Abstract
The role of central and peripheral neuronal nitric oxide synthase (nNOS) splice variants in the development of inflammatory hyperalgesia was investigated using the formalin test. Supraspinal administration of the NOS inhibitor NOArg lowered both the first and second phase of the formalin response. An oligodeoxynucleotide targeting four nNOS isoforms given supraspinally also reduced the formalin response of both phases. Supraspinal antisense mapping suggested that this effect results from the nNOS-1 splice variant, implying that nNOS-1 is important in mediating formalin pain. At the spinal level, antisense mapping suggested a role of both the nNOS-1 and the nNOS-β variants in producing formalin pain. Conversely, an antisense selective against nNOS-2 had an opposing effect against the first phase, increasing its intensity. This result, which was similar to prior studies examining opioid actions, implies that endogenous nNOS-2 activity acted to minimize pain perception. Locally in the foot, arginine, the precursor for NO, increased the phase II response at low doses while higher doses reduced the response. This complex biphasic response suggested opposing NOS actions. Local antisense mapping again showed that nNOS-1 is involved in producing phase II of the formalin response while nNOS-2 had an opposite effect similar to that seen spinally. Finally, downregulation of nNOS-1 by antisense prevented tolerance to morphine in both the tailflick and the formalin test. Together, these observations illustrate the complexity of nNOS in pain perception and the existence of opposing nNOS systems likely due to splice variants of nNOS.
Keywords: Nitric oxide synthase, antisense mapping, neuropathic pain, opiate
Introduction
Neuronal nitric oxide sythase has been widely implicated in thermal and mechanical hyperalgesia, neuropathic and inflammatory pain (Dolan, S. et al. 2003; Inoue, T. et al. 1998; Kolesnikov, Y. A. et al. 1993; Kolesnikov, Y. A. et al. 1997; Malmberg, A. B. & Yaksh, T. L. 1993; Meller, S. T. et al. 1992; Meller, S. T. & Gebhart, G. F. 1993; Roche, A. K. et al. 1996). Inhibition of NO production prevents NMDA receptor-induced hyperalgesia and the formalin-induced excitation of dorsal horn neurons (Reeve, A. J. et al. 1998). Injection of formalin into the hind paw increases the number of dorsal horn neuronal NOS containing neurons at the L4-L5 dorsal horn area (Lam, H. H. et al. 1996). Peripheral nerve injury may result in an increased excitability of spinal cord neurons (central sensitization) through activation of nociceptive afferents, leading to activation of N-methyl-D-aspartate receptors and subsequent spinal production of NO. Immunocytochemical staining reveals a significant increase (6 to 7-fold) of nNOS-immunoreactive neurons and fibers in the dorsal root ganglia of L4–L6 after chronic constriction injury in rats (Cizkova, D. et al. 2002).
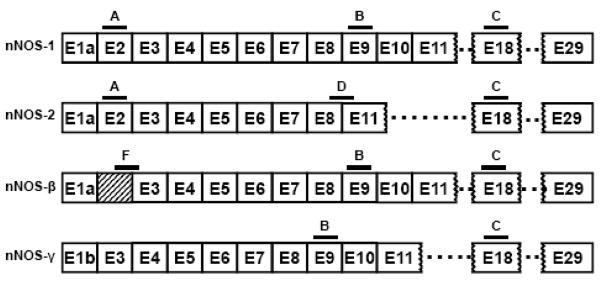
The gene encoding nNOS has been cloned (Bredt, D. S. et al. 1991). The mouse nNOS gene has two closely linked promoters and generates more than 10 differentially spliced nNOS transcripts (Brenman, J. E. et al. 1996; Fujisawa, H. et al. 1994; Hall, A. V. et al. 1994; Ogilvie, P. et al. 1995; Silvagno, F. et al. 1996; Xie, J. et al. 1995), including two lacking exon 2 (nNOS-β and nNOS-γ), which contains the PDZ domain, and another lacking exons 9 and 10 (nNOS-2) (Brenman, J. E. et al. 1997; Iwasaki, T. et al. 1999; Ogura, T. et al. 1993) (Fig. 1). In nNOS-2, the deletion of exon 9 and 10 causes an inframe 315-bp deletion, leading to the elimination of 105 amino acids from a highly conserved internal region, but it does not affect an essential co-factor binding region and is presumed to encode a functional enzyme. Homologous splice variants have been observed in human, mouse and rat. Other nNOS proteins with NH2-terminal truncations include the mouse nNOSβ and nNOSγ. The absence of exon 2 in both these mouse nNOS variants lead to a lack of the PDZ domain, implying a cytosolic localization. nNOSβ has an extended sequence in exon 1a that is not contained within the other variants and in vitro catalytic activity similar to the full-length nNOS. nNOSβ is still expressed in an exon 2 nNOS knockout mouse (Eliasson, M. J. L. et al. 1997). Thus, distinct molecular splice variants of nNOS are expressed in neuronal tissue, raising the possibility that these alternatively spliced isoforms may mediate pharmacologically and physiologically distinct actions.
Figure 1. Schematic of nNOS splicing.
Some of the splice variants of the nNOS gene are represented, along with the locations of the antisense oligodeoxynucleotides used in the reported studies.
Morphine-tolerance involves the NMDA/NO/PKC cascade at many levels of nociceptive neuronal pathways (Babey, A. M. et al. 1994; Mao, J. et al. 1995) and the non-selective NOS inhibitor N-nitro-L-arginine (NOArg) given systemically prevents and reverses morphine tolerance (Kolesnikov, Y. A. et al. 1993). However, a different picture emerged when NOArg was given centrally. Supraspinal NOArg significantly enhanced morphine analgesia and prevented opioid tolerance while spinal NOArg markedly reduced systemic morphine analgesia in a dose-dependent manner (Kolesnikov, Y. A. et al. 1997). Using an antisense mapping approach, we demonstrated opposing actions for nNOS-1 and nNOS-2 in morphine analgesia and tolerance (Kolesnikov, Y. A. et al. 1997). Whereas nNOS-1 was important in the production of tolerance that decreases morphine’s analgesic sensitivity, nNOS-2 activity has an opposite action, facilitating morphine analgesia. In the current study, we have examined the role of several nNOS splice variants in formalin-induced hyperalgesia, a mixed inflammatory-neuropathic model of pain.
Results
NOS inhibitors and L-Arginine
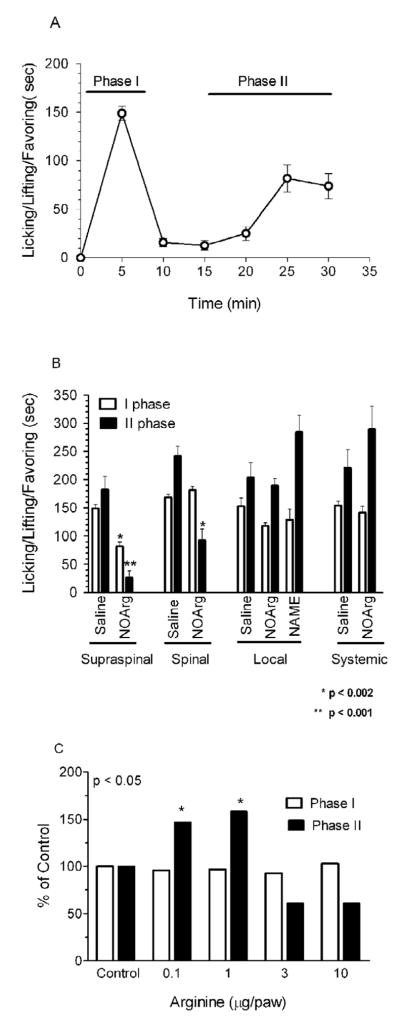
Formalin elicits two phases of nociceptive behavior (Fig. 2a). NOArg, a potent inhibitor of NOS, did not significantly affect formalin-induced pain behavior in the mouse when given systemically (Fig 2b). Similarly, local administration of NOArg did not significantly influence the formalin actions. Central administration showed quite different effects. Given supraspinally, NOArg (6 μg, i.c.v.) significantly lowered both phases of the formalin response. Spinal NOArg (6 μg, i.t.), on the other hand, significantly inhibited only phase 2, lowering it by 38% (Fig 2b, Table 1).
Figure 2. Effect of NOArg and L-arginine on formalin-induced pain.
A) A group of mice (n=10) received formalin (5%–10 μl) and the amount of time the mouse spent licking, lifting and/or favoring the injected paw was recorded in 5 min bins for 30 min (see Methods). B) Groups of mice (n=20) received NOArg (6 μg) spinally (i.t), supraspinally (i.c.v.) or locally 15 min before formalin (5%–10 μl) administration. Nociceptive behavior was recorded 0–5 min (first phase) and 15–30 min (second phase) after the formalin. C) Groups of mice (n=10) received L-arginine at the indicated doses locally 5 min before formalin (5%–10μl). Nociceptive behavior was recorded 0–5 min (first phase) and 15–30 min (second phase) after the formalin.
Table 1.
Effects of nNOS inhibitors on formalin - induced pain in mouse
| Nociceptive score (sec) | P value | |||
|---|---|---|---|---|
| Treatment / Route | I Phase | II Phase | I | II |
| Systemic | ||||
| Saline | 154 ±18 | 221 ± 11 | ||
| NOArg | 131 ±14 | 289 ± 41 | NS | NS |
| Local | ||||
| Saline | 153 ± 15 | 204 ± 26 | ||
| NOArg | 118 ±16 | 189 ± 13 | NS | NS |
| Spinal | ||||
| Saline | 169 ±15 | 242 ± 17 | ||
| NOArg | 181 ±17 | 92 ± 20 | NS | <0.001 |
| Supraspinal | ||||
| Saline | 149 ±17 | 182 ± 24 | ||
| NOArg | 82 ± 8 | 26 ± 13 | <0.002 | <0.0001 |
Groups of mice (n ≥10) received NOArg (6 μg), spinally, supraspinally (i.c.v) or locally 15 min before formalin administration. In this paradigm, NOArg significantly reduced phase I and II L/L/F behavior and phase II L/L/F after i.c.v. and i.t administration, respectively (Table 1). NOArg given systemically (4 mg/kg s.c. n=10, 15 min before formalin) or locally in hind paw (6 μg, n=10; 15 min before formalin) was ineffective.
Arginine is the substrate for nNOS, and its administration increases NO formation (Babey, A. M. et al. 1994). Although local administration of NOArg was without effect, L-arginine administered to achieve local delivery was active in phase II of the formalin response, yielding a biphasic response. Low doses of L-arginine (0.1 and 1.0 μg) given into the paws were pro-nociceptive, increasing phase II of the formalin response by 47% and 58%, respectively (Fig 2c). Higher doses (3–10 μg) saw a loss of this increased nociceptive effect and appeared to have a modest antinociceptive action. Although this did not achieve statistical significance compared to the control group that did not receive any argenine (Fig 2c), it was clearly different from the phase II response seen at the lower argenine doses. This biphasic response suggests opposing pro-nociceptive and anti-nociceptive roles for NOS locally and raises the possibility that the inactivity of the NOS antagonist reflected blockade of the two opposing systems.
Antisense Studies
The L-arginine study suggested opposing NO systems in the periphery, consistent with earlier antisense mapping work from our laboratory on opioid analgesia showing a pro-nociceptive role for the nNOS-1 isoform and an opposite pain decreasing role for the nNOS-2 splice variant. We therefore used a similar antisense mapping approach to assess the role of NO in the formalin assay. We designed several antisense oligdeoxynucleotides targeting four nNOS isoforms: nNOS-1, nNOS-2, nNOSβ and nNOSγ (Fig. 1; Table 1). Most of the targeted exons are contained within more than one variant. However, two probes, D and F, downregulate only one mRNA species, as previously shown (Kolesnikov, Y. A. et al. 1997). Probe D is selective for nNOS-2 by bridging the splice site between exons 8 and 11. Although the oligodeoxynucleotide can potentially bind separately to the exons 8 and 11 sequences in the other variants, the matching sequences of each exon alone are too short for activity. Probe F is selective for nNOS-β, targeting a extended sequence from exon 1a which is not present in any of the other variants.
Supraspinal antisense
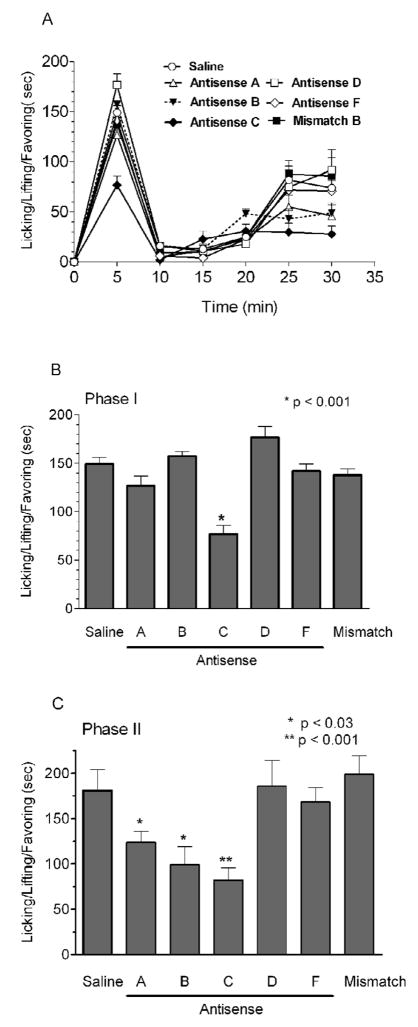
Mice were treated with the indicated antisense for 5 days and tested in the formalin test on the 6th day. Antisense C, which targets exon 18 in all the variants, was similar in its actions to NOArg, which antagonizes all nNOS activity. It significantly lowered phase I and phase II of the formalin response by 51% (p < 0.001) and 45% (p < 0.001) respectively (Fig 3). Targeting exon 9 with probe B lowered the second phase in the formalin response, as did the antisense to exon 2 (probe A; <0.03). A mismatch probe based upon the exon 18 was inactive, confirming the selectivity of these responses. The antisense probes selective for only nNOS-2 (probe D) and nNOS-β (probe F) were without effect in all the assays.
Figure 3. Effect of supraspinal antisense treatment on formalin-induced pain.
Groups of mice received the stated antisense (10 μg, i.c.v.) on days 1, 2 and 5 (n=18) and then were examined in the formalin test (5%–10μl) for pain behavior on day 6. Other groups were given either saline (n=12, 2 μl) or mismatch C against exon 18 (10 μg, n=12) on days 1, 3 and 5 and were tested in the formalin test on day 6. A) Nociceptive behavior was score as the amount of the time spent licking, lifting and favoring of the injected paw (mean ± S.E.M) in 5 min bins for up to 30 min following the formalin administration. B,C) Nociceptive behavior was recorded 0–5 min (first phase) and 15–30 min (second phase) after the formalin. Antisense C significantly lowered both phases of the formalin response by 51% (p<0.001) and 45% (p<0.001), respectively.
Spinal antisense
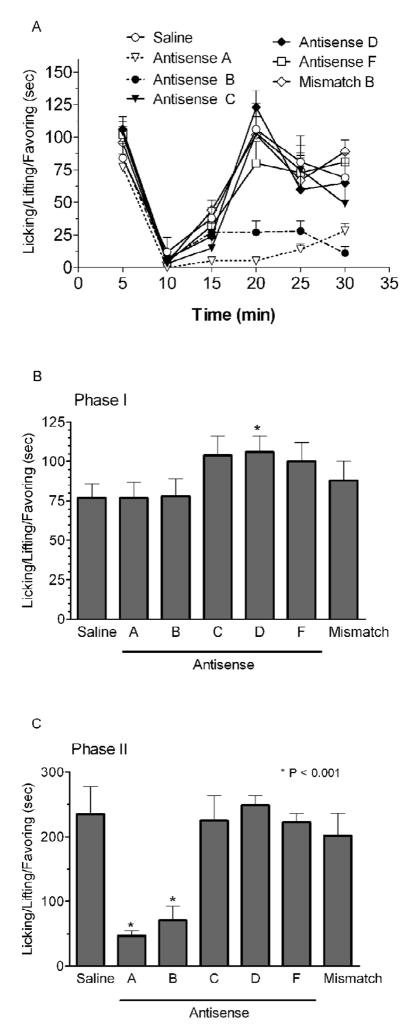
Next, we examined antisense mapping at the spinal level. Intrathecal administration of antisense targeting exons 2 (probe A) or 9 (probe B) of nNOS significantly inhibited the second phase of the formalin-induced inflammatory pain (Fig 4). As before, the mismatch probe based upon the exon 18 probe C was inactive. Phase I was not significantly decreased by any of the probes. However, the nNOS-2-selective probe D increased the response, suggesting that this isoform may oppose some of the other isoforms and normally act to suppress pain. A slight increase also was seen in the supraspinal study, but it did not achieve statistical significance. This is consistent with our prior work looking at the isoforms (Kolesnikov, Y. A. et al. 1997).
Figure 4. Effect of spinal antisense treatment on formalin-induced pain.
Mice were given the antisense on the days 1, 3 and 5 (10 μg, n=16) and were examined in the formalin (5%–10 μl) test for pain behavior on day 6. Nociceptive behavior was scored as has described earlier. Antisense targeting exon 2 (A) and 9 (B) as well nNOSβ (F) significantly lowered the second phase of formalin response by 27%, 33% and 55%, respectively, without affecting the first phase. The mismatch B probe was inactive in this model.
The pattern of inhibition of the formalin response on the second phase differed from the supraspinal study. The exon 18 probe (C) was ineffective, but the exon 2 probe (A) lowered the response significantly. Interestingly, the nNOS-β-selective probe (F) also lowered the response, differing from the supraspinal study. Antisense, which selectively targets nNOS-2 (D) significantly increased Phase I. Although there was also an increase in Phase II, it did not achieve significance. Thus, nNOS-2 may be an important component of an endogenous antinociceptive system.
Local antisense
Finally, we examined the role of peripheral nNOS isoforms in formalin induced inflammatory pain by administering the antisense subcutaneously into the hind paw of the mouse for two days and testing the animals on the third day (Fig. 5). The antisense probes selectively targeting exons 2 (A) and exon 9 (B) significantly lowered by the 30% and 20% the second phase of formalin response without affecting the first phase. None of the other antisense oligodeoxynucleotides showed any activity, with the exception of the nNOS-2 antisense (D), which again revealed a small, but significant increase in Phase I. A mismatch antisense nNOS-1 probe was inactive in this model, ensuring the specificity of the study.
Figure 5. Effect of local antisense treatment on formalin-induced pain.
Mice were given antisense (10 μg) s.c. on the days 1 and 2 in the left paw and were examined in formalin test for pain behavior on the day 3. Antisense that selectively targets exon-9 (B) and exon 2 (A) of nNOS-1 significantly lowered by 30% and 20% the second phase of formalin response, respectively. Antisense that selectively targets nNOS-2 (D) had no effect on the second phase of formalin response.
Morphine tolerance and formalin-induced hyperalgesia
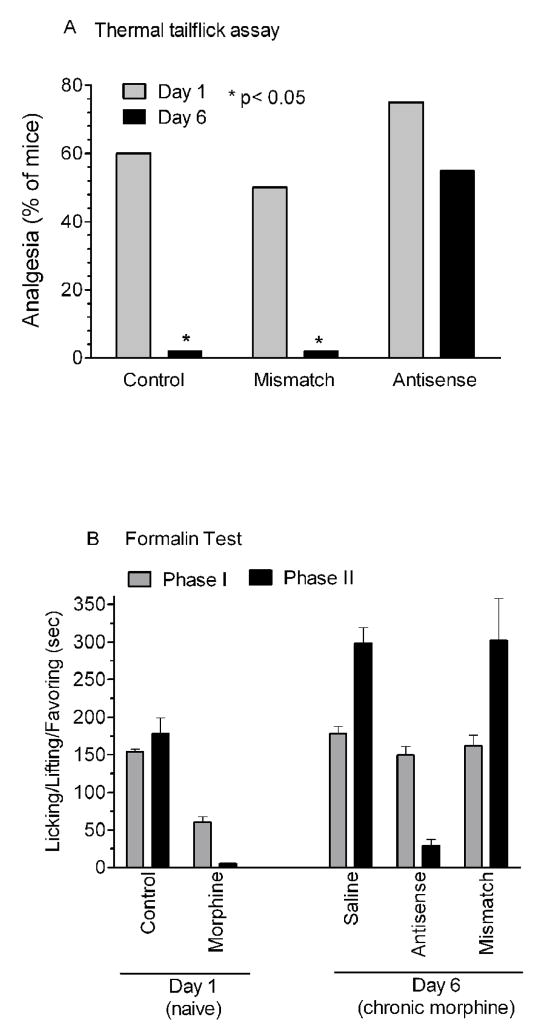
Morphine is analgesic in both the tailflick and the formalin test. Earlier studies revealed that nNOS inhibitors and antisense probes targeting nNOS-1 blocked the development of analgesic tolerance in the tailflick assay (Kolesnikov, Y. A. et al. 1993; Kolesnikov, Y. A. et al. 1997). We next examined the effects of the exon 9 antisense (B) in morphine tolerant mice in the both in the tailflick and formalin test. Morphine (5 mg/kg, s.c.) produced analgesia in 60% of naïve mice in the tailflick assay (Fig 6A) and significantly lowered by 43% (p < 0.01) the first phase of formalin response and virtually eliminated the second phase (Fig 6B). Chronic morphine given daily followed by testing on the 6th day showed a complete loss of analgesia in the tailflick assay. In the formalin test, morphine appeared to show an increased response in Phase II in the saline and mismatch groups compared to the naïve control group, but it was not significant. Antisense treatment restored the analgesic activity of morphine in the second phase of the formalin assay, eliminating the tolerance.
Figure 6. Effect of spinal antisense on morphine tolerance.
Groups of mice (n=10) received either antisense B (10 μg, i.t.) or mismatch (10 μg, i.t.) at 10: 00 a.m. on days 1, 3 and 5 in conjunction with daily (4:00 p.m.) systemic morphine (5 mg/kg, s.c.) for 6 days. The control group received spinal saline alone. Analgesia was determined on days 1 and 6 in the A) radiant heat tailflick assay and B) in the formalin test 30 min following morphine administration. By day 6, the morphine response in the control and mismatch groups was lost compared to the response on day 1 in the tailflick assay (p < 0.05). In the formalin study (B), the morphine response in both phase I (p < 0.02) and phase II (p < 0.001) were significantly different from saline in the acute study. Following chronic administration of morphine, the morphine response in both phase I (p < 0.02) and phase II (p<0.0001) were statistically different from the morphine response in naïve animals. Following chronic morphine, there was no statistical difference between the phase I results in these chronic morphine groups. The phase II response seen with morphine in the naïve animals was lost in the saline and mismatch groups while it was maintained in the antisense group (p < 0.0001).
Discussion
Neuronal nitric oxide plays an important role in nociception and opioid action in the central and peripheral nervous systems and the importance of the NMDA/nitric oxide cascade in opioid tolerance has been extensively studied. Early studies quickly established the ability of NOS inhibitors to block morphine tolerance (Kolesnikov, Y. A. et al. 1992; Kolesnikov, Y. A. et al. 1993). They also influence morphine analgesia directly. However, these actions are complex. Supraspinal NOArg enhances morphine analgesia whereas spinal NOArg decreases it (Kolesnikov, Y. A. et al. 1997). Although useful, enzymatic inhibitors have limitations, particularly when examining isoforms of a protein. Antisense mapping provides a valuable approach that can downregulate individual isoforms by targeting specific exons (Standifer, K. M. et al. 1994). Splice variants containing the targeted exon may be downregulated while those without the exon are unaffected. However, the approach does have some limitations, including limited downregulation of the mRNA being targeted. While activity of an antisense can be helpful, the absence of an effect might reflect the lack of involvement of the isoforms containing the targeted exon or it may simply be due to inactivity of the probe in the assay. Thus, these studies must be interpreted cautiously. In our earlier study, we observed opposing activities of nNOS-1 and nNOS-2 in morphine actions. Downregulation of nNOS-1 prevented the appearance of morphine tolerance. In contrast, selectively downregulating nNOS-2 with an antisense probe to a unique splice junction impaired morphine’s analgesic activity. Our current studies have extended this approach to the formalin test.
The current studies illustrate the complexity of the role of NO in the mediation of formalin–induced pain. Local application of NOArg did not affect either phase of the formalin assay. Yet, increasing NO production with the administration of the precursor L-arginine yielded a biphasic response, with low doses pro-nociceptive and higher doses anti-nociceptive. Thus, the lack of an effect with the inhibitor NOArg might reflect the simultaneous blockade of the opposing systems. Antisense studies illustrated the ability of antisense probes targeting exons 2 and 9 applied locally to lower the second phase of the response. There was a slight elevation in the phase I response that was significant with an antisense selective for nNOS-2, but this effect was modest. Together, these antisense results lend support to the possibility of opposing NOS systems in the periphery.
Supraspinal blockade of nNOS with NOArg lowered both phases in the formalin assay, implying a causal role for supraspinal NO in the production of the formalin response. Supraspinal antisense treatment also revealed a reduction in the pain response. The exon 18 antisense probe lowered both phases while the exon 2 and 9 antisense probes lowered only the second phase. The pro-nociceptive role of supraspinal NO is similar to the ability of supraspinal NOArg to enhance morphine analgesia (Kolesnikov, Y. A. et al. 1997).
A number of spinal antisense oligodeoxynucleotide probes significantly lowered the second phase of the formalin response without affecting the first phase, including the antisense unique to nNOS-β. Thus, nNOS at the spinal level has a pro-nociceptive activity. However, the antisense probe targeting the exon 8–11 splice site that is unique to nNOS-2 showed a different response. This antisense probe increased both the first and second phase of the formalin response, implying that the endogenous activity of this isoform diminishes the intensity of formalin nociception, an anti-nociceptive action, and provides further evidence for opposing nNOS systems in pain modulation. Thus, at the spinal level, nNOS-2 and nNOS-β have opposing actions. The role of nNOS-1 in these actions remains a bit unclear since the other antisense probes are not selective for a single isoform.
These observations emphasize the importance of peripheral, spinal and supraspinal nNOS in mediating formalin-induced hyperalgesia. However, these enzymes also are involved with morphine tolerance. Earlier studies had implicated nNOS-1 in morphine tolerance with the tailflick assay, a thermal measure of nociception (Babey, A. M. et al. 1994; Kolesnikov, Y. A. et al. 1992; Kolesnikov, Y. A. et al. 1993; Kolesnikov, Y. A. et al. 1997). The current studies reveal similar effects on tolerance with the formalin assay. Chronic morphine dosing produced tolerance in both the thermal and formalin assays. Spinal antisense treatment blocked the development of tolerance in both tests. Thus, the NMDA/nitric oxide cascade is important in the development of tolerance in both pain models.
The current study separates the functional roles of alternatively spliced isoforms of nNOS in formalin–induced hyperalgesia. nNOS-1 appears to play a major role in the second phase of formalin pain supraspinally, spinally and peripherally while nNOS-β does the same spinally. In contrast, the nNOS-2 isoform has an opposite effect, as shown by the enhanced formalin response with the isoform was downregulated. These results correlate with the morphine study, in which we have shown that nNOS-1 diminishes the analgesic actions of morphine, while nNOS-2 enhances them (Kolesnikov 1997). These studies demonstrate complex effects of NO in pain modulation and the utility of antisense mapping in exploring functional roles of NOS isoforms in the central nervous system.
Experimental Procedure
All studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Memorial Sloan-Kettering Cancer Center. Male CD-1 mice (24–30 g; Charles River Laboratories) were used in all studies. Animals were maintained on a 12h light/dark cycle and had free access to food and water. N-nitro-L-Arginine (NOArg) and L-arginine were purchased from Sigma (St. Louis, MO). Oligodeoxynucleotides were purchased from Midland Reagent Co. (Midland, TX). All antisense sequences were based upon the mouse nNOS sequence (from GenBank accession #D14552). Mismatch oligodeoxynucleotides were designed by switching the sequence of two sets of adjacent bases, keeping the remaining sequences and the overall base composition the same. All oligodeoxynucleotides were purified before use and dissolved in saline before injection (2 μl), as previously described (Kolesnikov, Y. A. et al. 1997). Intracerebroventricular (i.c.v.) and intrathecal (i.t.) injections were performed under light halothane anesthesia, as previously reported (Kolesnikov, Y. A. et al. 1997).
The formalin assays was performed as previously described (Kolesnikov, Y. et al. 2004). In brief, mice were individually acclimated to the observation chamber for 40 min before injecting formalin (5% in 10 μl) subcutaneously into the left hind paw using a Hamilton microsyringe with a 30G needle. The amount of time (sec) the animal spent licking, lifting and/or favoring the injected paw was recorded in 5 min bins for 30 minutes after the formalin injection. The first phase corresponded to the first five minutes after the injection of formalin and the second phase corresponded to the interval between 15 and 30 minutes after the formalin.
Analgesic tolerance to systemic morphine was studied using a daily injection paradigm, as previously described (Kolesnikov, Y. et al. 1998; Kolesnikov, Y. A. et al. 1992; Kolesnikov, Y. A. et al. 1993). A fixed morphine dose (5 mg/kg, s.c.) was given daily and analgesia was assessed using the tail-flick assay (D’Amour, F. E. & Smith, D. L. 1941; Kolesnikov, Y. et al. 1998; Kolesnikov, Y. A. et al. 1992; Kolesnikov, Y. A. et al. 1993). In the tail flick assay, baseline latencies ranged from 2.5 to 3 seconds and analgesia was assessed quantally as a doubling or greater of the baseline for that individual mouse. A maximal cutoff latency of 10 sec was used to minimize tissue damage.
All graded response data are expressed as the mean ± S.E.M. Statistical analysis was determined using either ANOVA or Student’s t test, depending upon the comparison. Quantal data were compared using the Fisher exact test.
Table 2.
Selectivity of the antisense mapping sequences for nNOS variants
| Presence of antisense targeting sequence | |||||
|---|---|---|---|---|---|
| Antisene | Antisense Target | nNOS-1 | nNOS-2 | nNOS-β | nNOS-γ |
| A | Exon 2 | Yes | Yes | No | No |
| B | Exon 9 | Yes | No | Yes | Yes |
| D | Exon 8/11 | No | Yes | No | No |
| F | Exon 1e/3 | No | No | Yes | No |
| C | Exon 18 | Yes | Yes | Yes | Yes |
The sequences of the oligonucleotides are as follows: antisense A: CGC TGT CAT AGC TGA GGT CTA CCA (nt 313–336); antisense B: CGA ACG CCA ATC TCC GTG CC (nt 1866–1885); C: GAA TCC TCT CCC CGC CCA (nt 2815–2833); antisense D: TCT TGG CTA CTT CCT GTG TGA ACT CC (NT 1595–1608, 1922–1935); antisense F: CTC TGC TGC CGA GCC CGC GCA G; antisense mismatch B: CAG ACG CAC ATC CTC GTG CC; mismatch C: GAA TCT CCT CCC GCC CCA (Gene ID no. 18125).
Acknowledgments
This work was supported, in part, by research grants DA07242 and DA02615 and a Senior Scientist Award (DA00220) from the National Institute on Drug Abuse to GWP and by a core grant from the National Cancer Institute to MSKCC (CA08748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW. Nitric oxide and opioid tolerance. Neuropharmacology. 1994;33:1463–1470. doi: 10.1016/0028-3908(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin medaited by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci. 1997;19:224–231. doi: 10.1159/000111211. [DOI] [PubMed] [Google Scholar]
- Cizkova D, Lukacova N, Marsala M, Marsala J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull. 2002;58(2):161–171. doi: 10.1016/s0361-9230(02)00761-x. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. Journal of Pharmacology and Experimental Therapeutics. 1941;72:74–79. [Google Scholar]
- Dolan S, Kelly JG, Huan M, Nolan AM. Transient up-regulation of spinal cyclooxygenase-2 and neuronal nitric oxide synthase following surgical inflammation. Anesthesiology. 2003;98(1):170–180. doi: 10.1097/00000542-200301000-00027. [DOI] [PubMed] [Google Scholar]
- Eliasson MJL, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: Prominent functional localizations in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Ogura T, Kurashima Y, Yokoyama T, Yamashita J, Esumi H. Expression of two types of NOS mRNA in human neuroblastoma cell lines. Journal of Neurochemistry. 1994;63:140–145. doi: 10.1046/j.1471-4159.1994.63010140.x. [DOI] [PubMed] [Google Scholar]
- Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. Structural organization of the human neuronal nitric oxide synthase gene (NOS1) Journal of Biological Chemistry. 1994;269:33082–33090. [PubMed] [Google Scholar]
- Inoue T, Mashimo T, Shibata M, Shibuta S, Yoshiya I. Rapid development of nitric oxide-induced hyperalgesia depends on an alternate to the cGMP-mediated pathway in the rat neuropathic pain model. Brain Res. 1998;792(2):263–270. doi: 10.1016/s0006-8993(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Hori H, Hayashi Y, Nishino T, Tamura K, Oue S, Iizuka T, Ogura T, Esumi H. Characterization of mouse nNOS2, a natural variant of neuronal nitric-oxide synthase produced in the central nervous system by selective alternative splicing. Journal of Biological Chemistry. 1999;274(25):17559–17566. doi: 10.1074/jbc.274.25.17559. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Cristea M, Oksman G, Torosjan A, Wilson R. Evaluation of the tail formalin test in mice as a new model to assess local analgesic effects. Brain Research. 2004;1029(2):217–223. doi: 10.1016/j.brainres.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: Evidence for a NMDA receptor defect. Journal of Pharmacology and Experimental Therapeutics. 1998;284(2):455–459. [PubMed] [Google Scholar]
- Kolesnikov YA, Pan YX, Babey AM, Jain S, Wilson R, Pasternak GW. Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: Evidence for opposing NO actions on morphine analgesia and tolerance. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(15):8220–8225. doi: 10.1073/pnas.94.15.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Pasternak GW. NG-Nitro-L-arginine prevents morphine tolerance. European Journal of Pharmacology. 1992;221:339–340. doi: 10.1016/0014-2999(92)90732-j. [DOI] [PubMed] [Google Scholar]
- Lam HH, Hanley DF, Trapp BD, Saito S, Raja S, Dawson TM, Yamaguchi H. Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neuroscience Letters. 1996;210:201–204. doi: 10.1016/0304-3940(96)12702-6. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and hyperalgesia. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- Ogilvie P, Schilling K, Billingsley ML, Schmidt HH. Induction and variants of neuronal nitric oxide synthase type I during synaptogenesis. FASEB Journal. 1995;9(9):799–806. doi: 10.1096/fasebj.9.9.7541381. [DOI] [PubMed] [Google Scholar]
- Ogura T, Yokoyama T, Fujisawa H, Kurashima Y, Esumi H. Structural diversity of neuronal nitric oxide synthase mRNA in the nervous system. Biochemical and Biophysical Research Communications. 1993;193:1014–1022. doi: 10.1006/bbrc.1993.1726. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: Modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. Journal of Neurophysiology. 1998;79(3):1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. Pain. 1996;66(2–3):331–341. doi: 10.1016/0304-3959(96)03025-4. [DOI] [PubMed] [Google Scholar]
- Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-μ, an alternatively spliced isoform expressed in differentiated skeletal muscle. Journal of Biological Chemistry. 1996;271:11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- Standifer KM, Chien CC, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of δ opioid analgesia and binding by antisense oligodeoxynucleotides to a δ opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Xie J, Roddy P, Rife TK, Murad F, Young AP. Two closely linked but separable promoters for human neuronal nitric oxide synthase gene transcription. Proc Natl Acad Sci USA. 1995;92(4):1242–1246. doi: 10.1073/pnas.92.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]