Abstract
Synaptically activated, rapid and dendritic synthesis of new proteins has long been proposed to mediate long-lasting changes at the synapse [1]. Studies of group 1 metabotropic glutamate receptor-dependent long-term depression (mGluR-LTD) have provided new insight into dendritic or local translation and plasticity. Here we highlight these exciting results and discuss how synaptic activity controls local translation, the proteins that are synthesized in dendrites, how they affect synaptic function and how altered local translational control contributes to a form of human mental retardation, Fragile X Syndrome.
Rapid postsynaptic translation is required for mGluR-dependent LTD
Principal neurons possess on average 10,000 excitatory synapses. Plasticity of individual or localized regions of synapses is necessary for the information storage capacity of the brain. Upon the discovery of polyribosomes within dendritic shafts and spines, it was suggested that rapid dendritic protein synthesis, triggered by synaptic activity, may serve as a mechanism for long-term plasticity at specific synapses. Empirical data support the role of dendritic protein synthesis in the maintenance of long-term potentiation (LTP) and depression (LTD) [2,3]. Recent advances in our understanding of localized protein synthesis in synaptic plasticity stem from studies of a form of LTD induced by activation of the Gq coupled metabotropic glutamate receptors (mGluR1 and 5; mGluR-LTD). At excitatory synapses onto CA1 pyramidal neurons, LTD can be induced pharmacologically with the group 1 mGluR agonist, DHPG, or by synaptic stimulation of mGluRs via paired-pulse low frequency stimulation of Schaffer collaterals [4,5]. MGluR-LTD is induced in mature neurons with either paradigm where it requires rapid protein synthesis (~15 minutes) from preexisting mRNA [4,6]. In CA1, mGluR-LTD can be elicited in transected dendrites and affects only active synapses, suggesting that newly required proteins are synthesized in the dendrite and locally weaken synapses [4]. More recently, it was demonstrated that activation of Gq-coupled M1 muscarinic acetylcholine receptors (mAChRs) elicits a protein synthesis dependent LTD that occludes mGluR-LTD in CA1 [7,8]. Therefore, activation of multiple Gq coupled receptors converges upon a single common LTD mechanism that utilizes rapid, postsynaptic protein synthesis. In contrast, another well characterized form of LTD at CA1 synapses is induced by activation of NMDA receptors but persists in protein synthesis inhibitors for an hour or more [4]. From this work, it has been hypothesized that mGluRs trigger the rapid synthesis of new proteins in dendrites that function to cause LTD at locally active synapses. For the purpose of this review, we will define these newly synthesized proteins as “LTD proteins”.
mGluR-LTD is altered in the Fragile X Syndrome mouse model
Understanding the mechanisms and function of mGluR-LTD has relevance for human neurological disease because mGluR-LTD is altered in a mouse model of mental retardation and autism, Fragile X Syndrome (FXS). Fragile X Syndrome results from loss of function mutations in Fmr1, which encodes an RNA binding protein, Fragile X Mental retardation protein (FMRP) [9]. FMRP associates with dendritic mRNAs and RNA granules, as well as translating polyribosomes, while Fmr1 mRNA is itself present in dendrites and translated in response to mGluR activation [10] (reviewed in [9]). In addition to Fmr1, many of the mRNAs translated in response to group 1 mGluRs interact with FMRP, including Psd-95 [11], amyloid precursor protein (App) [12], elongation factor 1a (Ef1a) [13], microtubule associated protein 1B (Map1b) [14,15], and activity regulated cytoskeleton-associated protein (Arc) [16,17]. Consequently, FMRP is emerging as a major regulator of mGluR-dependent protein synthesis and plasticity. In Fmr1 KO mice, mGluR- and M1 mAChR-dependent LTD are enhanced and persist independently of new protein synthesis and upstream activators of protein synthesis [15,18,19]. Based on these results and the known molecular function of FMRP, it has been proposed that FMRP translationally suppresses mRNAs encoding the “LTD proteins”. Therefore, in the absence of FMRP, as in FXS, “LTD proteins” are available or even enhanced in the dendrite. While LTD must be triggered by mGluR activation in Fmr1 KO mice, the availability of “LTD proteins” may enhance the magnitude of LTD at KO synapses and relieve the requirement for de novo synthesis (Fig. 1).
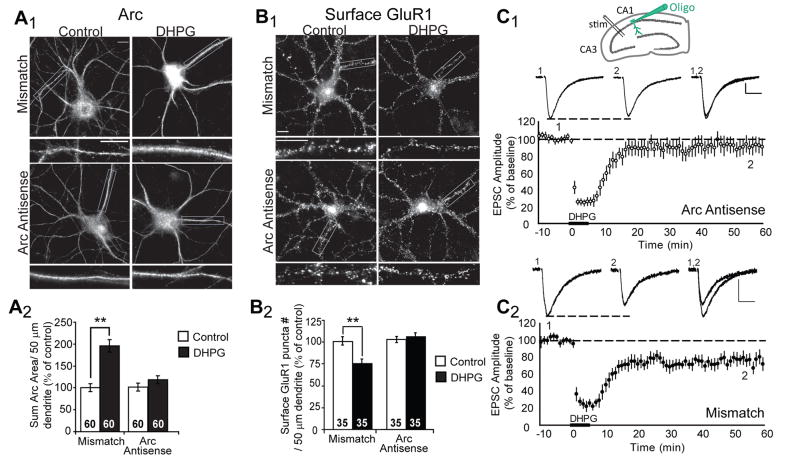
Figure 1. Acute blockade of Arc translation blocks mGluR-induced decreases in surface GluR1 and LTD of synaptic transmission.
A1, Representative images of Arc immunofluorescence in dissociated hippocampal neurons. Antisense oligonucleotides directed against Arc mRNA, or mismatch oligonucleotides were introduced into 19–21 DIV neurons via a lipid-based delivery system. Neurons were treated with media (control) or DHPG and fixed 10 min after onset of treatment. A2, Quantification of the area of the dendritic Arc fluorescence reveal that either one of two unique Arc antisense oligonucleotides (Arc antisense and Arc antisense 2) block DHPG-induced increases in Arc protein without affecting basal Arc levels (in control mismatch oligo treated cultures). Data from 2 cultures per condition. B1, Representative images of surface GluR1 in neurons treated with antisense or mismatch oligonucleotides 30 min prior to media (control) or DHPG treatment. One hour after DHPG, neurons were fixed and processed for surface GluR1 immunofluorescence. B2, Quantified group data reveal that DHPG fails to induce long-term decreases in surface GluR1 in neurons pretreated with either Arc antisense oligonucleotides, in contrast to neurons treated with mismatch oligonucleotides. Data from 2–3 cultures/condition. In all images scale bars = 10 μm. C1 Inset: Schematic of recording configuration in a rat hippocampal slices. Antisense oligo was infused into the postsynaptic CA1 neuron. EPSCs were evoked by an extracellular stimulating electrode placed in the Schaffer collateral axons from CA3. Average normalized EPSC amplitudes of CA1 neurons from acute hippocampal slices recorded with pipettes containing 250 μM Arc antisense or mismatch oligonucleotide. DHPG (100 μM, 5 min) applied to the bath resulted in LTD of EPSC amplitudes in cells filled with mismatch oligonucleotide. In contrast, intracellular introduction of Arc antisense oligonucleotide via patch pipette blocks DHPG-induced LTD. N = 7 for each group. Above each plot are representative EPSCs from cells filled with Arc antisense oligonucleotide or mismatch oligonucleotide taken during pre-DHPG baseline (1) or 50 min after LTD induction (2; as indicated in group plot). Scale bars = 50 pA/10 msec.
Expression Mechanisms of mGluR-LTD and candidate “LTD proteins”
Determining the molecular mechanisms of mGluR-LTD is essential to understanding how newly synthesized proteins in dendrites mediate plasticity as well as how and why plasticity is altered in Fragile X Syndrome. Activation of mGluRs causes a long-term decrease in surface AMPARs, both GluR1 and GluR2 subunits, lasting at least an hour [20,21]. AMPAR endocytosis is required for mGluR-LTD given that postsynaptic injection of D15, a peptide which interferes with dynamin-amphiphysin interactions and AMPAR endocytosis, blocks mGluR-LTD in slices [22]. New protein synthesis is not necessary for mGluRs to initiate AMPAR endocytosis, but instead are required for the decreases in surface AMPARs observed one hour after DHPG [16,20]. Therefore, candidate “LTD proteins” likely play a role in regulation of AMPAR trafficking.
How does mGluR activation lead to the persistent decreases in surface AMPAR expression levels that mediate LTD? Receptor biotinylation and ratiometric immunocytochemical studies of primary hippocampal cultures reveal that mGluR-LTD results in a protein synthesis-dependent increase in AMPAR endocytosis rate that lasts for at least one hour [16]. Interestingly, NMDAR- LTD is also mediated by decreases in surface AMPARs, but unlike mGluR-LTD, is not associated with persistent increases in AMPAR endocytosis rate. These data suggest that mGluR-LTD, specifically, is maintained by new protein(s) that increase AMPAR endocytosis rate. To maintain the steady state level of surface AMPARs observed during mGluR-LTD in the face of a persistently elevated endocytosis rate, the requisite exocytosis rate must also increase. This model also implies that AMPARs recycle more rapidly during mGluR-LTD [16].
Recent studies have identified several mRNAs that interact with FMRP and encode proteins that regulate AMPAR trafficking [12,14,16,17]. Of these, activity-regulated cytoskeleton-associated protein, (Arc) (also termed Activity regulated gene of 3.1kb or Arg3.1) is a leading candidate “LTD protein”, in part because it increases AMPAR endocytosis rate through interactions with endophilin 2/3 and dynamin 2 [23]. Arc is a particularly fascinating gene because it is induced in response to neuronal activity and is then rapidly transported to dendrites, where Arc mRNA accumulates at active synapses [24–27]. These results prompted the idea that local translation of Arc at synapses may mediate plasticity and encoding of Arc-inducing experience. In support of this idea, mGluR activation triggers rapid translation of Arc in dendrites [16,17] (Fig. 1). Transgenic knockout or constitutive knockdown of Arc with short-hairpin (sh) RNA blocks mGluR-induced LTD and long-term decreases in surface GluR1, illustrating a necessary role for Arc in LTD [16,17]. Surprisingly, Arc KD inhibited the early mGluR-triggered AMPAR endocytosis that is independent of new protein synthesis indicating a role for existing Arc protein. Because Arc KD did not hinder NMDAR-induced trafficking of AMPARs, demonstrating Arc specificity for mGluR-mediated AMPAR endocytosis. By utilizing constitutive knockdown or KO of proteins, such as Arc, it is difficult to discern the requirement for existing protein, versus newly synthesized protein, in LTD. Therefore, acute manipulations that inhibit mGluR-induced translation of specific proteins, such as antisense oligonucleotides (oligos) or small-interfering RNAs, have been used. Acute (20–30 min) introduction of antisense oligos into either dissociated hippocampal cultures or acute slices (via a patch pipette) blocks mGluR-induced increases in Arc protein without affecting basal Arc levels (Fig. 1). Such treatments also prevented mGluR-induced LTD, decreases in surface AMPAR expression, and persistent increases in AMPAR endocytosis rate (Fig. 1) [16]. In summary, existing Arc protein is required for mGluRs to trigger AMPAR endocytosis. Whereas, mGluR-induced synthesis of new Arc is necessary to maintain LTD, likely through increases in GluR1 endocytosis rate. In support of a role for Arc in mGluR-LTD maintenance, Arc levels remain elevated for at least one hour after LTD induction [17]. Because Arc directly interacts with dynamin and endophilin, Arc may act as a scaffold, localizing endocytosis machinery to its effectors and/or increasing the function of dynamin and endophilin to enhance endocytosis [23]. Postranslational modification of Arc or AMPARs by mGluRs may play a role in triggering AMPAR endocytosis. In support of this possibility, tyrosine phosphatase activity is required for mGluRs to dephosphorylate and trigger endocytosis of GluR2 [21].
Related to this work, translation of a specific tyrosine phosphatase, striatal-enriched protein tyrosine phosphatase (STEP), has been implicated in mGluR-driven AMPAR endocytosis (Fig. 2). Levels of STEP protein are increased in hippocampal synaptoneurosomes within minutes following DHPG treatment, a process that requires translation from pre-existing mRNA [28]. Inhibition of STEP function using a cell permeable substrate-trapping protein or genetic deletion of STEP increases AMPAR surface expression and blocks DHPG-triggered AMPAR endocytosis as well as Tyr dephosphorylation of GluR2. Synthesis of new STEP may function to maintain GluR2 in a Tyr dephosphorylated state, which, together with Arc, may maintain the increased endocytosis rate associated with mGluR-LTD. Future experiments are important to establish whether Step mRNA is present in dendrites and whether it interacts with FMRP.
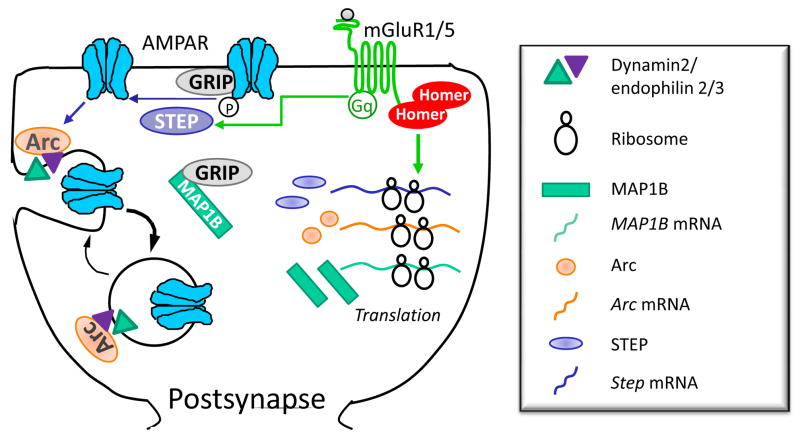
Figure 2. Proposed mechanisms by which newly synthesis proteins mediate persistent decrease in surface AMPA receptors during mGluR-LTD.
Brief activation of mGluR1/5 triggers rapid endocytosis of AMPARs that requires activity of the Tyr phosphatase STEP and basal levels of Arc. Tyr dephosphorylation of the GluR2 subunit of the AMPAR is correlated with mGluR-triggered AMPAR endocytosis, suggesting that this is the relevant phosphatase substrate. MGluRs also rapidly increase translation of Step, Map1b and Arc mRNA. MAP1B is required for mGluRs to induce long-term decreases in surface AMPARs perhaps by sequestering the AMPAR binding protein GRIP. New synthesis of Arc, and perhaps STEP, may maintain mGluR-LTD by causing persistent increases in AMPAR endocytosis rate.
A third candidate “LTD protein” is microtubule-associated protein 1B (MAP1B). Map1b is a well characterized, dendritic, FMRP interacting mRNA, but its role in mGluR-triggered AMPAR endocytosis has only been recently elucidated. DHPG treatment of hippocampal neurons increases MAP1B levels in dendrites, and constitutive knockdown of MAP1B prevents DHPG-induced AMPAR endocytosis [14,15]. MAP1B interacts with the GluR2 interacting protein and scaffold GRIP1, and DHPG increases this association [14,29]. Because GRIP1 stabilizes surface GluRs, the synthesis of MAP1B may serve to sequester GRIP1 away from the synapse and destabilize GluR surface expression. Although NMDAR activation induces synthesis of MAP1B, MAP1B is dispensible for NMDAR- triggered decreases in surface AMPARs.
This data suggests that mGluRs stimulate a coordinated synthesis of multiple, functionally related proteins that together mediate persistent decreases in surfaces AMPARs and LTD. However, in contrast to this idea, overexpression of Arc or STEP alone results in decreased surface AMPAR expression and only Arc occludes subsequent mGluR-induced decreases, whereas STEP does not [16,28]. One possibility is that STEP may play a somewhat permissive role in mGluR-LTD, such that Tyr dephosphorylation of GluR2 allows AMPAR endocytosis, perhaps by multiple pathways, but is not sufficient to cause endocytosis to the level that saturates and occludes mGluR-LTD. Importantly, these results also suggest that Arc is sufficient to mimic LTD in the absence of new synthesis of STEP and perhaps MAP1b. To better understand if and how synthesis of multiple proteins mediate LTD, experiments utilizing antisense oligos to block new synthesis of STEP and MAP1b during mGluR-LTD are required. In addition, determining if Arc overexpression is sufficient to induced LTD in STEP or MAP1B knockout neurons, and vice versa, may further this goal.
Understanding the role and interactions of the multiple “LTD proteins” may also further our understanding of how mGluR-LTD proceeds without new protein synthesis in Fmr1 KO mice [15,18]. MGluR-LTD in Fmr1 KO mice still relies on Arc because crossing Arc KO to Fmr1 KO mice leads to smaller LTD [17]. However, we do not detect elevated levels of Arc protein n hippocampal synaptoneurosomes of Fmr1 KO mice (J. Wilkerson and Huber, unpublished observations) suggesting that either posttranslational modifications of Arc or increased levels of other “LTD proteins” contribute to altered LTD in Fmr1 KO mice.
Protein Synthesis dependent mGluR-LTD in other brain regions
Gq coupled receptor and protein synthesis dependent LTD is observed in multiple brain regions including the neocortex, cerebellum, ventral tegmental area (VTA) and dentate gyrus [8,30–33]. MGluR-LTD has been most well characterized at the cerebellar granule cell-Purkinje cell synapse where strong evidence links LTD to cerebellar dependent learning. Like hippocampal mGluR-LTD, cerebellar mGluR-LTD is mediated by decreases in surface AMPARs and requires new protein synthesis, suggesting it may similarly rely on synthesis of Arc, MAP1b or STEP [30,31,34]. In contrast, mGluR-LTD in the VTA is mediated by distinct synaptic mechanisms from LTD in CA1, but still relies on rapidly synthesized proteins [32]. Elegant work performed by Luscher and colleagues demonstrated that mGluR-LTD of excitatory inputs onto dopaminergic neurons in the VTA occurs by replacing GluR2-lacking AMPARs with lower conducting GluR2-containing AMPARs, which requires new synthesis of GluR2 [32]. Interestingly, mGluR-LTD reverses the synaptic AMPAR subunit changes that are induced by cocaine, and therefore may be important for understanding mechanisms that reverse addiction-related behaviors [35]. Targeted inhibition of new GluR2 synthesis using an antisense olignucleotide or small interfering RNA rapidly (~ 5–10 min) abolished mGluR-LTD [32]. GluR2 may be rapidly turned over in dopaminergic neurons such that inhibition of new synthesis during the “baseline” period, prior to LTD induction, prevents LTD. Alternatively mGluR stimulation during the LTD induction protocol triggers a rapid synthesis of GluR2 that remarkably is functional within 5–10 min. In support of the latter idea, mGluR stimulation induces synthesis of GluR2 in dendrites of cultured hippocampal neurons [36,37].
mGluRs regulate translation at multiple stages
mGluR activation of translation initiation
Studies of mGluR-LTD have provided critical knowledge of how synaptic activity activates rapid protein synthesis. Evidence supports the view that mGluRs regulate translation at multiple levels, through regulation of general translation factors as well as RNA binding proteins such as FMRP (Fig. 3). MGluR activity stimulates translation initiation through 2 major signaling pathways, the ERK-MAPK and PI3K-mTOR pathways (Fig. 3). To initiate translation, mGluRs trigger phosphorylation of eukaryotic initiation factor 4E (eIF4E), and eIF4E binding protein (4EBP) as well as stimulate formation of the translation intiation (eIF4F) complex [19,38]. ERK phosphorylates and activates MAPK-interacting kinase (Mnk1), which in turn phosphorylates, eIF4E [3]. Consequently, MGluR-induced phosphorylation of Mnk1 and eIF4E require ERK [38]. mGluRs also stimulate the PI3 kinase-mTOR pathway of which one downstream effector is 4EBP [19,38,39]. Upon phosphorylation by mTOR, 4EBP reduces its affinity for eIF4E which may allow eIF4E phosphorylation by ERK as well as stimulate eIF4F initiation complex assembly [38]. Activation of translation initiation appears necessary for mGluR-LTD because inhibition of PI3 kinase, mTOR, ERK or translation initiation (by inhibition of 5′ CAP binding) prevents mGluR-LTD [4,39,40]. Furthermore, mice with a knockout of 4EBP2, an endogenous inhibitor of eIF4F complex assembly, display enhanced mGluR-LTD, suggesting that inhibition of translation initiation normally limits the magnitude of mGluR-LTD.
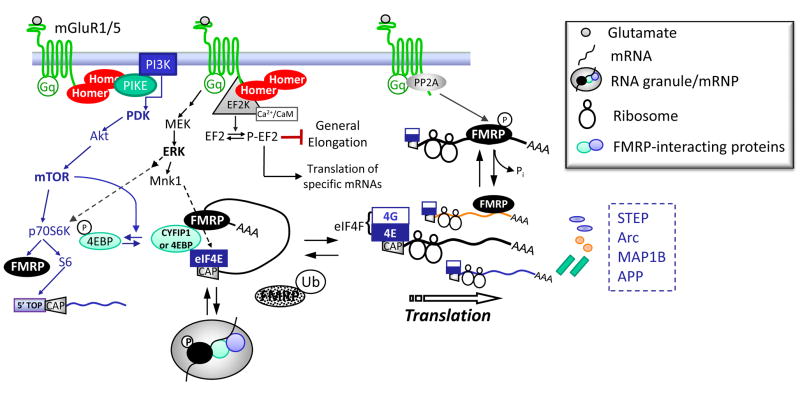
Figure 3. Group 1 mGluRs stimulate translation through multiple pathways in neurons.
mGluR5 regulates translation initiation through activation of the PI3K, mTOR and ERK pathways that converge on the initiation factor, eIF4E. mGluRs also stimulate p70S6K which is thought to generally enhance translation rate through phosphorylation of the S6 ribosomal subunit and translation of 5′TOP containing mRNAs encoding components of the translation apparatus, such as EF1a. mGluRs rapidly stimulate PP2A which results in a dephosphorylation of FMRP and translation of FMRP interacting mRNAs. mGluRs also inhibit elongation generally through activation of EF2K. The submaximal inhibition of general elongation is thought to make available initiation factors for translation of specific mRNAs, such as Arc and Map1b. Interestingly, mGluR5 complexes directly (EF2K and PP2A) or indirectly through Homer (EF2K and PIKE) with many signaling molecules that regulate translation. Interactions of mGluR5 with Homer are required for the ability of mGluRs to regulate translation initiation and perhaps elongation as well.
MGluRs enhance the overall translational capacity of dendrites through activation of mTOR, p70 S6 kinase, and phosphorylation of the S6 ribosomal subunit [41]. S6 phosphorylation correlates with enhanced translation rates and translation of 5′ terminal oligopyrimidine tract (5′TOP) containing mRNAs that encode ribosomes, initiation and elongation factors [2,41]. MGluRs stimulate phosphorylation of p70 S6K and S6 in hippocampal slices through activation of PI3K, mTOR as well as ERK [19,41]. Furthermore, mGluRs stimulate translation of a 5′ TOP containing mRNA encoding Elongation Factor 1a (EF1a) [13,19,41]. Surprisingly, constitutive deletion of the p70S6K isoforms S6K1 and S6K2 does not abolish mGluR-induced LTD and phosphorylation of S6, suggesting that alternate pathways for S6 phosphorylation are intact or compensate in these mice [41]. Like group 1 mGluRs, M1 mAChRs stimulate phosphorylation of ERK and p70 S6K and mAChR-induced LTD relies on ERK and mTOR [7].
mRNA specific translational control through FMRP
In addition to stimulation of general translation, mGluRs may activate translation of specific mRNAs through FMRP protein interactions or posttranslational regulation of FMRP (Fig. 3). Cytoplasmic FMRP interacting protein (CYFIP1) is a newly identified eIF4E binding protein (4EBP) which forms a complex with FMRP and target mRNAs such as MAP1b and Arc. Upon synaptic mGluR stimulation, CYFIP1 dissociates from eIF4E which presumably facilitates formation of the eIF4F initiation complex. Consistent with this idea, a reduction in CYFIP1 levels in neurons is correlated with increased protein expression of FMRP target mRNAs [42].
FMRP may regulate translation of its target mRNAs at the elongation step. Phosphorylation of FMRP on a conserved serine residue (Ser500 in human FMRP) alters the association of FMRP with stalled or translating polyribosomes, with dephosphorylated FMRP being more associated with translating polysomes [43]. Upon mGluR stimulation, FMRP is rapidly dephosphorylated by PP2A and then rephosphorylated by p70 S6K in an mTOR and ERK dependent manner. FMRP dephosphorylation is associated with rapid synthesis of a FMRP target mRNA, SAPAP3, observed 2 minutes after mGluR stimulation [44,45] (reviewed in [9]). Precisely how FMRP regulates translation is not understood. It is posited that the rapid dephosphorylation of FMRP may allow or stimulate movement of polyribosomes along mRNA [9]. Furthermore, FMRP is also ubiquitinated and rapidly degraded via the proteosome after mGluR stimulation which may function to relieve any translational suppression [15]. These posttranslational modifications of FMRP would provide a rapid mechanism to couple synaptic activity to rapid translational activation of specific FMRP interacting mRNAs.
mGluR5-signaling complex to translational machinery
To mediate rapid and localized translational control, mGluRs physically interact with molecules that signal to translation machinery (Fig. 3). mGluR5 directly interacts with PP2A and elicits a rapid and bidirectional control of PP2A activity and FMRP phosphorylation [44,46]. Interaction of the mGluR5 C-terminal tail with the scaffold and signaling molecule Homer forms a critical link between mGluRs and activation of the translational apparatus as well as LTD induction. Dimerization of Homer molecules scaffolds mGluR5 to PI3K enhancer (PIKE), a small GTPase that binds PI3K and stimulates its lipid kinase activity [47]. Acute disruption of mGluR5-Homer interactions in hippocampal slices blocks mGluR-LTD, as well as mGluR stimulation of PI3K-mTOR, translation initiation and synthesis of EF1a [19]. These findings may have important implications for understanding altered mGluR signaling in FXS. mGluR5 is less associated with Homer in Fmr1 KO mice [48], which may contribute to the inability of mGluRs to activate PI3K-mTOR and translation in these mice [19].
Recent findings reveal a role for mGluRs in control of translation elongation which also occurs through Homer interactions [14,17]. Surprisingly, mGluR5 inhibits translation elongation and this occurs through direct and indirect (via Homer) association with Ca2+/calmodulin-dependent eukaryotic elongation factor 2 kinase (EF2K) [17]. EF2K binds mGluRs under basal conditions. Following mGluR stimulation and Ca2+ increases, EF2K dissociates from the GPCR and phosphorylates eukaryotic elongation factor 2 (EF2). Although phosphorylation of EF2 generally inhibits elongation, it also leads to increased translation of specific mRNAs, including Arc and MAP1b, perhaps by making more initiation factors available. In support of this hypothesis, EF2K knockdown or KO abolishes mGluR-stimulated Arc and MAP1b synthesis, as well as mGluR-LTD [14,17]. Therefore, Homer forms an important link for mGluR5 to multiple translational control pathways which may contribute to the rapid and localized control of translation at synapses.
Concluding Remarks
The study of mGluR-dependent LTD has provided mechanistic insight into how synaptic activity rapidly activates translation in neurons and in turn, how newly synthesized proteins alter synaptic function. This basic knowledge, in turn guided fruitful experiments into the neuronal function of the mental retardation linked gene Fmr1, the neurobiological deficits in FXS, and the development of novel therapies for the disease [49]. Important questions remain, such as: How are the various translational control mechanisms coordinated by mGluRs and how do newly synthesized proteins affect specific synapses? What is role of mGluR- and translation-dependent LTD in hippocampal-dependent learning or behavioral plasticity? Because Arc translation is required for mGluR-LTD maintenance, this suggests that mGluR-LTD would be elicited during Arc-inducing experiences such as novelty, spatial learning or stress [50–52].
Although Arc is required for LTD in Fmr1 KO mice, the new synthesis of Arc is not, indicating that a level of regulation is lost in FXS. Does this property contribute to mental retardation and behavioral alterations observed in FXS? How does plasticity in Fragile X escape the requirement of new Arc and other protein synthesis? A better mechanistic understanding of Arc function in neurons, the contribution of other newly synthesized proteins to LTD and how the processes are altered in FXS is required to answer these critical questions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 2.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 4.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 5.Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 6.Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massey PV, Bhabra G, Cho K, Brown MW, Bashir ZI. Activation of muscarinic receptors induces protein synthesis-dependent long-lasting depression in the perirhinal cortex. Eur J Neurosci. 2001;14:145–152. doi: 10.1046/j.0953-816x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 9.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25:7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. This work demonstrated that the well known FMRP target mRNA, MAP1B, is required for mGluR-dependent AMPAR endocytosis. Notably, they also demonstrated elongation factor 2 kinase (EF2K) was required for mGluR-dependent AMPAR endocytosis and MAP1B synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. Here the authors demonstrated that FMRP is rapidly ubiquitinated and degraded via the proteosome during mGluR-LTD. This finding suggested a mechanism by which mGluRs activated translation of FMRP-interacting mRNAs through degradation of FMRP. In support of this idea, proteosome inhibitors block mGluR-LTD wildype, but not in Fmr1 KO mice. [DOI] [PubMed] [Google Scholar]
- 16**.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. The authors demonstrated that Arc is rapidly synthesized in dendrites in response to group 1 mGluR activation and new Arc synthesis is required to maintain mGluR-LTD. Data is also presented that provides a mechanism by which Arc maintains LTD through long-term increases in AMPAR endocytosis rate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. Here, the authors present data that mGluRs induce rapid synthesis of Arc which requires EF2K activation and inhibition of general elongation. Hence, synaptically and pharmacologically induced mGluR-LTD is absent in both Arc and EF2K KO mice. The authors also present novel findings that EF2K interacts with mGluR5 directly and indirectly through Homer. The authors link FMRP to Arc functionally and demonstrate that mGluR-LTD in Fmr1 KO mice depends on Arc, but it does not require synthesis of new Arc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile x syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 19*.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. The authors demonstrated that an acute, peptide-mediated disruption of mGluR5-Homer interactions blocks mGluR activation of the PI3K-mTOR pathway and phosphorylation of translation initiation factors and LTD. Because mGluR5 and Homer are less associated in Fmr1 KO mice [42], findings in this paper predicted and demonstrated a deficit in mGluR activation of PI3-mTOR and translation initiation in Fmr1 KO mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 21.Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao MYZQ, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 26.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 27.Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 28**.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. Previous work [21] demonstrated that Tyr phosphatase activity was required for mGluRs to trigger endocytosis of AMPARs, likely through dephosphorylation of GluR2. Here, the authors provide evidence that this phosphatase is Striatal-enriched Tyr Phosphatase (STEP). Surprisingly, STEP is synthesized in response to mGluRs and is required for mGluRs to mediate long-term decreases in surface AMPARs. This data provide a link between the Tyr dephosphorylation and protein synthesis in regulation of mGluR-LTD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seog DH. Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Biosci Biotechnol Biochem. 2004;68:1808–1810. doi: 10.1271/bbb.68.1808. [DOI] [PubMed] [Google Scholar]
- 30.Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- 31.Karachot L, Shirai Y, Vigot R, Yamamori T, Ito M. Induction of long-term depression in cerebellar Purkinje cells requires a rapidly turned over protein. J Neurophysiol. 2001;86:280–289. doi: 10.1152/jn.2001.86.1.280. [DOI] [PubMed] [Google Scholar]
- 32**.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. The authors infuse antisense oligonucleotides or small-interfering RNAs against GluR2 into postsynaptic dopaminergic neurons through a patch pipette to demonstrate that rapid synthesis and synaptic insertion of GluR2 is necessary for mGluR-dependent LTD in the VTA. To our knowledge, this is the first use of this technique to block synthesis of specific proteins during plasticity. Importantly, the authors demonstrate a physiological role for mGluR-LTD in the VTA i.e., to reverses synaptic changes elicited by cocaine. [DOI] [PubMed] [Google Scholar]
- 33.Naie K, Manahan-Vaughan D. Investigations of the protein synthesis dependency of mGluR-induced long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 2005;49 (Suppl 1):35–44. doi: 10.1016/j.neuropharm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 35.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 36.Kacharmina JE, Job C, Crino P, Eberwine J. Stimulation of glutamate receptor protein synthesis and membrane insertion within isolated neuronal dendrites. Proc Natl Acad Sci U S A. 2000;97:11545–11550. doi: 10.1073/pnas.97.21.11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 38.Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. This paper identifies Cytoplasmic-FMRP interacting protein (CYFIP1) as a new eIF4E binding protein and presents data to support a model where FMRP-CYFIP interactions suppress translation initiation of FMRP target mRNAs by preventing eIF4G interactions with eIF4E and formation of the eIF4F initiation complex. MGluR stimulation reduces CYFIP1 interactions with eIF4E which would be expected to relieve this suppression and may play a role in mGluR-dependent translational activation of FMRP target mRNAs. [DOI] [PubMed] [Google Scholar]
- 43.Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- 44**.Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. Here the authors demonstrate a role of FMRP in mGluR-induced, rapid translational activation. They identify PP2A as the FMRP phosphatase and demonstrate that mGluRs rapidly (~2 min) increase PP2A activity, dephosphorylate FMRP and increase translation of an FMRP target mRNA, SAPAP3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates FMRP with the neuronal protein synthesis-dependent mTOR signaling cascade. J Biol Chem. 2008 doi: 10.1074/jbc.C800055200. Here the authors identify S6K as the FMRP kinase and demonstrate that mGluR bidirectionally regulate FMRP phosphorylation. mGluRs activate a mTOR and ERK dependent activation of S6K that rephosphorylates FMRP after mGluR stimulated dephosphorylation. This would provide a mechanism by which mGluRs could elicit a rapid, but brief, translational activation of FMRP interacting mRNAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- 48.Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Mikkelsen JD, Larsen MH. Effects of stress and adrenalectomy on activity-regulated cytoskeleton protein (Arc) gene expression. Neurosci Lett. 2006;403:239–243. doi: 10.1016/j.neulet.2006.04.040. [DOI] [PubMed] [Google Scholar]