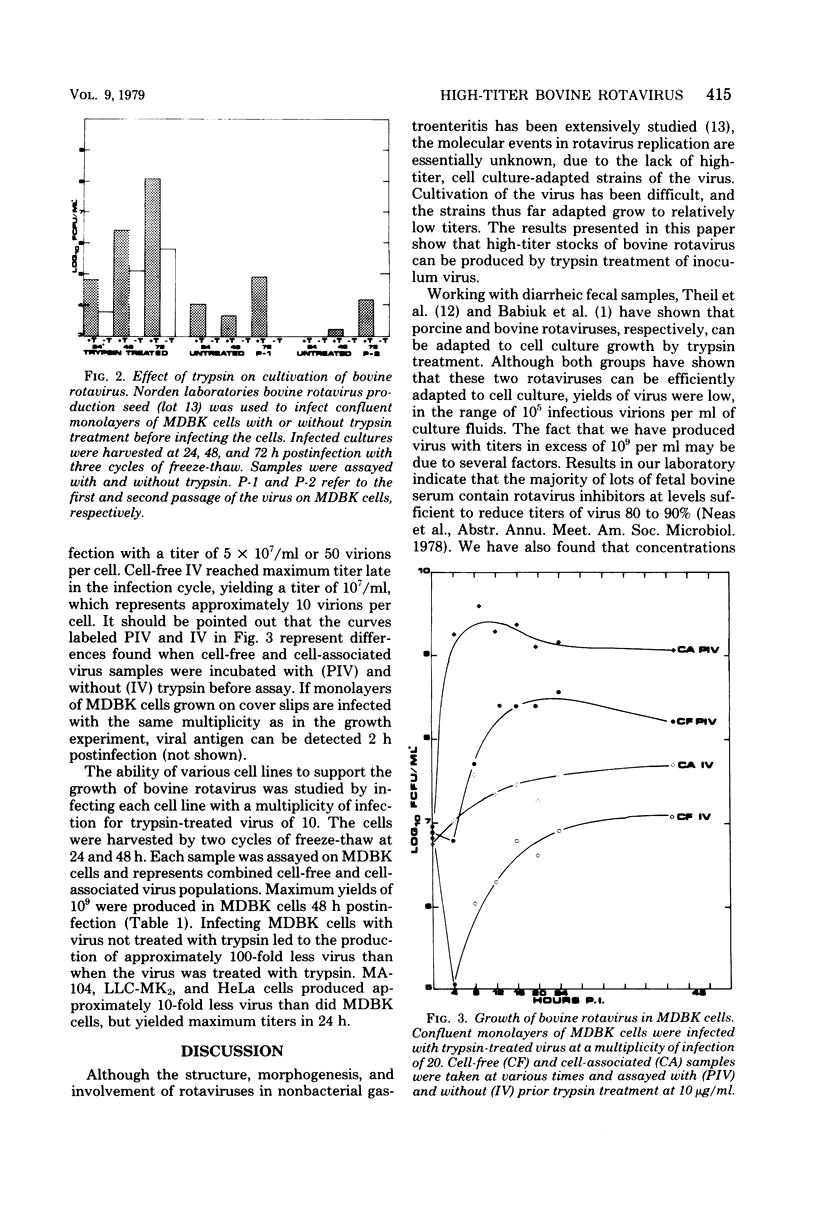
Titers of bovine rotavirus in excess of 10(9) immunofluorescent infectious units per ml of culture fluids have been produced, using trypsin treatment of the virus. Infectivity of preparations of the virus can be increased with as little as 1 ng of trypsin per ml, with maximum increases of 1 to 2 log10 with 1 microgram of trypsin per ml. The virus grows to titers in excess of 10(5) immunofluorescent units per ml in MDBK, LLC-MK2, MA-104, and HeLa cells. When MDBK cells are infected with a multiplicity of infection of 20, maximum yields of cell-associated, trypsin-enhanceable virus are obtained 4 to 8 h postinfection. Maximum yields of cell-free, trypsin-enhanceable virus are produced 16 to 20 h postinfection. The results presented here indicate that trypsin can be used to produce high-titer stocks of bovine rotavirus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Egbert L. N., Spendlove R. S. Characteristics of neonatal calf diarrhea virus ribonucleic acid. Can J Comp Med. 1978 Jan;42(1):46–53. [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Peterson M. W., Hsu L. Y., LaSalle V. A., Egbert L. N. Immunofluorescent cell assay of neonatal calf diarrhea virus. Can J Comp Med. 1975 Oct;39(4):462–465. [PMC free article] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S., Allan G. M., McFerran J. B. Isolation of a cytopathic calf rotavirus. Res Vet Sci. 1976 Jul;21(1):114–115. [PubMed] [Google Scholar]
- Mebus C. A., Kono M., Underdahl N. R., Twiehaus M. J. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971 Mar;12(3):69–72. [PMC free article] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., Lennette E. H., Knight C. O., Chin J. N. Production in FL cells of infectious and potentially infectious reovirus. J Bacteriol. 1966 Oct;92(4):1036–1040. doi: 10.1128/jb.92.4.1036-1040.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Hall G., Dennis M. J. The isolation of a reovirus-like agent associated with diarrhoea in colostrum-deprived calves in Great Britain. Res Vet Sci. 1974 Jan;16(1):102–105. [PubMed] [Google Scholar]


