Summary
The dermal papilla comprises the specialised mesenchymal cells at the base of the hair follicle. Communication between dermal papilla cells and the overlying epithelium is essential for differentiation of the hair follicle lineages. We report that Sox2 is expressed in all dermal papillae at E16.5, but from E18.5 onwards expression is confined to a subset of dermal papillae. In postnatal skin, Sox2 is only expressed in the dermal papillae of guard/awl/auchene follicles, whereas CD133 is expressed both in guard/awl/auchene and in zigzag dermal papillae. Using transgenic mice that express GFP under the control of the Sox2 promoter, we isolated Sox2+ (GFP+) CD133+ cells and compared them with Sox2- (GFP-) CD133+ dermal papilla cells. In addition to the `core' dermal papilla gene signature, each subpopulation expressed distinct sets of genes. GFP+ CD133+ cells had upregulated Wnt, FGF and BMP pathways and expressed neural crest markers. In GFP- CD133+ cells, the hedgehog, IGF, Notch and integrin pathways were prominent. In skin reconstitution assays, hair follicles failed to form when dermis was depleted of both GFP+ CD133+ and GFP- CD133+ cells. In the absence of GFP+ CD133+ cells, awl/auchene hairs failed to form and only zigzag hairs were found. We have thus demonstrated a previously unrecognised heterogeneity in dermal papilla cells and shown that Sox2-positive cells specify particular hair follicle types.
Keywords: Sox2, Dermal papilla, Hair follicle, Mouse
INTRODUCTION
The hair follicle is made up of approximately eight distinct epithelial lineages (Schmidt-Ullrich and Paus, 2005). It is established and maintained through reciprocal signalling between the epithelium and a group of mesenchymal cells at the base of the follicle that constitute the dermal papilla (Ehama et al., 2007; Fuchs and Horsley, 2008; Horne and Jahoda, 1992; Jahoda, 1992; Muller-Rover et al., 2001; Paus et al., 1999; Watt and Hogan, 2000; Wu et al., 2004). Key signalling pathways that mediate the effects of the dermal papilla include the Wnt and BMP pathways (Kishimoto et al., 2000; Rendl et al., 2008; Shimizu and Morgan, 2004).
In mouse skin there are several distinct hair follicle types that differ in length, thickness and in the presence or absence of kinks in the hair shaft (Schlake, 2007). Although there has been considerable progress in identifying the factors that regulate whether epidermal stem cell progeny select the hair follicle, sebaceous gland or interfollicular epidermal lineages (Watt et al., 2006), much less is known about how different hair follicle types are specified. Modulation of BMP, Shh, β-catenin or Eda/Edar activity in the epidermis can influence hair follicle phenotype (Botchkarev et al., 2002; Ellis et al., 2003; Huelsken et al., 2001; Mikkola and Thesleff, 2003) and epidermal Igfbp5 expression is required to maintain the kinks in zigzag hair follicles (Schlake, 2005).
One of the dermally expressed genes that regulate hair follicle type is Sox18. Loss of Sox18, which is expressed in the dermal papilla, results in a reduction in zigzag hairs (James et al., 2003; Pennisi et al., 2000). Sox2, like Sox18, is a SRY transcription factor (Wegner and Stolt, 2005) that is expressed in the dermal papilla (Rendl et al., 2005). These observations led us to investigate whether Sox2, like Sox18, is involved in specifying hair follicle types.
Sox2 is already known to play a key role in organ development and in determining stem cell properties. Sox2 is involved in maintaining embryonic stem cell pluripotency (Niwa, 2007), in regulating neural and retinal cell progenitors (Graham et al., 2003; Taranova et al., 2006) and in the development of the sensory organs of the inner ear and taste buds (Kiernan et al., 2005; Okubo et al., 2006). Sox2 is expressed in the endoderm of foregut-derived organs, including tongue, oesophagus and stomach, and plays a role in establishing the boundary between the fore- and hind-stomach (Que et al., 2007). In humans, heterozygosity for SOX2 is associated with anophthalmia-esophageal-genital (AEG) syndrome (Williamson et al., 2006). Our studies now reveal a previously unknown role for Sox2 in the skin.
MATERIALS AND METHODS
Transgenic mice
Sox2eGFP mice, in which eGFP expression is under the control of a 5.5 kb fragment of the upstream regulatory element of the Sox2 promoter (D'Amour and Gage, 2003), were a kind gift from Fred H. Gage (Salk Institute). CAG-dsRed mice, in which dsRed is expressed under the control of the chicken β-actin promoter, were a kind gift from Jose Silva (Wellcome Trust Centre for Stem Cell Research, Cambridge University).
Histology, whole mounts and immunostaining
Immunostaining for Sox2 and keratin 14 (K14) was performed on paraffin sections after citrate epitope retrieval. Sections were blocked in 0.5% Triton X-100, 0.1% fish skin gelatin, 10% donkey serum and 0.1% Tween 20 in PBS for 1 hour at room temperature, then incubated overnight in primary antibodies at 4°C. Antibodies were diluted 1:100 (Sox2, R&D Systems) or 1:1000 (K14, Covance) in blocking solution. Secondary antibodies (Alexa 555- and Alexa 488-conjugated anti-rabbit and anti-goat, Invitrogen) were added at a dilution of 1:500 for 1 hour at room temperature together with DAPI to label nuclei.
Immunostaining of cryosections, which had been fixed for 15 minutes in 4% paraformaldehyde at room temperature, was performed as described for paraffin sections. Primary antibodies were used at the following dilutions: 1:50 (CD133, eBiosciences), 1:50 (α8 integrin, R&D Systems), 1:100 (Sox10, Santa Cruz), 1:50 (HhiP, R&D Systems) and 1:50 (Dcc, R&D Systems).
For whole-mount preparations, embryonic or neonatal mouse skin from whisker, dorsal and tail regions was dissected, paraformaldehyde fixed and incubated for 1 hour at room temperature in a blocking buffer consisting of 0.1% fish skin gelatin, 0.5% Triton X-100 and 0.5% skimmed milk powder in PBS. Skin was incubated overnight with rabbit anti-GFP antibodies (Invitrogen) (1:500) at 4°C. The next day, the tissue was washed in PBS for 12 hours at 4°C with periodic changes in wash buffer. Skin was subsequently labelled with Alexa 555-conjugated K14 antibodies and Alexa 488-conjugated anti-rabbit secondary antibodies overnight at 4°C. Final washes were performed at room temperature for 3 hours in PBS.
Whole mounts and sections were examined using a Leica TCS SP5 confocal microscope. z-stacks were acquired at 100 Hz with an optimal stack distance and 2048×2048 dpi resolution. z-stack projections were generated using the LAS AF software package (Leica Microsystems).
Isolation of neonatal mouse dermal and epidermal cells for grafting and flow cytometry
Postnatal day 2 (P2) mouse skin was incubated in a 1:1 solution of 5% dispase/0.5% trypsin in calcium-free (-Ca) FAD (one part Ham's F12, three parts DMEM, 18 mM adenine) at 37°C for 1 hour. The epidermis was then peeled from the dermis and incubated in 0.2% collagenase in -Ca FAD for 2 hours at 37°C to yield a single-cell suspension. The dermis was dissociated by vigorous pipetting and filtered through a 70-μm cell strainer, resulting in a single-cell suspension.
Dermal cells were labelled with CD133 antibody conjugated to APC (eBiosciences) using the manufacturer's recommended concentrations. Cell sorting was performed using a MoFlo high-speed sorter (Dako Cytomation). Dead cells were excluded by gating out DAPI-positive cells. Sox2GFP dermal preparations and wild-type dermal preparations labelled with APC-conjugated anti-CD133 were used to set gates for non-specific labelling. Gating was used to separate three distinct cell populations based on GFP and CD133 expression, and DAPI was used to exclude dead or dying cells. The dermal papilla-negative population was CD133- GFP-, the zigzag dermal papilla population was CD133+ GFP-, and the guard/awl/achene dermal papilla population was CD133+ GFP+. Transgene-negative cells, unlabelled cells, and cells labelled with omission of primary antibody were used to verify all gates.
Gene expression analysis of flow-sorted cell populations
Cell sorting was used to collect 50,000 cells belonging to each of the dermal populations (CD133- GFP-, CD133+ GFP- and CD133+ GFP+) from a single embryo/neonate. mRNA was isolated using an Invitrogen Purelink Micro-Midi Total RNA Isolation Kit and cDNA was prepared with the Invitrogen Superscript III First-Strand Synthesis Supermix. Expression analysis of selected genes was performed using Applied Biosystems Taqman predesigned probe sets. The following probes were used: Sox2, CD133, Corin, Sox18, Sox10, Dcc, Itga8, Bmp4 and Alpl. The standard amplification protocol was used with the Applied Biosystems 7900HT Real-Time PCR System to amplify selected genes. Samples were normalised to β-actin expression using the ΔCt method.
For Affymetrix analysis, mRNA was isolated as described above from dermal populations of P2 mice. Ten nanograms of column-purified RNA was amplified to 10 μg of cDNA and hybridised to Affymetrix MG430.2A arrays by the Paterson Institute for Cancer Research Cancer Research UK Affymetrix Genechip Microarray Service. Arrays were performed in triplicate using RNA from three separate mice. The data are deposited in the NIH GEO repository (accession number GSE16801): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16801.
Array images produced by the Affymetrix PICR 3000 scanner were analysed as Cel files using Genespring X10.0 (Agilent Technologies). RMA normalisation was used and the bottom twentieth percentile of genes (i.e. the 20% of genes with the lowest expression levels) were excluded from subsequent analysis. One-way ANOVA with an asymptotic P-value cut-off of <0.05 without multiple testing correction was used to generate the final list of genes analysed.
To explore the different dermal papilla signatures in depth, we constructed three entity lists: (1) genes differentially expressed 2-fold or more between GFP+ CD133+ (guard/awl/auchene) dermal papilla cells and GFP- CD133- cells (dermal papilla depleted); (2) genes differentially expressed 2-fold or more between GFP- CD133+ (zigzag) dermal papilla cells and GFP- CD133- cells; and (3) genes differentially expressed 2-fold or more between GFP+ CD133+ and GFP- CD133+ dermal papilla cells. These lists were used for both gene ontology and network analysis by directly importing the lists into the Ingenuity Pathway Analysis program through Genespring GX.
Epidermal-dermal reconstitution in graft chambers
Grafting studies were performed as previously described (Ehama et al., 2007), except for the following modifications. On the first day of the procedure, 6-mm diameter chambers (Renner, Dannstadt, Germany, Cat. # -30 900) were implanted onto nu/Balb/c mice. Cells for grafting were injected into the chambers the following day. Each graft consisted of 5×106 dermal cells combined with 8×106 epidermal cells. The chamber tops were cut off to expose grafts to air 1 week after the chambers had been implanted. Two weeks after the tops had been removed, the chambers themselves were removed from the mouse. Hair follicle growth could be seen as early as 3 weeks after seeding the chambers. Grafts were harvested at 4 weeks.
RESULTS
Sox2 is expressed in the dermal papilla of a subset of hair follicles
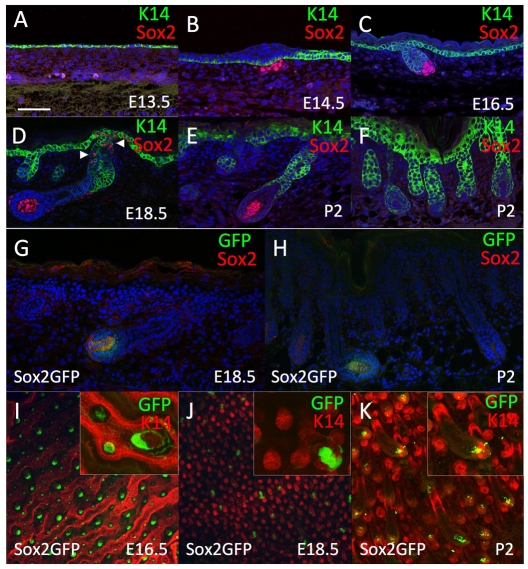
Sox2 was not detected in embryonic mouse skin prior to dermal condensate formation (Fig. 1A). At E14.5 and E16.5, Sox2 was expressed in the dermal condensates of all developing hair follicles (Fig. 1B,C). From E18.5 to P2, when the base of the developing hair follicles had ensheathed the dermal condensate to form the dermal papilla, Sox2 expression was confined to a subset of follicles (Fig. 1D-F). Sox2 expression could also be detected in the Merkel cells of the touch cone in the infundibulum of the follicles (Fig. 1D, arrowheads).
Fig. 1.
Sox2 expression is localised to the dermal papilla of specific hair follicles. (A-F) Paraffin sections of E13.5 (A), E14.5 (B), E16.5 (C), E18.5 (D) and P2 (E,F) wild-type skin immunolabelled with antibodies to Sox2 (red) and keratin 14 (green), with DAPI nuclear counterstain (blue). Arrows in D indicate Sox2-positive Merkel cells. (G,H) Cryosections of Sox2GFP skin immunolabelled for Sox2 and GFP at E18.5 (G) and P2 (H), with DAPI nuclear counterstain (blue). (I-K) Confocal micrographs of Sox2GFP transgenic mouse skin whole mounts at E16.5 (I), E18.5 (J) and P2 (K) immunolabelled with anti-keratin 14 (red). Inserts show higher magnification views of dermal papillae. Scale bar: 100 μm.
Transgenic expression of eGFP under the control of a 5.5 kb fragment of a proximal Sox2 regulatory element (Sox2GFP) (D'Amour and Gage, 2003) showed specific expression of GFP in the dermal papilla that correlated with endogenous Sox2 protein expression (Fig. 1G-K). Thus, GFP expression was found in all dermal condensates of the developing hair follicle at E16.5, whether visualised in skin whole mounts (Fig. 1I) or in conventional sections (data not shown). After E18.5, GFP expression was confined to a subset of dermal papillae (Fig. 1H,J,K).
We conclude that Sox2 expression is a marker of a subset of dermal papilla cells in developing skin and that Sox2GFP is a faithful reporter of expression of the endogenous protein.
Sox2 is expressed in guard/awl/auchene dermal papillae but not in zigzag dermal papillae
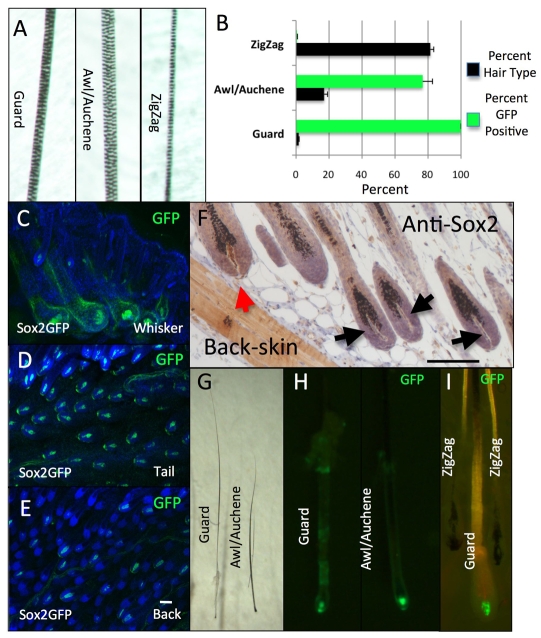
There are six major types of murine hair follicle: whisker, guard, awl, auchene, zigzag and tail (Muller-Rover et al., 2001; Paus et al., 1999). The four hair types found in back skin (guard, awl, auchene and zigzag) can be distinguished on the basis of the length of the hair shaft, the number of medulla cells, and the presence of kinks (bends) in the shaft. Guard hairs are the longest, do not contain kinks, and develop during the first wave of hair follicle morphogenesis at E13-14.5 (Schlake, 2007). They contain two rows of medulla cells, have two associated sebaceous glands, and are thought to play a sensory role (Fig. 2A,G). Awl hairs are also straight but are shorter than guard hairs, contain three or four rows of medulla cells, and form in the second wave of hair morphogenesis at E15-16.5 (Fig. 2A,G). Auchene hairs are identical to awl hairs, except that they have one kink in the hair shaft. Since auchene hairs are a modified type of awl hair they are often classified together (Falconer et al., 1951; Sundberg, 1994). Finally, zigzag hairs have one row of medulla cells and at least two bends in the hair shaft, giving the hair a characteristic `z' shape (Fig. 2A; data not shown). Zigzag hairs are the most abundant follicle type in mouse back skin, making up ∼81% of hairs (Fig. 2B). Guard hairs make up ∼1% of the back skin hairs, whereas awl/auchene together make up ∼17% (Fig. 2B).
Fig. 2.
Sox2 expression is restricted to guard/awl/auchene dermal papillae. (A) Bright-field images showing layers of medulla cells at the thickest point of the shaft of different hair types. (B) Percentage of hairs of each type in P10 Sox2GFP back skin (black bars) and percentage hairs with GFP-positive dermal papillae. n=200 hairs per mouse. Data are mean±s.e.m. from three mice. (C-E) Whole-mount GFP immunostaining of whisker (C), tail (D) and back (E) skin from P10 Sox2GFP mice. (F) Paraffin section of P10 wild-type skin stained with antibody to Sox2. Red arrow, Sox2-positive dermal papilla; black arrows, Sox2-negative dermal papillae. (G-I) Bright-field (G) and fluorescence (H,I) images of isolated hair follicles. Guard/awl/auchene hairs express GFP in their dermal papillae (H,I), whereas zigzag hairs do not (I). The same follicles are shown in G and H. Scale bars: 200 μm.
The developmental timing of hair follicle morphogenesis and the dynamic expression pattern of Sox2 in the dermis led us to investigate whether Sox2 expression was associated with a specific hair type. We examined the skin of Sox2GFP mice at postnatal day 10 (P10), when all hair types are fully developed and are in the anagen growth phase of the hair cycle. By whole-mount immunostaining, we found GFP expression in the dermal papilla of all whisker and tail follicles (Fig. 2C,D), whereas in back skin GFP expression was restricted to a subset of follicles (Fig. 2E). The heterogeneous expression of Sox2 was confirmed by immunolabelling back skin sections with antibodies to Sox2 (Fig. 2F). Within individual Sox2-positive dermal papillae, all the cells expressed Sox2 (Fig. 2; data not shown).
Hairs plucked from the back skin of Sox2GFP mice retained the dermal papilla (Fig. 2H,I). We classified 1000 individual hairs from five different Sox2GFP P10 mice (200 hairs per mouse) on the basis of total hair length, kinks, medulla cell width and GFP expression (Fig. 2B). One hundred percent of guard hairs expressed GFP, whereas 75% of awl/auchene hairs were GFP positive (Fig. 2H,I). By contrast, zigzag hairs never had GFP-positive dermal papillae (Fig. 2B,I).
We conclude that Sox2 expression is present in guard/awl/auchene dermal papillae and absent from zigzag hair dermal papillae. This fits with the timing of Sox2 expression during hair follicle morphogenesis, as guard/awl/auchene are the first hair follicles to form, between E14.5 and E16.5, whereas zigzag hair follicles, which do not express Sox2, develop from E18.5 (Fig. 1A-F).
Isolation of two different populations of dermal papilla cells
We hypothesised that Sox2-positive and -negative dermal papilla cells might have different gene expression signatures that could in turn impact on signalling to the epidermis and thereby influence hair follicle type. In order to investigate this, we developed a method to isolate Sox2-positive and -negative dermal papilla cells. We isolated cells from P2 Sox2GFP skin because all hair types are undergoing morphogenesis at this stage. In agreement with a recent report (Ito et al., 2007b), we found that CD133 (Prom1 - Mouse Genome Informatics) was expressed in all dermal papillae throughout morphogenesis of the hair follicle, including P2 (Fig. 3A; data not shown).
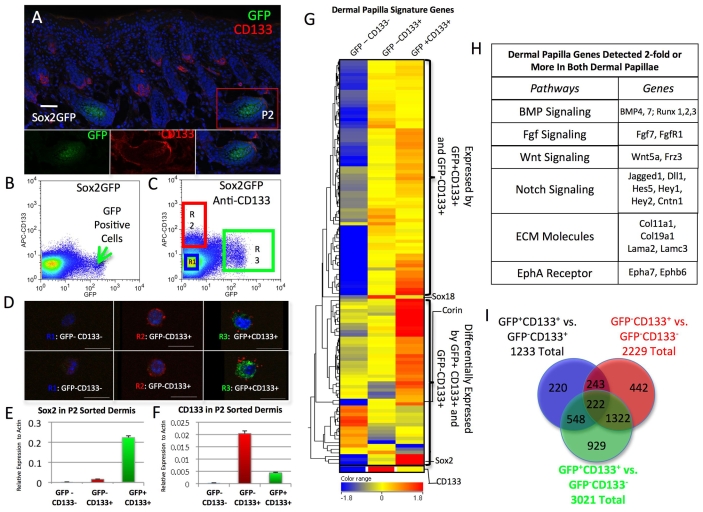
Fig. 3.
Isolation and gene expression profiling of different dermal papilla cell populations. (A) Immunostaining of cryosection of P2 Sox2GFP mouse skin to show the presence of two types of dermal papilla: CD133+ Sox2+ and CD133+ Sox2-. Lower panels are merged (right) and de-merged (left and middle) images of the Sox2-positive follicle boxed in the main panel. Note that CD133 staining of Sox2-positive dermal papillae is less intense than in Sox2-negative dermal papillae and dermal sheath cells. Scale bar: 50 μm. (B,C) Flow sorting of P2 Sox2GFP dermal cells, on the basis of GFP expression only (B) or GFP and CD133 expression (C). (C) R1 gate, GFP- CD133-; R2 gate, GFP- CD133+; R3 gate, GFP+ CD133+. (D) Live cells sorted on the basis of CD133 and GFP expression were examined by confocal microscopy. Scale bar: 10 μm. (E,F) Q-PCR of Sox2 (E) and CD133 (Prom1) (F) mRNA levels in the different sorted populations. mRNA levels are expressed relative to β-actin. Data are mean±s.e.m. from three mice. (G) Heat map of expression levels of 225 previously published dermal papilla genes (Rendl et al., 2005) in the three populations of dermal cells isolated on the basis of CD133 and GFP expression. Hierarchical clustering was performed and the range indicates a log-2. (H) Examples of genes differentially expressed in both GFP+ CD133+ and GFP- CD133+ dermal papillae relative to GFP- CD133- dermal cells. (I) Venn diagram constructed from lists of entities differentially expressed (2-fold or more) in the different dermal populations.
Sox2-positive dermal papillae showed uniform expression of CD133, albeit at a lower level than in neighbouring dermal sheath cells and Sox2-negative dermal papillae (Fig. 3A). This was confirmed by disaggregating dermal cells from five individual P2 Sox2GFP mice and labelling them with anti-CD133 antibodies. When analysed using a digital flow cytometer (Becton Dickinson CyAn), we found that 1% of cells were Sox2 positive and, of those, 85±1% (±s.e.m.) were also positive for CD133.
We next sorted dermal cells into three categories: GFP+ CD133+ (guard/awl/auchene dermal papilla), GFP- CD133+ (zigzag dermal papilla) and GFP- CD133- (depleted of dermal papilla cells). When sorted cells were analysed by confocal microscopy, almost all Sox2-positive cells were also CD133+ (Fig. 3D). Nevertheless, Sox2GFP-positive cells expressed lower levels of CD133 protein and mRNA than Sox2-negative cells (Fig. 3A-F).
We isolated 50,000 cells of each population from a single P2 mouse and performed microarray analysis using the Affymetrix M430A platform. The analysis was performed in triplicate using cells isolated from three different mice. We analysed the data using Genespring GX10 and RMA normalisation. The isolation of each cell population was validated by the relative levels of CD133 and Sox2 expression detected on the arrays (Fig. 3G).
We compared our data with the expression profile of 225 signature dermal papilla genes reported by Rendl et al. (Rendl et al., 2005) (Fig. 3G). In the GFP- CD133- population, roughly two thirds of dermal papilla genes were under-represented, consistent with the cells being depleted of dermal papilla cells. By contrast, most of the signature genes were upregulated in the GFP+ CD133+ and GFP- CD133+ populations (Fig. 3G). Examples of genes that were highly expressed in both types of dermal papilla are Corin, Bmp4, Fgfr1 and Wnt5a, all of which are known to be expressed specifically in dermal papillae (Fig. 3G,H) (Botchkarev et al., 2002; Enshell-Seijffers et al., 2008; Kawano et al., 2005; Reddy et al., 2001; Rendl et al., 2005; Rendl et al., 2008).
Profiling different dermal papilla populations
To explore the different dermal papilla signatures in depth, we constructed three entity lists from our microarray data: (1) genes that were differentially expressed 2-fold or more between GFP+ CD133+ (guard/awl/auchene) dermal papilla cells and GFP- CD133- cells (dermal papilla depleted); (2) genes that were differentially expressed 2-fold or more between GFP- CD133+ (zigzag) dermal papilla cells and GFP- CD133- cells; and (3) genes that were differentially expressed 2-fold or more between GFP+ CD133+ and GFP- CD133+ dermal papilla cells. As each gene is covered by multiple probes, the number of entities per list is greater than the number of genes. The extent of overlap of the three lists is represented in the Venn diagram in Fig. 3I. In total, 1322 entities were common to GFP+ CD133+ and GFP- CD133+ cells but lacking in GFP- CD133- cells. There were 1477 entities (929+548) that were specific for GFP+ CD133+ (guard/awl/auchene) dermal papillae, whereas 685 entities (442+243) were specific for GFP- CD133+ (zigzag) dermal papillae. For the full gene lists, see Tables S1-S3 in the supplementary material. The microarray data are deposited in the NIH GEO repository (accession number GSE16801).
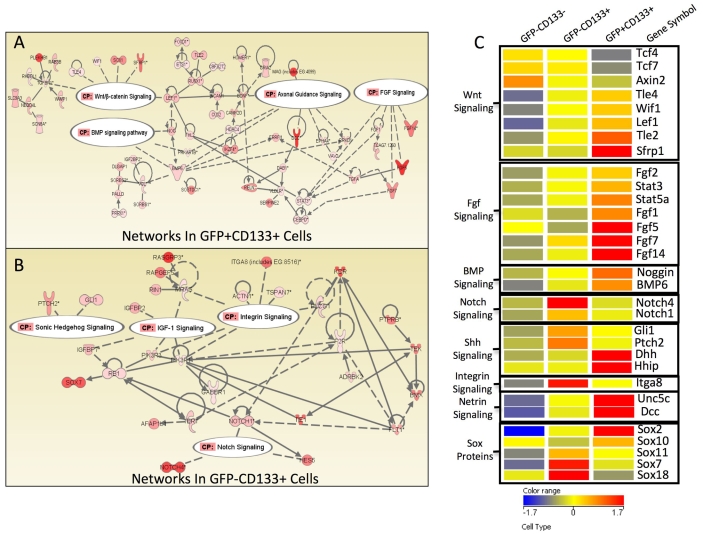
We next performed network analysis on the entity lists of GFP+ CD133+-specific and GFP- CD133+-specific genes. Using Ingenuity Pathway Analysis, we found that specific, interconnected, networks were upregulated in the different dermal papilla types (Fig. 4A,B). When we entered the GFP+ CD133+ list, we found networks that represented axonal guidance genes and genes involved in Wnt, BMP and FGF signalling (Fig. 4A). Some of these genes have previously been associated with guard/awl/auchene hairs (Fig. 4C) (Botchkarev et al., 2002; Kishimoto et al., 2000), while the upregulation of neuronal genes is consistent with Sox2 expression in neural crest and peripheral nerve (Morrison et al., 1999; Wegner and Stolt, 2005).
Fig. 4.
Genes differentially expressed in the different dermal papilla cell populations. (A,B) Ingenuity Pathway Analysis was performed on genes and pathways specifically upregulated in GFP+ CD133+ (A) and GFP- CD133+ (B) dermal papillae. The level of upregulation compared with GFP- CD133- cells is indicated by a scale from pink to red (lowest to highest upregulation). CP, canonical pathway. (C) Heat map showing examples of genes upregulated or downregulated at least 2-fold compared with GFP- CD133- cells.
The GFP- CD133+ (zigzag dermal papilla) gene lists featured a high representation of genes in the Shh, IGF, Notch and integrin signalling pathways (Fig. 4B). Upregulation of these pathways is also in agreement with previous studies (Ellis et al., 2003). Sox18, which is known to be required for zigzag hair formation (Graham et al., 2003; Taranova et al., 2006), was specifically detected in zigzag dermal papillae (Fig. 4C).
The GFP+ CD133+ (guard/awl/auchene) dermal papilla gene signature is present in both embryonic and postnatal follicles
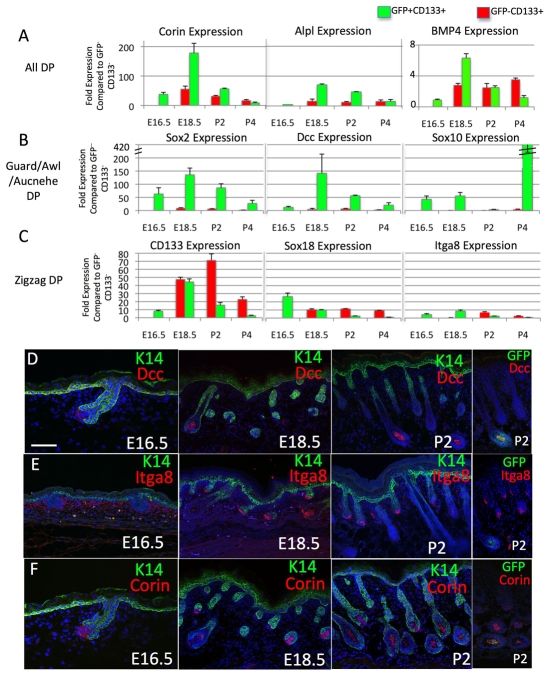
To validate the results of the microarray analysis and to investigate whether the gene expression signatures changed during development, we used flow sorting for Sox2GFP and CD133 (Fig. 3C) to isolate dermal papilla cells at E16.5, E18.5 and P4, and compared them with P2 dermal papillae. Hair follicle morphogenesis occurs in a proximal-distal wave beginning with guard hairs at E14.5, the other hair types developing at later time points. Corin, Bmp4 and Alpl, which were detected in both GFP+ CD133+ and GFP- CD133+ datasets and have previously been reported to be ubiquitous dermal papilla markers (Enshell-Seijffers et al., 2008; Rendl et al., 2005; Rendl et al., 2008), were detected at the mRNA and protein levels at all time points in both types of dermal papilla (Fig. 5A,F; data not shown). Sox2 mRNA was only detected in the GFP-positive populations, as expected, and peaked at E18.5 (Fig. 5B). CD133 expression peaked later, at P2 (Fig. 5C). Consistent with the data in Fig. 3, CD133 levels were lower in GFP+ than GFP- dermal papilla cells at P2; however, at E18.5, CD133 was equally abundant in both cell populations (Fig. 5C).
Fig. 5.
Kinetics of dermal papilla gene expression during embryonic development. (A-C) Q-PCR of specific mRNA levels at E16.5, E18.5, P2 and P4 in GFP+ CD133+ (green) and GFP- CD133+ (red) populations compared with unfractionated dermal cells. Error bars indicate the s.e.m. of three mice. (D-F) Cryosections of embryonic and neonatal mouse tissue immunolabelled for keratin 14 (green), Dcc (red, D), α8 integrin (red, E) and Corin (red, F). Scale bar: 100 μm.
Deleted in colorectal carcinoma (Dcc) and Sox10 were identified as markers of guard/awl/auchene dermal papillae (GFP+ CD133+) in the microarrays (Fig. 4C). Dcc and Sox10 are neural crest markers that play a role in maintaining or promoting migrating neural crest cells during development (Kim et al., 2003; Young et al., 2004). Dcc and Sox10 mRNA levels were elevated in GFP+ dermal papillae at all stages of development, Dcc levels peaking at E18.5 and Sox10 at P4 (Fig. 5B). As predicted, Dcc protein was specifically expressed in GFP+ CD133+ (guard/awl/auchene) dermal papillae (Fig. 5D). GFP expression was used to mark guard/awl/auchene dermal papillae (right-hand panel of Fig. 5B; data not shown).
Sox18 and α8 integrin (Itga8) were part of the gene expression signature of zigzag dermal papillae (GFP- CD133+) (Fig. 4C). At E16.5, Itga8 protein was widely expressed in the superficial dermis, but from E18.5 onwards it was specifically expressed in zigzag dermal papillae (Fig. 5E). At P2 and P4, Itga8 and Sox18 mRNA were enriched in GFP- CD133+ cells (Fig. 5C).
We conclude that genes identified in the microarrays as being upregulated specifically in GFP+ CD133+ or GFP- CD133+ dermal papillae show dynamic regulation during development and are not restricted to P2 skin. They therefore represent distinct gene expression signatures.
Sox2-positive dermal papilla cells are required for formation of awl/auchene hairs
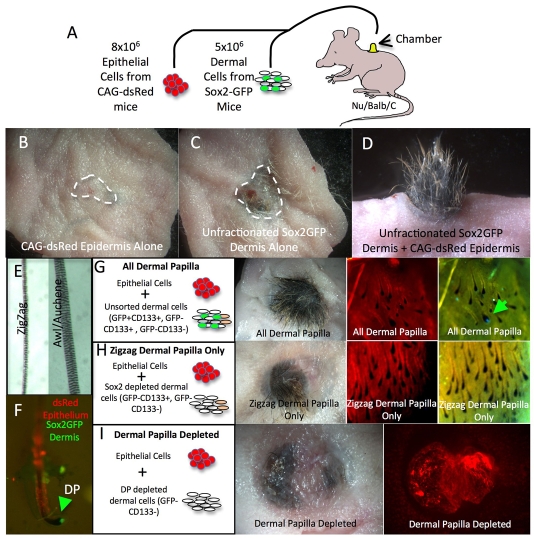
To examine whether Sox2GFP dermal papilla cells were necessary for formation of guard/awl/auchene hairs, we performed skin reconstitution assays (Fig. 6A). P2 dermal cells (5×106) from Sox2GFP mice were combined with epidermal cells (8×106) in a chamber implanted into a full-thickness wound on the back of a nude mouse. In order to distinguish graft from host hairs, we used epithelial cells from mice that constitutively expressed dsRed in all tissues (CAG-dsRed).
Fig. 6.
Hair reconstitution with different dermal cell populations. (A) Schematic representation of the hair reconstitution assay. (B-D) Macroscopic appearance of skin reconstituted with CAG-dsRed epidermal cells only (B), unfractionated dermal cells from Sox2GFP mouse with no added epidermal cells (C), or combination of epidermal cells and unfractionated dermal cells (D). (E) Bright-field images of hair types found in grafts. (F) Hair follicle isolated from graft combination (D) showing dsRed-positive outer root sheath and GFP-positive dermal papilla (DP, arrowhead). (G-I) Examples of grafts reconstituted with the different dermal cell populations shown. Shown are macroscopic views of (left to right) skin viewed under natural light, epidermis upwards, or under fluorescent light with red or green excitation, dermis upwards. Arrow in G indicates GFP-positive dermal papilla.
When CAG-dsRed epidermal cells were engrafted without dermal cells, no hair follicles formed (Fig. 6B), confirming the known requirement for dermal cells (Ehama et al., 2007). Conversely, when Sox2GFP dermis was engrafted without epithelial cells, a few hairs formed, indicating low-level contamination of dermal cells with epidermal cells (Fig. 6C). When unfractionated dermal cells from a Sox2GFP mouse were combined with epidermal cells, ectopic hairs formed in the graft after 3 weeks (Fig. 6D).
In a graft reconstituted with unfractionated dermal papilla cells (containing GFP+ CD133+, GFP- CD133+ and GFP- CD133- cells), awl/auchene hairs comprised ∼10-15% of follicles, the rest being zigzag hairs (Fig. 6E; Fig. 7). We never observed any guard hairs in the grafts. GFP expression was confined to the dermal papillae of hair follicles in the grafts (Fig. 6F,G; Fig. 7C,D).
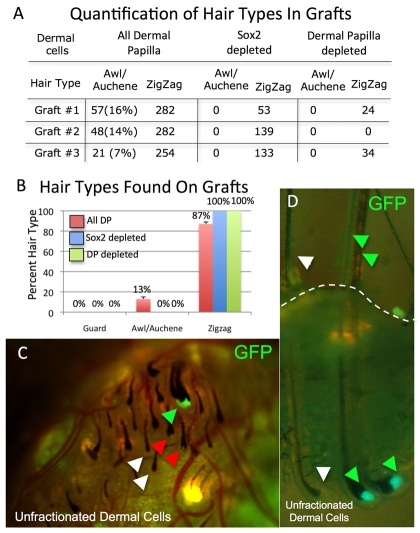
Fig. 7.
Requirement of Sox2-positive dermal papilla cells for awl/auchene hair formation. (A) Number of hairs of each type formed in the presence of different dermal populations. Data from three separate grafts with each dermal population are shown. (B) Percentage of each hair type (mean±s.e.m.) in the grafts shown in A. (C,D) Relationship between presence of Sox2GFP-positive dermal papilla cells and hair follicle type. Intact grafts are viewed dermis side up (C) or from the side (D). Dashed line in D indicates the skin surface. Green arrows indicate intense GFP labelling associated with large bulbs (C,D) and awl/auchene shafts (D). White arrows indicate GFP-negative dermal papillae associated with small bulbs (C,D) and zigzag shaft (D). Red arrows indicate small dermal papillae with weakly GFP-positive bulbs (C).
To determine whether Sox2GFP dermal papilla cells were necessary for the formation of awl/auchene hairs, we prepared grafts in which epidermal cells were recombined with GFP+ CD133+-depleted dermal cells (Fig. 6H) or dermal preparations depleted of both GFP+ CD133+ and GFP- CD133+ dermal cells (Fig. 6I). In grafts lacking GFP+ CD133+ and GFP- CD133+ dermal cells, the number of follicles that formed was reduced to ∼10% of that of grafts with unfractionated dermal cells, and the only hairs that formed were zigzag (Fig. 6I; Fig. 7A,B). When grafts were specifically depleted of GFP+ CD133+ cells, the total number of follicles was ∼50% of that of grafts with unfractionated dermal cells, and the only hairs to form were zigzag (Fig. 6H; Fig. 7A,B).
In skin reconstituted with unfractionated dermal cells, greater than 95% of large dermal papillae (corresponding to awl/auchene hairs; green arrows in Fig. 7C,D) were strongly GFP positive. Although most small dermal papillae (corresponding to zigzag hairs) lacked GFP expression (white arrows in Fig. 7C,D), ∼27% had weak, but detectable, GFP expression (red arrows in Fig. 7C). Thus, although only awl/auchene follicles had dermal papillae with high Sox2 expression, some Sox2-positive cells were associated with zigzag follicles.
We conclude that Sox2-positive dermal papilla cells are necessary to induce awl/auchene hairs and that Sox2+ CD133+ and Sox2- CD133+ cells together constitute the majority of dermal papilla cells in P2 dermis.
DISCUSSION
It has long been appreciated that hair follicle formation and maintenance depend on reciprocal interactions between the dermal papilla and overlying epithelial cells (Jahoda et al., 1984; Jahoda, 1992; Millar, 2002; Oshima et al., 2001). Progress has been made in identifying genes that are specifically expressed in the dermal papilla compared with other dermal fibroblasts (Enshell-Seijffers et al., 2008; Rendl et al., 2005). However, the potential heterogeneity of dermal papilla cells is largely unexplored. We have now isolated two distinct populations of dermal papilla cells on the basis of the presence or absence of Sox2 expression. We show that they have unique gene expression profiles and that Sox2-expressing cells are required for formation of awl/auchene follicles in a hair reconstitution assay. In the minority of reconstituted zigzag follicles with detectable Sox2GFP labelling, the GFP signal was substantially lower than in awl/auchene follicles; this would be consistent with a dose-dependent effect of Sox2 in specifying hair follicle type, as observed in Sox2-dependent patterning and differentiation of anterior foregut endoderm (Que et al., 2007).
Both the Sox2+ and Sox2- CD133+ populations expressed many previously described dermal papilla markers. However, they had, in addition, unique gene expression signatures, with the levels of expression of individual genes showing dynamic changes at different developmental stages. Wnt signalling maintains the hair inductive ability of all dermal papilla cells (Kishimoto et al., 2000), but Wnt pathway genes were more prominent in the Sox2+ gene list (Fig. 4A,C). High Sox2 expression lies downstream of canonical Wnt signalling (Okubo et al., 2006), and the bulbs of guard/awl/auchene follicles are larger than zigzag bulbs (Fig. 2F), which could be indicative of higher β-catenin activation (Silva-Vargas et al., 2005). Nevertheless, although upregulation of Lef1 and downregulation of Axin2 would be consistent with elevated signalling, there was also increased expression of the Wnt inhibitors Sfrp1 and Wif1 and of the transcriptional co-repressor Tle2 (Fig. 4C). It has previously been shown that differences in hair follicle dermal papilla size reflect differences in cell number and extracelllular matrix volume (Elliott et al., 1999).
Upregulation of the BMP antagonist noggin in Sox2+ dermal papillae is intriguing because administration of Bmp4 causes selective anagen arrest in zigzag hairs (Botchkarev et al., 2001) and epidermal overexpression of noggin results in replacement of zigzag with awl-like hairs (Sharov et al., 2006). In addition to Wnt and BMP signalling, FGF signalling was more prominent in Sox2+ dermal papilla cells; this pathway is known to regulate hair follicle cycling and the length of hair shafts (Hebert et al., 1994; Petiot et al., 2003; Rosenquist and Martin, 1996).
Within Sox2- dermal papillae, the most prominent gene regulatory networks were those involved in hedgehog, integrin, Notch and IGF signalling (Fig. 4B,C), all of which are known to regulate hair follicle development or cycling (Lorenz et al., 2007; Schlake, 2005; Schlake, 2007; Watt, 2002; Watt et al., 2008). IGF signalling plays a role in determining the bent structure of zigzag hairs (Schlake, 2005). Overexpression of Shh results in a lack of guard/awl/auchene hair follicles, with only zigzag hairs being formed (Ellis et al., 2003). Consistent with this, GFP- CD133+ (zigzag) dermal papillae had elevated patched 2 (Ptch2) and Gli2 mRNAs, whereas the hedgehog antagonist Hhip was more prominent in the Sox2+ population.
The relationship between the two populations of dermal papilla cells and the neural crest lineages of the skin remains to be explored. Markers of neural crest stem cells, such as p75 (neurotrophin receptor), S100b and Sox10, were specifically upregulated in GFP+ CD133+ (guard/awl/auchene) dermal papillae, consistent with the known expression of Sox2 in cell types of the neural crest lineage (Joseph et al., 2004). It therefore seems likely that Sox2-positive cells encompass the neural crest stem cell population previously reported to lie in the dermal papilla of whisker follicles (Sieber-Blum and Grim, 2004). It will be interesting to determine whether or not Sox2-positive dermal papilla cells have multi-lineage differentiation potential in vitro, and to investigate their relationship to skin-derived precursors (SKPs), which are known to reside in the dermal papilla (Fernandes et al., 2004; Toma et al., 2001).
The heterogeneity of dermal fibroblasts is becoming increasingly apparent. Fibroblasts in skin from different anatomical locations along the proximal-distal and anterior-posterior body axes have intrinsic differences in gene expression, as reflected in the expression of Hox genes (Rinn et al., 2008a; Rinn et al., 2008b). The dermis is profoundly altered upon injury, and in this context it is striking that when hair follicles are induced through wounding in adult skin only zigzag hairs form (Ito et al., 2007a). It is also evident that fibroblasts in the stroma of skin tumours express distinct genes from fibroblasts in the normal tissue (Rinn et al., 2006; Sneddon et al., 2006). These findings lead us to speculate that the differences between Sox2+ and Sox2- dermal papilla cells reflect differences in the differentiated state of dermal fibroblasts at the stages in development when the dermal papillae form (Fig. 8). Characterising different fibroblast populations in the skin and investigating their interactions with the distinct epidermal stem cell populations (Watt et al., 2006) will lead to a fuller understanding of skin function in health and disease.
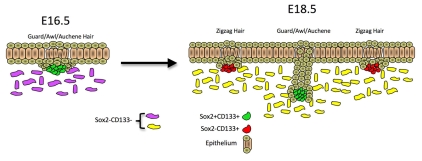
Fig. 8.
Origin of dermal papilla populations during mouse development. At E16.5, when guard/awl/auchene hairs are induced, all dermal papillae are Sox2 positive. At E18.5, zigzag hairs form in association with Sox2-negative dermal papillae. These differences persist in postnatal skin.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/16/2815/DC1
Supplementary Material
We thank Fred Gage for the generous gift of Sox2eGFP mice, and Jose Silva and Ornella Barrandon for the generous gift of CAG-dsRed mice. We acknowledge the expert technical assistance of Rachael Walker, Margaret McLeish and Peter Humphreys (Wellcome Trust Centre for Stem Cell Research) and Stuart Pepper (Paterson Institute, Manchester). This work was supported by funds from the Wellcome Trust, Medical Research Council and Cancer Research UK. K.W.M. is the recipient of a European Union FP7 Marie Curie Fellowship (PIEF-GA-2008-220642). We acknowledge the support of the University of Cambridge and Hutchison Whampoa. Deposited in PMC for release after 6 months.
References
- Botchkarev, V. A., Botchkareva, N. V., Nakamura, M., Huber, O., Funa, K., Lauster, R., Paus, R. and Gilchrest, B. A. (2001). Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 15, 2205-2214. [DOI] [PubMed] [Google Scholar]
- Botchkarev, V. A., Botchkareva, N. V., Sharov, A. A., Funa, K., Huber, O. and Gilchrest, B. A. (2002). Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J. Invest. Dermatol. 118, 3-10. [DOI] [PubMed] [Google Scholar]
- D'Amour, K. A. and Gage, F. H. (2003). Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc. Natl. Acad. Sci. USA 100Suppl. 1, 11866-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehama, R., Ishimatsu-Tsuji, Y., Iriyama, S., Ideta, R., Soma, T., Yano, K., Kawasaki, C., Suzuki, S., Shirakata, Y., Hashimoto, K. et al. (2007). Hair follicle regeneration and human cells using grafted rodent and human cells. J. Invest. Dermatol. 127, 2106-2115. [DOI] [PubMed] [Google Scholar]
- Elliott, K., Stephenson, T. J. and Messenger, A. G. (1999). Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J. Invest. Dermatol. 113, 873-877. [DOI] [PubMed] [Google Scholar]
- Ellis, T., Smyth, I., Riley, E., Bowles, J., Adolphe, C., Rothnagel, J. A., Wicking, C. and Wainwright, B. J. (2003). Overexpression of Sonic Hedgehog suppresses embryonic hair follicle morphogenesis. Dev. Biol. 263, 203-215. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers, D., Lindon, C. and Morgan, B. A. (2008). The serine protease Corin is a novel modifier of the Agouti pathway. Development 135, 217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer, D. S., Fraser, A. S. and King, J. W. B. (1951). The genetics and development of `crinkled', a new mutant in the house mouse. J. Genet. 50, 324-344. [DOI] [PubMed] [Google Scholar]
- Fernandes, K. J., McKenzie, I. A., Mill, P., Smith, K. M., Akhavan, M., Barnabe-Heider, F., Biernaskie, J., Junek, A., Kobayashi, N. R., Toma, J. G. et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 6, 1082-1093. [DOI] [PubMed] [Google Scholar]
- Fuchs, E. and Horsley, V. (2008). More than one way to skin. Genes Dev. 22, 976-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, V., Khudyakov, J., Ellis, P. and Pevny, L. (2003). SOX2 functions to maintain neural progenitor identity. Neuron 39, 749-765. [DOI] [PubMed] [Google Scholar]
- Hebert, J. M., Rosenquist, T., Gotz, J. and Martin, G. R. (1994). FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell 78, 1017-1025. [DOI] [PubMed] [Google Scholar]
- Horne, K. A. and Jahoda, C. A. B. (1992). Restoration of hair-growth by surgical implantation of follicular dermal sheath. Development 116, 563-571. [DOI] [PubMed] [Google Scholar]
- Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. and Birchmeier, W. (2001). beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533-545. [DOI] [PubMed] [Google Scholar]
- Ito, M., Yang, Z., Andl, T., Cui, C., Kim, N., Millar, S. E. and Cotsarelis, G. (2007a). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316-320. [DOI] [PubMed] [Google Scholar]
- Ito, Y., Hamazaki, T. S., Ohnuma, K., Tamaki, K., Asashima, M. and Okochi, H. (2007b). Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J. Invest. Dermatol. 127, 1052-1060. [DOI] [PubMed] [Google Scholar]
- Jahoda, C. A. B. (1992). Induction of follicle formation and hair-growth by vibrissa dermal papillae implanted into rat ear wounds-vibrissa-type fibers are specified. Development 115, 1103-1109. [DOI] [PubMed] [Google Scholar]
- Jahoda, C. A., Horne, K. A. and Oliver, R. F. (1984). Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560-562. [DOI] [PubMed] [Google Scholar]
- James, K., Hosking, B., Gardner, J., Muscat, G. E. O. and Koopman, P. (2003). Sox18 mutations in the ragged mouse alleles ragged-like and opossum. Genesis 36, 1-6. [DOI] [PubMed] [Google Scholar]
- Joseph, N. M., Mukouyama, Y. S., Mosher, J. T., Jaegle, M., Crone, S. A., Dormand, E. L., Lee, K. F., Meijer, D., Anderson, D. J. and Morrison, S. J. (2004). Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131, 5599-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, M., Komi-Kuramochi, A., Asada, M., Suzuki, M., Oki, J., Jiang, J. and Imamura, T. (2005). Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Invest. Dermatol. 124, 877-885. [DOI] [PubMed] [Google Scholar]
- Kiernan, A. E., Pelling, A. L., Leung, K. K. H., Tang, A. S. P., Bell, D. M., Tease, C., Lovell-Badge, R., Steel, K. P. and Cheah, K. S. E. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031-1035. [DOI] [PubMed] [Google Scholar]
- Kim, J., Lo, L., Dormand, E. and Anderson, D. J. (2003). SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17-31. [DOI] [PubMed] [Google Scholar]
- Kishimoto, J., Burgeson, R. E. and Morgan, B. A. (2000). Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 14, 1181-1185. [PMC free article] [PubMed] [Google Scholar]
- Lorenz, K., Grashoff, C., Torka, R., Sakai, T., Langbein, L., Bloch, W., Aumailley, M. and Fassler, R. (2007). Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 177, 501-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola, M. L. and Thesleff, I. (2003). Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 14, 211-224. [DOI] [PubMed] [Google Scholar]
- Millar, S. E. (2002). Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, 216-225. [DOI] [PubMed] [Google Scholar]
- Morrison, S. J., White, P. M., Zock, C. and Anderson, D. J. (1999). Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96, 737-749. [DOI] [PubMed] [Google Scholar]
- Muller-Rover, S., Handjiski, B., van der Veen, C., Eichmuller, S., Foitzik, K., McKay, I. A., Stenn, K. S. and Paus, R. (2001). A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3-15. [DOI] [PubMed] [Google Scholar]
- Niwa, H. (2007). How is pluripotency determined and maintained? Development 134, 635-646. [DOI] [PubMed] [Google Scholar]
- Okubo, T., Pevny, L. H. and Hogan, B. L. M. (2006). Sox2 is required for development of taste bud sensory cells. Genes Dev. 20, 2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. and Barrandon, Y. (2001). Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233-245. [DOI] [PubMed] [Google Scholar]
- Paus, R., Muller-Rover, S., van der Veen, C., Maurer, M., Eichmuller, S., Ling, G., Hofmann, U., Foitzik, K., Mecklenburg, L. and Handjiski, B. (1999). A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol. 113, 523-532. [DOI] [PubMed] [Google Scholar]
- Pennisi, D., Gardner, J., Chambers, D., Hosking, B., Peters, J., Muscat, G., Abbott, C. and Koopman, P. (2000). Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 24, 434-437. [DOI] [PubMed] [Google Scholar]
- Petiot, A., Conti, F. J., Grose, R., Revest, J. M., Hodivala-Dilke, K. M. and Dickson, C. (2003). A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development 130, 5493-5501. [DOI] [PubMed] [Google Scholar]
- Que, J., Okubo, T., Goldenring, J. R., Nam, K. T., Kurotani, R., Morrisey, E. E., Taranova, O., Pevny, L. H. and Hogan, B. L. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, S., Andl, T., Bagasra, A., Lu, M. M., Epstein, D. J., Morrisey, E. E. and Millar, S. E. (2001). Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107, 69-82. [DOI] [PubMed] [Google Scholar]
- Rendl, M., Lewis, L. and Fuchs, E. (2005). Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 3, e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl, M., Polak, L. and Fuchs, E. (2008). BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 22, 543-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Bondre, C., Gladstone, H. B., Brown, P. O. and Chang, H. Y. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Wang, J. K., Allen, N., Brugmann, S. A., Mikels, A. J., Liu, H., Ridky, T. W., Stadler, H. S., Nusse, R., Helms, J. A. et al. (2008a). A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 22, 303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, J. L., Wang, J. K., Liu, H., Montgomery, K., van de Rijn, M. and Chang, H. Y. (2008b). A systems biology approach to anatomic diversity of skin. J. Invest. Dermatol. 128, 776-782. [DOI] [PubMed] [Google Scholar]
- Rosenquist, T. A. and Martin, G. R. (1996). Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 205, 379-386. [DOI] [PubMed] [Google Scholar]
- Schlake, T. (2005). Segmental Igfbp5 expression is specifically associated with the bent structure of zigzag hairs. Mech. Dev. 122, 988-997. [DOI] [PubMed] [Google Scholar]
- Schlake, T. (2007). Determination of hair structure and shape. Semin. Cell Dev. Biol. 18, 267-273. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich, R. and Paus, R. (2005). Molecular principles of hair follicle induction and morphogenesis. BioEssays 27, 247-261. [DOI] [PubMed] [Google Scholar]
- Sharov, A. A., Sharova, T. Y., Mardaryev, A. N., di Vignano, A. T., Atoyan, R., Weiner, L., Yang, S., Brissette, J. L., Dotto, G. P. and Botchkarev, V. A. (2006). Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc. Natl. Acad. Sci. USA 103, 18166-18171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, H. and Morgan, B. A. (2004). Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J. Invest. Dermatol. 122, 239-245. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum, M. and Grim, M. (2004). The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res. C Embryo Today 72, 162-172. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas, V., Lo Celso, C., Giangreco, A., Ofstad, T., Prowse, D. M., Braun, K. M. and Watt, F. M. (2005). Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell 9, 121-131. [DOI] [PubMed] [Google Scholar]
- Sneddon, J. B., Zhen, H. H., Montgomery, K., van de Rijn, M., Tward, A. D., West, R., Gladstone, H., Chang, H. Y., Morganroth, G. S., Oro, A. E. et al. (2006). Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc. Natl. Acad. Sci. USA 103, 14842-14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg, J. P. (1994). Handbook of Mouse Mutations with Skin and Hair Abnormalities. Bar Harbor, ME: CRC Press.
- Taranova, O. V., Magness, S. T., Fagan, B. M., Wu, Y. Q., Surzenko, N., Hutton, S. R. and Pevny, L. H. (2006). SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 20, 1187-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma, J. G., Akhavan, M., Fernandes, K. J., Barnabe-Heider, F., Sadikot, A., Kaplan, D. R. and Miller, F. D. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778-784. [DOI] [PubMed] [Google Scholar]
- Watt, F. M. (2002). The stem cell compartment in human interfollicular epidermis. J. Dermatol. Sci. 28, 173-180. [DOI] [PubMed] [Google Scholar]
- Watt, F. M. and Hogan, B. L. M. (2000). Out of Eden: stem cells and their niches. Science 287, 1427-1430. [DOI] [PubMed] [Google Scholar]
- Watt, F. M., Lo Celso, C. and Silva-Vargas, V. (2006). Epidermal stem cells: an update. Curr. Opin. Genet. Dev. 16, 518-524. [DOI] [PubMed] [Google Scholar]
- Watt, F. M., Estrach, S. and Ambler, C. A. (2008). Epidermal Notch signalling: differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 20, 171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, M. and Stolt, C. C. (2005). From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 28, 583-588. [DOI] [PubMed] [Google Scholar]
- Williamson, K. A., Hever, A. M., Rainger, J., Rogers, R. C., Magee, A., Fiedler, Z., Keng, W. T., Sharkey, F. H., McGill, N., Hill, C. J. et al. (2006). Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum. Mol. Genet. 15, 1413-1422. [DOI] [PubMed] [Google Scholar]
- Wu, P., Hou, L. H., Plikus, M., Hughes, M., Scehnet, J., Suksaweang, S., Widelitz, R. B., Jiang, T. X. and Chuong, C. M. (2004). Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 48, 249-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, H. M., Anderson, R. B. and Anderson, C. R. (2004). Guidance cues involved in the development of the peripheral autonomic nervous system. Auton. Neurosci. 112, 1-14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.