Abstract
Th1 cell responses to the variant surface glycoprotein (VSG) of African trypanosomes play a critical role in controlling infection through the production of IFN-γ, but the role of antigen presenting cells (APCs) in the induction and regulation of T cell mediated protection is poorly understood. In this study, we have investigated the antigen presentation capabilities of dendritic cells (DCs) and macrophages (MPs) during early trypanosome infection in relatively resistant responder and susceptible non-responder mouse strains. Splenic DCs appeared to be the primary cell responsible for activating naive VSG-specific Th cell responses in resistant responder animals through the coordinated up-regulation of costimulatory molecules, secretion of IL-12, and presentation of VSG peptides to T cells in vivo. Splenic DC depletion and the down-regulation of costimulatory markers on splenic MPs were observed in susceptible animals and may be associated with the inability of these animals to elicit a significant VSG-specific T cell response. In contrast to splenic APCs, peritoneal MPs secreted NO, failed to activate naïve Th cells in vitro, and presented relatively low levels of VSG peptides to T cells in vivo. Thus, VSG-specific Th1 cell responses may be determined by tissue- and cell-specific differences in antigen presentation. Additionally, all APCs from resistant and susceptible strains displayed a reduced ability to process and present newly encountered exogenous antigen, including new VSG molecules, during high parasitemia. Thus, initial uptake of VSG (or other trypanosome factors) may interfere with antigen presentation and have dramatic consequences for subsequent T cell responses to other proteins.
Keywords: Antigen Processing Cells, Parasitic Protozoa, T Cell Activation
Introduction
African trypanosomes express an immunogenic surface coat that is composed of variant surface glycoprotein (VSG) homodimers (1). VSG-specific B and T cell responses provide temporal variant-specific protection during infection by eliminating distinct variant antigenic types (VATs) present in the blood and extravascular tissues (2–5). However, trypanosomes may express up to 1000 different VSG genes and surface coats that ultimately enables the organisms to evade immune destruction (6, 7). The characterization of VSG-specific CD4+ Th cell responses, coupled with earlier functional and genetic studies on the failure of B cells to provide relative resistance, revealed the comparative importance of T cell responses in providing VAT-specific protection in trypanosomiasis (4, 5, 12–15). VSG-specific T cell responses of Trypanosoma brucei rhodesiense infected animals were MHC II restricted and required processing of the VSG molecule by APCs in order to activate T cells (4); the production of IFN-γ by polarized Th1 cells was shown to contribute to early host resistance during infection (5, 16), likely through the activation of macrophages to produce trypanolytic factors such as ROI, RNI and TNF-α (16–22). Recently we have demonstrated that VSG-specific Th1 cells generated during infection recognize peptides distributed throughout N-terminal domain sequences of the VSG molecule, but do not recognize peptide sequences from the conserved C-terminal domain (23).
Historically little is known about the role of APCs in the development of VSG-specific Th cell responses during trypanosome infection beyond MHC II restriction and processing-dependent T cell activation (4). However, the picture is much more complex due to evidence that cells of the innate immune system, including macrophages (MPs) and dendritic cells (DCs), may be dramatically altered in function following trypanosome infection. For example, MPs are known for their ability to suppress, in a pan-specific manner, the adaptive T cell responses of infected animals and to release a number of immunomodulatory factors including NO, TNF-α, PGE2 and IL-10 (19, 24–38). Furthermore, MPs and DCs are activated by glycosylinositolphosphate (GPI) substituents present on the C-terminal domain of shed VSG molecules (39–42), and trypanosome CpG DNA released during infection also has immunomodulatory effects on cells of the innate immune system (43). Both parasite molecules also have been shown to down-regulate IFN-γ-induced activation of the innate immune system, however, dependent on timing of exposure to these trypanosome factors (15, 43–45), so it is difficult to envision how cells of the innate immune system process and present VSG peptides in a manner sufficient to activate host Th cells. Given that there are differences in VSG-specific Th1 cell activation in relatively resistant compared to susceptible mice, that there is tissue-specific regulation of Th cell responses during infection, that Th cell specificity is limited to variable region subsequences of the VSG molecule, and that there is gradual loss of T cell responsiveness during progressive infection, we hypothesize that APC functions are substantially altered during trypanosome infection.
Supportive evidence for this hypothesis includes an earlier study showing that MPs from T. b. brucei-infected mice exhibited reduced presentation of exogenous (non-parasite) antigens to T cells; this was presumed to be due to a defect in the display of antigenic peptide:MHC II complexes (27). A similar reduction in antigen presentation for exogenous non-parasite antigen previously had been noted for T. b. rhodesiense-infected mice (46). These types of studies point to infection-related changes in APC function, although it is still unclear how antigen presentation capabilities develop among different APC subsets during early infection and the extent to which they may affect VSG-specific T cell responses. Moreover, DCs and their functions have not been examined in the context of African trypanosome infection, despite extensive evidence that DCs are responsible for initiating naive T cell responses to many protein antigens.
Thus, to better understand the role of MPs and DCs in the development and specificity of VSG-specific Th1 cell responses, we have characterized phenotypic and functional parameters of antigen presentation during early T. b. rhodesiense infection in both relatively resistant responder (B10.BR; B10) and susceptible non-responder (C3HeB/FeJ; C3H) mice. These host strains have been previously characterized to show that B10 mice make VSG-specific antibodies and develop VSG-specific Th1 cell responses, whereas C3H mice lack effective B cell or T cell responses to trypanosome antigens (3–5, 12). We examined multiple time points during early infection (prior to, during and after the first wave of parasitemia) for APC function as well as the activation of VSG-specific Th1 cell responses within the lymphoid tissues. Overall, our results show that DCs play a pivotal role in orchestrating the early development of VSG-specific Th1 cell responses in resistant mice through up-regulation of surface co-stimulatory markers, secretion of IL-12, ability to process and present antigen for the activation of naïve T cells, and presentation of VSG peptides during infection. However, altered functional capabilities were noted for infected mouse APCs in terms of processing and presenting exogenous non-parasite and parasite antigens to T cells, suggesting that exposure to trypanosomes in vivo may have dramatic consequences for Th cell reactivity to other antigens and, perhaps, to VSG determinants of new variants arising in infection.
Materials and Methods
Mice
H-2k compatible female B10.BR/SgSnJ (B10.BR) and C3HeB/FeJ (C3H) mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and used between 6 and 12 weeks of age for studies of APC function. A colony of 3A9 TCR transgenic mice on the B10.BR background was established from a breeding pair kindly provided by Dr. Matyas Sandor (University of Wisconsin, Madison). Additionally, C57BL/6 mice (H-2b ) which express a resistant VSG-responder phenotype when infected with trypanosomes of the T. b. rhodesiense LouTat 1 serodeme were used (55, 64–67). Female C57BL/6J wt mice, C57BL/6-IL-12p35 knockout mice and C57BL/6-IL-12p40 knockout mice (6 to 8 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, ME) and used in the present study for a determination of the role of IL-12 in development of VSG-specific Th1 responses. Outbred Swiss mice (Harlan Spraque-Dawley, Madison, WI) were used for initial growth of trypanosome stabilates in order to provide organisms for infection of experimental animals. All animals were handled according to National Institutes of Health and University of Wisconsin-Madison guidelines, and were housed in American Association for Accreditation of Laboratory Animal Care-accredited facilities.
Trypanosomes
Trypanosoma brucei rhodesiense clones LouTat 1 and LouTat 1.5 were used for infections and were grown from frozen stabilates as described previously (47). Briefly, Swiss mice were immunosuppressed with cyclophosphamide treatment (300 mg/kg body weight; Sigma, St. Louis, MO) (47) concurrent with i.p. injection of trypanosomes. After five days, blood was collected and diluted in PBS/1% glucose (PBSG) and experimental mouse groups were infected by i.p. injection of 105 trypanosomes.
Variant surface glycoprotein
Trypanosomes harvested for VSG purification were passed over a DEAE cellulose column (DE52; Fisher Scientific, Hanover Park, IL) equilibrated with PBSG to bind cellular components of host blood (48). Eluted trypanosomes were washed three times in PBSG, and VSG purification was performed as described previously (1, 4, 24, 45). Briefly, trypanosomes were resuspended in 0.3 mM zinc acetate containing 0.1 mM tosyl lysine chloromethyl ketone to 109/ml and incubated for 15 min at 4°C prior to centrifugation at 3000 × g at 4°C for 10 min. Supernatant fluid was reserved and kept at 4°C. The pellet was resuspended in an equal volume of 10 mM phosphate buffer containing 0.1 mM tosyl lysine chloromethyl ketone and incubated for 20 min at 37°C, then cooled to 4°C and spun at 10,000 × g for 15 min. Supernatant and previously reserved fluids were combined and spun at 300,000 × g for 1 hr at 4°C. The resulting supernatant was concentrated by centrifugation through a Centriprep-30 column (Amicon Corp, Danvers, MA) and passed over a DEAE-cellulose column equilibrated with 10 mM phosphate buffer, pH 8. VSG detected in the first peak eluted off the column was subjected to electrophoretic analysis and appeared as a single 62 kDa band on SDS-polyacrylamide gels under reducing conditions.
Isolation of macrophages and dendritic cells
Adherent splenic MPs (SMPs) and peritoneal MPs (PMPs) were isolated from uninfected or infected mice at different times during infection. Spleens were disrupted in complete RPMI medium (RPMI-1640, Sigma; supplemented with 10% FBS, 2mM L-glutamine, 16 mM HEPES, 50 µg/ml gentamycin) and passed through 40 µm nylon filters to obtain a single cell suspension. Peritoneal exudates cells collected in ice-cold PBS/heparin (10U/ml) and splenocytes were treated with ACK lysis buffer to remove erythrocytes. Cells were washed twice with complete RPMI media, plated at 1×106 (peritoneal) or 2.5×106 (splenocyte) viable cells/ml, and incubated for 2 hours at 37°C in 5% CO2. Plates were washed thoroughly with PBS to remove non-adherent cells. SMPs were collected after incubation at 4°C for 15 min in ice-cold PBS, followed by scraping with a rubber policeman. PMPs were lifted from plates using Accutase (Innovative Cell Technologies, Inc., San Diego, CA). MPs were washed and resuspended in complete DMEM (DMEM, Sigma; supplemented with 10% FBS, 2mM L-glutamine, 1% MEM nonessential amino acids, 16mM HEPES, 50µM 2-ME, and 50 µg/ml gentamycin) prior to use.
Splenic DCs were isolated by enzymatic digestion of tissues with Dnase I (Invitrogen, Carlsbad, CA)/Liberase (Roche Applied Science, Indianapolis, IN) in complete RPMI media for 30 min at 37°C, 5% CO2. Dnase I and Liberase concentrations were empirically determined for each day of infection to account for changes in spleen size and composition. After 5 min incubation with EDTA (0.01M final), spleen fragments were passed through 40 µm nylon filters and washed with Ca2+/Mg2+-free PBS/0.5% BSA/5mM EDTA (MACS buffer); erythrocytes were lysed with ACK lysis buffer. The remaining cells were overlaid onto 14.1% Nycodenz (Sigma) and spun at 1900 rpm for 20 min at 4°C. For flow cytometry, low density -enriched cells were resuspended in PBS/0.5% BSA/2mM EDTA/0.05% azide (flow buffer) and treated as described below. For DC isolation, low density cells collected from the interface were washed twice with MACS buffer, labeled with anti-CD11c magnetic beads (Miltenyi Biotec Inc., Auburn, CA), and passed over two consecutive MACS columns (Miltenyi Biotec Inc.) according to a standard protocol provided by the manufacturer. CD11c+ DCs were washed and resuspended in complete DMEM.
Flow cytometry
FITC-conjugated anti-CD11c, anti-CD11b, anti-CD4 and anti-CD3; PE-conjugated anti-CD40, anti-CD54, anti-CD80, anti-CD86, and anti-I-Ak; and APC-conjugated anti-CD8 were purchased from BD Biosciences Pharmingen (San Diego, CA). Alexa 647-conjugated F4/80 was purchased from Serotec (Raleigh, NC). Isotype matched Abs with the appropriate fluorochromes were used as controls. Peritoneal cells, splenocyte suspensions, or low density-enriched cells were incubated with FcBlock (BD Biosciences) for 15 min on ice to block nonspecific staining. Cells were then incubated with Abs to cell surface antigens for 30 min, washed twice with flow buffer, and stained with a live/dead fixable stain (Invitrogen) according to manufacturer protocols. All cells were washed twice with flow buffer, fixed in 1% paraformaldehyde (Polysciences), and analyzed immediately on an LSR II using BD FACSDiva (BD Biosciences) and FlowJo (Tree Star, San Carlos, CA) software. Live MPs and DCs were identified based on dead cell exclusion, SSC/FSC profiles, and CD11chigh CD8+/− (DC), F4/80 high CD11c− (SMP), or F4/80 high CD11b high (PMP) expression. Rainbow fluorescent particles (Spherotech, Libertyville, IL) were used to set fluorochrome voltages so that quantitative analysis of MHC II and costimulatory molecule expression could be performed across multiple days of infection.
TCR transgenic T cell isolation
Naïve T cells were enriched from the spleen and lymph nodes (inguinal, axillary, brachial) of H-2k compatible 3A9 TCR transgenic mice by adherence to nylon wool as described previously. Cell depletion was performed on the T cell-enriched population using Low-Tox rabbit complement (Cedarlane, Burlington, NC) and monoclonal antibodies 16-1–2 (anti-H2k), 31M (anti-CD8α) and M1/70 (TIB128, American Type Culture Collection (ATCC), Manassas, VA). Live cells were collected on a Percoll (Sigma) gradient and washed twice in complete DMEM before use.
Cytokine assays
MPs (2 ×105) or DCs (105) from B10 or C3H mice were incubated in 96-well plates in complete DMEM for 24 hr at 37°C, 5% CO2. Supernatants were stored at –20°C prior to analysis. For cytokine secretion and antigen presentation assays (see below), IL-2, IL-4, TNF-α, IFN-γ, IL-18, IL-12p40, and IL-10 were measured using OptiEIA ELISA kits (BD Biosciences) unless otherwise noted. Nitric oxide was determined by adding an equal volume of supernatant to 1% sulfanilamide (Sigma) in 2.5% H3PO4 and 0.1% N-(1-Naphthyl)ethylenediamine dihydrochloride (Sigma) in 2.5% H3PO4 and measuring absorbance at 550 nm. A standard curve was prepared using sodium nitrite. The ability of infected wt and IL-12 knockout mice to generate VSG-specific Th1 responses was determined by preparing spleen cell suspensions and stimulating 106 viable cells with 50 µg purified VSG for 48 to 72 hr, as we have described previously (4, 38, 39); supernatant fluids were harvested, stored and tested as above for IL-12 levels.
Antigen presentation assays
Irradiated splenocytes (106), MPs (105), or DCs (5 ×104) from naïve and infected B10 or C3H mice were combined with T cell hybridomas (105) or naïve T cells isolated from 3A9 transgenic mice (105) and varying doses of either hen egg lysozyme (HEL; Sigma) or LouTat 1 VSG at 37°C, 5% CO2. HEL and VSG were incubated for 30 min at 37°C with and without polymyxin B (20ug/ml, Sigma) prior to dilution and addition as a standard control for LPS contamination. Supernatants were collected at 24 hr from T cell hybridoma stimulations. For analysis of naïve T cell activation, 50 µl supernatant was collected at 48 hr (for cytokine analysis) and replaced with 50 µl [3H]thymidine (1uCi/ml in complete DMEM) for the final 16 hr of culture. IL-2 was measured by [3H]thymidine incoporation of CTLL-2 cells (TIB-214, ATCC) or by ELISA.
VSG-specific Th cell hybridomas
Trypanosome infected B10 mice were euthanized at day 7 and pooled spleen cells passed over nylon wool columns to enrich for T lymphocytes. T cells were combined in a 1:1 ratio with BW5147 TCRα-β- thymoma cells and 1 ml of 50% polyethylene glycol 1500 (Sigma) to promote fusion and washed with 9 ml FBS-free DMEM. After centrifugation, cells were combined with thymocytes (feeder cells) in DMEM-20, aliquoted into 96-well plates, and incubated at 37°C, 5% CO2. DMEM-20 supplemented with HAT was added at intervals after fusion to deplete non-hybrid cells, and proliferating cell wells were expanded. Hybridoma populations possessing reactivity to VSG were cloned by several rounds of limiting dilution and reselected for VSG reactivity and CD3/CD4 expression, as we have described (4, 23).
Statistical Analysis
Data were analyzed by the Student t-test (significance was defined as p < 0.05), where appropriate, using GraphPad Prism software v5.
Results
Membrane phenotype of APCs
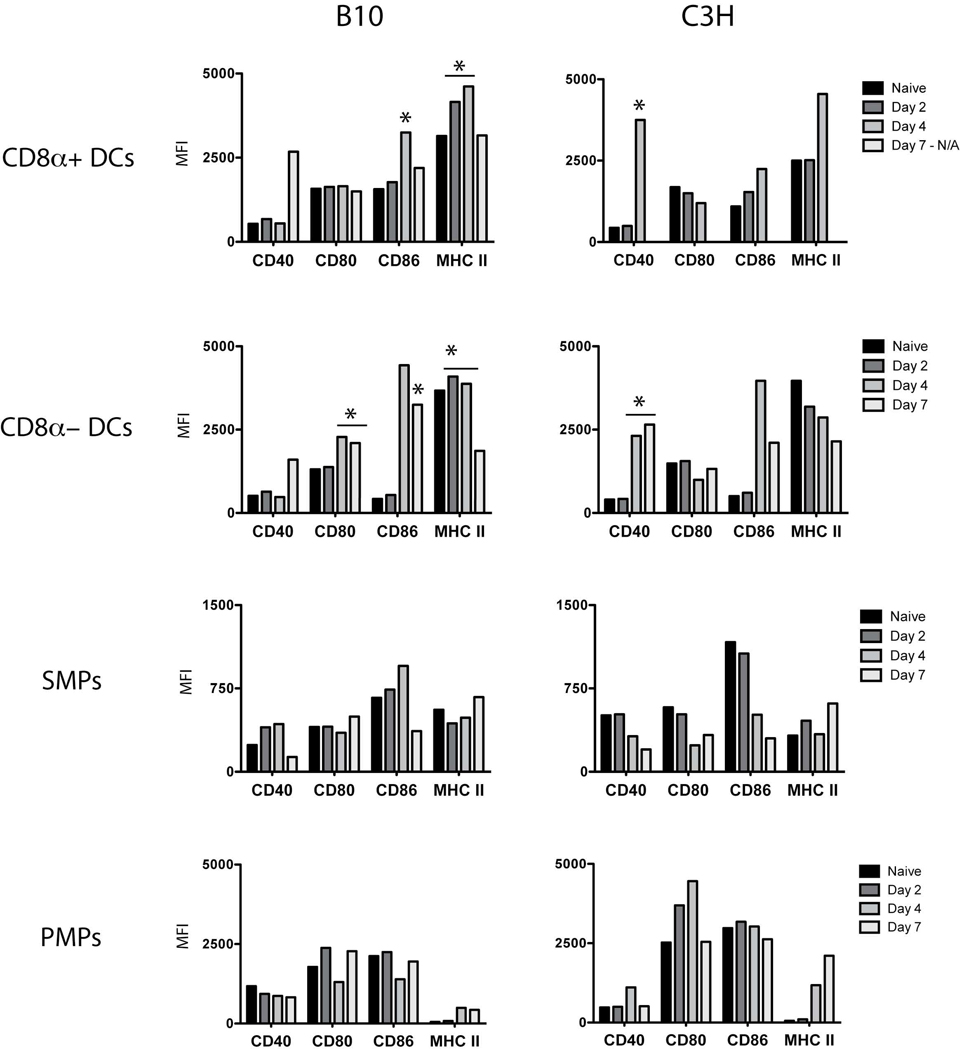
Th cell activation depends on the recognition of antigenic peptide:MHC II complexes and secondary signals from costimulatory molecules on APCs. In order to test the hypothesis that APC function was altered by trypanosome infection, we first examined for the expression patterns of these molecules on CD11chighCD8α+ and CD11chigh CD8α- splenic DCs, CD11c–F4/80high SMPs, and CD11bhigh F4/80high PMPs from resistant (B10) and susceptible (C3H) mice. Mean fluorescence intensities (MFIs) for CD40, CD80, CD86, and MHC II were determined for DCs and MPs and are shown in Figure 1.
Figure 1. Expression of MHC II and costimulatory molecules by DCs and MPs from uninfected and Trypanosoma brucei rhodesiense LouTat 1-infected animals.
Peritoneal exudate cells and splenocytes were isolated from B10 or C3H mice at 0, 2, 4 and 7 days post-infection. The data points shown represent MFI values derived from flow cytometry for multiple phenotypic markers expressed by the different tissue-specific cell populations; pooled cells from 5 mice per time point per strain were examined in these studies, and the data set shown is from one of five separate representative experiments. MPs were gated based on viability, SSC/FSC profiles, and finally lineage-specific markers to identify peritoneal MPs (F4/80high CD11bhigh) and splenic MPs (F4/80highCD11c− ). A low density-enriched fraction of splenocytes was prepared for DC analysis; DCs were gates as above using CD11chighCD8+/− for DC identification. Asterisks highlight data showing phenotypic changes in CD8a+ and CD8a− DCs that are significantly different between resistant and susceptible mice on the specific days of infection shown.
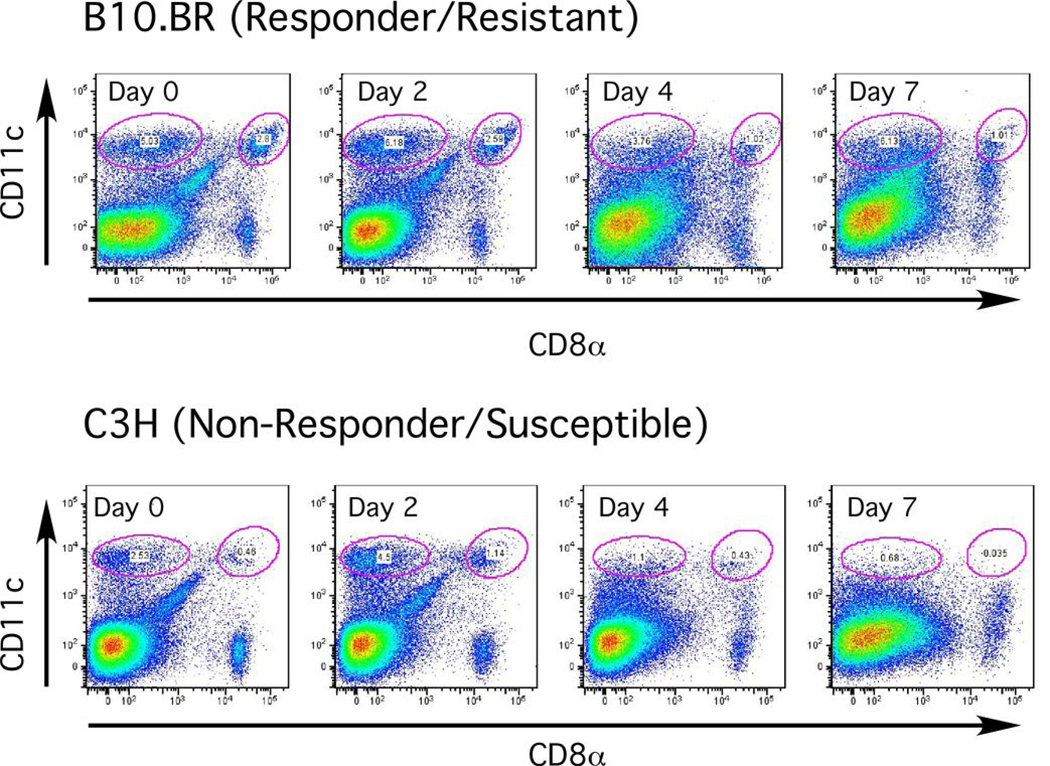
CD8α+ and CD8α- DCs from B10 mice up-regulated or maintained basal levels of expression of all costimulatory molecules examined throughout infection (Figure 1). MHC II levels were maintained on both DC subsets through day 4 and down-regulated by day 7. In contrast, CD8α- DCs from C3H animals immediately and continuously down-regulated MHC II upon infection, despite up-regulation of costimulatory molecules such as CD40 (Figure 1). CD80 expression was also down-regulated on both DC subsets from C3H mice. The most striking difference between strains was evident within the DC population: CD8α+ DCs were not detectable through day 7 of infection in C3H animals, and the relative proportion of CD8α- DCs was also reduced (Figure 2).
Figure 2. CD8α− and CD8α+ DCs are selectively depleted in susceptible C3H but not resistant B10 mice infected with African trypanosomes.
CD11chigh CD8α− or CD11chigh CD8α+ DCs in live, low density-enriched splenocytes from B10 or C3H animals (5 mice/group) on days 0–7 of infection; percentage of each subset is listed within the gated cell subpopulations. Results of a representative experiment are shown. The values shown in this figure for different time points of infection are: B10.BR (Day 0) CD11chighCD8α− = 5%; CD11chighCD8α+ = 2.8%. (Day 2) CD11chighCD8α− = 6.2%; CD11chighCD8α+ = 2.6%. (Day 4) = CD11chighCD8α− = 3.8%; CD11chighCD8α+ = 1%. (Day 7) = CD11chighCD8α− = 6.1%; CD11chighCD8α+ = 1%. C3H (Day 0) CD11chighCD8α− = 2.5%; CD11chighCD8α+ = 0.5%. (Day 2) CD11chighCD8α− = 4.5%; CD11chighCD8α+ = 1.1%. (Day 4) = CD11chighCD8α− = 1.1%; CD11chighCD8α+ = 0.4%. (Day 7) = CD11chighCD8α− = 0.7%; CD11chighCD8α+ = 0.03%.
With the exception of CD80, costimulatory molecules were up-regulated on SMPs from B10 animals by day 4 of infection, although both CD40 and CD86 decreased by day 7 (Figure 1). In contrast, SMPs from C3H animals down-regulated CD40, CD80, and CD86 by day 4. MHC II expression levels fluctuated slightly on SMPs from both strains during infection. In both resistant and susceptible models, SMP expression of all costimulatory markers examined was significantly lower than those on activated DCs.
In B10 mice, MHC II levels increased on PMPs but costimulatory molecules fluctuated with decreases in CD40, CD80, and CD86 at day 4 (Figure 1). Unexpectedly, PMPs from C3H animals displayed increased expression of costimulatory molecules and MHC II by day 4, though CD40 and CD80 were down-regulated by day 7 (Figure 1). MHC II expression was several fold higher in C3H mice compared to B10 animals. ICAM-1 expression was also monitored and increased similarly on all APCs from both resistant and susceptible animals throughout infection (data not shown). Overall, these data illustrate temporal, tissue-specific, and strain-specific differences in phenotype during infection that may represent altered APC functions that contribute to the development or modulation of Th cell responses.
Cytokine production
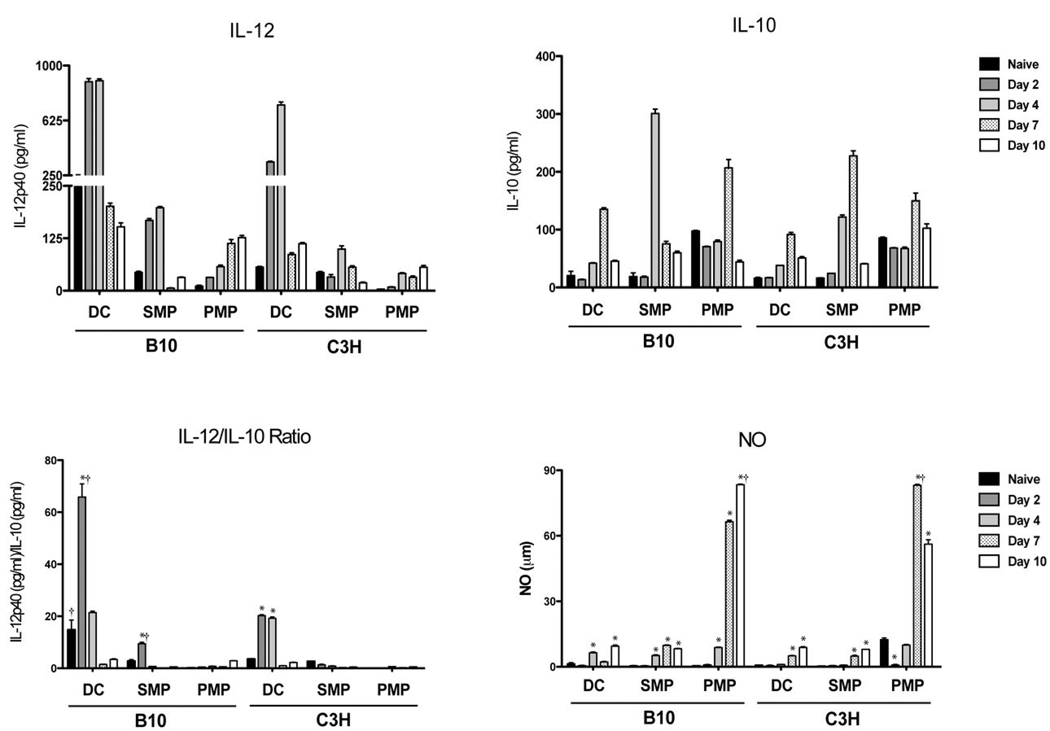
Since local cytokine environment influences the activation and polarization of Th cells, we examined cytokine production by APCs from naïve and infected mice cultured ex vivo. As seen in Figure 3, all APCs produced IL-10 and IL-12p40 at varying levels throughout infection. The ratio of Th1-associated IL-12 to IL-10, a regulatory cytokine, demonstrates the significant phenotypic potential of DCs to elicit Th1 responses. The timing of IL-12 production by DCs also coincided with up-regulation of surface markers. Although the general pattern of cytokine production was similar between B10 and C3H animals, APCs from B10 animals generally secreted higher levels of IL-12 at earlier times of infection. SMPs produced less IL-12 but more IL-10 than DCs, and PMPs produced little IL-12 or IL-10 but high levels of nitric oxide, which has been shown to suppress T cell proliferative responses to both trypanosome antigens and mitogens (26, 35, 38). TNF-α, IL-4, and IL-18 were not detected (data not shown). These quantitative and temporal differences in cytokine responses by APCs during infection may be a contributing factor to the relative ability of B10 animals to mount a polarized Th1 cell response. The role of IL-12 in contributing to the Th1 phenotype was clearly demonstrated using knockout mice on a resistant VSG responder genetic background. As shown in Figure 4, the early development and expression of VSG-specific Th1 cytokine responses (IFN-gamma and IL-2) were absent in IL-12 knockout mice from day 4 through day 10 post-infection; there was no compensatory Th2 cell response in IL-12 knockout mice as measured by IL-4 secretion. Interestingly the VSG-specific Th1 cytokine phenotype emerged in IL-12 knockout mice by day 14 of infection, demonstrating that even in the absence of IL-12 there are significant alternative signal(s) for mounting this protective response to infection.
Figure 3. Splenic APCs produce IL-12 upon infection, whereas NO is produced by peritoneal MPs.
Cytokine production by DCs and MPs taken from naïve and infected animals (5 mice/group) and cultured ex vivo; supernatants were collected after 24 hr. IL-12p40 and IL-10 production were assessed by ELISA; NO was determined by Griess reaction. Results are mean +/− SEM of triplicate wells in a representative experiment. *, p < 0.05 as compared to naive control; †, p < 0.05 as compared to same measurement in opposite mouse strain.
Figure 4. IL-12 induces the early generation and polarization of VSG-specific Th1 cells during trypanosome infection.
Relatively resistant C57BL/6 mice with the IL-12p35 gene deleted were infected with T. b. rhodesiense LouTat 1. SPC were harvested at the time points shown and tested for the secretion of IFN-γ, IL-2 and IL-4 following stimulation in vitro with purified LouTat 1 VSG. Identical results were obtained with IL-12p40 knockout mice and wt mice treated with neutralizing Ab to IL-12 (data not shown; manuscript in preparation). Results presented as +/− SEM of three separate experiments; *,p < 0.05 knockout mouse responses as compared to wt controls.
APC function
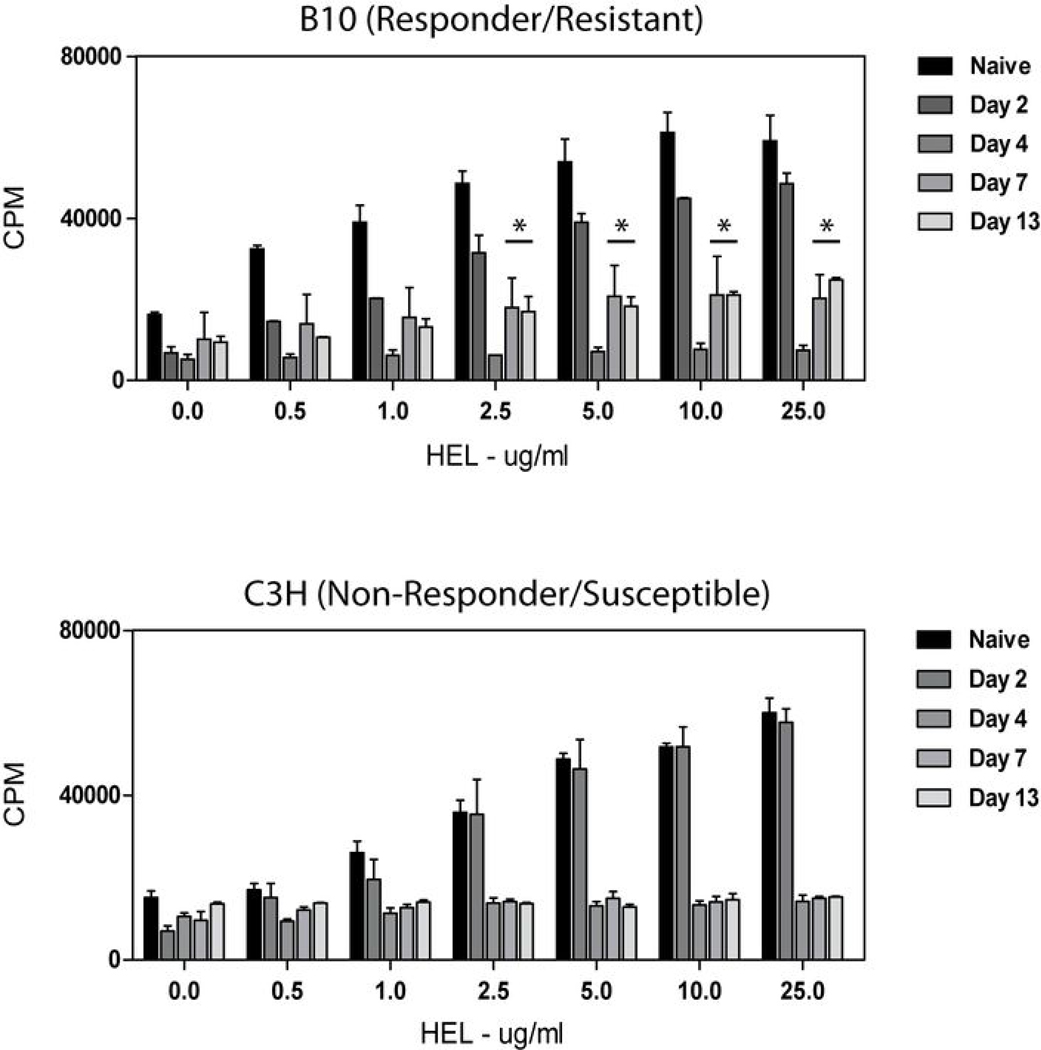
Cytokine secretion and MHC II/costimulatory molecule expression appeared to be temporally regulated in APCs during trypanosome infection. To relate APC phenotype to function, we first tested irradiated splenocytes for their ability to process and present a well-defined non-parasite antigen, HEL, to the HEL-specific 3A9 T cell hybridoma as a functional measure of antigen presentation capability. Splenic APCs isolated two days post-infection processed and presented HEL in a dose-dependent manner, similar to APCs from naïve animals (Figure 5). As infection progressed, splenic APCs elicited only weak T cell responses when combined with exogenous antigen, regardless of mouse strain tested. However, it may be worthwhile noting that APCs from resistant mice recovered a measurable ability to process and present exogenous antigen during the test period compared to APCs from susceptible mice.
Figure 5. Splenocytes from infected B10 and C3H mice display impaired ability to process and present newly encountered antigen.
Splenocytes from uninfected and infected animals (days 0–13 post infection; 5 mice/group) were irradiated and combined with the 3A9 T cell hybridoma and varying concentrations of HEL antigen. Supernatants were collected after 24 hr and assayed for IL-2 production by [3H] thymidine incorporation of IL-2-dependent CTLL-2 cells. T cell hybridomas cultured alone or with HEL did not produce IL-2 (data not shown). Results are mean +/− SEM of triplicate wells in a representative experiment.
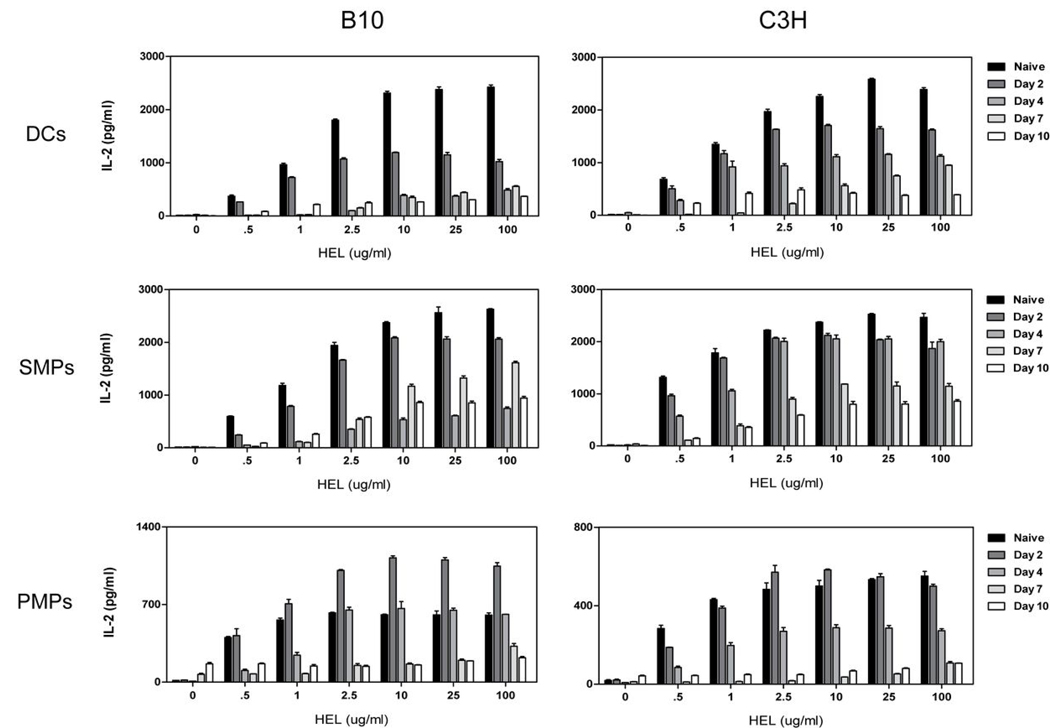
To determine whether reduced antigen presentation extended to specific cell types or lymphoid compartments, DCs, SMPs, and PMPs were isolated from naïve and infected animals and incubated with HEL and the 3A9 T cell hybridoma. As shown in Figure 6, all APCs displayed dose-dependent HEL processing and presentation capability early in infection, whereas by day 4 or thereafter the ability to present antigen diminished. Antigen presentation could not be rescued with high doses of antigen (Figure 6; up to100 µg/ml concentrations). A reduction in DC processing and presentation of exogenous antigen during infection may be explained by their maturation state, as maturation halts endocytic activity. Our observations of increased IL-12 production and up-regulation of costimulatory molecules during the early period of infection support the contention that DCs undergo maturation during this time. Decreased endocytosis could not explain the decline in antigen presenting function by MPs, as FITC-dextran uptake was similar in both naïve and infected animals (Freeman et al., data not shown).
Figure 6. DCs and MPs from infected animals display impaired processing and presentation of newly encountered antigen.
DCs, SMPs, or PMPs isolated from uninfected and infected mice (days 0–10 post infection; 5 mice/group) were combined with the 3A9 T cell hybridoma and varying concentrations of HEL antigen. Supernatants were collected after 24 hr and assayed for IL-2 production by ELISA. Results are mean +/− SEM of triplicate wells in a representative experiment.
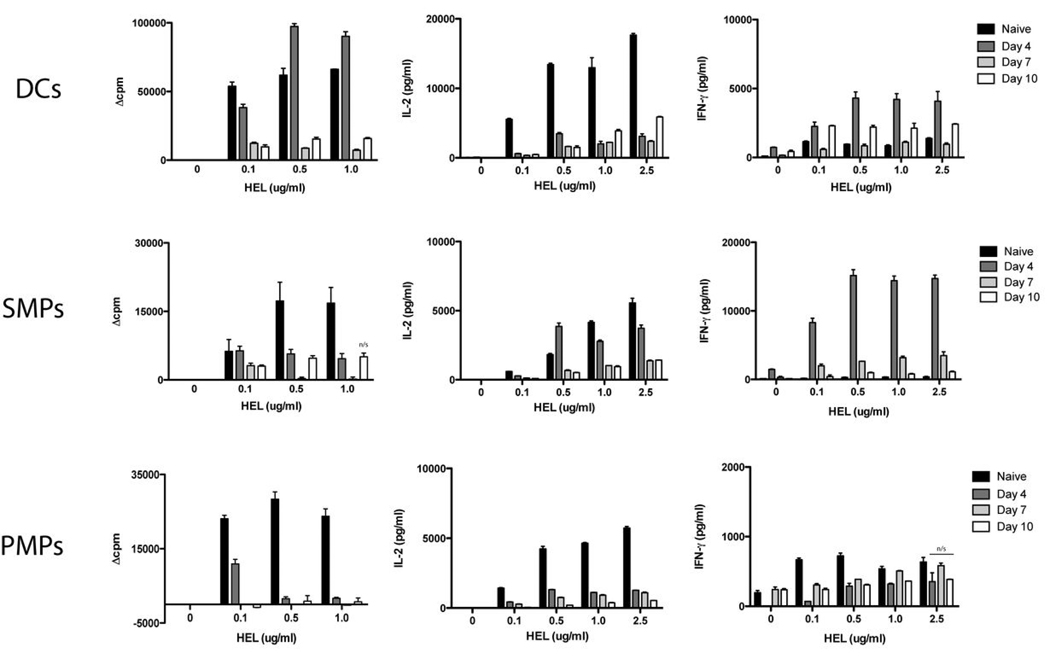
Processing and presentation of exogenous antigen to T cell hybridomas reflects only the amount of HEL peptide:MHC II complexes expressed by APCs. To determine the additional contributions of costimulatory molecule expression and/or cytokine production, we tested the ability of APCs from control and infected animals to activate naïve HEL-specific T cells from 3A9 transgenic mice. Similar to T cell hybridoma activation, naïve TG HEL-specific T cells produced less IL-2 in response to APCs from infected animals (Figure 7). DCs isolated at day 4 post-infection were, however, able to elicit high levels of IFN-γ secretion and T cell proliferation in the absence of IL-2. SMPs at day 4 elicited IFN-γ production as well as IL-2 from T cells but were unable to induce proliferation. These data suggest that T cell proliferation observed in response to DCs may be IL-2-independent or, alternatively and probably, that rapidly replicating T cells in these 48 hr cultures have taken up and utilized the IL-2 produced during activation. However, PMPs were not capable of inducing T cell proliferation or cytokine secretion, and similar results were obtained with APCs from C3H mice (data not shown). APCs from infected animals preferentially polarized naïve T cells toward a Th1 phenotype, as we did not detect IL-4 in the cell supernatant fluids of any cultures tested (data not shown); this is in complete agreement with earlier published data (4, 5, 49) and our findings above with respect to polarization of the Th1 cell response in wt and IL-12 knockout mice. Overall, these results reveal that DCs isolated at distinct times of infection from either resistant or susceptible mice are functionally capable of activating Th1 cells. SMPs may also contribute to the development of Th1 responses, but PMPs from either B10 or C3H animals clearly lack the ability to activate Th cells. Thus APC function is selectively altered in specific tissues and at specific times of infection with African trypanosomes.
Figure 7. Splenic APCs activated during trypanosome infection induce naïve CD4+ T cell proliferation and/or IFN-γ production.
DCs, SMPs, or PMPs isolated at different times of infection (days 0–10 post infection; 5 mice/group) were incubated with naïve TG T cells and varying doses of HEL. (A) T cell proliferation was determined by [3H]thymidine incorporation during the last 18 hr of a 64 hr culture period, and results are expressed as Δcpm (cpm of wells with HEL–cpm of wells without HEL). (B) IL-2 and (C) IFN-γ were assessed from 48 hr culture supernatants by ELISA. Results are mean +/− SEM of triplicate wells in a representative experiment. At the highest HEL concentration given, all measurements except those noted were significantly different from the naive control. n/s, no significant difference (p > 0.05) as compared to naive control.
Processing and presentation of VSG to T cells ex vivo
Although DCs and MPs displayed similar functional characteristics for non-parasite antigen regardless of host resistance phenotype, a protective VSG-specific Th1 cell response occurs only in resistant B10 and C57BL/6 mice. We might predict based on the findings above, therefore, that only APCs from B10 mice process and present VSG during infection, or that APCs from resistant animals display a different VSG peptide repertoire than C3H mice. To survey the presentation of VSG-derived peptides displayed by APCs ex vivo from infected animals, we combined APCs with VSG-specific T cell hybridomas (originally derived from Th cells activated during infection) and monitored activation by IL-2 production; prior analyses had demonstrated that the Th cell hybridomas tested represent clones with unique TCRs specific for peptides distributed throughout the N-terminal domain of the LouTat 1 VSG molecule (23).
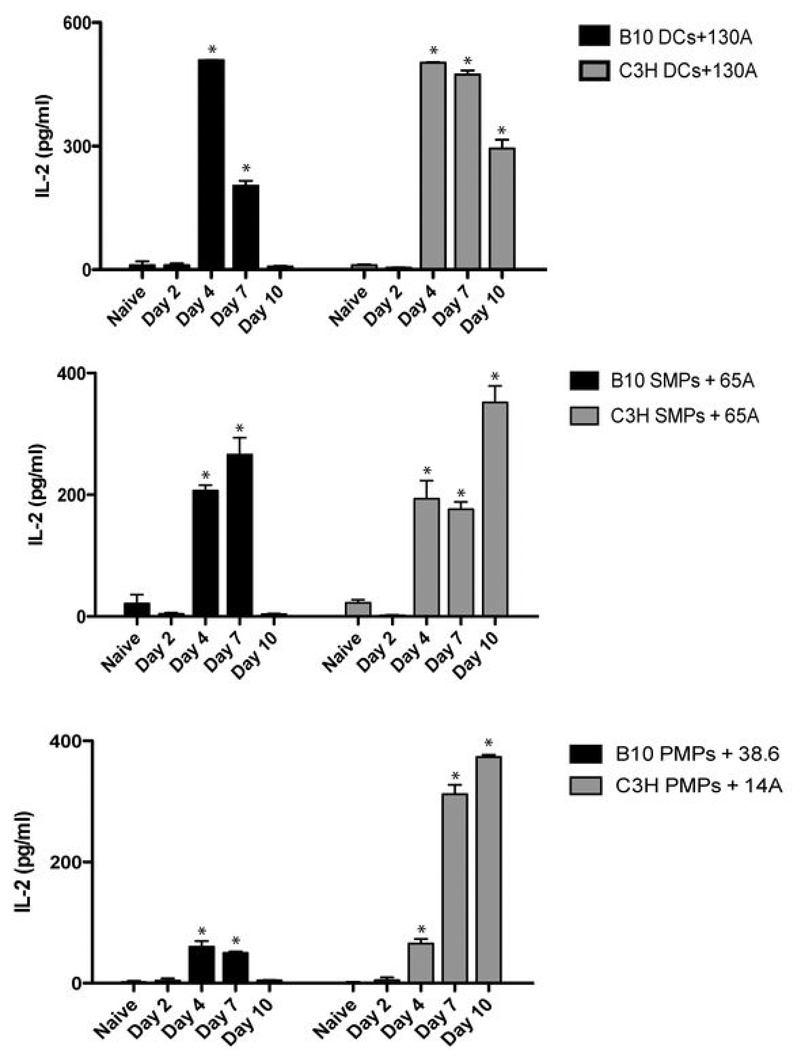
VSG-specific T hybridomas were combined with APCs from naïve and infected animals to determine the extent and kinetics of VSG presentation during infection. Figure 8 shows representative hybridoma responses to DCs and MPs ex vivo. All APCs displayed the highest detectable levels of VSG peptide:MHC II complexes by day 4 of infection. However, VSG presentation by B10 APCs diminished by day 7, whereas C3H APCs continued to present VSG and activate VSG-specific T hybridomas through day 10, the endpoint of the experiment. These patterns were observed in all of the VSG-specific T hybridoma responses to APCs (Figure 8 and data not shown). Since parasite burden remains high and uncontrolled in C3H mice whereas B10 mice exhibit antibody- and T cell-mediated clearance of VATs by day 5 or 6 of infection, we hypothesized that the failure to clear parasites, resulting in continuous exposure to VSG, led to an extended period of presentation due to greater levels of VSG peptide:MHC II complexes expressed. To test this hypothesis, APCs from B10 mice at day 10 of infection (following clearance of the first wave of parasitemia and at a time when these cells were expressing no or low VSG peptide:MHC II complexes) were combined with VSG-specific T hybridomas with or without administration of exogenous VSG. APCs from infected B10 animals were able to process and present additional exogenous VSG (Figure 9), supporting the notion that VSG availability in vivo dictates a level of VSG peptide presentation. Additionally, the ability of APCs from infected animals to process and present exogenous VSG suggests a different mechanism of processing and presentation from that of HEL. This may be due to the scavenger-dependent mechanism by which VSG enters APCs and initiates cell signaling and subsequent cellular functions (43, 44, 50). APCs from C3H animals activated VSG-specific T hybridomas in the absence or presence of exogenous VSG (Figure 8 and data not shown) but are unable to generate a VSG-specific T cell response in vivo during infection (5). These results suggest that the potential for C3H APCs to activate VSG-specific T cells during infection exists, but that mechanisms unrelated to the ability to process and present antigenic peptide:MHC II complexes alone are effective at blocking the T cell response.
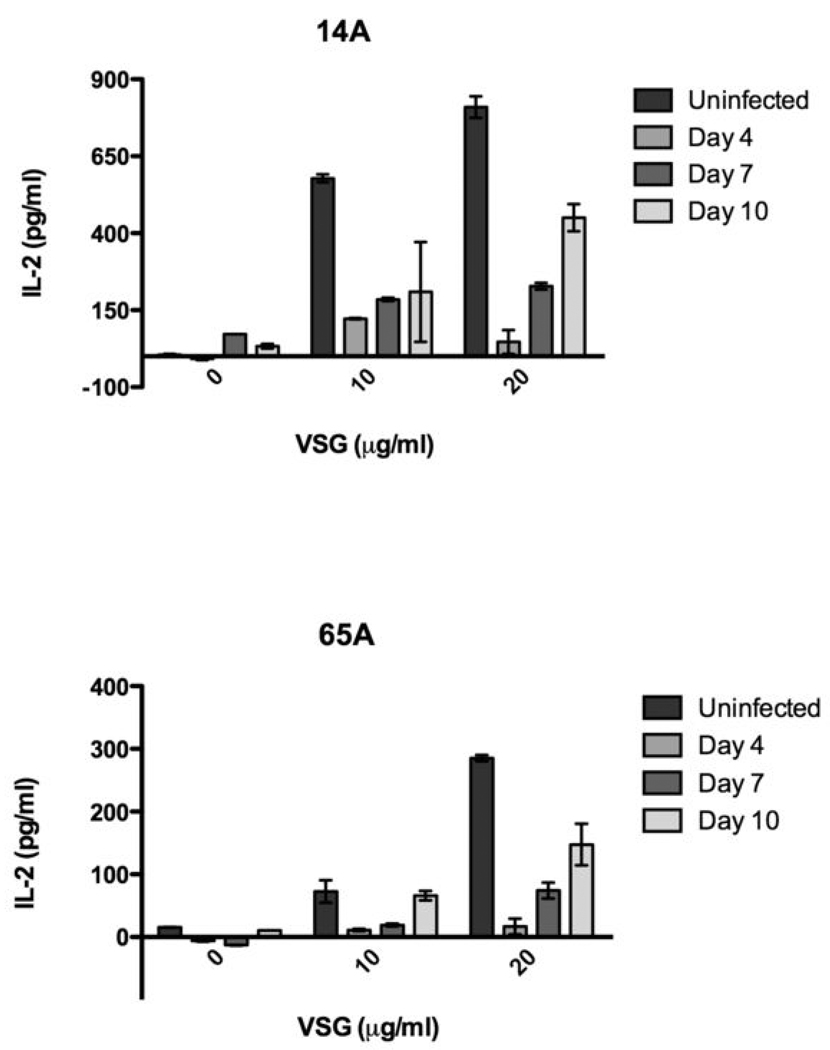
Figure 8. APCs ex vivo from infected mice present VSG peptides to T cells.
DCs or MPs isolated from uninfected and infected animals (days 0–10 post infection; 5 mice/group) were combined with VSG-specific T hybridomas without the addition of exogenous VSG (50 ug/ml). IL-2 production was measured in 24 hr supernatants by ELISA. Shown are representative responses from four of thirteen T hybridomas. The APC type and T hybridoma used are shown for each graph (note: unlike splenic DCs and MPs, PMPs from resistant and susceptible animals activated different subsets of T hybridomas). Results are mean +/− SEM from triplicate wells in a representative experiment. *, p < 0.05 as compared to naive control.
Figure 9. VSG availability contributes to the sustained presentation of VSG peptides during trypanosome infection.
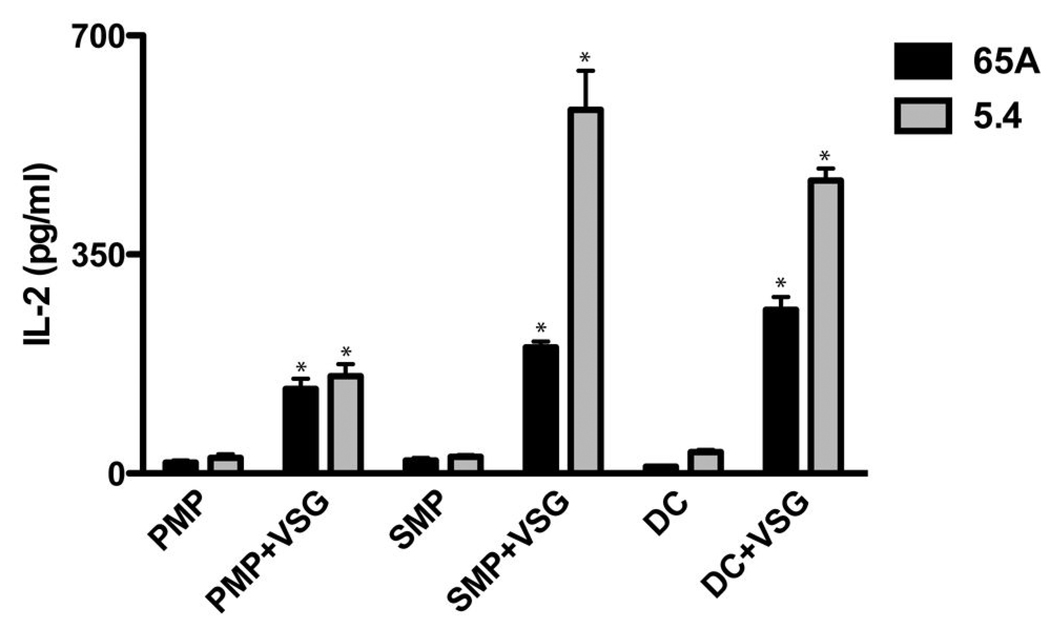
B10 APCs isolated at day 10 of infection (following clearance of LouTat 1 trypanosomes and at a time when such APCs ex vivo were no longer able to present peptides to the T hybridomas; see Figure 8) were supplemented with additional (exogenous) VSG and cultured with VSG-specific T cell hybridomas. Two representative T hybridoma responses are shown. PMPs were also able to activate these hybridomas when provided with exogenous VSG, though they were unable to do so at any time during infection without additional VSG added to cultures. IL-2 was measured by ELISA; results are displayed as mean +/− SEM of triplicate wells in a representative experiment. *, p < 0.05 as compared to control.
The relative level of VSG peptide:MHC II molecules expressed and the variety of peptides displayed were determined by T hybridoma responses and the specific T hybridoma activated, respectively (it should be noted that the VSG peptide specificities of T hybridomas are matched precisely by Th cells activated in situ during infection, as we have recently demonstrated (23)). DCs from B10 and C3H animals elicited IL-2 production from all but one VSG-specific T hybridoma (Table 1), reflecting the variety of VSG epitopes that are presented for Th cell recognition. DCs also expressed comparatively higher levels of VSG peptide:MHC II molecules, as indicated by the number of hybridomas producing high amounts of IL-2 relative to responses from MPs. SMPs from both B10 and C3H mice elicited IL-2 production from the same VSG-specific hybridomas highly activated by DCs, but IL-2 responses were moderate or low (Table 1). SMPs failed to activate the remaining hybridomas and therefore by inference express fewer and less varied VSG peptide:MHC II molecules on their surface during infection compared to DCs. Regardless of host strain, DCs and SMPs activated the same hybridomas to a similar extent during peak parasitemia (Table 1 and data not shown). Splenic APCs of both resistant and susceptible animals therefore appear equally capable of processing and presenting VSG during infection.
TABLE 1.
VSG Presentation by APCs During Infection
| APCs | Max. Hybridoma Response* | Hybridomas |
|---|---|---|
| B10/C3H DCs | +++ | 5.4, 38.6, 40.6, 28A, 130A, 65A, 14A |
| ++ | − | |
| + | 4.6, 17.5, 116A, 100A, Q7 | |
| −− | 12.2 | |
| B10/C3H SMPs | +++ | 38.6, 28A |
| ++ | 40.6, 130A, 65A, 14A | |
| + | 5.4, 116A | |
| −− | 4.6, 17.5, 100A, Q7, 12.2 | |
| B10 PMPs | +++ | − |
| ++ | 28A | |
| + | 38.6, 116A | |
| −− | 5.4, 40.6, 130A, 65A, 14A, 4.6, 17.5, 100A, Q7, 12.2 | |
| C3H PMPs | +++ | − |
| ++ | 28A, 38.6, 40.6 | |
| + | 14A, 5.4 | |
| −− | 130A, 65A, 116A, 4.6, 17.5, 100A, Q7, 12.2 |
The maximum hybridoma response elicited by APCs isolated from infected animals. +++ = >400pg/ml IL-2, ++ = 200–400pg/ml IL-2, + = 40–200pg/ml, −− = <40pg/ml.
In contrast to splenic APCs, PMPs activated comparatively few VSG-specific T hybridomas (Table 1). The limited variety of peptides presented within the peritoneum may reflect the amounts or form of VSG encountered throughout infection. Indeed, PMPs from infected animals supplied with exogenous VSG were capable of activating a wider array of VSG-specific T hybridomas than when tested ex vivo (Figure 9 and data not shown). Additionally, some hybridomas were uniquely activated by PMPs from either B10 or C3H mice, suggesting differences in PMP processing of VSG between strains (Figure 8).
Processing and presentation of newly encountered VSG
The results above demonstrate that APCs from T. b. rhodesiense LouTat 1 infected mice present LouTat 1 VSG peptides to T cells coincident with a failure to process and present additional exogenous non-parasite antigens such as HEL to T cells. These experiments also show that sufficient concentrations of VSG peptide:MHC II complexes, in which the peptides are derived from the surface coat of the infecting trypanosome strain, are presented only as long as the parasites remain in host tissues. What is not known is whether subsequent exposure to a newly encountered trypanosome VSG (e.g., that of a different VAT) results in processing and presentation, like the initial VSG, or whether such molecules are not processed or presented, similar to HEL. To test this B10 mice were first infected with LouTat 1.5, a distinct VAT of the LouTar serodeme which displays a unique VSG (51, 52); VSG-specific T cells from LouTat 1.5 infected mice do not cross-react with LouTat 1 VSG and LouTat 1 VSG-specific T cells from LouTat 1 infected mice do not cross-react with LouTat 1.5 VSG ((4, 23), and data not shown). DCs were isolated from both naive and LouTat 1.5-infected animals and combined with LouTat 1 VSG-specific Th cell hybridomas in the presence or absence of exogenous LouTat 1 VSG. As shown in Figure 10, DCs from LouTat 1.5-infected animals were unable to stimulate LouTat 1 VSG-specific T hybridoma cells (or HEL-specific T hybridomas, data not shown) during the period of time when LouTat 1.5 parasite burden was high (days 4–7). This data shows that active infection alters the ability of APCs to process or present exogenous antigens, including the VSG molecules of other trypanosome VATs. The data also reinforces previous evidence from this lab that VSG-specific T cells activated during infection do not cross-react with other VSGs and therefore do not contribute towards protective immunity against subsequent waves of infection, despite the presence of conserved sequences (4, 23, 49). The reason why conserved VSG sequences are not processed to peptides for presentation to T cells remains to be determined.
Figure 10. DCs from mice infected with LouTat 1.5 display impaired processing and presentation of newly encountered variant LouTat 1 VSG.
B10 animals were infected with LouTat 1.5 and DCs were collected on days 0, 4, 7, and 10 of infection. DCs were combined with different LouTat 1 VSG-specific T cell hybridomas (shown in upper left of each graph) and with or without the addition of LouTat 1 VSG. IL-2 was measured by ELISA; results are displayed as mean +/− SEM of triplicate wells in a representative experiment.
Discussion
Trypanosome VSG molecules are members of a protein superfamily that exhibits extensive variable sequences as well as defined conserved amino acid subsequences. Conserved invariant sequences permit VSGs to fold and orient in the same manner within the surface coat structure and also provide a scaffold for membrane anchoring via GPI residues; this preservation of structure is likely important to preserve a physical coat barrier that protects plasma membrane components from exposure to innate and adaptive immune attack during infection. However, most of the VSG molecule contains variable sequences that distinguish different VSGs and trypanosome VATs from one another. It has been suggested that B and T cell reactivity against VSGs resulted in selective pressure to alter amino acids within variable subregions of the molecule; thus antigenic variation of B and T cell epitopes within such regions provides a means to escape immune elimination. However our recent studies of VSG-specific T cell responses in vivo demonstrated that infected responder mouse Th1 cells recognize multiple variant peptides distributed throughout the N-terminal domain of the VSG molecule (23), but there were no T cell responses detectable to invariant residues including those in the C-terminal domain suggesting that peptides may not be generated for MHC II binding and TCR presentation from these sites.
In order to examine the role of APCs in processing and presentation of VSG molecules during trypanosomiasis, and to address known issues regarding the activation and regulation of VSG-specific T cell responses during infection, we tested the hypothesis that APC functions are functionally altered by trypanosome infection. Thus we have broadly examined the phenotype and function of MPs and DCs during experimental African trypanosomiasis in the context of activation of T cells for both VSG peptides and exogenous non-parasite peptides. Analysis of splenic APC phenotypes revealed some intriguing differences between responder B10 and non-responder C3H animals that may contribute to the relative resistance and susceptibility of these strains. DCs and SMPs from responder B10 animals up-regulated MHC II and/or costimulatory molecules and produced IL-12 that contributes to development of the Th1 cell phenotype during early infection. In contrast, SMPs from nonresponder C3H animals down-regulated CD40, CD80, and CD86 by day 4, at which time their expression would be essential for providing proper costimulation to naive Th cells. Although some markers were up-regulated on DCs from C3H mice and cells produced IL-12 levels similar to responder B10 mice, both CD8α+ DCs and CD8α- DCs displayed decreased levels of CD80 expression, and CD8α- DCs continuously down-regulated MHC II during infection.
Perhaps most striking, we observed a reduction in the proportion of CD8α- DCs and complete depletion of CD8α+ DCs by day 7 in C3H animals. Loss of CD8α- and CD8α+ DCs may be the result of changes in migration or apoptosis of DCs. Apoptosis-induced depletion of CD8α+ DCs by an unknown mechanism was recently observed in Plasmodium chabaudi-infected mice (53). A growing body of evidence suggests that mature DC are susceptible to MHC II-mediated apoptosis, resulting from interactions with other DCs, lymphocytes, or even microbial ligands (54, 55). MHC II-mediated apoptosis may therefore play a role in CD8α+ DC depletion, as CD8α+ DCs from C3H mice appear phenotypically mature prior to depletion. Parasite-mediated apoptosis may also drive DC death, a phenomenon observed in human DCs cultured in vitro with Brugia malayi microfilariae (56). Given the known activating and deactivating effects of GPI residues associated with the VSG molecule, it is possible that GPI residues preferentially promote DC maturation and augment apoptosis during infection of susceptible animals.
It is difficult to predict how the diminished proportion of CD8α+ and/or CD8α- DCs may affect the development of Th1 responses to trypanosomes, as we have not included functional assays for each distinct CD8 DC subset. However, the ability to process and present antigen ex vivo and present VSG peptides in vivo was similar between total CD11c+ DCs of B10 and C3H mice throughout infection. It is possible that CD8α-DCs remaining within C3H mice when CD8α+ have been depleted provide a similar functional contribution as the combination of subsets. Substantial evidence supports functional plasticity among DC subsets, such that environmental cues (e.g., microbial products such as GPI residues) can induce similar cytokine responses and antigen presentation capabilities from different subsets (57–59). Regardless of any subset-specific functions, a reduction in the number of DCs, combined with down-regulation of MHC II, would limit productive Th interactions and could impair the development of Th cell-mediated immunity in susceptible animals.
APC maturation and activation within the peritoneum seem to be regulated differently than in the spleen, perhaps because of differences in the local cytokine environment and the degree of interaction with parasites or parasite antigens. Unexpectedly, only susceptible PMPs coordinately up-regulated both MHC II and costimulatory molecules at the time of splenic APC activation and peak parasitemia. However, we have observed that total peritoneal cells and the proportion of PMPs in C3H mice decrease dramatically during early infection, whereas the number and proportion of PMPs in B10 animals increases (unpublished observations). A reduction in the number of PMPs would thus significantly limit productive interactions with naïve or effector Th cells. Aside from phenotypic differences observed, PMPs produced little IL-12 and were unable to induce naïve T cell proliferation or cytokine production when isolated from infected B10 or C3H mice. In fact, trypanosome infection may preferentially impart regulatory or suppressive capabilities on PMPs. We observed high levels of NO production by PMPs one week after infection, at which time VSG-specific Th1 cytokine but not proliferative responses are consistently detected within the peritoneum; NO has been shown to contribute to the suppression of Th cell responses during early trypanosomiasis and may limit T cell clonal expansion (15, 38, 60).
Further support for this theory on Th cell responses comes from our analysis of VSG presentation in vivo. Fewer (or different) VSG peptides are presented by PMPs, in comparison to the broader variety and higher level of presentation of VSG peptides by splenic APCs, as noted by the selective activation of different VSG-specific T hybridomas. Restricted processing or presentation of VSG may thereby only elicit effector functions from a limited number of VSG-specific Th1 cells. We might speculate that a variety of VSG-specific Th1 cells initially becomes activated in the spleen or lymph nodes, but migration to the peritoneum results in suppression through soluble mediators (NO, PGE) that limit clonal expansion and reduce VSG peptide presentation in a site-specific context of reduced costimulation (resistant PMPs) or fewer APC contacts (susceptible PMPs). These compartment-specific differences in APC activation may underlie the limited Th cell responses that we previously have observed in resistant responder animals (4, 13, 38).
Regardless of cell type or mouse strain, APCs from infected animals displayed a reduced ability to process and present exogenous antigen to antigen-specific T cell hybridomas and naïve T cells. This includes newly encountered exogenous VSGs which may help explain why, despite the continuous generation of new VATs during infection and the potential for T cell priming by exposure to such minor VATs, the host adaptive Th cells “see” only the VSG expressed by the predominant trypanosome VAT. DCs may be taking up less antigen as a result of maturation, but altered endocytosis does not explain the reduction in antigen presentation by MPs. Reduced presentation of exogenous non-parasite antigen has previously been observed in MPs from T. b. brucei-infected animals and was attributed to a potential defect in loading of exogenous antigenic peptides onto MHC II (27). It is possible that trypanosome antigens such as VSG with its GPI residues or trypanosome CpG DNA affect intracellular processing events. Preliminary experiments pre-treating bone marrow-derived DCs with VSG or trypanosome DNA resulted in a reduced ability to present HEL to the 3A9 T cell hybridoma, though the mechanism of inhibition has yet to be defined (unpublished observations). However, more recent results from our lab show that APCs taken from infected mice fail to generate new stable intracellular VSG peptide:α and β chain MHC complexes and that recycling of MHCII:VSG peptide complexes from the cell membrane is altered (61).
Differences in VSG processing and presentation among DCs, SMPs, and PMPs are likely associated with the form and/or amount of VSG seen within different lymphoid compartments. We have established that splenic DCs and SMPs from both B10 and C3H animals displayed a greater array of VSG-derived peptides than PMPs. However, PMPs from infected mice provided with additional exogenous VSG were able to activate T cell hybridomas that only splenic APCs could activate during infection, thus suggesting that VSG exposure or uptake differs among lymphoid compartments. One theory would be that splenic APCs encounter a greater proportion of VSG filtered from the blood than whole parasites, whereas PMPs may ingest a greater proportion of parasites or take up less VSG. We also observed that PMPs from B10 and C3H mice process VSG to present a different array of peptides. Since only PMPs from B10 animals would encounter parasites opsonized with anti-VSG antibodies, the route of antigen uptake may reflect differential processing. Extended encounter with the parasite and/or parasite antigens (or the GPI residues of VSG) in C3H mice may also alter proteolytic capabilities of PMPs. Studies are currently underway to explore the trafficking of eGPF+ LouTat 1 trypanosomes in vivo to determine the extent of parasitemia within the tissues compared to the vasculature, so that we might better understand how parasites interface with cells of the innate immune system.
In conclusion, we have tested the hypothesis that trypanosome infection alters APC function using a variety of classical approaches. Our results reveal that APC phenotype and antigen processing/presentation capacity are altered by infection, but that the overall picture is quite complex and is dependent on multiple factors: APC type (DCs vs MPs); tissue source (lymphoid organs vs peritoneum); maturation state relative to time of infection (surface expression of MHC II and costimulatory factors); availability of parasite antigen (levels of parasitemia in resistant vs susceptible mice at the time of sampling); and prior exposure to VSG GPI determinants or other parasite factors. In particular our results demonstrate that splenic DCs are the primary APC population responsible for eliciting Th1 cell responses during trypanosome infection in resistant responder B10 animals. DCs clearly undergo maturation within the first few days of infection, as evidenced by up-regulation of MHC II and costimulatory molecules, secretion of IL-12, and an enhanced ability to activate naïve T cells to a Th1 phenotype. Coincident with their maturation, DCs present high levels of VSG peptides in vivo and are able to activate VSG-specific T hybridomas as well as infected mouse Th cells ex vivo. The timing of DC maturation and VSG presentation correlate with peak parasite tissue burden and the presence of VSG shed into plasma (44). DCs thus take up trypanosome antigen early in infection and present high and varied levels of VSG peptide:MHC II complexes, along with proper costimulatory and cytokine signals, for development of VSG-specific Th1 cells. SMPs may play only a supporting role in the development of T cell-mediated immunity by providing additional IL-12 and costimulatory signals for a select number or subset of VSG epitope-specific Th cells. Although VSG-specific Th cell responses have been detected within the peritoneum, PMPs exhibit reduced antigen processing and presentation capabilities, secretion of cytokines and factors such as NO that inhibit T cell clonal expansion, and limited VSG presentation during infection that likely reflect regulatory or suppressive functions. The finding that, independent of the APC populations studied, cells expressing peak levels of VSG peptide:MHC II complexes are unable to process and present newly encountered exogenous antigen, including new VSG molecules. This has far-reaching implications for control of a parasite population that constantly expresses new surface coats, and this observation may also impact on attempts to vaccinate trypanosome infected hosts against any antigen.
ACKNOWLEDGEMENTS
We thank Dr. P. Marrack, Departments of Microbiology and Immunology and Medicine, University of Colorado Health Sciences Center, Denver, CO, for the BW5147 TCRα-β- cells. Jim Schrader supplied invaluable animal care, trypanosome infection and harvesting support.
Footnotes
Supported by USPHS grants AI-22441 and AI-073346 (JMM), AI-048242 and AI-051421 (DMP)
REFERENCES
- 1.Cross GA. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71:393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey WL, Mansfield JM. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130:405–411. [PubMed] [Google Scholar]
- 3.Levine RF, Mansfield JM. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J Immunol. 1984;133:1564–1569. [PubMed] [Google Scholar]
- 4.Schleifer KW, Filutowicz H, Schopf LR, Mansfield JM. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J Immunol. 1993;150:2910–2919. [PubMed] [Google Scholar]
- 5.Hertz CJ, Filutowicz H, Mansfield JM. Resistance to the African trypanosomes is IFN-gamma dependent. J Immunol. 1998;161:6775–6783. [PubMed] [Google Scholar]
- 6.Van-der-Ploeg LH, Gottesdiener K, Lee MG. Antigenic variation in African trypanosomes. Trends Genet. 1992;8:452–457. doi: 10.1016/0168-9525(92)90330-7. [DOI] [PubMed] [Google Scholar]
- 7.Borst P. Antigenic variation and allelic exclusion. Cell. 2002;109:5–8. doi: 10.1016/s0092-8674(02)00711-0. [DOI] [PubMed] [Google Scholar]
- 8.Blum JL, Down JA, Gurnett AM, Carrington M, Turner MJ, Wiley DC. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature. 1993;362:603–609. doi: 10.1038/362603a0. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf P, Blum M, Freymann D, Turner M, Wiley DC. Two variant surface glycoproteins of Trypanosoma brucei of different sequence classes have similar 6 A resolution X-ray structures. Nature. 1987;325:84–86. doi: 10.1038/325084a0. [DOI] [PubMed] [Google Scholar]
- 10.Reinitz DM, Aizenstein BD, Mansfield JM. Variable and conserved structural elements of trypanosome variant surface glycoproteins. Mol Biochem Parasitol. 1992;51:119–132. doi: 10.1016/0166-6851(92)90207-z. [DOI] [PubMed] [Google Scholar]
- 11.Field MC, Boothroyd JC. Sequence divergence in a family of variant surface glycoprotein genes from trypanosomes: coding region hypervariability and downstream recombinogenic repeats. Journal of Molecular Evolution. 1996;42:500–511. doi: 10.1007/BF02352280. [DOI] [PubMed] [Google Scholar]
- 12.De Gee AL, Levine RF, Mansfield JM. Genetics of resistance to the African trypanosomes. VI. Heredity of resistance and variable surface glycoprotein-specific immune responses. J Immunol. 1988;140:283–288. [PubMed] [Google Scholar]
- 13.De Gee AL, Mansfield JM. Genetics of resistance to the African trypanosomes. IV. Resistance of radiation chimeras to Trypanosoma rhodesiense infection. Cell Immunol. 1984;87:85–91. doi: 10.1016/0008-8749(84)90132-1. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield JM. T-cell responses to the trypanosome variant surface glycoprotein: A new paradigm? Parasitol Today. 1994;10:267–270. doi: 10.1016/0169-4758(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield JM, Paulnock DM. Regulation of innate and acquired immunity in African trypanosomiasis. Parasite immunology. 2005;27:361–371. doi: 10.1111/j.1365-3024.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 16.Drennan MB, Stijlemans B, Van Den Abbeele J, Quesniaux VJ, Barkhuizen M, Brombacher F, De Baetselier P, Ryffel B, Magez S. The Induction of a Type 1 Immune Response following a Trypanosoma brucei Infection Is MyD88 Dependent. J Immunol. 2005;175:2501–2509. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 17.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck JP, Rampelberg M, Sablon E, De Baetselier P. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 18.Magez S, Geuskens M, Beschin A, Delfavero H, Verschueren H, Lucas R, Pays E, Debaetselier P. Specific Uptake of Tumor Necrosis Factor-Alpha Is Involved in Growth Control of Trypanosoma Brucei. Journal of Cell Biology. 1997;137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magez S, Radwanska M, Beschin A, Sekikawa K, De Baetselier P. Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections. Infect Immun. 1999;67:3128–3132. doi: 10.1128/iai.67.6.3128-3132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daulouede S, Bouteille B, Moynet D, De Baetselier P, Courtois P, Lemesre JL, Buguet A, Cespuglio R, Vincendeau P. Human macrophage tumor necrosis factor (TNF)-alpha production induced by Trypanosoma brucei gambiense and the role of TNF-alpha in parasite control. Journal of Infectious Diseases. 2001;183:988–991. doi: 10.1086/319257. [DOI] [PubMed] [Google Scholar]
- 21.Mnaimneh S, Geffard M, Veyret B, Vincendeau P. Albumin Nitrosylated By Activated Macrophages Possesses Antiparasitic Effects Neutralized By Anti-No-Acetylated-Cysteine Antibodies. Journal of Immunology. 1997;158:308–314. [PubMed] [Google Scholar]
- 22.Vincendeau P, Daulouede S, Veyret B, Darde ML, Bouteille B, Lemesre JL. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol. 1992;75:353–360. doi: 10.1016/0014-4894(92)90220-5. [DOI] [PubMed] [Google Scholar]
- 23.Dagenais TT, Demick KP, Bangs JD, Paulnock DM, Mansfield JM. T Cell Responses to the Trypanosome Variant Surface Glycoprotein are Not Limited to Hypervariable Subregions. Infect. Immunity. 2009;77:141–151. doi: 10.1128/IAI.00729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellhausen SR, Mansfield JM. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J Immunol. 1979;122:818–824. [PubMed] [Google Scholar]
- 25.Wellhausen SR, Mansfield JM. Characteristics of the splenic suppressor cell--target cell interaction in experimental African trypanosomiasis. Cell Immunol. 1980;54:414–424. doi: 10.1016/0008-8749(80)90221-x. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg J, McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 27.Namangala B, Brys L, Magez S, De Baetselier P, Beschin A. Trypanosoma brucei brucei infection impairs MHC class II antigen presentation capacity of macrophages. Parasite immunology. 2000;22:361–370. doi: 10.1046/j.1365-3024.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 28.Mabbott N, Sternberg J. Bone marrow nitric oxide production and development of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1995;63:1563–1566. doi: 10.1128/iai.63.4.1563-1566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borowy NK, Sternberg JM, Schreiber D, Nonnengasser C, Overath P. Suppressive macrophages occurring in murine Trypanosoma brucei infection inhibit T-cell responses in vivo and in vitro. Parasite immunology. 1990;12:233–246. doi: 10.1111/j.1365-3024.1990.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 30.Sileghem M, Darji A, Hamers R, Van de Winkel M, De Baetselier P. Dual role of macrophages in the suppression of interleukin 2 production and interleukin 2 receptor expression in trypanosome-infected mice. Eur J Immunol. 1989;19:829–835. doi: 10.1002/eji.1830190508. [DOI] [PubMed] [Google Scholar]
- 31.Grosskinsky CM, Askonas BA. Macrophages as primary target cells and mediators of immune dysfunction in African trypanosomiasis. Infect Immun. 1981;33:149–155. doi: 10.1128/iai.33.1.149-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaushik RS, Uzonna JE, Zhang Y, Gordon JR, Tabel H. Innate resistance to experimental African trypanosomiasis: differences in cytokine (TNF-alpha, IL-6, IL-10 and IL-12) production by bone marrow-derived macrophages from resistant and susceptible mice. Cytokine. 2000;12:1024–1034. doi: 10.1006/cyto.2000.0685. [DOI] [PubMed] [Google Scholar]
- 33.Ogunremi O, Tabel H. Genetics Of Resistance to Trypanosoma Congolense In Inbred Mice - Efficiency Of Apparent Clearance Of Parasites Correlates With Long-Term Survival. Journal of Parasitology. 1995;81:876–881. [PubMed] [Google Scholar]
- 34.Uzonna JE, Kaushik RS, Gordon JR, Tabel H. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite immunology. 1999;21:57–71. doi: 10.1046/j.1365-3024.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 35.Beschin A, Brys L, Magez S, Radwanska M, De Baetselier P. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. Journal of Leukocyte Biology. 1998;63:429–439. doi: 10.1002/jlb.63.4.429. [DOI] [PubMed] [Google Scholar]
- 36.Darji A, Lucas R, Magez S, Torreele E, Palacios J, Sileghem M, Bajyana Songa E, Hamers R, De Baetselier P. Mechanisms underlying trypanosome-elicited immunosuppression. Ann Soc Belg Med Trop. 1992;1:27–38. [PubMed] [Google Scholar]
- 37.Lucas R, Magez S, Songa B, Darji A, Hamers R, de-Baetselier P. A role for TNF during African trypanosomiasis: involvement in parasite control, immunosuppression and pathology. Res Immunol. 1993;144:370–376. doi: 10.1016/s0923-2494(93)80082-a. [DOI] [PubMed] [Google Scholar]
- 38.Schleifer KW, Mansfield JM. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 39.Tachado SD, Schofield L. Glycosylphosphatidylinositol toxin of Trypanosoma brucei regulates IL-1 alpha and TNF-alpha expression in macrophages by protein tyrosine kinase mediated signal transduction. Biochem Biophys Res Commun. 1994;205:984–991. doi: 10.1006/bbrc.1994.2763. [DOI] [PubMed] [Google Scholar]
- 40.Tachado SD, Gerold P, Schwarz R, Novakovic S, McConville M, Schofield L. Signal Transduction In Macrophages By Glycosylphosphatidylinositols Of Plasmodium, Trypanosoma, and Leishmania - Activation Of Protein Tyrosine Kinases and Protein Kinase C By Inositolglycan and Diacylglycerol Moieties. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4022–4027. doi: 10.1073/pnas.94.8.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schofield L, Tachado SD. REGULATION OF HOST CELL FUNCTION BY GLYCOSYLPHOSPHATIDYLINOSITOLS OF THE PARASITIC PROTOZOA [Review] Immunology & Cell Biology. 1996;74:555–563. doi: 10.1038/icb.1996.89. [DOI] [PubMed] [Google Scholar]
- 42.Radwanska M, Magez S, Michel A, Stijlemans B, Geuskens M, Pays E. Comparative analysis of antibody responses against HSP60, invariant surface glycoprotein 70, and variant surface glycoprotein reveals a complex antigen-specific pattern of immunoglobulin isotype switching during infection by Trypanosoma brucei. Infection & Immunity. 2000;68:848–860. doi: 10.1128/iai.68.2.848-860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris TH, Cooney NM, Mansfield JM, Paulnock DM. Signal transduction, gene transcription, and cytokine production triggered in macrophages by exposure to trypanosome DNA. Infect Immun. 2006;74:4530–4537. doi: 10.1128/IAI.01938-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coller SP, Mansfield JM, Paulnock DM. Glycosylinositolphosphate soluble variant surface glycoprotein inhibits IFN-gamma-induced nitric oxide production via reduction in STAT1 phosphorylation in African trypanosomiasis. J Immunol. 2003;171:1466–1472. doi: 10.4049/jimmunol.171.3.1466. [DOI] [PubMed] [Google Scholar]
- 45.Paulnock DM, Coller SP. Analysis of macrophage activation in African trypanosomiasis. J Leukoc Biol. 2001;69:685–690. [PubMed] [Google Scholar]
- 46.Paulnock DM, Smith C, Mansfield JM. New York: Alan R Liss, Inc; 1989. Antigen presenting cell function in African trypanosomiasis; pp. 135–144. [Google Scholar]
- 47.Smith CJ, Levine RF, Mansfield JM. Cloning of African trypanosomes in mice immunosuppressed by cyclophosphamide treatment. Am J Trop Med Hyg. 1982;31:1098–1102. doi: 10.4269/ajtmh.1982.31.1098. [DOI] [PubMed] [Google Scholar]
- 48.Lanham SM, Godfrey DG. Isolation of salivarian trypanosomes from man and other mammals using DEAE cellulose. Experimental Parasitology. 1970;28:412–416. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 49.Schopf LR, Filutowicz H, Bi XJ, Mansfield JM. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect Immun. 1998;66:451–461. doi: 10.1128/iai.66.2.451-461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leppert BJ, Mansfield JM, Paulnock DM. The soluble variant surface glycoprotein of African trypanosomes is recognized by a macrophage scavenger receptor and induces I kappa B alpha degradation independently of TRAF6-mediated TLR signaling. J Immunol. 2007;179:548–556. doi: 10.4049/jimmunol.179.1.548. [DOI] [PubMed] [Google Scholar]
- 51.Theodos CM, Mansfield JM. Regulation of B cell responses to the variant surface glycoprotein molecule in trypanosomiasis. II. Down-regulation of idiotype expression is associated with the appearance of lymphocytes expressing antiidiotypic receptors. J Immunol. 1990;144:4022–4029. [PubMed] [Google Scholar]
- 52.Theodos CM, Reinitz DM, Mansfield JM. Regulation of B cell responses to the variant surface glycoprotein (VSG) molecule in trypanosomiasis. I. Epitope specificity and idiotypic profile of monoclonal antibodies to the VSG of Trypanosoma brucei rhodesiense. J Immunol. 1990;144:4011–4021. [PubMed] [Google Scholar]
- 53.Sponaas AM, Cadman ET, Voisine C, Harrison V, Boonstra A, O’Garra A, Langhorne J. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J Exp Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertho N, Drenou B, Laupeze B, Berre CL, Amiot L, Grosset JM, Fardel O, Charron D, Mooney N, Fauchet R. HLA-DR-mediated apoptosis susceptibility discriminates differentiation stages of dendritic/monocytic APC. J Immunol. 2000;164:2379–2385. doi: 10.4049/jimmunol.164.5.2379. [DOI] [PubMed] [Google Scholar]
- 55.Leverkus M, McLellan AD, Heldmann M, Eggert AO, Brocker EB, Koch N, Kampgen E. MHC class II-mediated apoptosis in dendritic cells: a role for membrane-associated and mitochondrial signaling pathways. Int Immunol. 2003;15:993–1006. doi: 10.1093/intimm/dxg099. [DOI] [PubMed] [Google Scholar]
- 56.Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, Gilden JK, Nutman TB. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol. 2003;171:1950–1960. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 57.De Smedt AC, Van Den Heuvel RL, Zwi Berneman N, Schoeters GE. Modulation of phenotype, cytokine production and stimulatory function of CD34+-derived DC by NiCl(2) and SDS. Toxicol In Vitro. 2001;15:319–325. doi: 10.1016/s0887-2333(01)00029-7. [DOI] [PubMed] [Google Scholar]
- 58.Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 59.Qi H, Denning TL, Soong L. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect Immun. 2003;71:3337–3342. doi: 10.1128/IAI.71.6.3337-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wellhausen SR, Mansfield JM. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J Immunol. 1979;122:818–824. [PubMed] [Google Scholar]
- 61.Freeman BE, Demick KP, Mansfield JM, Paulnock DM. Instability of peptide:MHC II complexes underlies modulation of APC function in African trypanosomiasis. Submitted for publication. 2009 [Google Scholar]