Abstract
Objective
Decreased dopamine transporters (DAT) in the basal ganglia were shown in patients with human immunodeficiency virus (HIV) associated dementia. Therefore, we assessed the relationship between striatal DAT and dopamine D2 receptors (D2R) availability and cognitive performance, and whether cocaine abuse, a common co-morbid condition in HIV patients, would be associated with further decreases in DAT and D2 receptors.
Methods
35 HIV-positive subjects [24 without (HIV) and 11 with a history of cocaine-dependence (HIV+Coc)] and 14 seronegative controls (SN) were evaluated with PET to measure DAT using [C-11]cocaine and D2R using [C-11]raclopride (availability of DAT or D2R estimated with Bmax/Kd), and a battery of neuropsychological tests.
Results
Compared to SN controls, both HIV subject groups had lower DAT in putamen (HIV+Coc: −16.7%, p=0.003; HIV: −12.2%, p=0.02) and only HIV+Coc showed lower DAT in caudate (−12.2%, p=0.04). Lower D2R in both regions of both HIV groups were accounted by the greater nicotine use. Lower DAT, but not D2R, in putamen and caudate were associated with poorer performance on multiple neuropsychological tests, corrected for the effects of age, education, intelligence, mood, and nicotine use. Furthermore, a structural equation model (SEM) indicated that lower average dopamine function (both DAT and D2R) were related to poorer overall function on neuropsychological tests (p=0.05).
Interpretation
Reduced dopaminergic function may contribute to cognitive dysfunction in HIV patients with or without additional cocaine abuse. These findings suggest that these HIV patients may benefit from treatments that enhance dopamine function or protection from dopamine cell injury.
Keywords: dopamine, HIV, cognition, dementia, PET, transporters
Human immunodeficiency virus (HIV) associated dementia is characterized by cognitive as well as motor deficits, hence the term HIV-cognitive motor complex (HIV-CMC)(American Academy of Neurology AIDS Task Force Working Group, 1991).Clinical observations suggested that patients with HIV-CMC may have dopaminergic deficits, such as decreased attention and working memory (Law et al., 1994; Miller et al., 1990), hypersensitivity to dopamine blockers (Factor et al., 1994), and decreased cerebrospinal fluid (CSF) dopamine (Berger et al., 1994) and its metabolite homovallinic acid (Larsson et al.). Some patients with HIV dementia also developed acute onset parkinsonism and dystonia when treated with dopamine antagonists (Hriso et al., 1991). Collectively, these findings suggest that dopaminergic system might be especially vulnerable to the effects of HIV brain infection.
Neuropathology confirmed that HIV has the propensity to invade the basal ganglia (Kure et al., 1990), which have the highest density of dopaminergic synapses. Neuronal injury may result from HIV neurotoxic proteins and a complex cascade of cytokines, excitotoxins, and free radicals triggered by HIV-infected or immune-stimulated brain macrophages and astrocytes (Kaul et al., 2001). Magnetic resonance imaging (MRI) also demonstrated atrophy in the caudate (Jernigan et al., 2005), and positron emission tomography (PET) illustrated hypermetabolism during early stages but hypometaoblism at later stages in the basal ganglia of HIV patients with dementia (Rottenberg et al., 1996).
A preliminary PET study found that HIV patients with mild dementia, but not those without dementia, showed decreases in presynaptic dopamine transporters (DAT) (Wang et al., 2004). The current study aims to determine the relationship between decreased DAT and cognitive function in HIV patients, and whether a history of cocaine-dependence might further impact the dopaminergic system in HIV-infected individuals.
Since the neurotoxic effects of HIV might lead to injury in the dopaminergic terminals, we hypothesized that: 1) HIV patients will show decreased DAT availability, and only moderately decreased postsynaptic D2 receptor (D2R) binding, which would validate the prior observations (Wang et al., 2004); 2) the decreased DAT would be associated with poorer cognitive performance in these HIV patients; 3) HIV patients with a history of cocaine-dependence would show further decreases in DAT and D2R availability compared to those without a history of drug abuse.
Methods
Subjects
Participants were recruited from the local community and each signed a written informed consent approved by our institution. We enrolled 35 HIV subjects, 24 without a history of drug use (HIV) and 11 with a history of cocaine-dependence (HIV+Coc), and 14 healthy seronegative controls (SN). Each subject was evaluated with a neuropsychiatric examination [including assessments for HIV disease severity (see Table 1)], screening blood tests (complete blood count, chemistry panel, thyroid hormones, protime/prothrombin time, hepatitis A, B & C serology, and HIV test if the status is not known), urine tests (urinalysis, drug screen and pregnancy test if female), and an electrocardiogram.
Table 1.
Clinical characteristics of participants (mean ± SD)
| SN(n = 14) | HIV(n = 24) | HIV+Coc (n=11) | P value* | ||||
|---|---|---|---|---|---|---|---|
| ANOVA | SN vs. HIV | SN vs. HIV+Coc | HIV vs. HIV+Coc | ||||
| Age (years) | 40.8±12.0 | 44.5±11.9 | 39.6±5.2 | ns | ns | ns | ns |
| Female/Male | 4/10 | 7/17 | 3/8 | ||||
| Education (years) | 15.0±2.3 | 13.4±1.9 | 12.8±2.0 | 0.02 | 0.03 | 0.02 | ns |
| Center for Epidemiological Scale-Depression | 6.6±5.2 | 15.3±7.0 | 14.8±12.1 | 0.006 | 0.0003 | 0.03 | ns |
| Thyroid stimulating hormone | 1.9±1.1 | 1.7±1.0 | 1.3±0.7 | ns | ns | ns | ns |
| Folstein Mini-Mental State examination (MMSE, maximum 30) | 29.6±0.5 | 27.8±1.9 | 28.2±1.9 | 0.01 | 0.003 | 0.02 | ns |
| Duration of HIV diagnosis (mo) | 88. 3±63.2 | 110.91±71.20 | ns | ||||
| CD4 (#/mm3) | 230±158 | 240±171 | ns | ||||
| Nadir CD4 (#/mm3) | 99±104 | 87±70 | ns | ||||
| Plasma Viral Load(copies/mL) | 90,288±179,056 | 142,122±275,009 | ns | ||||
| Log Viral Load | 3.7±1.4 | 3.6±1.5 | ns | ||||
| HIV Dementia Scale (maximum 16) | 13.8±2.5 | 13.1±2.0 | ns | ||||
| Karnofsky score (maximum 100) | 85.8±8.8 | 92.7±4.7 | 0.02 | ||||
| AIDS dementia complex (ADC) Stage | 1.02±0.50** | 0.96±0.57*** | ns | ||||
P values are run separately for each variable in StatView from unpaired t-tests (HIV vs. HIV+Coc) or one-way ANOVA (all three groups).
ADC stage in HIV subjects: 7 with stage 0.5 (correspond to minor cognitive motor disorder), 13 with stage 1 and 4 with stage 2 dementia
ADC stage in HIV+Coc subjects: 5 with stage 0.5 and 4 with stage 1, and 2 with stage 2
All HIV subjects fulfilled these inclusion criteria: 1) age ≥20 years; 2) seropositive for HIV and had some cognitive deficits either by subjective complaints, clinical evaluations or on neuropsychological testing; 3) CD4<500/mm3 within the past six months; 4) on no antiretrovirals or stable on a regimen for at least 8 weeks prior to the study; 5) able to provide written informed consent; 6) education level≥8th grade. 7) HIV+Coc subjects also fulfilled DSM-IV criteria for cocaine-dependence for at least 2 years, but had been abstinent from psychostimulant use ≥one month. In contrast, HIV subjects did not fulfill DSM-IV criteria for any drug dependence. SN subjects fulfilled inclusion criteria 1, 5, 6 and had to be seronegative for HIV, and on no medications except for vitamins. Subjects were excluded if they had: 1) past or present history of psychiatric illness which may confound the analysis of the study (e.g., schizophrenia, major depression); 2) presence of opportunistic brain lesions; 3) confounding neurological disorder; 4) severe hepatic or renal dysfunction; 5) present or past history of drug dependence (except for cocaine in the HIV+Coc group) or nicotine usage more than one pack per day; 6) positive urine toxicology screen or positive pregnancy test if female; 7) head trauma with loss of consciousness >30 minutes.
PET scanning
PET scans were performed with a Siemens EXACT HR+ (Knoxville, TN) tomograph (resolution 4×4×4.5 mm FWHM, 63 slices). Each subject received two tracers, [11C]raclopride and [11C]cocaine. Procedures for subjects positioning and scanning protocol were described previously11. Briefly, for [C-11]raclopride, dynamic scans were started immediately after intravenous injection of 3–8 mCi (specific activity, >0.25 Ci/µmol, at time of injection) for 60 minutes11. For [C-11]cocaine, dynamic scans were started immediately after intravenous injection of 3–8 mCi (specific activity, >0.25 Ci/µmol, at time of injection) for 54 minutes.
Data Analysis
Regions of interest (ROI) in basal ganglia (caudate, putamen and ventral striatum) and cerebellum in both hemispheres were drawn directly on an emission image that represented the sum of images obtained 10–60 minutes for [C-11]raclopride, and 10–54 minutes for [C-11]cocaine. ROIs for basal ganglia were obtained bilaterally from three planes where they were best identified. Right and left cerebellar regions were drawn in three planes, 1.0, 1.4 and 1.8 cm, above the canthomeatal line. These regions were then projected onto the dynamic images to generate time activity curves for striatum and cerebellum. Average values for the basal ganglia and cerebellar regions were computed from three slices, and the two hemispheres were averaged. The measure Bmax/Kd, the ratio of the distribution volume in basal ganglia to that in cerebellum minus 1, was obtained using a graphical analysis method without blood sampling technique for reversible systems [Logan Plots (Logan et al., 1994)].
Neuropsychological evaluations
The neuropsychological tests were performed within one month of the PET studies and evaluated: 1) gross motor function (Timed Gait); 2) fine motor coordination (Grooved Pegboard Test); 3) immediate and delay memory function: Rey Auditory Verbal Learning Tests; 4) attention and psychomotor speed: Symbol Digit Test, Trail-making Tests A and B; 5) executive function: Stroop Color Interference Tests; 6) California Computerized Assessment Package (CalCAP–customized version, which includes evaluation of working memory, psychomotor speed and reaction times). In addition, mood and depressive symptoms were assessed with Center for Epidemiologic Studies-depression scale (CES-D), and premorbid verbal intelligence quotient (VIQ) was estimated from the National Adult Reading Test (NART).
Statistical Analysis
Analyses of covariance (ANCOVAs) were performed to evaluate group differences in clinical variables (Table 1), using StatView and SAS packages (both SAS Institute, Cary, NC). These analyses were adjusted for possible confounders (age, education, VIQ, CES-D, MMSE, and nicotine use). To determine whether PET and neuropsychological variables showed increasing abnormalities with the number of brain insults (from SN to HIV to HIV+Coc), a “trend test” was performed, by adjusting p-values for correctly-ordered variables for the probability of obtaining the predicted ordering by chance (i.e. dividing p-values by 6, since there are 6 possibilities of ordering 3 values) (Howell, 2001). For these trend-tests, a correction for multiple comparisons using the Simes procedure (Simes, 1986) was applied for neuropsychological measures, but not for the dopamine variables, because of a priori knowledge (Wang et al., 2004). For variables that showed a significant effect on the trend test, multiple linear regression analyses were also performed, to explore the relationships between DAT and D2R availabilities (Bmax/Kd), clinical variables (CD4, viral load, length of abstinence (log), HIV dementia scale and ADC stage), and neuropsychological measures. Age, VIQ and nicotine use were included as covariates in these analyses, since they may affect neuropsychological and dopamine measures (Volkow et al., 1996a; Volkow et al., 2000).
Furthermore, a structural equation model (SEM) was created in SAS to evaluate the possible effect of dopamine variables on neuropsychological function. To minimize the number of variables in the SEM, principal component analyses (PCA) were performed for the PET (left and right hemisphere combined) and the neuropsychological data. The first two of the PET, and the first four of the neuropsychological, principal components were then used as “measured variables” in the SEM. The SEM model involved two “latent” variables, one of which (“dopamine function”) which was assumed to have a causal relation to the dependent latent variable “cognitive function”. A chi-square test was used to determine how well the experimental data were modeled by the SEM.
Results
Subject characteristics and clinical assessments
Table 1 shows no differences in the three subject groups with respect to age or gender proportion. However, SN subjects had higher education than HIV subjects. The two HIV groups had similar duration of HIV diagnosis, CD4 count, nadir CD4 count, plasma viral loads, HIV dementia scale scores and the AIDS dementia staging. Although none of the participants were clinically depressed or required antidepressants, both HIV groups had more depressive symptoms than the SN group, as measured by the CES-D (p=0.006). Compared to the SN, both HIV groups had slightly lower scores on the MMSE. The HIV group also had marginally lower Karnofsky scores than HIV+Coc.
All but one HIV subjects, who was medication-naïve, were treated on stable antiretroviral medications. The average duration of treatment on current regimen was 24.5±22.8 months. Nine HIV subjects had undetectable plasma viral load (4 in HIV+Coc group and 5 in HIV group). Two HIV subjects and one HIV+Coc subject also had history of hepatitis C but were treated and had no liver dysfunction. None of the subjects had resting tremor or postural instability. However, 3 HIV and 3 HIV+Coc subjects had mild bradykinesia and 2 had mildly increased rigidity.
All HIV+Coc subjects had used cocaine daily, with average use of 158±93 months (median: 136 months; range: 60–384 months), mean usage of 1.45±1.2 grams/day (median: 1 gram/day; range: 0.2–4 grams/day), and last used cocaine 59.8±74 months ago (median: 24 months; range: 1–204 months). One HIV+Coc subject also used methamphetamine on the weekends (2–3 days/week) for 5 years and another HIV+Coc subject used methamphetamine and MDMA on rare occasions. Five HIV+Coc, one HIV and one SN subjects also used marijuana (≤once/week). Six HIV+Coc, 5 HIV, and 5 SN subjects also used alcohol regularly but did not fulfill criteria for abuse or dependence. Lastly, half of the HIV subjects smoked nicotine [HIV: 12/24 (4 were past smokers); HIV+Coc: 6/11] and only 2 SN smoked nicotine (1 past smoker).
Dopamine transporter and receptor levels (Table 2, Figure 1 and Figure 2)
Table 2.
Dopamine transporters and receptors in seronegative controls and HIV subjects
| P – values (F or t values)* | |||||||
|---|---|---|---|---|---|---|---|
| SN(n=14) | HIV (n=24) | HIV+Coc (n=11) | Trend-test df=(2, 46) | SN vs. HIV df = 36 | SN vs. HIV+Coc df = 23 | HIV vs. HIV+Coc df = 33 | |
| C-11 cocaine (Dopamine Transporter availability) | |||||||
| Caudate | |||||||
| Bmax /Kd | 0.74±0.08 | 0.68±0.14 | 0.65±0.15 | 0.04 (F=1.5) | ns | 0.04 (t=1.8) (−12.2%) | ns |
| Putamen | |||||||
| Bmax /Kd | 0.90±0.11 | 0.79±0.17 | 0.75±0.14 | 0.005 (F=3.9) | 0.02 (t=2.2) (−12.2%) | 0.003 (t=3.1) (−16.7%) | ns |
| Ventral Striatum | |||||||
| Bmax /Kd | 0.70±0.09 | 0.66±0.11 | 0.64±0.14 | ns | ns | ns | ns |
| C-11 raclopride (Dopamine D2 Receptor availability) | |||||||
| Caudate | |||||||
| Bmax /Kd | 2.41±0.26 | 2.19±0.39 | 2.18±0.42 | 0.03 (F=2.0) | 0.03 (t=1.9) (−9.1%) | 0.05 (t=1.7) (−9.5%) | ns |
| Putamen | |||||||
| Bmax/Kd | 2.97±0.25 | 2.70±0.49 | 2.64±0.55 | 0.02 (F=2.2) | 0.03 (t=1.9) (−9.1%) | 0.03 (t=2.0) (−11.1%) | ns |
| Ventral Striatum | |||||||
| Bmax/Kd | 2.26±0.34 | 2.27±0.39 | 2.22±0.53 | ns | ns | ns | ns |
P values for the trend tests are predictions for the values to be SN > HIV > HIV+Coc
P values for the post hoc unpaired t-tests predicts SN>HIV, SN>HIV+Coc or HIV>HIV+Coc.
Percentages are calculated as the corresponding deviations from SN controls.
Figure 1.
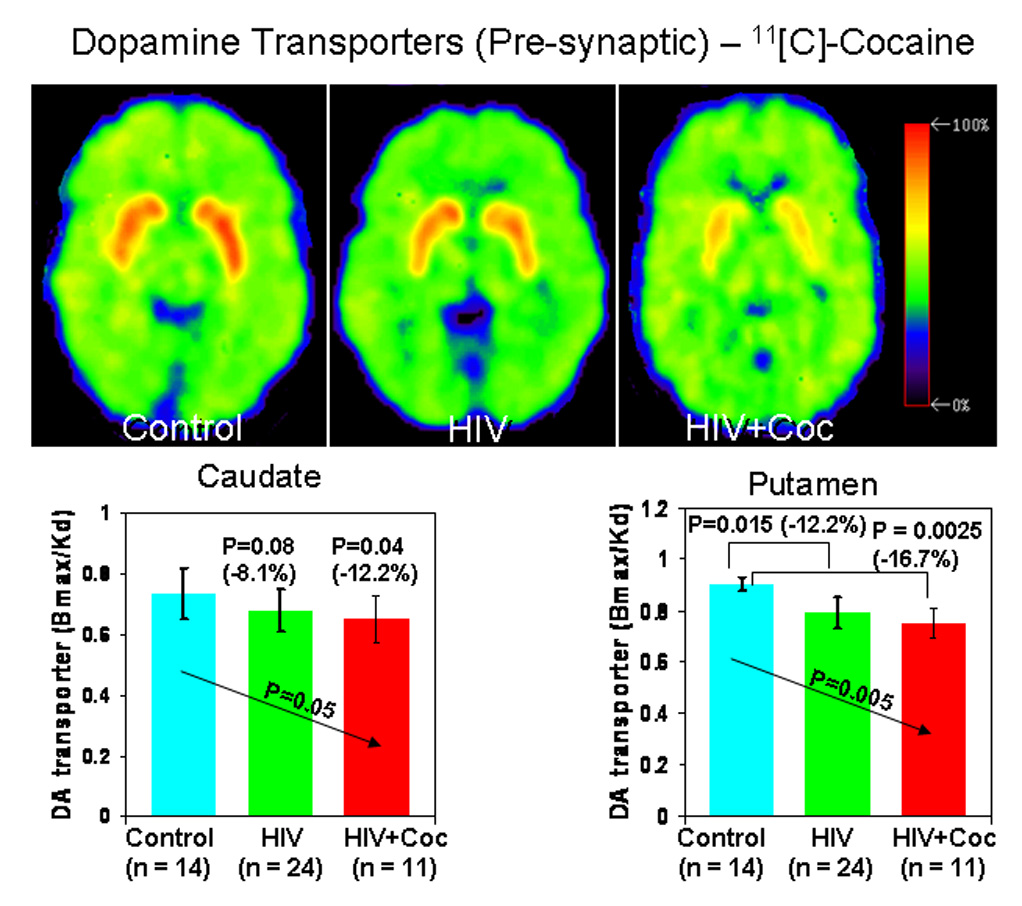
Distribution volume ratio images of PET with [11C]cocaine (DA transporter) in a seronegative control subject (SN), an HIV subject (HIV), and an HIV subject with a history cocaine-dependence (HIV+Coc). The images are shown at the level of the basal ganglia and are scaled with respect to the maximum value obtained in the control subject and presented using the rainbow scale (red color - high value, violet – low value). Bargraphs below show the DA transporter availability (Bmax/Kd) in the two HIV subject groups and control subjects.
Figure 2.
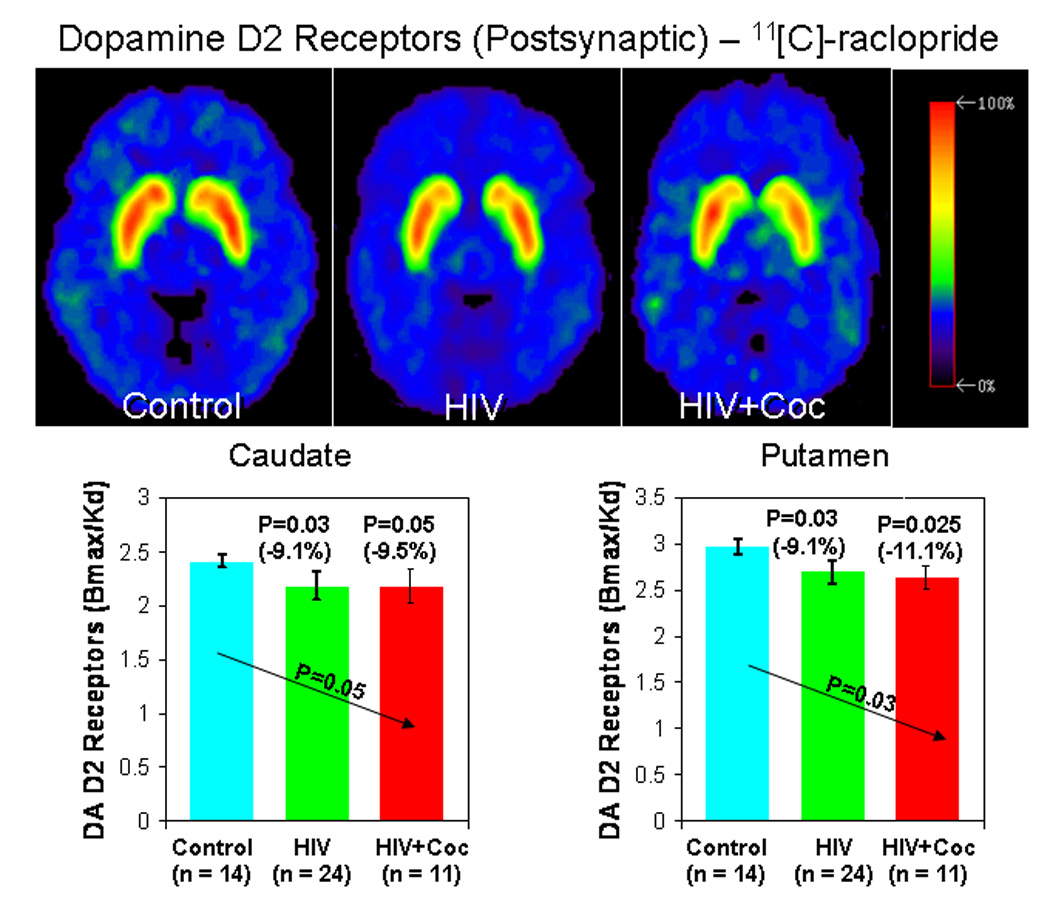
Distribution volume ratio images of PET with [11C]raclopride (DA D2 receptor) in a seronegative control subject, an HIV subject, and an HIV subject with a history of cocaine-dependence (HIV+Coc). Bargraphs below show the DA D2 receptor availability (Bmax/Kd) in the two HIV subject groups and control subjects.
Since the left and right hemisphere DAT and D2R densities were not different, values from the two sides were averaged to minimize multiple comparisons. The trend tests demonstrated a significant compounding effect of HIV or HIV+Coc on the DAT and D2R densities in the putamen and to a lesser degree in the caudate, but not in the ventral striatum (Figure 1 and Figure 2, and Table 2). These findings were essentially unchanged when we covaried for age, education, VIQ, CES-D and MMSE. We also evaluated the possible effect of nicotine smoking on these PET variables. While nicotine use had no effect on DAT in these three brain regions, the group effect on D2R became non-significant in the caudate and putamen (p-value=0.11 for both regions). The p-values for nicotine use independent of the group status were 0.08 and 0.02 for the caudate and putamen, respectively. The same results were obtained when we controlled for each covariate one at a time and when all the covariates were included in the model at once.
Correlation between dopamine transporters and clinical variables
These correlations were limited to variables that showed a significant group effect or trend test in Table 1 and Table 2 (at p≤0.05). Subjects with higher DAT (Bmax/Kd) in the putamen performed better on the MMSE (p=0.01). Additionally, caudate D2R density declined with age (p=0.03). DA variables did not correlate with other clinical variables, including CES-D, Karnofsky or ADC scores, length of abstinence from cocaine use (log), or measures of systemic disease (CD4 counts, log viral load).
Neuropsychological test performance (Table 3 and Table 4)
Table 3.
Neuropsychological test performance in seronegative (SN) controls, HIV subjects and HIV subjects that used Cocaine (HIV+Coc)
| P-values (F-value, t-values and % difference) | |||||||
|---|---|---|---|---|---|---|---|
| SN Controls (n=14) | HIV (n=24) | HIV+Coc (n=11) | Trend test df= (2,46) | SN vs. HIV df = 36 | SN vs. HIV+Coc df = 23 | HIV vs. HIV+Coc df = 33 | |
| Gross motor function | |||||||
| Timed Gait (s) | 8.9±1.4 | 10.4±1.6 | 10.6±2.3 | 0.004* (F=4.2) | 0.003 (t=2.9; 16.8%) | 0.01 (t=2.3; 19.1%) | ns |
| Fine motor function (Grooved Pegboard) | |||||||
| Dominant hand (s) | 58.3±7.9 | 70.1±19.3 | 67.7±12.1 | ns | 0.02 (t=2.2; 20.2%) | 0.01 (t=2.3; 16.1%) | ns |
| Non-Dominant hand (s) | 68.8±16.9 | 82.9±26.2 | 75.5±9.8 | ns | 0.05 (t=1.7; 20.5%) | ns | ns |
| Verbal Memory (Auditory Verbal Learning Test) | |||||||
| Immediate (# words) | 7.0±2.2 | 5.9±2.1 | 6.1±1.4 | ns | ns | ns | ns |
| After 5 trials (# words) | 11.8±2.0 | 11.2±2.3 | 10.5±2.6 | ns | ns | ns | ns |
| Following interference(#words) | 10.6±3.0 | 8.9±3.6 | 8.0±2.8 | 0.02* (F=2.1) | ns | 0.02 (t=2.4; −24.5%) | ns |
| Delayed (# words) | 9.7±3.5 | 8.3±3.2 | 7.2±3.9 | 0.03* (F=1.7) | ns | 0.05 (t=1.7; −25.8%) | ns |
| Psychomotor speed | |||||||
| Trail A (s) | 24.2±6.5 | 30.4±11.7 | 30.5±9.9 | 0.03* (F=1.9) | 0.04 (t=1.8; 25.6%) | 0.03 (t=1.9; 26.0%) | ns |
| Trail B (s) | 54.6±11.8 | 80.2±36.6 | 84.7±37.5 | 0.006* (F=3.7) | 0.01 (t=2.5; 46.9%) | 0.005 (t=2.8; 55.1%) | ns |
| Symbol Digit Modalities (# correct) | 58.7±3.6 | 47.9±13.0 | 43.2±10.2 | 0.0003* (F=7.5) | 0.002 (t=3.0; −18.6%) | <0.0001 (t=5.3; 26.4%) | ns |
| Executive function | |||||||
| Stroop Color (s) | 56.2±9.4 | 66.8±16.9 | 72.7±21.6 | 0.008* (F=3.2) | 0.02 (t=2.1; 18.4%) | 0.01 (t=2.5; 29.6%) | ns |
| Stroop Word (s) | 43.4±8.9 | 49.8±13.4 | 60.2±21.6 | 0.004* (F=4.1) | ns | 0.007 (t=2.6; 38.7%) | 0.05 (t=1.7) |
| Stroop Interference (s) | 107.0±30.3 | 127.5±50.4 | 143.6±43.2 | 0.02* (F=2.2) | ns | 0.01 (t=2.5; 34.2%) | ns |
| Estimated verbal Intelligence Quotient | |||||||
| NART-VIQ | 116.7±5.4 | 106.2±13.2 | 104.8±13.1 | 0.02 (F=4.3) | 0.01 (t=2.7; −8.1%) | 0.007 (t=3.0; −10.2%) | ns |
P values for the trend tests are predictions for the values to be SN > HIV > HIV+Coc (asterisks indicate significance after correction for multiple comparisons)
P values for the post hoc unpaired t-tests predicts SN>HIV, SN>HIV+Coc or HIV>HIV+Coc.
Percentages are calculated as the deviations from SN controls.
Table 4.
Reaction times on CalCAP tasks (milliseconds) in SN, HIV subjects and HIV subjects who used cocaine
| Tasks | SN Controls (n=14) | HIV (n=24) | HIV+Coc (n=11) | P-values (F or t-values and % change) | |||
|---|---|---|---|---|---|---|---|
| Trend test df= (2, 46) | SN vs. HIV df = 36 | SN vs. HIV+Coc df = 23 | HIV vs. HIV+Coc df = 33 | ||||
| Simple reaction | 293±31 | 325±86 | 350±79 | 0.03* (F=1.9) | ns | 0.01 (t=2.5; 19.5%) | ns |
| Choice reaction("7") Single digit recognition | 388±30 | 408±47 | 417±55 | 0.04* (F=1.4) | ns | ns | ns |
| 1-back cued response (“X” only after “A”) | 388±55 | 461±122 | 452±113 | ns | 0.02 (t=2.1; 18.8%) | 0.04 (t=1.9; 16.5%) | ns |
| Sequential # (1-back) | 503±119 | 614±125 | 606±99 | ns | 0.006 (t=2.7; 22.3%) | 0.02 (t=2.3; 20.5%) | ns |
| Sequential # (1-increment) | 531±105 | 638±112 | 651±118 | 0.002* (F=4.9) | 0.003 (t=2.9; 20.2%) | 0.008 (t=2.6; 22.6%) | ns |
| Sequential # (2-back) | 670±156 | 843±175 | 817±165 | ns | 0.002 (t=3.0; 25.9%) | 0.02 (t=2.2; 21.8%) | ns |
| Degraded words with distracters | 517±60 | 596±133 | 537±73 | ns | 0.02 (t=2.1; 15.1%) | ns | ns |
| Response reversal / visual scanning | 640±140 | 703±124 | 733±114 | 0.03* (F=1.8) | ns | 0.05 (t=1.7; 14.5%) | ns |
| Form discrimination | 675±111 | 854±159 | 774±127 | ns | 0.0004 (t=3.7; 26.5%) | 0.03 (t=2.0; 14.7%) | ns |
P values for the trend tests are predictions for the values to be SN > HIV > HIV+Coc (asterisks indicate significance after correction for multiple comparisons)
P values for the post hoc unpaired t-tests predicts SN>HIV, SN>HIV+Coc or HIV>HIV+Coc.
Percentages are calculated as the corresponding deviations from SN controls
The two HIV-infected groups performed more poorly on many cognitive tasks, typically worst in the HIV+Coc group; 13/22 of these tests showed group effects even after correction for multiple comparisons (Table 3 and Table 4). Worsening performance were observed for Symbol Digit Modality task (trend test: p=0.0003) and Stroop Color-Word Interference tasks (Color: p=0.008; Word: p=0.004; Interference: p=0.02), with the slowest performance in the HIV+Coc group. Worsening cognitive performance also was observed on Timed Gait, two of the Auditory Verbal Learning tests (after interference and delayed), Trail A & B, and several CalCAP tests (that involve working memory and greater attention). No differences were found between HIV subjects who were smokers and HIV non-smokers on any of the cognitive tests.
Correlation between dopamine transporters and cognitive function
The correlation analyses were limited to variables that showed significant group trend tests (at p≤0.05). Figure 3 shows the simple regression analyses that showed the strongest relationship between DAT and the cognitive measures. When age, VIQ and nicotine were included as covariates, lower DAT in the putamen remain significantly correlated with poorer performance on multiple neuropsychological tests: verbal memory (AVLT recall after 5 trials: p=0.037, AVLT recall after interference: p=0.005*; AVLT delayed recall: p=0.011), and psychomotor tasks (Symbol Digit: p=0.010; Trail A: p=0.044). Lower putamen DAT also was associated with slower reaction times on several CalCAP tasks that involve working memory (sequential numbers: 1-back, p=0.002*, 1-increment, p=0.011, 2-back, p=0.004*). Similarly, lower caudate DAT density was associated with poorer performance on AVLT-interference (p=0.019) and Symbol Digit Modalities (p=0.032), and two CalCAP tasks that involved working memory (sequential numbers: 1-back, p=0.020 and 2-back, p=0.030). P-values marked with a asterisk were significant after correction for multiple correlations.
Figure 3.
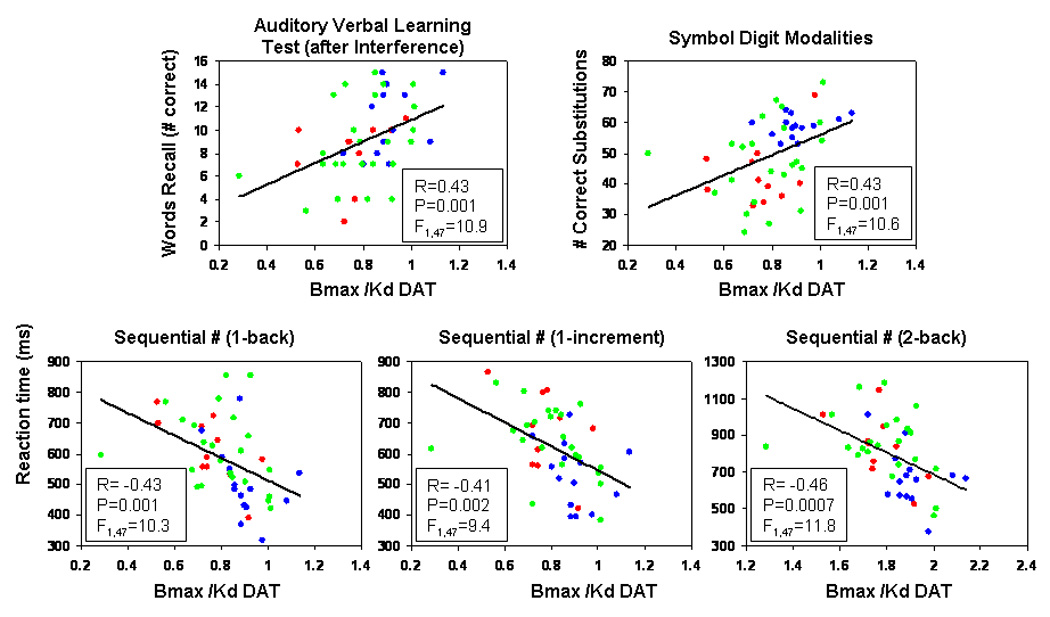
Correlations between measures of DA transporter availability (Bmax/Kd) in the putamem and neuropsychological test performance. R and p-values reflect statistical values from simple linear regression analyses; however, the results remained significant after covarying for age, VIQ and nicotine use (see results). Blue dots: seronegative controls (SN); Green dots: HIV subjects without a history of drug abuse (HIV); Red dots: HIV subjects with a history of cocaine-dependence (HIV+Coc).
SEM of PET and neuropsychological data
The SEM analysis was based on the results of the PCAs (Figure 4). The DA measures were represented by the first two PCs of the PET data, which captured approximately 86% of the total variance. The first PC had near-identical loadings for all six DA variables, and therefore reflects the average DAT and D2R level (“DA_status”). The second PC essentially represents the difference between DAT and D2R (“DA_balance”).
Figure 4.
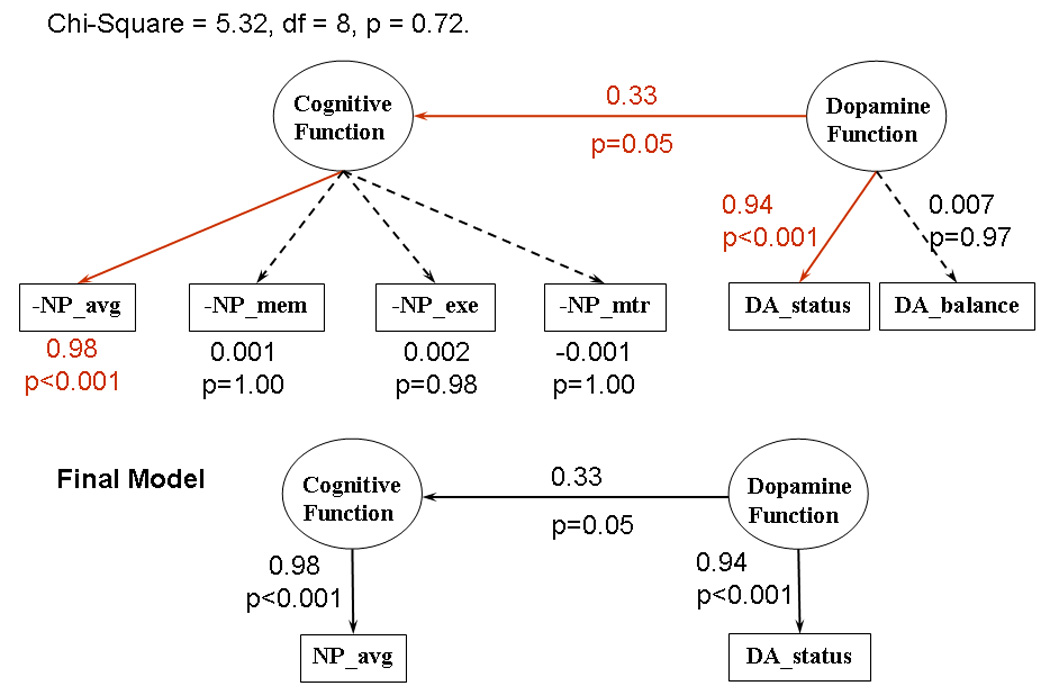
Structural equation modeling showing that the dopamine function is expressed as the dopamine status (average of dopamine transporters and dopamine receptors), which in turn affects the cognitive function of the subjects, specifically the average performance of all neuropsychological tests. Abbreviations for the principal components: NP-avg = average performance of all cognitive tasks, NP-mtr = motor tasks, NP-exe = executive function, NP-mem = memory tasks.
The first 4 PCs of the 22 neuropsychological variables captured 69% of the total variance (PC1: 41%, PC2: 13%, PC3: 8%, PC4: 7%). The first PC reflected the “average performance” (“NP_avg”) across all NP tasks, with better performance on a given task resulting in a higher value on the first PC. The second PC had strong weightings from variables in Memory and Learning tasks, and was denoted NP_mem. The third PC represented a measure of executive function (NP_exe), while the fourth represented motor function (PC_mtr).
The SEM model involved the independent “latent” variable “dopamine function”, which was assumed to have a causal relation to the dependent latent variable “cognitive function” (Figure 3). The independent latent variable was measured by the two PCs DA_Status and DA_Balance, while the dependent latent variable was measured by the 4 PCs NP_avg, NP_mem, NP_exe and NP_mtr.
The model showed an excellent fit to the data (goodness of fit index = 0.958, Chi-Square = 5.32, df = 8, p = 0.72). Two paths involving measured variables, from “Cognitive Function” to NP_avg, and from “Dopamine Function” to DA_Status, were significant (t = 8.61, p<0.001, and t = 8.33, p<0.001). The causal relation from “dopamine function” to “cognition function” was significant at p=0.05. The positive path coefficients from DA_Status via dopamine and cognitive function to NP_Avg indicate that reduced dopamine transporters and receptors are related to poorer overall cognitive function.
Discussion
Lower densities of dopamine transporters and receptors are related to poorer cognitive function, even after accounting for the independent effects of age, nicotine use and VIQ on these measures. Therefore, decreased dopaminergic function may contribute to HIV-associated cognitive deficits. The current study confirms prior observation of lower presynaptic DAT availability in the putamen of HIV patients (Wang et al., 2004). We additionally found lower postsynaptic D2 receptors in both the caudate and putamen in our HIV subjects, which were accounted for by the greater nicotine use in these subjects. Psychostimulant abuse (primarily cocaine), however, led only to a trend for further minimal decreases in DAT, and no additional decreases in D2R, in the caudate and putamen of these HIV subjects. Regardless of HIV status or cocaine use history, lower DAT availability was associated with poorer performance on tests that involve motor, psychomotor, attention and working memory function, while lower binding for D2R which occurs with aging and with nicotine use also contributed to slower motor function. Together, these findings suggest that HIV infection is associated with dopaminergic deficit that underlie the poorer cognitive performance.
Neuropathology of decreased dopamine markers in HIV
Decreased DAT indicates injury or alteration to presynaptic dopaminergic nerve terminals, while decreased D2R reflect changes postsynaptically (Volkow et al., 1996b), primarily on striatal GABAergic and cholinergic neurons (Scheel-Kruger, 1986) as well as on astroglia (Khan et al., 2001). Postmortem studies demonstrated decreased dopamine and homovallinic acid in the caudate of HIV patients, suggesting loss of nigrostriatal dopaminergic neurons (Sardar et al., 1996), and neuronal loss (up to 25%) in the substantia nigra (Reyes et al., 1991). Decreased dopamine in the putamen and frontal cortex, but not in the substantia nigra, were also observed in a Simian immunodeficiency virus (SIV) model during the early asymptomatic phase (Czub et al., 2001). In contrast, a postmortem study of 6 individuals with HIV-encephalitis found increased presynaptic messenger RNA for DAT (Gelman et al, 2006); however, the protein level may not reflect the binding capacity of the transporter as measured in our PET study. Furthermore, this postmortem study also found decreased postsynaptic D2R in HIV patients as observed in the current study, although nicotine use status was not discussed in the study (Gelman et al, 2006).
HIV proteins (e.g. Tat and gp120) also can cause toxicity to dopaminergic neurons in vitro and in rodent models (Nath et al., 2000). Imaging studies of HIV patients demonstrated that basal ganglia, brain reigons with the highest density of dopaminergic synapses, had altered glucose metabolism (Rottenberg et al., 1996), reduced volumes (Jernigan et al., 2005), and increased gadolinium-enhancement, which suggested increased blood-brain barrier permeability and viral entry in these regions (Avison et al., 2004). Neuropathological indeed showed high HIV viral burden, multinucleated giant cells and glial-microglial infiltrations, in the basal ganglia (Kure et al., 1990) which may injure the dopaminergic neurons and hence decreased dopaminergic synapses. Unlike the earlier study (Wang et al., 2004), plasma viral load did not correlate with DAT levels in the current study. The lack of correlation might be due to the undetectable plasma viral loads in 9 of the HIV subjects, who may not have equally effective viral suppression in their brains. As reported, plasma or even CSF viral load may no longer correlate with cognitive function in HIV patients treated with potent antiretrovirals (Cysique et al., 2005). Surrogate markers for brain injury, such as these dopaminergic tracers that correlate well with cognitive function may be more useful to monitor brain injury and treatment effects in HIV patients with cognitive deficits.
Relationship between decreased dopamine markers and cognitive dysfunction
The greater reduction of DAT in the putamen than in the caudate of HIV patients parallels that observed in patients with Parkinson’s disease (Miller et al., 1997), although the magnitude of the DAT loss is significantly less in HIV patients. In contrast, cocaine users without HIV do not show decreased DAT in either regions (Wang et al., 1997), while methamphetamine users showed greater decreases of DAT in caudate than in putamen, both on PET (caudate:−28%; putamen:−21%)(Volkow et al., 2001) and in postmortem studies (caudate:−61%; putamen:−50%) (Moszczynska et al., 2004). Because of the mildly decreased DAT, only few HIV patients had signs of mild parkinsonism; as a group, however, they performed slower on tasks that involve motor, psychomotor function, attention and working memory. Similar to patients with Parkinson’s disease (Marie et al., 1999), the severity of cognitive deficits correlated with reduced DAT in both putamen and caudate. Our findings are also consistent with neuropathology findings that density of microtubule-associated protein (reflecting neuronal cell bodies and dendrites) and synaptophysin (measure of presynaptic terminals) in the putamen predicted antemortem cognitive impairment (Moore et al., 2006). Additionally, decreased D2R in the caudate and putamen in our HIV patients correlated with slower gross and fine motor function, which appears to be partly related to aging.
Using structural equation modeling (SEM), we evaluated a model in which a latent variable “cognitive function”, assessed by neuropsychological testing, was assumed to be driven by the latent variable “dopamine function”, assessed by the PET measurements (Figure 3). The analysis demonstrated that the overall structural equation model was consistent with the experimental data. The latent variable “dopamine function” was represented by the average of all six dopamine variables measured, and the latent variable “cognitive function” was represented by the average of all individual neuropsychological z-scores. In contrast, none of the three specific cognitive domains extracted by the PCA (memory/learning, executive function, and motor) showed a major influence of the dopamine variables. Therefore, across these three different groups, the level of subcortical dopamine status appears to affect predominantly the overall cognitive function, rather that any specific cognitive domain.
Cocaine abuse may further contribute to dopamine deficits and cognitive dysfunction
Compared to SN and HIV subjects, the HIV+Coc group showed only a small trend for further reduction of DAT and DA D2R in both the caudate and putamen. The further reduction in dopamine markers also associated with greater cognitive dysfunction in the HIV+Coc subjects, despite comparable plasma viral burden and degree of immunosuppression (CD4 count) relative to the HIV subjects without drug abuse. These further small reductions in dopamine markers might have resulted from both direct and indirect effects from cocaine. Although cocaine abuse is associated typically with decreased D2R (Volkow et al., 1999), but no change or even upregulation of DAT (Mash et al., 2002; Wang et al., 1997), it has been shown to enhance HIV viral replication both in vitro and in vivo in mice (Roth et al., 2002; Scheller et al., 2000) and in an SIV model (Czub et al., 2004), hence leading to greater neuronal toxicity and decreased binding of DAT. Lastly, cocaine can also suppress the immune system (macrophage killing (Lefkowitz et al., 1996), down regulation of cytokines (Mao et al., 1996), which indirectly allow the virus to replicate and cause brain injury.
Limitations and possible treatment approaches
First, the small sample size of the HIV+Coc subgroup coupled with the small effect size led to the non-significant further reduction in DAT compared HIV subjects without a history of cocaine user. Future study with a larger comparison group of HIV+Coc subjects is needed to determine whether cocaine (or psychostimulant) abuse is associated with further reduction in DAT in HIV patients. Second, two of the HIV+Coc subjects also abused methamphetamine regularly and MDMA occasionally. Methamphetamine might lead to even greater decrease in DAT; however, the subjects with the greatest decrease in DAT had used cocaine only. Third, our cocaine users had been abstinent for almost two years on average; more recent cocaine use may demonstrate a larger effect on the decreased DAT in HIV patients. Fourth, our HIV subjects had greater usage of nicotine compared to SN which appears to have accounted for the decreased D2R, although nicotine use did not appear to affect DAT or cognitive performance. Larger sample sizes for the subgroups (with or without nicotine use) and preclinical models are needed to further delineate the contributory effects of nicotine smoking on dopaminergic function in HIV patients. Lastly, some of the subjects also used marijuana and alcohol recreationally; however, we excluded those who abused or tested positive for any of these drugs on the days of the PET or cognitive tests.
Reduced dopaminergic function in HIV patients, with and without additional psychostimulant abuse, in relation to poorer cognitive function suggests that these patients may benefit from treatments that enhance dopamine function. One study found improvement on cognitive performance in HIV patients after treatment with methylphenidate (Hinkin et al., 2001). However, the enhancement of HIV viral replication by several dopaminergic agents (including cocaine) suggests that cessation of psychostimulant use and alternative means to improve dopaminergic function are needed. Memantine, an NMDA receptor antagonist, also have been shown to prevent dopaminergic deficits, by upregulating neurotrophic factor brain-derived neurotrophic factor (BDNF), in SIV-infected macaques (Meisner et al., 2007). However, a 16-week trial of memantine was conducted in HIV patients and found no significant improvement in their cognitive function, but the dopaminergic system was not evaluated (Schifitto et al, 2007). Dopamine agonists or combined treatments with antioxidants (e.g. glutathione, N-acetylcysteine) that block the effects of dopamine-mediated HIV activation and neurotoxicity may be useful alternatives for adjunctive or preventive therapy for HIV-associated cognitive deficits.
Acknowledgments
This research was carried out at Brookhaven National Laboratory (BNL) and support by the U. S. Department of Energy, Office of Biological and Environmental Research (DE-ACO2-76CH00016) and the National Institute on Drug Abuse (DA K24-DA16170; K02-DA16991). We thank David Schlyer and Michael Schueller for Cyclotron operations; Donald Warner and David Alexoff for PET operations; Richard Ferrieri, Colleen Shea, Youwen Xu, Victor Garza and Payton King for radiotracer preparation and analysis; Dana Carasig, Lisa Zimmerman and Naomi Pappas for subject recruitment, Noelwah Netusil, Pauline Carter and Millard Jayne for nursing support, Chris Wang, Xuena Wang and Caroline Jiang for data management and analyses. We also thank Dr. Jack Fuhrer (SUNY-Stony Brook Medical Center) for HIV subject referrals, and we are especially grateful to the research participants in this study. None of the authors has any competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Neurology AIDS Task Force Working Group. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type-1. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR. Neuroimaging correlates of HIV-associated BBB compromise. Journal of Neuroimmunology. 2004;157:140–146. doi: 10.1016/j.jneuroim.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez J, Levin B. Cerebrospinal fluid dopamine in HIV-1 infection. Acquired Immunodeficiency Syndrome. 1994;8:67–71. doi: 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ, Halman M, Catalan J, Sacktor N, Price RW, Brown S, Atkinson JH, Clifford DB, Simpson D, Torres G, Hall C, Power C, Marder K, McArthur JC, Symonds W, Romero C. Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr. 2005;39:426–429. doi: 10.1097/01.qai.0000165799.59322.f5. [DOI] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller J, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol (Berl) 2004;107:216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller J, Pedersen V, Gsell W, Heeney J, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta of Neuropathology (Berlin) 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- Factor S, Podskalny G, Barron K. Persistent neuroleptic-induced rigidity and dystonia in AIDS dementia complex: a clinico-pathological case report. Journal of Neurological Sciences. 1994;127:114–120. doi: 10.1016/0022-510x(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Gelman B, Spencer J, Holzer C, Soukup V. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. Journal of Neuroimmune Pharmacology. 2006;1(4):410–420. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- Hinkin C, Castellon S, Hardy D, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;13:248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- Howell D. Statistical Methods for Psychology. 4th Edition ed. Wadsworth Publishing; 2001. [Google Scholar]
- Hriso E, Kuhn T, Masdeu J, Grundman M. Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. American Journal of Psychiatry. 1991;148:1558–1561. doi: 10.1176/ajp.148.11.1558. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Gamst A, Archibald S, Fennema-Notestine C, Mindt M, Marcotte T, Heaton R, Ellis R, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden G, Lipton S. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Khan Z, Koulen P, Rubinstein M, Grandy D, Goldman-Rakic P. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proceedings of the National Academy of Sciences, U S A. 2001;98:1964–1969. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. ACTA Neuropathologica (Berlin) 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagberg L, Forsman A, Norkrans G. Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. Journal of Neuroscience Research. 1991;28:406–409. doi: 10.1002/jnr.490280313. [DOI] [PubMed] [Google Scholar]
- Law WA, Martin A, Mapou RL, Roller TL, Salazar AM, Temoshok LR, Rundell JR. Working Memory in individuals with HIV infection. Journal of Clinical and Experimental Neuropsychology. 1994;16:173–182. doi: 10.1080/01688639408402628. [DOI] [PubMed] [Google Scholar]
- Lefkowitz SS, Vaz A, Lincoln J, Cain T, Brown DJ, Lefkowitz DL. Alteration of macrophage functions by cocaine. Advances in Experimental Medicine and Biology. 1996;402:135–144. doi: 10.1007/978-1-4613-0407-4_19. [DOI] [PubMed] [Google Scholar]
- Logan J, Volkow N, Fowler J, Wang G, Dewey S, MacGregor R, Schlyer D, Gatley S, Pappas N, King P, et al. Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. Journal of Cerebral Blood Flow and Metabolism. 1994;14:995–1010. doi: 10.1038/jcbfm.1994.132. [DOI] [PubMed] [Google Scholar]
- Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunology. 1996;172:217–223. doi: 10.1006/cimm.1996.0235. [DOI] [PubMed] [Google Scholar]
- Marie R, Barre L, Dupuy B, Viader F, Defer G, Baron J. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson's disease. Neuroscience Letters. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. Journal of Neurochemistry. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, Sheridan K, Machado AM, Van Gorp WG, Visscher B. Neuropsychological performance in HIV-1 infected homosexual men: the Multicenter AIDS Cohort Study (MACS) Neurology. 1990;40:197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- Miller G, Staley J, Heilman C, Perez J, Mash D, Rye D, Levey A. Immunochemical analysis of dopamine transporter protein in Parkinson's disease. Annals of Neurology. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. Aids. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, Meulen V, Koutsilieri E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: A novel pharmacological action of memantine. Neuropsychopharmacology. 2007 Oct 31; doi: 10.1038/sj.npp.1301615. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser K, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. Journal of Psychopharmacology. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Reyes M, Faraldi F, Senseng C, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathologica (Berlin) 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. Journal of Infectious Diseases. 2002;185:701–705. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. Journal of Nuclear Medicine. 1996;37:1133–1141. [PubMed] [Google Scholar]
- Sardar A, Czudek C, Reynolds G. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport. 1996;7:910–912. doi: 10.1097/00001756-199603220-00015. [DOI] [PubMed] [Google Scholar]
- Scheel-Kruger J. Dopamine-GABA interactions: evidence that GABA transmits, modulates and mediates dopaminergic functions in the basal ganglia and the limbic system. Acta Neurologic Scandanavia Suppl. 1986;107:1–54. [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E. Dopamine activates HIV in chronically infected T lymphoblasts. Journal of Neural Transmission. 2000;107:1483–1489. doi: 10.1007/s007020070012. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Navia B, Yiannoutsos C, Marra C, Chang L, Ernst T, Jarvik J, Miller E, Singer E, Ellis R, Kolson D, Simpson D, Nath A, Berger J, Shriver S, Millar L, Colquhoun D, Lenkinski R, Gonzalez R, Lipton S Adult AIDS Clinical Trial Group (ACTG) 301; 700 Teams; HIV MRS Consortium. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21(14):1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, Sedler M, Gatley S, Hitzemann R, Ding Y, Logan J, Wong C, Miller E. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow N, Ding Y, Fowler J, Wang G, Logan J, Gatley S, Hitzemann R, Smith G, Fields S, Gur R. Dopamine transporters decrease with age. Journal of Nuclear Medicine. 1996a;37:554–559. [PubMed] [Google Scholar]
- Volkow N, Fowler J, Gatley S, Logan J, Wang G, Ding Y, Dewey S. PET evaluation of the dopamine system of the human brain. Journal of Nuclear Medicine. 1996b;37:1242–1256. [PubMed] [Google Scholar]
- Volkow N, Logan J, Fowler J, Wang G, Gur R, Wong C, Felder C, Gatley S, Ding Y, Hitzemann R, Pappas N. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. Journal of Psychopharmacology. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wang G, Chang L, Volkow N, Telang F, Logan J, Ernst T, Fowler J. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Fischman M, Foltin R, Abumrad NN, Logan J, Pappas NR. Cocaine abusers do not show loss of dopamine transporters with age. Life Sciences. 1997;61:1059–1065. doi: 10.1016/s0024-3205(97)00614-0. [DOI] [PubMed] [Google Scholar]


