Abstract
The serine-threonine kinase RIP1 was originally identified through its ability to bind to the death domain of Fas (CD95). RIP1 has been shown to be recruited to the Fas Death-Inducing Signaling Complex (DISC) and is required for the induction of necrotic cell death. Here we show that in Jurkat T lymphocytes, RIP1 is also necessary for the most efficient activation of downstream caspases by Fas when treated with membrane-bound Fas Ligand (memFasL), but not with agonistic antibodies or crosslinked soluble Fas Ligand. RIP1 participates in the FADD-mediated recruitment of caspase-8 to the Fas receptor complex in a manner that promotes caspase-8 activation. Crosslinking antibodies, such as CH11, bypass the requirement for RIP1 in caspase activation by initiating larger, though less efficient, DISC complexes, while memFasL initiates a smaller but more efficient DISC that functions, in part, by effectively incorporating more RIP1 into the complex. Consequently, RIP1 is likely a more integral part of physiological signaling through the Fas/CD95 receptor complex than previously recognized; at least when the signal is mediated by full-length membrane bound Fas Ligand. Crosslinked Soluble FasL, which also occurs physiologically, behaves similarly to the CH11 antibody, and may therefore be more likely to initiate non-apoptotic Fas signaling due to less RIP1 in the receptor complex. Thus, agonists that bind the same Fas receptor initiate mechanistically distinct pathways resulting in differential cytotoxicity.
Keywords: Fas/CD95, Caspase-8, Fas Ligand, RIP1
INTRODUCTION
The Fas/CD95 receptor is a member of the TNF receptor superfamily and one of the most studied of the death receptors, a subfamily of receptors that contain an intracellular death domain and are capable of mediating cell death and a variety of other signals in their regulatory role of the immune system. The physiological ligand for Fas/CD95 (FasL) is produced by several types of cells, including T cells, as a type II transmembrane protein. Cleavage of the ligand by metallo-proteases in the extracellular portion results in the generation of a soluble portion of FasL (sFasL) that lacks the transmembrane domain, but is still capable of trimerization and binding to the receptor, and therefore retains biological activity (1, 2).
Many different agonists have been used to stimulate the Fas/CD95 pathway, and though it is known that all do not cause the same degree of response (3–7), they are often used interchangeably in the literature. Fas was initially discovered as the target of an IgM monoclonal antibody (now termed CH11) that had cytolytic activity (8). Therefore, antibodies of various kinds have historically been used to ligate Fas and induce apoptosis. Antibodies to Fas have been shown to be active in vivo, as injection of the Jo2 antibody into mice results in significant liver toxicity due to apoptosis (9). Soluble Fas ligand (sFasL) may be the predominant form of the ligand in vivo that participates in non-apoptotic Fas signaling, and many Fas-expressing cells types do not die in response to sFasL in the absence of a crosslinking agent of some kind. However, the apoptotic potency of sFasL is increased in association with matrix proteins (10). Therefore, when sFasL has been used for cytotoxicity assays, it is usually tagged and crosslinked with an antibody (i.e. Flag-sFasL), or is expressed as a fusion protein that naturally trimerizes (i.e. leucine zipper sFasL). The full-length, membrane-bound Fas ligand (memFasL) is a potent cell death agonist in many cell types and could represent the most physiological ligand for inducing cell death through Fas (2, 11).
Though it has since been mostly studied as part of other pathways, the serine-threonine kinase RIP1 (also known as RIP or RIPK1) was originally identified through its ability to bind to the death domain of Fas (CD95) (12). RIP1 is recruited to the Fas DISC and is required for necrotic cell death initiated by Fas (13). RIP1 is also essential for necrotic cell death initiated by TNFα, and we have recently shown that that this is due to its participation in the formation of a superoxide–producing complex with the NADPH oxidase NOX1, its adaptor protein NOXO1, TRADD, and Rac1 (14). The role of RIP1 in apoptosis has mostly been described as a protective one, since it is important in the activation of NF-kB and its pro-survival target genes. However, RIP1 has recently been shown to play a critical pro-apoptotic role in TNF-induced caspase-8 activation in the presence of Smac mimetic, where it nucleates a secondary non-receptor protein complex containing FADD and caspase-8 (15).
Using Jurkat cells deficient in RIP1, we show here that while RIP1 is not absolutely essential for caspase-8 activation and cell death induced by memFasL, it plays a role in the recruitment or organization of caspase-8 in such a way that this caspase is efficiently activated. Crosslinking of the receptor using agonistic antibodies or crosslinked soluble FasL bypasses the requirement for RIP1 by producing larger, less efficient complexes that can activate caspase-8 by virtue of the enormous amount of caspase-8 present. Thus, memFasL organizes the Fas DISC in a slightly different way than antibody crosslinking does, resulting in effective RIP1 recruitment and more efficient caspase-8 activation, and leading to a differential sensitivity to cell death by agonists of the same receptor.
MATERIALS AND METHODS
Reagents
MemFasL and Neutralizing anti-Fas (ZB4) were from Upstate. Mouse anti-Fas IgM (CH11), MBL. Goat (C20, for IP) and rabbit anti-caspase-8 (H134, western), mouse anti-Fas (B-10), and rabbit anti-Xpress were from Santa Cruz. Mouse anti-RIP1, anti-FADD, and anti-Bcl-2 were from BD Transduction Laboratories. Soluble flag-FasL, mouse anti-actin, anti-B-tubulin and anti-Flag antibodies, and staurosporine were from Sigma. zVAD was from MP Biomedicals. Rabbit anti-Caspase-3, rabbit anti-cleaved caspase-8, mouse anti-Bid, rabbit anti-FLIP, were from Cell Signaling. Rabbit anti-caspase- 8 (cleavage western, supplemental) was from BD Pharmingen. Pre-designed Lamin A/C siRNA and RIP1 siRNA pools were obtained from Dharmacon.
Cell culture
Jurkat cells were maintained in RPMI with 10 % fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin/ streptomycin. Reconstituted cell lines were established by electroporation of RIP1 deficient Jurkat cells with Xpress-RIP1 plasmid and maintained in media containing 20mg/ml G418. Clones were isolated using limiting dilution and screened by western blotting for RIP1 expression and equal expression of DISC components.
Co-immunoprecipitation
~100 Million Jurkat cells were treated with the appropriate agonist. After treatment, cells were lysed in M2 buffer (as in ref (14)). Lysates were cleared and precipitated with 1 μg antibody and protein G-agarose beads at 4°C overnight. Beads were washed 5X with buffer, and boiled in SDS buffer, then resolved in 4–20 % PAGE gels for western analysis.
Cytotoxicity assays
DNA fragmentation was measured using the methodology of (16). Unless otherwise indicated, all other cell death (or lack of survival) was quantitated using MTS reagent (Promega) according to manufacturers directions. Statistical analysis was performed using a Student's t test (two-tailed).
siRNA knockdown
Oligos were transfected with an Amaxa Biosystems Nucleofector II electoporator with Nucleofector solution V at a concentration of 200 nM, according to manufacturer’s protocol.
Caspase-8 assay
Caspase -8 activity was measured using a colormetric assay kit (Chemicon) according to manufacturer’s protocol. Activity was normalized to untreated cells of the same type.
RESULTS
RIP1 deficient Jurkat cell are resistant to memFasL
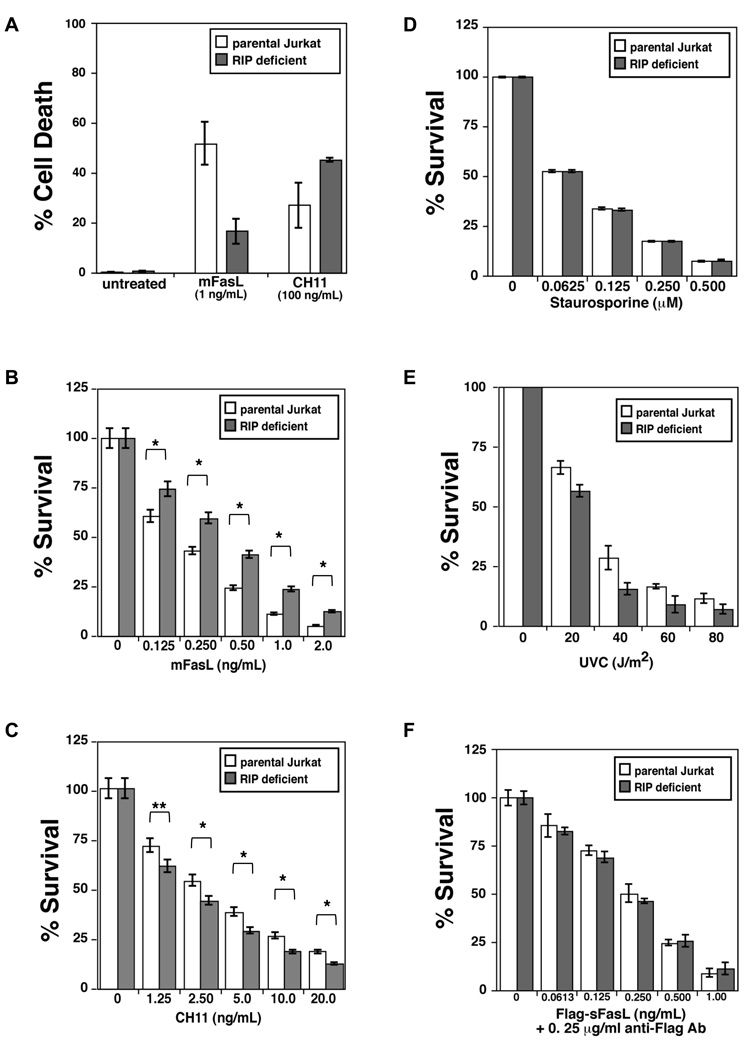
We found that while RIP1-deficient Jurkat cells were resistant to membrane bound Fas ligand (memFasL) when compared to the parental Jurkat cell line, they were equally or slightly more sensitive to a crosslinking IgM antibody (CH11) as seen under the microscope, by propidium iodide exclusion (data not shown), or as measured by DNA fragmentation (Fig. 1A). This was surprising as both agents act on the Fas receptor. To confirm that the cytotoxicity was Fas-mediated, we pre-treated cells with a Fas-neutralizing antibody (ZB4), and this effectively inhibited all toxicity due to treatment with either memFasL or CH11, even at high concentrations (data not shown). Although the DNA fragmentation measures the amount of apoptotic cells in a culture at any given time, it often does not account for the many cells that have previously died and fragmented, and are gated out by the flow cytometer as debris. In order to further confirm the differential sensitivity to the different Fas agonists using a more cumulative measurement of cell death and survival, we did a dose curve comparison of memFasL and CH11 cytotoxicity using the MTS assay. Measurement of death using MTS confirmed what we had found previously, namely that the parental cell line was more sensitive to memFasL (Fig. 1B), while the RIP1-deficient cell line was slightly more sensitive to CH11 treatment (Fig. 1C). Although RIP1-deficient Jurkat cells were derived from the parental Jurkat cell line, we questioned whether there were cell line differences in the downstream caspase-amplification loop. We therefore treated cells with apoptotic stimuli dependent on the intrinsic pathway, namely, staurosporine and UV-C radiation. The dose curve of toxicity to staurosporine was identical (Fig. 1D), suggesting that the mitochondrial pathway was equally proficient in both cell types. RIP1-deficient Jurkat cells were actually slightly more sensitive to UV radiation (Fig. 1E). Therefore, we conclude that the resistance of RIP1-deficient cells to memFasL is not due to a defect in the downstream mitochondrial amplification loop.
Figure 1. RIP1-deficient Jurkat cells are resistant to memFasL, but not to CH11 or other apoptotic stimuli.
(A) DNA fragmentation assay in parental and RIP1 deficient Jurkat cells treated with indicated amounts of memFasL or CH11 antibody for 24hrs. (B–F). Dose curves indicating survival (quantitated using MTS) of parental and RIP1 deficient Jurkat cells treated with (B) memFasL, (C) CH11 antibody, (D) staurosporine, (E) UV-C radiation, or (F) crosslinked Flag-tagged soluble Fas ligand overnight (~18 hrs). Error bars shown represent standard error of the mean, and two-tailed p values were calculated using the Student’s t-test. Comparisons with single asterisks indicate P value < 0.001; double asterisks indicate P value < 0.01.
We saw no toxicity of soluble Fas ligand (sFasL), even at extremely high dose (1 µg/mL, data not shown), but when crosslinked with an anti-Flag antibody, both cell lines underwent sFasL-induced apoptosis with a similar dose curve (Fig. 1F), suggesting that crosslinked sFasL behaved more like the CH11 antibody and that crosslinking of the receptor by antibodies overcomes the potential resistance of the RIP1-deficient cell line to FasL.
Agonist sensitivity correlates with caspase cleavage
As RIP1 has been shown to be essential for necrotic cell death induced by death receptors, including Fas, we first examined whether the Jurkat cell lines underwent cell death in the absence of caspase activity. In the presence of the pancaspase inhibitor zVAD, neither parental nor RIP1-deficient Jurkat cells died in response to memFasL, CH11, or crosslinked sFasL (Fig. S1, data not shown), suggesting no necrotic cell death was occurring during the treatments.
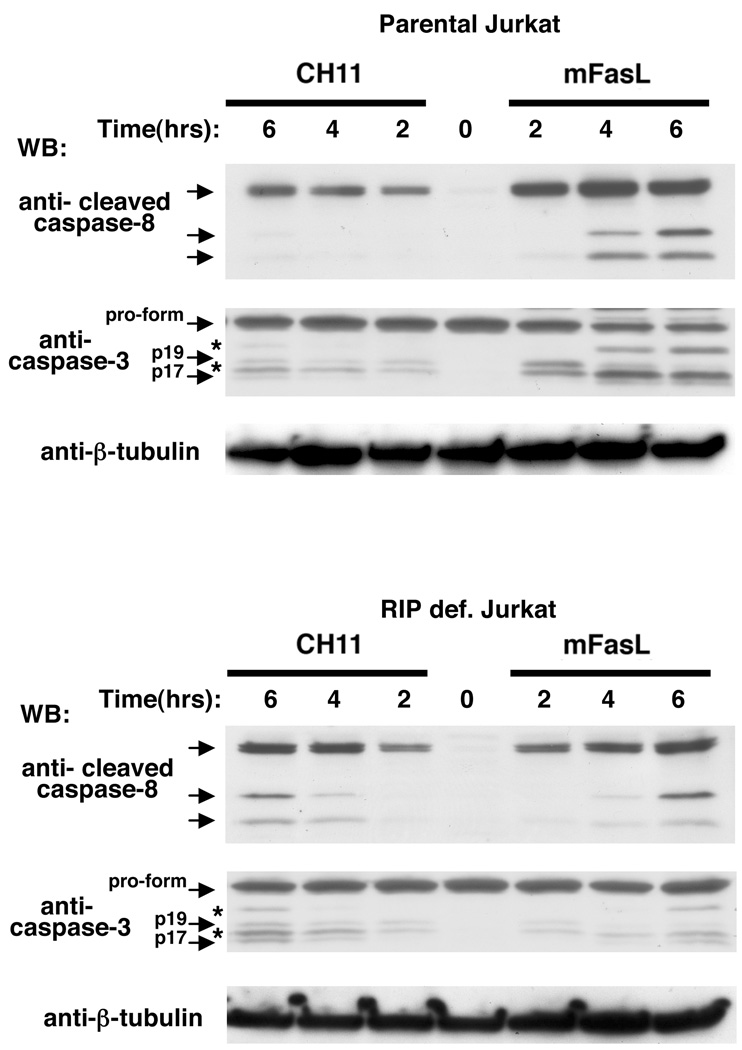
Cell lines were treated with memFasL or CH11 at three different time points, and cell lysates were probed with an antibody that detects the autocatalytically cleaved forms of caspase-8 that occur in response to stimuli, and which drive the stabilization of enzymatically active caspase dimers (17). In parental Jurkat cells at these concentrations of the two agonists (2 ng/mL FasL, 20ng/mL CH11), caspase-8 cleavage products were more efficiently generated in response to memFasL than to CH11, as shown by the western blots (Fig. 2, top panel, Fig. S2A). However, when the same concentrations were used to treat RIP1-deficient Jurkat cells, cleavage of caspase-8 occurred at comparable levels in both the CH11 and memFasL-treated cells (Fig. 2, bottom panel, Fig. S2A). Western blotting of caspase-3 indicated that caspase-3 cleavage occurred preferentially in response to memFasL in the parental Jurkat cells, while CH11 caused slightly more cleavage in the RIP1-deficient cells when compared to memFasL (Fig. 2, Fig. S2B). Thus, while memFasL activates caspases more efficiently than CH11 in the parental Jurkat cells, RIP1-deficient Jurkat cells respond similarly to both agonists at these concentrations, with a slight preference for CH11-mediated caspase activation. The caspase activation indicated by cleavage consequently corresponds to the levels of cell death observed in the two cell types at these doses of agonists.
Figure 2. Caspase cleavage correlates to cytotoxicity of Fas agonists.
Cell lysates from parental and RIP1 deficient Jurkat cells treated with either 2ng/mL memFasL or 20 ng/mL CH11 for the indicated times and immunoblotted with an antibody to cleaved caspase-8, then stripped and re-probed with caspase-3 and tubulin antibodies. Asterisks indicate bands from previous immunoblots. A fresh caspase-3 immunoblot to a different sample is shown in Figure S2.
RIP1 reconstitution restores memFasL sensitivity
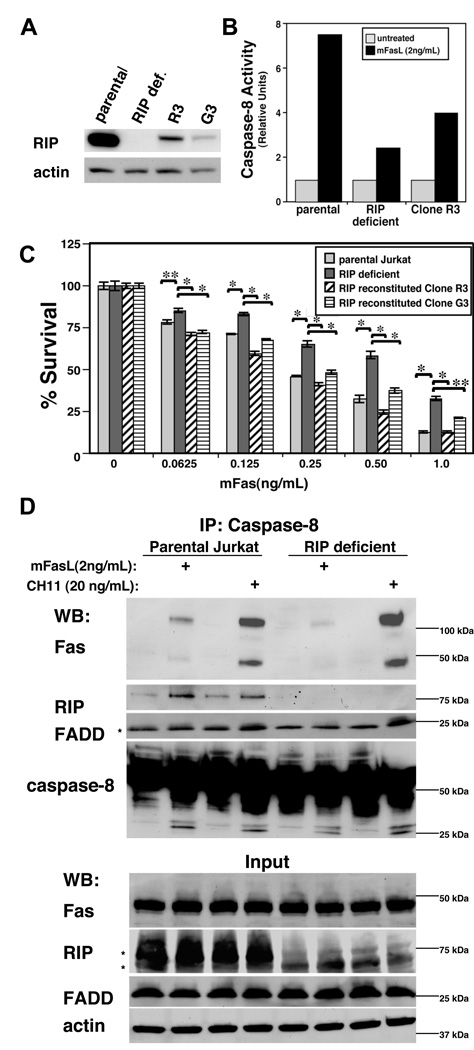
In order to verify the requirement for RIP1 in efficient memFasL-mediated caspase activation and cell death, we made stable cell lines in which RIP1 was reconstituted in the RIP1-deficient Jurkat cells. Since high expression of RIP1 was toxic to the cells, we screened a large number of clones to find clones that stably expressed RIP1. While some clones were isolated that expressed RIP1, none expressed RIP1 to the same extent as the parental Jurkat line (Fig. 3A). Nevertheless, caspase-8 activity was somewhat restored in a majority of them as measured by cleavage of a colorimetric substrate (Fig. 3B), and most RIP1-expressing clones were found to be more sensitive to memFasL than the original RIP1-deficient cell line (Fig. 3C). RIP1-expressing clones that were resistant to memFasL were typically also resistant to CH11 or were significantly deficient in Fas or FADD as detected by Western blot (data not shown). Clones that had no detectable expression of RIP1 were similar to the original RIP1-deficient cells in cytotoxicity assays, or were resistant to both CH11 and memFasL. No clones were isolated that lacked detectable RIP1 expression and that were more sensitive to memFasL than the original RIP1-deficient line. We verified that the RIP-reconstituted clones used for the cytoxicity assays had similar levels of many pro-apoptotic and anti-apoptotic proteins (Fig. S3), and while these clones were more sensitive to memFasL than the RIP-deficient cell line (Fig. 3C), they were not more sensitive to UV and staurosporine (Fig. S4), suggesting that the increased sensitivity upon RIP1 reconstitution was specific to memFasL-mediated apoptosis. Indeed, one clone was actually more resistant to staurosporine when compared to the RIP-deficient cell line, and yet was more sensitive to memFasL. Thus we conclude that the reconstitution of RIP1 expression in RIP1 deficient Jurkat cells increases their sensitivity to memFasL and that RIP1 is responsible for the differences seen in response to the different agonists.
Figure 3. RIP1 is partly responsible for recruitment of caspase-8 to the DISC upon memFasL treatment.
(A) Western blots of parental, RIP1-deficient, or RIP1-reconstituted Jurkat cell lines. (B) Caspase-8 activity assay in parental, RIP1-deficient, or RIP1-reconstituted Jurkat cell lines after 4hrs treatment with memFasL (C) Cell death assays in parental, RIP1-deficient, or RIP1-reconstituted Jurkat cell lines treated with memFasL overnight (~18 hrs). Error bars shown represent standard error of the mean, and two-tailed p values were calculated using the Student’s t-test. Comparisons with single asterisks indicate P value δ 0.001; double asterisks indicate P value < 0.01. (D) Western blots showing caspase-8 immunoprecipitation (IP) experiments in parental and RIP1-deficient Jurkat cells treated with the indicated amounts of memFasL or CH11 antibody overnight (~18 hrs).
To further validate the differential requirement for RIP1 in memFasL- and CH11-mediated cell death, we transfected parental Jurkat cells with siRNA targeting RIP1 or Lamin A/C, and then treated these cells with the two agonists. Although the differences were not as striking as that seen when comparing the RIP-deficient cell line with the parental cell line, the differences in sensitivity followed a similar pattern as that seen in Figure 1. Parental Jurkat cells transfected with the RIP1 siRNA were more resistant to memFasL-induced cell death compared to those that had been transfected with the Lamin A/C siRNA (Fig. S5, top). When these same cells were treated with the CH11 antibody, both the RIP1- and Lamin A/C- transfected cells showed nearly identical responses (Fig. S5, bottom), suggesting that RIP1 is not as important in CH11-induced cell death as it is in memFasL-induced cell death.
DISC composition varies with different agonists
As RIP1 activates MAP kinases in TNF signaling, we used pharmacological inhibitors of JNK, p38, and ERK kinases to see if they influenced Fas signaling by memFasL and CH11. None of these inhibitors affected the differences in toxicity curves (Fig. S6–S8). In addition, cycloheximide treatment did not prevent the differential cytotoxicity of the agonists, suggesting that changes in gene expression were not involved in the different responses (Fig. S9). The differences did not appear to involve lipid rafts, as treatment with methyl-β-cyclodextrin had no discernable effect (data not shown).
To further investigate mechanistic differences between the agonists, we performed a co-immunoprecipitation of the Fas DISC. Since immunoprecipitation of the receptor itself might not give an accurate comparison due to differences in epitope exposure between the agonists, we immunoprecipitated caspase-8 and probed for the presence of Fas and FADD in the DISC. We pretreated with zVAD in order to stabilize the complex and prevent caspase-8 release. When treated with the same levels of memFasL or CH11, more Fas was associated with caspase-8 in the memFasL-treated parental Jurkat cell line than in the memFasL-treated RIP1-deficient cell line, while slightly more Fas co-precipitated in the CH11-treated RIP1-deficient cells than in the CH11-treated parental cell line (Fig. 3D). An SDS-stable Fas aggregate was detected in the immunoprecipitates at well above 100 kDa, and followed the same pattern as monomeric Fas. FADD levels in the DISC followed the same pattern as Fas, but were less clear due to the presence of the IgG light chain band.
This data is consistent with the differences seen in caspase activation when comparing RIP1-deficient and parental cells with the different agonists. In the parental Jurkat cells, we also observed that RIP1 appeared to be more efficiently recruited to the memFasL-initiated DISC than to the CH11-initiated DISC when compared to the levels of Fas in the DISC. However, we were surprised to find that far more Fas was found in association with caspase-8 under CH11-treated conditions than under memFasL-treated conditions in both cell lines. Interestingly, the levels of death induced by 2ng/mL memFasL were comparable to the levels of cell death induced by 20ng/mL CH11 in the RIP1-deficient cells (see Fig. 1B,C). Therefore, at least in RIP1-deficient cells, these levels of agonists induced comparable amounts of cell death even though the amount of caspase-8 in the CH11-induced DISC was estimated to be ten-fold more than that found in the memFasL DISC. This result suggests that although less caspase-8 is recruited to the memFasL DISC, it is activated more efficiently.
Thus, the previous results suggest that memFasL more efficiently activates caspase-8 than CH11, even in the absence of RIP1, that caspase-8 recruitment in response to memFasL is more efficient in the presence of RIP1, and that antibody crosslinking by CH11 overcomes any requirement for RIP1 in recruitment, likely by mediating a large amount of caspase-8 to the DISC, where it is activated relatively inefficiently on a large scale.
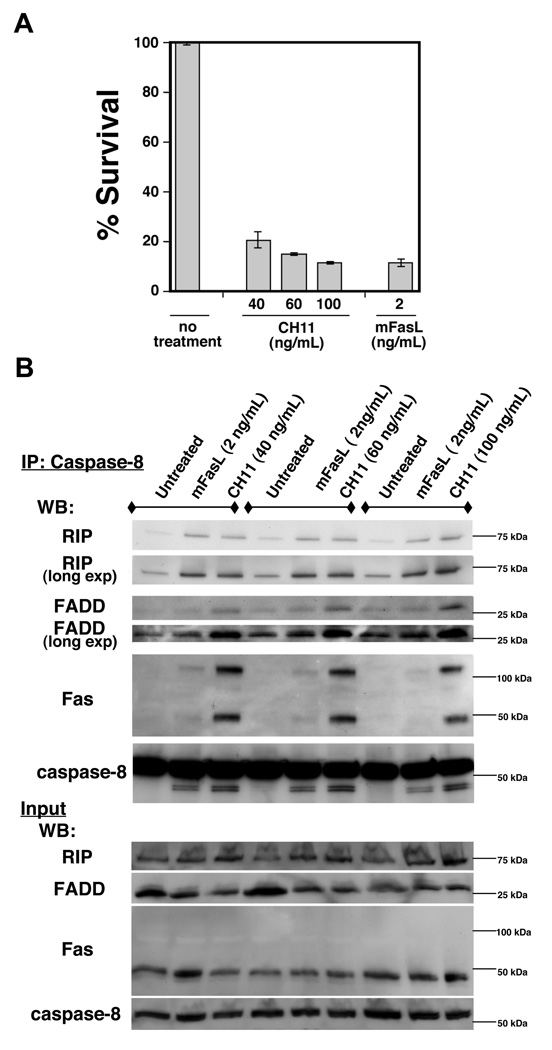
We wanted to further test these ideas by performing the experiments in the parental Jurkat cells under conditions where CH11 triggered similar amounts of cell death as memFasL (Fig. 4A). We performed Caspase-8 immunoprecipitations in parental Jurkat cells under several different concentrations. While the levels of RIP1 recruited to the DISC slightly increased with increasing concentrations of CH11 (40 ng/mL–100 ng/mL), RIP1 continued to be more efficiently recruited to the DISC by memFasL treatment when compared to the levels of Fas in the DISC (Fig. 4B). In these western blots we used an Fc-specific secondary antibody to more effectively visualize FADD. The levels of both FADD and Fas in the DISC with caspase-8 were consistently higher in CH11-treated DISCs than in memFasL-treated DISCs, even though the levels of cell death were similar at 100ng/mL CH11 or 2ng/mL memFasL. This supports the hypothesis that memFasL organizes the DISC in such a way that efficient caspase-8 activity is achieved, and also suggests that one of the ways in which efficiency is achieved, in part, is through efficient recruitment and involvement of RIP1. Though CH11 activates the DISC less efficiently, it recruits large amounts of Fas, FADD, and caspase-8, thus increasing the probability of obtaining the proper alignments or conformations of the molecular players within the DISC to trigger caspase-8 activation without RIP1 involvement.
Figure 4. At equivalent doses, memFasL is more efficient in RIP1 recruitment than CH11.
(A) Cell death assays of parental Jurkat cells treated with indicated amounts of memFasL or CH11 antibody overnight (~20 hrs). (B) Western blots showing caspase-8 immunoprecipitation (IP) experiments in parental Jurkat cells treated with the indicated amounts of memFasL or CH11 antibody overnight (~20 hrs).
DISCUSSION
Our data suggests that RIP1 is a more integral part of physiological signaling through the Fas/CD95 receptor complex than previously recognized; at least when the signal is mediated by full-length membrane bound Fas Ligand. We have shown here, among its many roles, RIP1 efficiently enables the FADD-mediated pathway for death induction. Though it is not absolutely essential for cell death, it is, however, likely involved somehow in orienting the molecules of the DISC (Fas, FADD, Caspase-8 and others) in such a way that efficient caspase activity is achieved. Thus, the amount of caspase-8 that is recruited to the DISC is not the sole determining factor in how well this caspase is activated, but activation is also dependent on the other DISC components in relation to the amount of caspase-8.
A number of studies in addition to our own clearly indicate that RIP1 is not absolutely essential for either Fas- or TNF-R1– mediated apoptosis (18, 19). Our data show that RIP1-deficient Jurkat cells die, albeit less efficiently, in response to memFasL (Fig. 1B), and other studies have shown that RIP1 knockout MEFs die more rapidly than wild type MEFs in response to TNFα (19). Nevertheless, our data is in consistent with recent studies where RIP1 has been proposed to play a significant positive role in caspase-8 activation within the TNF pathway. The remaining amount of TNF-induced death in Tradd−/− macrophages is attributed to RIP1 acting as an adaptor molecule to downstream caspase activation (20). In another study, RIP1 is essential for nucleating a secondary complex of FADD and caspase-8 after its release from the TNF receptor (15). Secondary complexes have only recently been postulated to form separately from the receptor in Fas signaling, in conjunction with caspase-8 ubiquitination by CUL3 (21), though CUL3 knockdown has less effect on Fas-induced caspase-8 processing than processing induced by other death receptors. As both RIP1 and caspase-8 are regulated by ubiquitination, it would be tempting to speculate that it is ubiquitin modification that could regulate RIP1--caspase-8 secondary complexes. But while caspase-8 is polyubiquitinated in these secondary complexes (21), only de-ubiquitinated RIP1 is found in secondary complexes associated with caspase-8 (15, 22), and RIP1 is ubiquitinated by cIAPs (22, 23), instead of CUL3. At higher exposure of our western blots, it appears that RIP1 may be ubiquitinated when co-immunoprecipitated with caspase-8 (Fig. 4B), but we did not probe with a ubiquitin antibody. However, polyubiquitinated caspase-8 secondary complexes are heavily enriched in the lipid raft/cytoskeleton subcellular fraction, and we saw no effect of methyl-β-cyclodextrin on the differences in caspase-8 activation by memFas and CH11, suggesting that raft localization of caspase-8 is not important in the RIP1-dependent differences. Nevertheless, it is possible that memFasL can induce secondary, RIP1-containing, complexes to form, while the CH11 antibody cannot, and this could explain the role of RIP1 in signaling by memFasL. Although we view this as unlikely, we cannot exclude the possibility that we are precipitating two separate complexes, since the ratio of RIP1 to Fas in our memFasL DISC immunoprecipitates would be consistent with this hypothesis.
RIP1 has also been proposed to bind directly to caspase-8 and/or to its non-catalytic homologue, c-Flip (22, 24–27), and has been postulated to even directly activate caspase-8 in the absence of FADD during TNF signaling (27). However, in our hands, FADD-deficient Jurkat cells do not die in response to memFasL (data not shown), and therefore FADD appears to be required for caspase-8 activation, even when it is potentiated by RIP1. This is consistent with our data that suggest that, even in the absence of RIP1, memFasL forms a more efficient DISC with regard to caspase-8 activation. Nevertheless, it is possible that direct interactions between RIP1 and caspase-8 facilitate a FADD-dependent process, especially within a complex where many protein interactions are likely to occur.
Our data from Figure 1F suggests that crosslinked soluble Fas ligand acts in a more similar fashion to the CH11 antibody than to the ligand's full-length, membrane bound form. Thus, it is likely that when sFasL is associated with matrix proteins in a physiological context, it initiates cell death much less efficiently that memFasL due to the recruitment of less RIP1 to the disc. Because of this, it is therefore more likely that sFasL is involved in non-death signaling pathways, which is consistent with the views of sFasL in the current literature.
That two different Fas agonists act in slightly different ways has implications in modulating death receptor signaling from an investigational therapeutic standpoint. Our data suggests that it is possible to manipulate death receptor pathways using different pharmacological agonists of the same pathway to achieve remarkably different outcomes. The manipulation of Fas signaling is potentially important in developing treatment of autoimmune disease, such as some patients with autoimmune lympho-proliferative syndrome (ALPS), which have predisposition to lymphoma development as a result of inherited mutations in Fas (28, 29). Other death receptors are also potential targets in the treatment of disease. TRAIL receptor agonists, for example, are currently being tested as anti-cancer agents in clinical trials either individually or in combination with other chemotherapeutics. One would predict, based on our data, that different TRAIL-receptor agonists may have different efficacies, and therefore one may manipulate a given chemotherapeutic window of treatment by selecting a different agonist. Indeed, it has recently been shown that different agonistic antibodies to DR5 may act mechanistically distinct from each other in inducing FADD-dependent caspase-8 activation (30). Thus, it seems likely that differential sensitivity of same-receptor agonists could be somewhat common, and may therefore be of benefit in pharmacological modulation of these pathways in the treatment of disease.
In conclusion, we feel that the difference in response to the different agonists in the presence or absence of RIP1 gives some insight into the molecular and structural workings of the Fas receptor complex. As much of the scientific community continues to use antibody and ligand interchangeably despite known differences (3–7), our data is not only a further reminder that the agonists act distinctly and differently, but also at least partially explains why this is the case.
Supplementary Material
Footnotes
This research was supported by the Intramural Research Program of Center for Cancer Research, NCI, NIH.
References
- 1.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 3.Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkler F, Behrle E, Dennehy KM, Wicovsky A, Peters N, Warnke C, Pfizenmaier K, Wajant H. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J Cell Biol. 2005;168:1087–1098. doi: 10.1083/jcb.200501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villunger A, Huang DC, Holler N, Tschopp J, Strasser A. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J Immunol. 2000;165:1337–1343. doi: 10.4049/jimmunol.165.3.1337. [DOI] [PubMed] [Google Scholar]
- 6.Legembre P, Beneteau M, Daburon S, Moreau JF, Taupin JL. Cutting edge: SDS-stable Fas microaggregates: an early event of Fas activation occurring with agonistic anti-Fas antibody but not with Fas ligand. J Immunol. 2003;171:5659–5662. doi: 10.4049/jimmunol.171.11.5659. [DOI] [PubMed] [Google Scholar]
- 7.Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–1220. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 10.Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- 11.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 13.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 17.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 18.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 19.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 20.Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, Liu Z. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–1054. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol. 2005;174:5270–5278. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 27.Jin Z, El-Deiry WS. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2006;26:8136–8148. doi: 10.1128/MCB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 29.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 30.Thomas LR, Johnson RL, Reed JC, Thorburn A. The C-terminal tails of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas receptors have opposing functions in Fas-associated death domain (FADD) recruitment and can regulate agonist-specific mechanisms of receptor activation. J Biol Chem. 2004;279:52479–52486. doi: 10.1074/jbc.M409578200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.