Abstract
Dysregulation of Ca2+ has long been implicated to be important in cell injury. A Ca2+-linked process important in necrosis and apoptosis (or necrapoptosis) is the mitochondrial permeability transition (MPT). In the MPT, large conductance permeability transition (PT) pores open that make the mitochondrial inner membrane abruptly permeable to solutes up to 1500 Da. The importance of Ca2+ in MPT induction varies with circumstance. Ca2+ overload is sufficient to induce the MPT. By contrast after ischemia-reperfusion to cardiac myocytes, Ca2+ overload is the consequence of bioenergetic failure after the MPT rather than its cause. In other models, such as cytotoxicity from Reye-related agents and storage-reperfusion injury to liver grafts, Ca2+ appears to be permissive to MPT onset. Lastly in oxidative stress, increased mitochondrial Ca2+ and ROS generation act synergistically to product the MPT and cell death. Thus, the exact role of Ca2+ for inducing the MPT and cell death depends on the particular biologic setting.
Keywords: calcium, cyclosporin A, mitochondrial permeability transition, necrapoptosis, oxidative stress
INTRODUCTION
Free Ca2+ is a key signaling agent in eukaryotic cells. Hormonal and other stimuli activate phospholipase C, isositol triphosphate formation and mobilization of Ca2+ from the endoplasmic reticulum [3,5]. In excitable tissues, membrane depolarization also causes Ca2+ entry through voltage gated Ca2+ channels. Increased cytosolic free Ca2+ then activates protein kinases and in striated muscle stimulates release of Ca2+ from sarcoplasmic reticulum to activate muscle contraction. Influx of Ca2+ into the cytosol also leads to mitochondrial accumulation of Ca2+ and activation of respiratory dehydrogenases and oxidative phosphorylation [35]. Most unstimulated cells tightly regulate free Ca2+ in the range of 100 to 200 nM in both the cytosol and the mitochondria through action of membrane Ca2+ pumps in the plasma membrane and endoplasmic/sarcoplasmic reticulum, Na/Ca exchange across the plasma membrane and, to a lesser extent, mitochondrial Ca2+ uptake. Thus, cytosolic free Ca2+ is substantially lower than the 2 mM free Ca2+ present in extracellular fluids. With physiologic stimulation, however, free Ca2+ in both cytosol and mitochondria can rapidly and transiently increases by 10 to 20-fold as occurs on a beat to beat basis in cardiac myocytes due to mitochondrial Ca2+ uptake [48].
Dysregulation of Ca2+ homeostasis has long been implicated to play an important role in cell injury. Pathological Ca2+ overload and calcification are frequently features of tissue ischemia and infarction, and increased Ca2+ activates a number of phosphatases, proteases and nucleases. Early experimental studies in hepatocytes showed that removal of extracellular Ca2+ protects against various hepatotoxicants, suggesting that influx of extracellular Ca2+ is responsible for irreversible cell injury [13,44]. Since maintenance of Ca2+ gradients across the plasma membrane and between cellular compartments depends on ATP-driven reactions, metabolic disruption by injurious stresses may quickly perturb cellular Ca2+ homeostasis. In particular, release of Ca2+ from the endoplasmic reticulum may flood the cytosol with free Ca2+, possibly leading to activation of degradative processes and dysfunction of other organelles, particularly mitochondria [39]. However, other early work indicated that cytosolic free Ca2+ does not rise preceding hypoxic cell death and therefore may not contribute to the pathogenesis of lethal cell injury after ATP depletion [32].
MITOCHONDRIAL PERMEABILITY TRANSITION
A Ca2+-linked process important in many instances of both necrotic and apoptotic cell death is the mitochondrial permeability transition (MPT). In the MPT, large conductance permeability transition (PT) pores open that make the mitochondrial inner membrane abruptly permeable to all solutes of molecular weight up to about 1500 Da [2,21,25]. Ca2+, numerous reactive chemicals and oxidative stress induce PT pore opening, whereas cyclosporin A (CsA) and pH less than 7 block opening. As a consequence of the MPT, mitochondrial depolarization, uncoupling of oxidative phosphorylation and large amplitude mitochondrial swelling driven by colloid osmotic forces occur.
The composition of PT pores remain controversial. In one model, the adenine nucleotide transporter (ANT) from the inner membrane, the voltage dependent anion channel (VDAC) from the outer membrane, the CsA binding protein cyclophilin D (CypD) from the matrix, and possibly other proteins form PT pores (reviewed in [10,17]). However, recent genetic knockout studies challenge the validity of this model by showing that the MPT still occurs in mitochondria that are deficient in ANT, VDAC and even CypD, although some properties of the MPT are altered [2,23,28,29]. For example in ANT deficient mitochondria, the ANT ligand, atractyloside, no longer promotes PT pore opening, whereas in CypD deficient mitochondria, MPT induction requires greater Ca2+ and is not blocked by CsA.
An alternative model proposes that damage by reactive chemicals, oxidative stress, thiol crosslinking and other perturbations cause misfolding of integral membrane proteins [18]. Misfolding creates hydrophilic surfaces that face the lipid bilayer, a thermodynamically unfavorable condition. Consequently, misfolded membrane proteins aggregate at these hydrophilic surfaces to create aqueous transmembrane channels, which are the nascent PT pores. To prevent catastrophic permeabilization and uncoupling of oxidative phosphorylation, CypD and other molecular chaperones acting from both sides of the inner membrane block conductance through these nascent PT pores. CypD like other cyclophilins is a cis-trans peptidyl proline isomerase, namely a foldase, which may have a physiological role of promoting refolding of the misfolded proteins back to their native state [51]. However, CypD binding also makes PT pores sensitive to reversible Ca2+-induced opening, an action inhibited by CsA. When nascent PT pores formed by misfolded protein aggregates exceed the content of chaperones available to block their conductance, pore opening occurs in an unregulated fashion. Such unregulated pore opening is insensitive to CsA inhibition and no longer requires Ca2+ to occur. In addition to CypD, other as yet poorly characterized chaperones are involved in regulating conductance by nascent PT pores. Indirect evidence suggest that the Rieske iron sulfur protein (RISP) and the heat shock protein Hsp27 may participate in regulating PT pore conductance [19,20]. In particular, dephosphorylation of RISP may be an important event promoting pore opening.
In the protein misfolding model, any membrane protein in principle might contribute to pore formation, but abundance and propensity to misfold determine the likelihood of a particular membrane protein to contribute to the MPT. As the most abundant inner membrane protein on a molar basis, ANT frequently is the target of damage causing misfolding. Moreover, ANT undergoes large conformation changes, and the atractyloside-stabilized conformation appears to be particularly vulnerable to damage leading to misfolding and PT pore formation (reviewed in [33]). Other proteins can also misfold and contribute to PT formation. For example, both old and recent evidence suggests that other anion transporters, such as the aspartate-glutamate and phosphate carriers, participate in formation of large conductance pores [11,12,33,34,45]. Because PT pores can form from these other membrane proteins, the MPT still occurs in ANT deficient mitochondria [28].
The misfolded protein model also accounts for why completely exogenous pore-forming peptides like mastoparan and alamethicin form CsA-sensitive PT pores [18,41]. Chaperones recognize and regulate mastoparan and alamethicin pores in the same way as endogenous PT pores. When pores formed by exogenous pore forming peptides are at low concentration, chaperones block their conductance, but these regulated pores open in response to increased matrix Ca2+ in a CsA sensitive fashion. However, when the concentration of exogenous pore formers exhaust the supply of chaperones, constitutively open CsA insensitive pores form even in the absence of Ca2+. Such behavior for mastoparan and alamethicin is confirmed experimentally [18]. Also as predicted by the misfolded protein model, mitochondria from CypD knockout mice are desensitized to Ca2+ induction of the MPT and require much higher Ca2+ to induce mitochondrial swelling [1], which is consistent with the proposed role of CypD as the Ca2+ sensor of the MPT.
Kinetic analysis of mitochondrial swelling after the MPT suggests that the diameters (conductances) of individual pores are heterogeneous [36]. Such variation is consistent with a variable protein composition for individual PT pores. Protein misfolding promoting PT pore formation should not be equated with irreversible protein denaturation. Indeed, reversal of protein misfolding may be an important physiological function of CypD. When ANT is the principal misfolded membrane protein contributing to PT pore formation, bongkrekic acid by inducing the more stable m-conformation of ANT may also be effective in reversing misfolding and pore opening [17].
MULTIPLE ROLES OF CALCIUM IN PERMEABILITY TRANSITION-DEPENDENT CELL INJURY
Many chemicals and radicals promote the MPT. Typically the effect of such inducers is to decrease the threshold amount of Ca2+ needed to cause PT pore opening. In pathological settings where the MPT contributes to cell killing, Ca2+ may have several roles. First, increased Ca2+ alone and its uptake into mitochondria may cause MPT onset. Second, other stressors may decrease the threshold for the Ca2+-induced MPT such that Ca2+ need not change but is still permissive for MPT onset. Lastly, stressors and increased Ca2+ may act synergistically to induce the MPT.
Induction of the MPT and cell death by calcium overload
That increased Ca2+ alone can cause the MPT and cell death is supported by experiments with Br-A23187, a Ca2+ ionophore [42]. In cultured rat hepatocytes, Br-A23187 causes rapid mitochondrial depolarization and inner membrane permeabilization, as shown by confocal microscopy from mitochondrial release of tetramethylrhodamine methylester (TMRM), a potential-indicating fluorophore, and mitochondrial uptake of calcein, a 623 Da fluorophore that is normally impermeant to the inner membrane. After the Br-A23187-induced onset of the MPT, necrotic cell death occurs from ATP depletion. However, if an alternative source of ATP as from glycolysis, then necrosis is prevented. Instead, caspase-dependent apoptosis occurs secondary to release of cytochrome c and other proapoptotic factors from the mitochondrial intermembrane space after outer membrane rupture. CsA prevents both necrosis and apoptosis induced by Br-23187, confirming the important role of the MPT in both phenotypes of cell death. Similarly after MPT-dependent ischemia-reperfusion injury, the presence or absence of ATP determines whether apoptosis or necrosis occurs [27]. The term, necrapoptosis, describes such death processes that begin with a common death signal or toxic stress, progress by shared pathways, and culminate in either plasma membrane failure (necrosis) or programmed cellular resorption (apoptosis) depending on other modifying factors [30,31].
Permissive role of calcium in cell death caused by Reye-related toxicants
Reye’s and Reye-related syndromes are often fatal illnesses characterized by high fever, fulminant hepatic failure and encephalopathy in children and sometimes adults in association with ingestion of aspirin, valproic acid, unripened fruit of the ackee tree and other toxicants [40]. Electron microscopy of liver and brain from these patients shows large-amplitude mitochondrial swelling, suggestive on onset of the MPT. In isolated liver mitochondria, Reye-related toxicants do not by themselves induce the MPT but instead lower the Ca2+ threshold for the MPT [49].
Salicylate, the active metabolite of aspirin, is directly cytotoxic to cultured rat hepatocytes [50]. CsA blocks this cytotoxicity, and confocal microscopy shows that CsA-sensitive mitochondrial depolarization and inner membrane permeabilization occur after salicylate exposure. A permissive role of Ca2+ in salicylate toxicity is shown by cytoprotection with the Ca2+ channel blocker, verapamil, an effect associated with decreased mitochondrial-free Ca2+ measured by confocal microscopy using the Ca2+ indicator, Rhod-2. Similarly in support of a permissive role for Ca2+, increased extracellular Ca2+ promotes salicylate-induced cell killing. Synergism between salicylate and Ca2+ may help account for the idiopathic nature of Reye’s syndrome. The vast majority of individuals taking aspirin experience no liver or brain toxicity, but children with a prodromal viral illness may incur higher mitochondrial Ca2+ loading, predisposing them to MPT-dependent illness after exposure to salicylate, valproic acid and other Reye-related toxicants.
CALCIUM AND OXIDATIVE STRESS-INDUCED INJURY
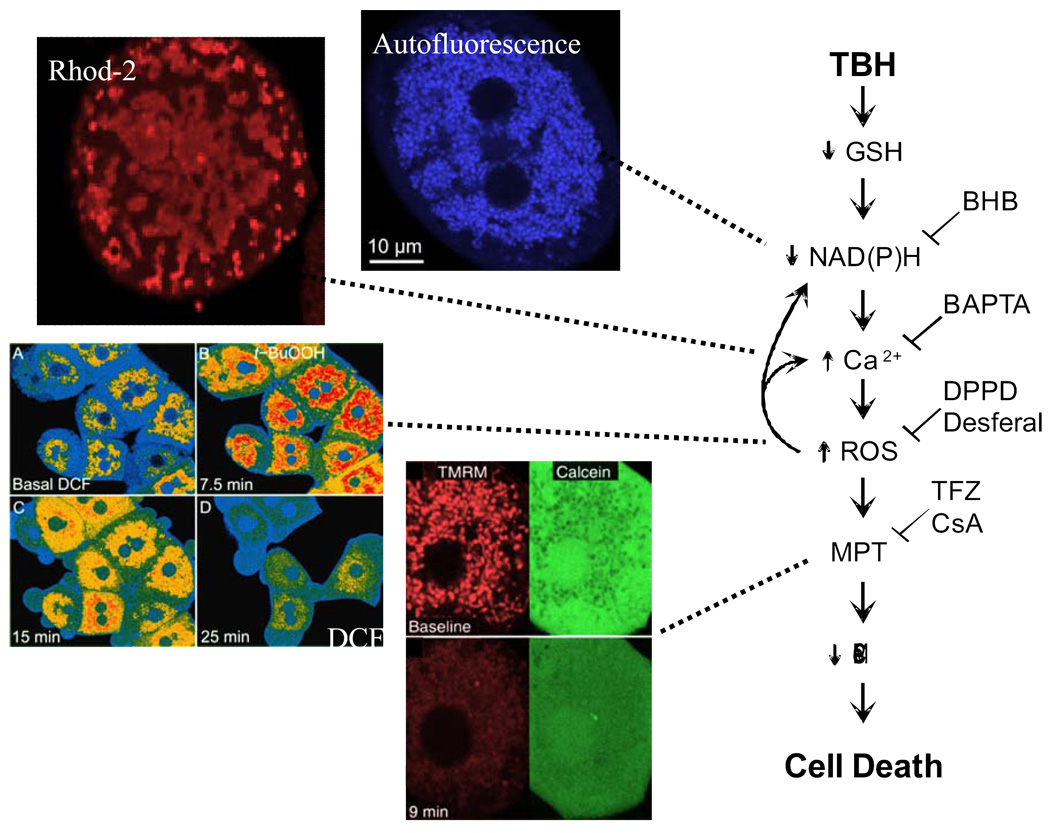
The short-chain hydroperoxide, tert-butylhydroperoxide (TBH), is an analog of the lipid hydroperoxides that form during oxidative stress and ischemia-reperfusion. TBH is detoxified to t-butanol with oxidation of glutathione, NADPH, and NADH by the concerted action glutathione peroxidase, glutathione reductase, and the mitochondrial NADP/NAD transhydrogenase [4,46]. In isolated mitochondria, TBH is a potent inducer of the MPT [16]. Similarly in cultured hepatocytes, TBH induces MPT-dependent cell killing [22]. After TBH, confocal microscopy reveals sequential mitochondrial NAD(P)H oxidation, increased mitochondrial free Ca2+, intramitochondrial generation of reactive oxygen species (ROS), mitochondrial depolarization and inner membrane permeabilization, as monitored with autofluorescence, Rhod-2, dichlorofluorescein, TMRM and calcein, respectively (Fig. 1) [6,37,38]. These events culminate in ATP depletion and cell death. Chelation of intramitochondrial Ca2+ with BAPTA-AM prevents the increase of mitochondrial free Ca2+ and also blocks increased mitochondrial ROS formation but not early oxidation of mitochondrial NAD(P)H. By contrast, inhibition of ROS generation with desferal or diphenylphenylene diamine delays, but does not block, increased mitochondrial Ca2+ after TBH. Thus, NAD(P)H oxidation leads to mitochondrial Ca2+ loading, which in turn promotes ROS formation and the MPT. Indeed, Ca2+ loading of isolated mitochondria promotes ROS formation, although the mechanism for this remains obscure [7]. In this way, increased mitochondrial Ca2+ and ROS generation act synergistically to induce the MPT in this model of oxidative stress.
Figure 1. Changes leading to onset of the MPT after oxidative stress to hepatocytes.
After exposure of hepatocytes to TBH, a sequence of events occurs: oxidation of GSH and NAD(P)H (autofluorescence), increased mitochondrial free Ca2+ (Rhod2), increased mitochondrial ROS formation (dichlorofluorescein [DCF]), the MPT with inner membrane permeabilization (calcein) and mitochondrial depolarization (TMRM), and cell death. The MPT is prevented or delayed by increased reduction of NAD(P)H with β-hydroxybutyrate (BHB), mitochondrial Ca2+ chelation with BAPTA, antioxidants (diphenylphenylene diamine [DPPD] and desferal) and blockers of the MPT (trifluoperazine [TFZ] and CsA). Adapted from [6,37,38].
CALCIUM, OXIDATIVE STRESS AND MPT-DEPENDENT ISCHEMIA-REPERFUSION INJURY IN CARDIAC MYOCYTES
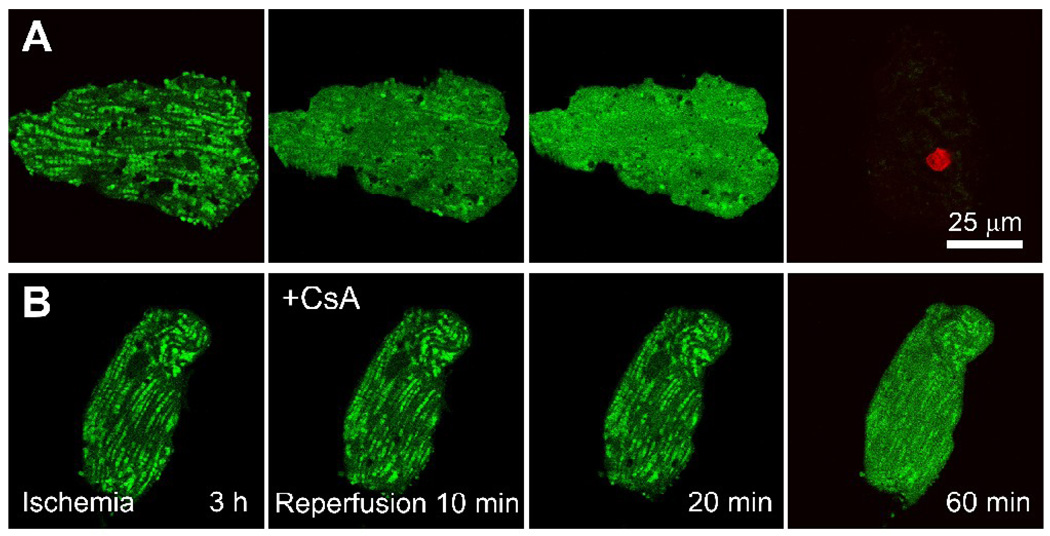
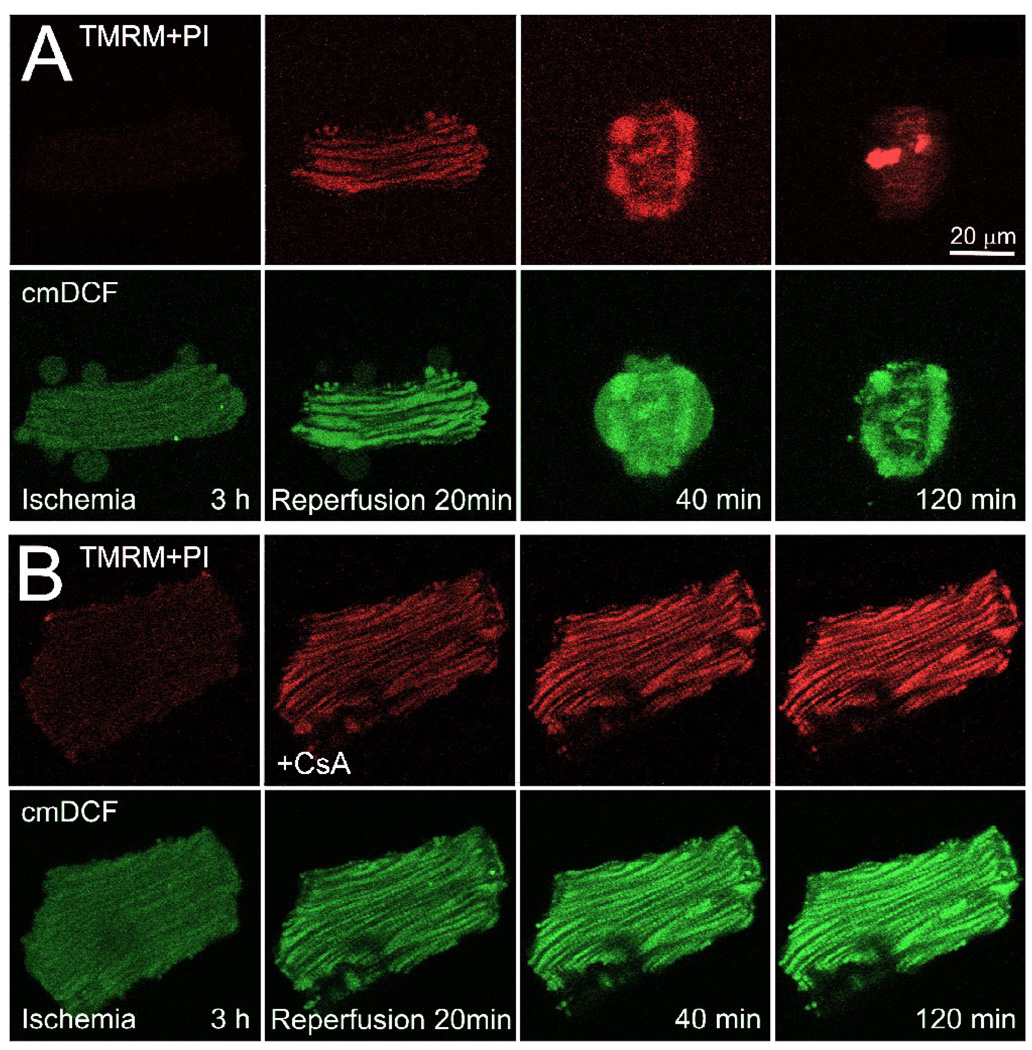
In cardiac myocytes, both cytosolic and mitochondrial free Ca2+ increase transiently during the contractile cycle as part of excitation-contraction coupling and possibly to stimulate mitochondrial ATP production on a beat to beat basis [8,48]. Since MPT-dependent ischemia-reperfusion injury is associated with Ca2+ overload and hypercontracture, a reasonable expectation is that Ca2+ dysregulation promotes MPT onset and ensuing cell death. After about 20 to 40 min of reperfusion of ischemic cardiac myocytes, the MPT occurs as shown by mitochondrial depolarization and movement of calcein through the mitochondrial inner membrane (Fig. 2) [26]. MPT onset after reperfusion is preceded by mitochondrial ROS formation and followed quickly by hypercontracture and cell death (Fig. 3). Antioxidants and MPT blockers like CsA and its nonimmunosuppressive analog, N-methyl-4-isoleucine cyclosporin (NIM811), prevent the MPT, depolarization, hypercontraction, and cell killing (Fig. 2). Antioxidants but not MPT blockers inhibit ROS formation after reperfusion, signifying that ROS generation is upstream of MPT induction (Fig. 3).
Figure 2. The MPT and cell death after ischemia-reperfusion to adult rat cardiac myocytes.
Mitochondria of myocytes were loaded with calcein by a cold-loading/warm incubation procedure, and the cells were subjected to 3 h of anoxia at pH 6.2 (ischemia) followed by reoxygenation at pH 7.4 (reperfusion) in the absence (A) and presence (B) of 1 µM CsA. Nuclear staining by red-fluorescing propidium iodide (PI) detected loss of cell viability. Note that green mitochondrial calcein fluorescence was retained at the end of ischemia, indicating that PT pores were closed. After reperfusion in the absence of CsA (A), mitochondria released calcein into the cytosol beginning after as early as 10 min. After 60 min, all cellular calcein was lost, and PI nuclear staining signified loss of cell viability. In the presence of CsA added at reperfusion (B), mitochondrial release of calcein was greatly diminished and viability was maintained for more than an hour. Adapted from [26].
Figure 3. Cyclosporin A-insensitive formation of mitochondrial ROS after reperfusion.
Myocytes were co-loaded with TMRM and chloromethyldihydrodichlorofluorescein (cmDCF) to monitor mitochondrial membrane potential and ROS formation, respectively, and subjected to ischemia-reperfusion as described in Fig. 2. Note loss of red TMRM fluorescence after 3 h of ischemia, signifying depolarization. After reperfusion, mitochondria accumulated TMRM, indicating repolarization, and green cmDCF fluorescence increased progressively inside mitochondria. In the absence of CsA (A), the myocyte hypercontracted and depolarized after 40 min, and lost viability after 120 min, as shown by nuclear PI labeling. When myocytes were reperfused in the presence of CsA (B), mitochondria repolarization was sustained, and cell death did not occur, but mitochondrial cmDCF still increased. Adapted from [26].
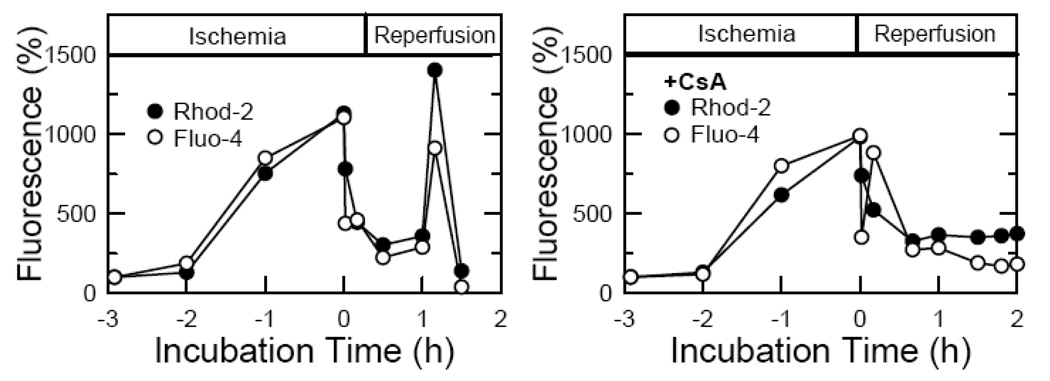
Although both cytosolic and mitochondrial free Ca2+ increase markedly during ischemia, both Ca2+ pools recover to near normal in the first few minutes after reperfusion (Fig. 4). Late after reperfusion, mitochondrial and cytosolic Ca2+ overloading again occur, but CsA and NIM811 block this late Ca2+ overloading (Fig. 4). Moreover, intramitochondrial Ca2+ chelation by cold loading/warm incubation of BAPTA did not decrease cell death after reperfusion. These data indicate that mitochondrial ROS generation after reperfusion promotes MPT onset and subsequent myocyte death. Unexpectedly, late Ca2+ overloading is not a factor inducing the MPT after reperfusion. Rather, Ca2+ overloading is a consequence of bioenergetic failure following MPT onset. Since mitochondrial BAPTA loading fails to suppress cell death after reperfusion, mitochondrial Ca2+ appears not to have even a permissive role for onset of the MPT after reperfusion of cardiac myocytes [26].
Figure 4. Change of cytosolic and mitochondrial free Ca2+ changes during ischemia-reperfusion to cardiac myocytes.
Relative changes of mitochondrial and cytosolic Ca2+ were monitored with Rhod-2 and Fluo-4, respectively, during ischemia-reperfusion to cardiac myocytes. Adapted from [26].
ROLE OF THE MITOCHONDRIAL PERMEABILITY TRANSITION IN STORAGE-REPERFUSION INJURY TO TRANSPLANTED LIVER GRAFTS
Because cardiac myocytes experience constant fluctuations of free Ca2+, their mitochondria may adapt to become resistant to Ca2+ induction of the MPT. Such an adaptation may not occur in non-excitable tissues, such as liver, which are also vulnerable to MPT-dependent ischemia-reperfusion injury [27,43]. A clinically important form of ischemia-reperfusion injury to liver is that associated with cold ischemic storage of livers for transplantation surgery and which can lead to liver graft dysfunction and failure. When rat livers are stored 18 h in University of Wisconsin cold storage solution and transplanted, intravital (in vivo) confocal/multiphoton microscopy reveals mitochondrial depolarization and loss of cell viability of hepatocytes at 4 h after implantation in association with transaminase release and subsequent development of histological necrosis [47]. Within individual hepatocytes, mitochondria depolarization is an all or nothing phenomena. Graft and recipient treatment with the non-immunosuppressive MPT blocker, NIM811, prevents mitochondrial depolarization, decreases cell killing and transaminase release and improves graft survival. NIM811 also decreases apoptosis after transplantation as assessed by deoxynucleotidyl transferase-mediated nick-end labeling(TUNEL) and caspase 3 measurements. Thus, the MPT plays an important role in storage-reperfusion injury to liver grafts.
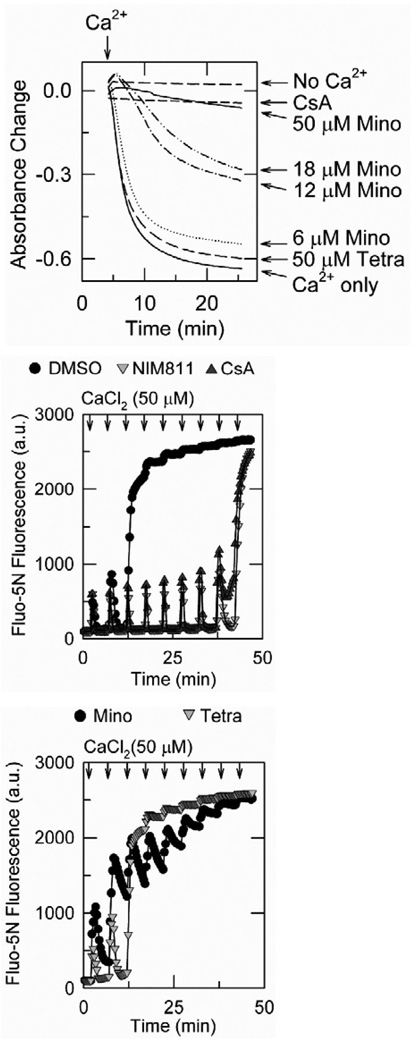
Minocycline is a semisynthetic tetracycline antibiotic shown to be protective in neurodegenerative disease, trauma, and hypoxia–ischemia [9,14,15,24,52–54,54,55]. Cytoprotective mechanisms proposed for minocycline include inhibition of apoptotic pathways, decreased mitochondrial release of pro-apoptotic factors such as cytochrome c [55], upregulation of antiapoptotic proteins [52], inhibition of caspase-1 and caspase-3 activation and suppression of cytochrome c release [52,55]. After cold storage, reperfusion and transplantation of rat livers, minocycline but not tetracycline decreases graft injury, improves graft survival, minimizes mitochondrial depolarization and decreases cell death as effectively as NIM811. In isolated rat liver mitochondria, minocycline and NIM811 but not tetracycline block the MPT. By contrast to NIM811 which does not block mitochondrial Ca2+ uptake, minocycline blocks onset of the MPT by inhibiting mitochondrial Ca2+ uptake (Fig. 5). Thus, minocycline represents a new inhibitor class for the mitochondrial electrogenic Ca2+ uniporter. An implication of minocycline protection against the MPT after storage-reperfusion graft injury is that Ca2+ plays a role in MPT induction in this model. So far, it has not been possible to visualize cytosolic and mitochondrial free Ca2+ in this in vivo model, but previous work in cultured hepatocytes indicates that cytosolic free Ca2+ does not increase during hypoxia [32]. Thus, Ca2+ may be permissive in MPT onset after storage-reperfusion with other factors such as ROS formation decreasing the threshold for a Ca2+ -induced MPT.
Figure 5. Minocycline inhibits calcium uptake and onset of the MPT in isolated rat liver mitochondria.
In the top panel, onset of the MPT was monitored by mitochondrial swelling as determined from decreased absorbance. CaCl2 (250 µM) was added as indicated in mitochondria pretreated with minocycline (Mino), tetracycline (Tetra), 1 µM CsA or no addition. In the middle and lower panels, extramitochondrial free Ca2+ was measured by Fluo-5N fluorescence. As indicated, mitochondria were pre-incubated with vehicle, 5 µM NIM811, 1 µM CsA, 18 µM minocycline or 18 µM tetracycline. CaCl2 was then added in 50 µM increments. Note that minocycline inhibits mitochondrial swelling (the MPT) in a dose-dependent fashion. Minocycline also inhibits mitochondrial accumulation after each Ca2+ pulse. In the absence of minocycline, mitochondria rapidly accumulate each pulse of Ca2+ until onset of the MPT when all accumulated Ca2+ is abruptly released. Adapted from [47].
CONCLUSION
While a role for the MPT in the pathobiology of cell death is strongly supported by experimental data for a number of models of cell injury, the importance of Ca2+ in MPT induction seems to vary with the particular set of circumstances. Certainly, Ca2+ overload when it occurs can be sufficient to induce the MPT, as occurs with cytotoxicity from the Ca2+ ionophore Br-A23187. By contrast after reperfusion of ischemic cardiac myocytes, Ca2+ overload is the consequence of bioenergetic failure after MPT onset rather than its cause. Even intracellular Ca2+ chelation does not suppress MPT onset and subsequent cell death after reperfusion of cardiac myocytes. In other models, such as cytotoxicity from Reye-related agents and storage-reperfusion injury to liver grafts, Ca2+ appears to be permissive to MPT onset. Reye-related toxicants lower the threshold for Ca2+ induction of the MPT, and modulation of cellular Ca2+ up or down has a corresponding effect on Reye toxicant-induced cell death. In liver grafts, minocycline protects against MPT onset and graft failure apparently by inhibiting mitochondrial Ca2+ uptake. The implication is that Ca2+ is at minimum permissive for MPT onset in the liver grafts. Lastly in a model of oxidative stress with TBH, increased mitochondrial Ca2+ and ROS generation act synergistically to product the MPT and cell death. Thus, Ca2+ can be involved in MPT onset in a number of ways, but the exact role of Ca2+ depends on the particular biologic setting.
ACKNOWLEDGMENTS
This work was supported, in part, by Grants DK37034, DK073336, DK070844 and CA119079.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi P, Forte M. The mitochondrial permeability transition pore. Novartis. Found. Symp. 2007;287:157–164. 157–164. discussion 164-9. [PubMed] [Google Scholar]
- 3.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 4.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid. Redox. Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandman O, Meyer T. Feedback loops shape cellular signals in space and time. Science. 2008;322:390–395. doi: 10.1126/science.1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne AM, Lemasters JJ, Nieminen AL. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology. 1999;29:1523–1531. doi: 10.1002/hep.510290521. [DOI] [PubMed] [Google Scholar]
- 7.Chacon E, Acosta D. Mitochondrial regulation of superoxide by Ca2+: an alternate mechanism for the cardiotoxicity of doxorubicin. Toxicol. Appl. Pharmacol. 1991;107:117–128. doi: 10.1016/0041-008x(91)90336-d. [DOI] [PubMed] [Google Scholar]
- 8.Chacon E, Ohata H, Harper IS, Trollinger DR, Herman B, Lemasters JJ. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996;382:31–36. doi: 10.1016/0014-5793(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 9.Chu HC, Lin YL, Sytwu HK, Lin SH, Liao CL, Chao YC. Effects of minocycline on Fas-mediated fulminant hepatitis in mice. Br. J. Pharmacol. 2005;144:275–282. doi: 10.1038/sj.bjp.0706079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem. Soc. Symp. 1999;66:167–179. 167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- 11.Dierks T, Salentin A, Heberger C, Kramer R. The mitochondrial aspartate/glutamate and ADP/ATP carrier switch from obligate counterexchange to unidirectional transport after modification by SH-reagents. Biochim. Biophys. Acta. 1990;1028:268–280. doi: 10.1016/0005-2736(90)90176-o. [DOI] [PubMed] [Google Scholar]
- 12.Dierks T, Salentin A, Kramer R. Pore-like and carrier-like properties of the mitochondrial aspartate/glutamate carrier after modification by SH-reagents: evidence for a performed channel as a structural requirement of carrier-mediated transport. Biochim. Biophys. Acta. 1990;1028:281–288. doi: 10.1016/0005-2736(90)90177-p. [DOI] [PubMed] [Google Scholar]
- 13.Farber JL. Membrane injury and calcium homeostasis in the pathogenesis of coagulative necrosis. Lab. Invest. 1982;47:114–123. [PubMed] [Google Scholar]
- 14.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 15.Gao W, Washington MK, Bentley RC, Clavien PA. Antiangiogenic agents protect liver sinusoidal lining cells from cold preservation injury in rat liver transplantation. Gastroenterology. 1997;113:1692–1700. doi: 10.1053/gast.1997.v113.pm9352874. [DOI] [PubMed] [Google Scholar]
- 16.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 18.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 19.He L, Lemasters JJ. Heat shock suppresses the permeability transition in rat liver mitochondria. J. Biol. Chem. 2003;278:16755–16760. doi: 10.1074/jbc.M300153200. [DOI] [PubMed] [Google Scholar]
- 20.He L, Lemasters JJ. Dephosphorylation of the Rieske iron-sulfur protein after induction of the mitochondrial permeability transition. Biochem. Biophys. Res. Commun. 2005;334:829–837. doi: 10.1016/j.bbrc.2005.06.170. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 22.Imberti R, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial and glycolytic dysfunction in lethal injury to hepatocytes by t-butylhydroperoxide: protection by fructose, cyclosporin A and trifluoperazine. J. Pharmacol. Exp. Ther. 1993;265:392–400. [PubMed] [Google Scholar]
- 23.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann. N. Y. Acad. Sci. 2008;1123:197–212. 197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am. J. Physiol Renal Physiol. 2004;287:F760–F766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am. J. Physiol Heart Circ. Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 28.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Lemasters JJ. V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am. J. Physiol. 1999;276:G1–G6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 31.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J. Gastroenterol. Hepatol. 2007;22 Suppl 1:S31–S37. S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, DiGuiseppi J, Nieminen AL, Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987;325:78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- 33.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim. Biophys. Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res. Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massari S. Kinetic analysis of the mitochondrial permeability transition. J. Biol. Chem. 1996;271:31942–31948. [PubMed] [Google Scholar]
- 37.Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997;272:C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 38.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orrenius S, McConkey DJ, Bellomo G, Nicotera P. Role of Ca in toxic cell injury. TIPS. 1989;10:281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- 40.Partin JC, Schubert WK, Partin JS. Mitochondrial ultrastructure in Reye's syndrome (encephalopathy and fatty degeneration of the viscera) N. Engl. J. Med. 1971;285:1339–1343. doi: 10.1056/NEJM197112092852402. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer DR, Gudz TI, Novgorodov SA, Erdahl WL. The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J. Biol. Chem. 1995;270:4923–4932. doi: 10.1074/jbc.270.9.4923. [DOI] [PubMed] [Google Scholar]
- 42.Qian T, Herman B, Lemasters JJ. The mitochondrial permeability transition mediates both necrotic and apoptotic death of hepatocytes exposed to Br-A23187. Toxicol. Appl. Pharmacol. 1999;154:117–125. doi: 10.1006/taap.1998.8580. [DOI] [PubMed] [Google Scholar]
- 43.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am. J. Physiol. 1997;273:C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 44.Schanne FAX, Kane AB, Young EA, Farber JL. Calcium dependence of toxic cell death, a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 45.Schroers A, Kramer R, Wohlrab H. The reversible antiport-uniport conversion of the phosphate carrier from yeast mitochondria depends on the presence of a single cysteine. J. Biol. Chem. 1997;272:10558–10564. doi: 10.1074/jbc.272.16.10558. [DOI] [PubMed] [Google Scholar]
- 46.Sies H, Moss KM. A role of mitochondrial glutathione peroxidase in modulating mitochondrial oxidations in liver. Eur. J. Biochem. 1978;84:377–383. doi: 10.1111/j.1432-1033.1978.tb12178.x. [DOI] [PubMed] [Google Scholar]
- 47.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys. J. 2000;79:39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trost LC, Lemasters JJ. The mitochondrial permeability transition: a new pathophysiological mechanism for Reye's syndrome and toxic liver injury. J. Pharmacol. Exp. Ther. 1996;278:1000–1005. [PubMed] [Google Scholar]
- 50.Trost LC, Lemasters JJ. Role of the mitochondrial permeability transition in salicylate toxicity to cultured rat hepatocytes: implications for the pathogenesis of Reye's syndrome. Toxicol. Appl. Pharmacol. 1997;147:431–441. doi: 10.1006/taap.1997.8313. [DOI] [PubMed] [Google Scholar]
- 51.Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Curr. Med. Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline upregulates Bcl-2 and protects against cell death in mitochondria. J. Biol. Chem. 2004;279:19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and - dependent mitochondrial cell death pathways in models of Huntington's disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells JEA, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 55.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]