Abstract
Smoking, diet and physical activity may impact chronic diseases, in part, by promoting or attenuating oxidative stress. We evaluated associations between lifestyle factors and urine F2a-isoprostanes, a marker of oxidative stress among 1610 participants of Study of Women’s Health Across the Nation (SWAN). Dietary intake and physical activity were assessed at baseline and year 05 (Y05). These data were related to Y05 urinary F2a-isoprostane concentration with regression analyses. Median urine F2a-isoprostane concentration was 433 ng/L overall, 917 ng/L in smokers (inter-quartile range: 467, 1832 ng/L) and 403 ng/L in non-smokers (inter-quartile range: 228, 709 ng/L; P<0.0001 for difference). Higher trans fat intake was associated with higher urine F2a-isoprostane concentration; partial Spearman correlations (ρx|y) between Y05 urine F2a-isoprostane concentration and trans fatty acids were 0.19 (P=0.03) and 0.13 (P <0.0001) in smokers and non-smokers, respectively. Increased log trans fat intake from baseline to Y05 was associated with higher concentration of logurine F2a-isoprostanes in non-smokers (β=0.131, SE=0.04, P =0.0003). In non-smokers, the partial correlation (ρx|y) between lutein and urine F2a-isoprostane concentration was −0.13 (P <0.0001). Increased intake of log lutein from baseline to Y05 was also associated with lower log urine F2a-isoprostane concentration (β= −0.096, SE=0.03, P =0.0005) in non-smokers. Increased zinc intake from baseline to Y05 was associated with lower log urine F2a-isoprostane concentration in smokers and non-smokers (β= −0.346, SE=0.14, P =0.01), and −0.117, 0.04 (P =0.001), respectively]. In conclusion, diet (fat subtypes, zinc, vegetable components) and smoking were associated with urine F2a-isoprostanes, a marker of oxidative stress.
Keywords: diet, physical activity, cigarette smoking, isoprostanes, oxidative stress
INTRODUCTION
Oxidative stress, an unfavorable balance between free radical generation and level of antioxidants, has been implicated in the pathogenesis of cancers, atherosclerosis, and diabetes (1, 2). Stable biomarkers of this oxidative stress include F2a-isoprostanes, the prostaglandin-like compounds formed in vivo from the free radical catalyzed peroxidation of arachidonic acid. (3–5). F2a-isoprostanes have been shown to be reliable biomarkers of lipid peroxidation. They are continuously formed under normal physiological conditions, and increased production has been observed with co-occurrence of smoking, alcohol intake, exercise, drug treatment. There is decreased production with dietary antioxidant supplementation, and fruit and vegetable intake (6).
Lifestyle factors, including dietary intake and physical activity, are thought to influence free radical proliferation. Antioxidants mediate oxidative damage by mitigating the progression of unpaired electron transfer associated with free radical formation (7), and they are identified as constituents responsible for the protective effects of fruits and vegetables (8–13).
Polyphenols may be effective in preventing oxidative-stress associated diseases (14, 15) but long-term investigations of their efficacy are sparse. Studies have focused on β-carotene, ascorbic acid and vitamin E, and while some evidence supports a protective effect against oxidative damage (16, 17), results from randomized, controlled trials in healthy populations are less convincing (18–20). Lutein, from sources including spinach and greens, has been associated with favorable levels of oxidative stress markers (21). While human studies are scarce, intake of saturated (22) and trans fats have been implicated in higher oxidative stress levels (23).
Cigarette smoking and environmental tobacco smoke in non-smokers has been associated with higher levels of oxidative stress (24–26). Cigarette smoke has been shown to increase requirements for several antioxidants (27, 28); however, smokers generally consume lower levels of antioxidants and higher amounts of saturated and trans fats than non-smokers (29, 30). Thus, it is clear that associations between dietary intake and oxidative stress are not comparable in smokers vs. non-smokers.
Physical activity has also been associated with oxidative stress, with indications that this association is apparently related to the intensity and duration of the activity. Physical activity of moderate intensity and duration has been associated with a favorable effect or no increase in F2a-isoprostanes (31, 32). The higher oxygen required for muscle activity in higher intensity or endurance exercise may lead to an accumulation of excess reactive oxygen species in anaerobic respiration and increased oxidative stress (33).
We evaluated the association between oxidative stress and lifestyle factors in women being studied longitudinally through the menopausal transition. We postulated that antioxidant intake would be inversely correlated with urine F2a-isoprostanes, a measure of oxidative stress, and that these relationships would differ by cigarette smoking status. We also hypothesized that low or moderate physical activity would be inversely associated with urine F2a-isoprostanes, while more vigorous activity would be linked to higher levels of oxidative stress. Although rates of certain cancers and cardiovascular disease differ by race (34, 35), no research on lifestyle factors and oxidative stress has focused on ethnically-diverse, midlife women. Inclusion of diverse women in our analysis adds a key strength, providing a wide range in nutrient intake and physical activity and differing rates of smoking.
MATERIALS AND METHODS
Sampling and Study Population
This report includes data from the baseline and fifth annual follow-up examination (Y05)2 of the Study of Women’s Health Across the Nation (SWAN), a community-based, longitudinal study of the menopausal transition (36). The analysis includes 1610 participants with baseline and Y05 dietary intake and physical activity data who were located in Boston MA, Chicago IL, the Detroit MI area, Los Angeles CA, Oakland CA, or Pittsburgh PA. Observations from the New Jersey SWAN site were excluded because of incomplete data at Y05. Study eligibility criteria at baseline for the SWAN longitudinal cohort were: age 42 to 52 years; intact uterus and at least one ovary; no current use of estrogens or other medications known to affect ovarian function; at least one menstrual period in the 3 mo before enrollment; and, self-identification as a member of a site-eligible racial/ethnic group. Caucasians were recruited at all study sites. African-American women were recruited in Boston, Chicago, Pittsburgh and the Detroit area, while Japanese and Chinese women were recruited at the Los Angeles and Oakland sites, respectively. The Chicago site only provided urine samples for 44 women for a SWAN substudy; these samples were analyzed for F2a-isoprostanes and represent the Chicago site contribution. Institutional Review Board approval was secured for the study protocol and informed consent obtained at each study site.
Assays
F2a-isoprostanes were assayed in urine specimens collected prior to 0900 during the Y05 visit and made available through the SWAN Repository (n=1606). Assays were completed in the CLASS laboratory at the University of Michigan.
Samples were applied to the F2a-isoprostane affinity column, washed with buffer and eluted with 95% ethanol. Following evaporation of the solvent, the dried samples were diluted 1:10 with 0.1 mol/L phosphate buffer and assayed using the F2a-isoprostane enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI). The range of the standard curve was 3.9–500 ng/L. Samples in a 96-well microplate were read at 405 nm. The post-extraction intra- and inter-assay coefficients of variation were 14.4% (51.2 ng/L, n=83 pairs) and 17.5% (51.2 ng/L, n=85) respectively. The intra-assay coefficient of variation for the concentration of F2a-isoprostanes was 5.8% (n=1707 pairs).
Diet and lifestyle data
Dietary and supplement data were obtained at baseline and Y05 from a modified food frequency interviewer-assisted questionnaire (FFQ). Originally developed by Block (37), the questionnaire was designed to obtain “usual” dietary patterns for the previous year and was administered in three languages (English, Chinese or Japanese). All FFQ contained a 103-item core food list; the Chinese and Japanese language versions included additional culturally-specific foods.
Number of daily servings of Brassica vegetables, including cabbage, broccoli, cauliflower, brussels sprouts and kale, were specifically assessed due to their association with lower F2a-isoprostane concentration, independent of micronutrient intake (8). For most fruit, a daily serving was quantified as one medium piece, except watermelon (one slice), cantaloupe (1/4 medium) and mangoes or papayas (½ medium). The serving size for prunes and strawberries was ½ cup. While most daily vegetable servings consisted of ½ cup, the exceptions were green salad (one medium bowl), French fries (3/4 cup) and white potatoes (½ cup). Polyphenol values from Manach (38) were assigned to foods and beverages by using the midpoint of the published range (in mg of the particular polyphenol per serving) for a mean daily serving estimated via FFQ. The contribution of coffee and caffeine were evaluated independently and not included in the total polyphenol value or in hydroxycinnamic acids. Genistein and daidzein intakes, based on database information from Reinli (39), were evaluated separately.
Baseline and Y05 dietary data were excluded from this report under the following conditions: too few or too many solid foods (or food groups) consumed per day (less than 4 or more than 17, respectively; n=104), missing data for more than 10 foods (n=4), and daily energy intake considered too low or too high for usual intake [less than 500 or greater than 20920 kJ/d3 (5000 kcal/d), respectively; n=14].
Physical activity was assessed usingan adaptation of the Kaiser Physical Activity Survey (40). This 38-questionsurvey is a self-administered questionnaire with establishedtest-retest reliability and validity (40, 41). Total possible physical activity scores ranged from 3 to 14 with higher numbers indicating greater activity.
Other lifestyle variables
Weight (kg) was measured using balance beam scales and height (m) was measured using stadiometers. Data on cigarette smoking and environmental tobacco smoke exposure were obtained from a self-administered questionnaire incorporating American Thoracic Society questions (42) and validated questions on environmental tobacco smoke exposure (43).
Statistical approach
SAS Version 9.1 statistical software was used for data management and analyses. The univariate distributions of continuous measures were examined and natural log transformations were employed, as appropriate, to meet the assumptions of normality and to reduce skewness.
Created variables
Due to almost negligible intakes of daidzein and genistein by Caucasian and African-American participants, only Chinese and Japanese participants were included in analyses of these variables. Daily intakes of daidzein and genistein in Chinese and Japanese women were categorized into tertiles. Thirty-third and 66th percentile cutoff values for daidzein intake were 906 μg and 3071 μg, respectively, in Chinese, and 2845 μg and 8992 μg, respectively in Japanese women. Thirty-third and 66th percentile cutoff values for genistein were 1757 μg and 6118 μg, respectively for Chinese and 4039 μg and 13286 μg, respectively in Japanese participants.
Most analyses were stratified based on a smoking status variable that classified participants as active smokers or not. A four-level variable that considered environmental tobacco smoke was used to compare the least squared means of urine F2a-isoprostane concentration. The levels were: 1) non-smokers with no active or environmental tobacco smoke exposure; 2) non-smokers with more than 1 h/wk of environmental tobacco smoke exposure; 3) smokers without environmental tobacco smoke exposure; or 4) smokers with more than 1 h/wk of environmental tobacco smoke exposure.
Physical activity variables were categorized to test the hypothesis that more intense physical activity would have a positive association with urine F2a-isoprostanes while moderate physical activity would have an inverse association. The categories were generated from the total physical activity questionnaire scores at Y05 (cutoffs: 40th and 80th percentiles, 7.25 and 9.4 points, respectively) Additionally, Y05 scores for the questionnaire’s “sport” sub-section that measures the frequency, intensity, and duration of two sports or exercise activities in the year prior to assessment had cutoffs at the 33rd and 66th percentiles, 2.25 and 3.5 points, and range from 1–5 points.
Statistical analyses
Medians and quartile values (Q1 and Q3), were calculated for continuous measures according to smoking status stratum. The Wilcoxon rank-sum, Kruskal-Wallis and chi-square tests were used to test differences in medians between race/ethnic groups and between smokers and non-smokers.
Partial Spearman correlations (ρx|y) were estimated to describe the statistical associations between Y05 urine F2a-isoprostanes and dietary/supplement intakes after controlling for age, BMI, physical activity, clinical site and race/ethnicity. Additionally, given that antioxidants attenuate oxidative damage caused by high dietary fat intake and because the beneficial effect of antioxidants is dependent on the amount of dietary fat a person consumes, partial Spearman correlations between urine F2a-isoprostanes and antioxidants were also adjusted for Y05 total fat intake.
Multiple variable regression analyses, with log transformed urine F2a-isoprostane concentration as the dependent variable, were used to estimate prospective associations with changes in dietary consumption over time. Significant p-values were two-sided at α<0.05. Dietary variables were evaluated singly rather than simultaneously to avoid collinearity.
Associations between individual dietary variables and urine F2a-isoprostane concentration are stratified by smoking status for three reasons. First, there is a strong relationship in our data between smoking status and urine F2a-isoprostane concentration, and this is supported by others (24, 44); second, the dietary intake of smokers differed from that of non-smokers in our data and in nationally representative data (30); finally, associations between dietary intake variables and F2a-isoprostanes differed in smokers versus non-smokers in our results and in other investigations (45, 46). Because smoking was a behavior rarely reported by Chinese or Japanese women, those few Asian women who reported smoking were excluded from selected analyses, as appropriate.
When total environmental smoke exposure was evaluated, there was a graded increase in the least squared means of urine F2a-isoprostane concentration according to cigarette smoke exposure. However, after adjustment for age, clinical site, BMI, physical activity and race, a significant difference in least squared means of urine F2a-isoprostane concentration was only observed between active smokers and non-smokers, regardless of environmental tobacco smoke exposure status. Thus, the analyses is stratified based on active smoking.
RESULTS
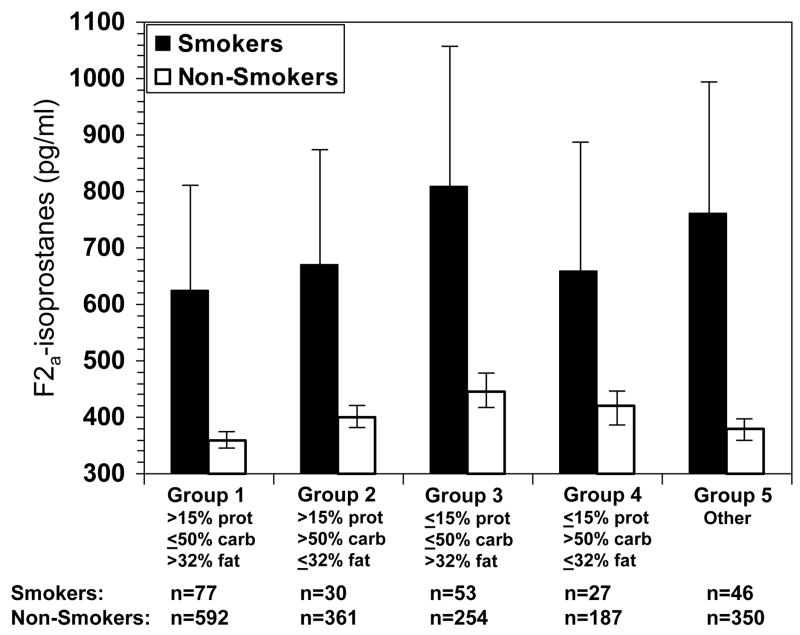
The overall median concentration of urine F2a-isoprostanes was 433 ng/L. Because cigarette smoking status differed by race, dietary intake and urine F2a-isoprostane concentration, results are reported by smoking status. Median urine F2a-isoprostane concentration was more than twice as high in women who smoked [917 ng/L, inter-quartile range (IQR): 467, 1832 ng/L] as in non-smokers (403 ng/L, IQR: 228, 709 ng/L; P <0.0001 for difference). Smoking was reported by 21% and 10% of African-American and Caucasian women, respectively, and by 1% and 8% of the Chinese and Japanese women, respectively (Table 1). Among non-smokers, Chinese and Japanese women had significantly lower median urine F2a-isoprostane concentration compared to the other two racial/ethnic groups (P<0.0001; Table 1).
TABLE 1.
Characteristics of SWAN participants according to smoking status and race1
| Smokers |
Non-Smokers |
|||||||
|---|---|---|---|---|---|---|---|---|
| African American | Caucasian | Chinese2 | Japanese2 | African American | Caucasian | Chinese | Japanese | |
| n | 76 | 81 | 2 | 17 | 283 | 747 | 194 | 206 |
| Age, y | 51 [48, 53] | 50 [48, 52] | - | - | 51 [49, 53] | 51 [49, 53] | 51 [49, 53] | 51 [49, 53] |
| BMI, kg/m2 | 31 [27, 37]* | 28 [24, 32] | - | - | 33 [27, 39]* | 27 [23, 32] | 23 [21, 25] | 23 [21, 26] |
| Physical activity score | 6.7 [5.4, 8.3]* | 7.1 [6.1, 8.7] | 7.3 [6.0, 8.7]* | 8.3 [6.8,9.5] | 7.3 [6.2, 8.4] | 7.9 [6.9, 9.0] | ||
| Urine F2a-isoprostane level, ng/L | 1226 [721, 2074]* | 803 [470, 1676] | - | - | 599 [361, 1021]* | 444 [242, 744] | 284 [175, 408] | 262 [167, 453] |
| Economic stress | Frequency (%) | |||||||
| Very hard to pay for basics | 13 (12)* | 2 (2) | - | - | 39 (9)* | 22 (2) | 1 (0) | 3 (1) |
| Somewhat hard to pay for basics | 41 (37) | 32 (31) | - | - | 115 (28) | 114 (13) | 29 (16) | 44 (21) |
| Not hard to pay for basics | 57 (51) | 68 (67) | - | - | 260 (63) | 772 (85) | 159 (84) | 165 (78) |
Values are medians and quartiles [Q1, Q3] collected at year 5, except economic stress, for which data collected at year 6 are presented; information is presented for those with urine F2a-isoprostanes data.
Values different between race/ethnicity among smokers, P<0.05 (Wilcoxon rank-sum or chi-square test) or non-smokers, P<0.05 (Kruskal-Wallis or chi-square test).
Values not estimated due to small cell sizes
Dietary intake and physical activity score at Y05 by smoking status
In general, women who smoked had dietary intakes lower in micronutrients, polyphenols, fruits and vegetables and higher in fat than non-smokers (Table 2). Women who smoked reported somewhat lower levels of physical activity than non-smokers [median physical activity questionnaire scores: 7.0 (IQR: 5.9, 8.5) vs. 7.9 (IQR: 6.5, 9.2), respectively; P <0.0001 for difference].
TABLE 2.
Dietary intake of SWAN participants by smoking status1
| Smokers2 | Non-Smokers3 | |
|---|---|---|
| n | 143 | 1385 |
| Energy4, kJ/d | 7824 [5908, 10305]* | 6849 [5452, 8556] |
| Total fat, g/d | 74.3 [54.4, 98.8]* | 61.2 [45.1, 79.8] |
| Saturated fatty acid, g/d | 25.4 [17.3, 34.8]* | 18.0 [13.0, 25.0] |
| Trans fatty acid, g/d | 5.9 [3.4, 8.9]* | 4.2 [2.6, 7.2] |
| Linoleic fatty acid, g/d | 14.0 [9.6, 18.0]* | 11.8 [8.3, 16.6] |
| Oleic fatty acid, g/d | 23.4 [21.0, 37.8]* | 24.0 [17.4, 31.4] |
| (n-3) fatty acid, g/d | 1.4 [1.0, 1.7] | 1.2 [0.9, 1.7] |
| Vitamin A, RE/d | 6703 [3782, 10573]* | 8586 [5684, 11892] |
| α-carotene, μg/d | 116.8 [31.3, 294.8]* | 190.4 [96.8, 386.2] |
| β-carotene, μg/d | 2357 [1311, 4647]* | 3134 [2050, 5078] |
| Lutein, μg/d | 1633 [872, 3546] | 1757 [982, 3338] |
| Lycopene, μg/d | 1011 [439, 1570]* | 1325 [738, 2244] |
| β-cryptoxanthin, μg/d | 96.5 [50.4, 157.3] | 93.7 [49.9, 151.7] |
| Vitamin C, mg/d | 136.1 [71.0, 226.1]* | 166.6 [103.4, 309.1] |
| Vitamin D, μg/d | 4.3 [2.3, 11.9]* | 7.6 [2.8, 12.4] |
| Vitamin E, α-TE/d | 15.5 [8.7, 31.6]* | 25.5 [10.5, 90.2] |
| Zinc, mg/d | 12.4 [7.8, 23.7] | 15.9 [8.8, 23.1] |
| Animal zinc, mg/d | 5.5 [4.2, 7.6]* | 4.4 [3.2, 6.1] |
| Fruit5, servings/d | 1.0 [0.5, 1.4]* | 1.1 [0.7, 1.7] |
| Vegetables6, servings/d | 1.5 [0.8, 2.1]* | 1.9 [1.2, 3.0] |
| Brassica vegetables, servings/d | 0.3 [0.2, 0.6] | 0.3 [0.2, 0.6] |
| Broccoli, servings/wk | 1.0 [0.25, 2.0]* | 1.0 [0.6, 2.0] |
| Cauliflower, Brussels sprouts, servings/wk | 0.25 [0, 0.6] | 0.25 [0, 0.6] |
| Greens, kale, servings/wk | 0 [0, 0.6]* | 0 [0, 0.3] |
| Coleslaw, cabbage, servings/wk | 0.25 [0, 0.6] | 0.25 [0, 0.6] |
| Total polyphenols, mg/d | 580.2 [269.7, 795.9]* | 356.3 [202.2, 630.7] |
| Anthocyanidins, mg/d | 22.6 [6.5, 58.2]* | 39.6 [17.6, 94.4] |
| Hydroxybenzoic acid, mg/d | 1.2 [0.3, 3.1]* | 1.5 [0.9, 5.4] |
| Hydroxycinnamic acid, mg/d | 530.7 [157.6, 559.3]* | 161.8 [24.5, 527.3] |
| Monomeric flavanols, mg/d | 23.4 [12.5, 44.6]* | 34.9 [16.7, 83.5] |
| Flavanones, mg/d | 10.7 [0, 33.0] | 10.7 [0, 33.0] |
| Flavonols, mg/d | 14.8 [8.3, 23.0] | 16.3 [10.0, 24.4] |
| Isoflavones, mg/d | 1.5 [1.0, 2.3]* | 2.5 [1.0, 12.4] |
| Quercetin, mg/d | 4.2 [2.2, 7.3]* | 5.8 [3.4, 10.3] |
Values are medians and quartiles [Q1, Q3] collected at Y05.
Different from non-smokers, P<0.05 (Wilcoxon rank-sum test)
Includes African Americans and Caucasians, n=143; Japanese and Chinese smokers were excluded due to small cell sizes
Includes all ethnic groups, n=1385
To convert kcal to kJ, multiply by 4.184
A ½ cup serving of fruit is approximately 123 grams
A ½ cup serving of chopped vegetables is approximately 90 grams
Overall, 52~61% women reported use of one of the following supplements: β-carotene, vitamins A, C, D, E or zinc; women who smoked were less likely to report dietary supplement (46~55%) use than non-smokers (56~67%; comparison of smokers vs. non-smokers taking at least one supplement vs. none P =0.001).
Concurrent associations between nutrients, foods, and urinary F2a-isoprostane concentration
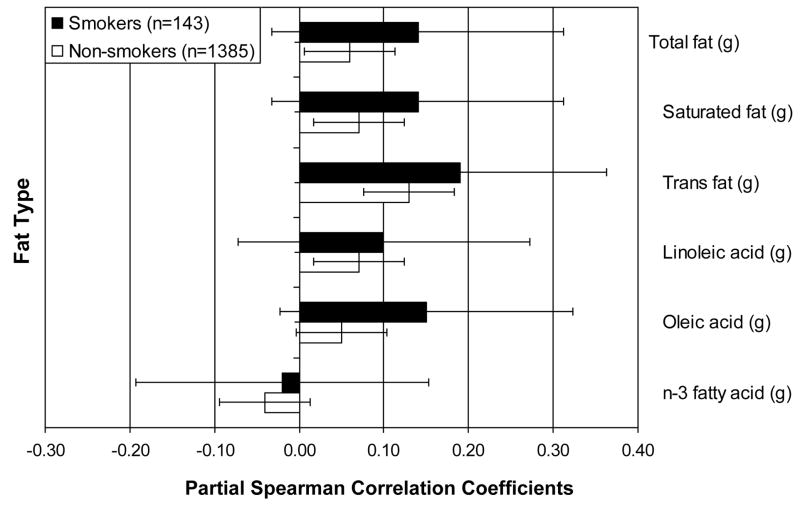
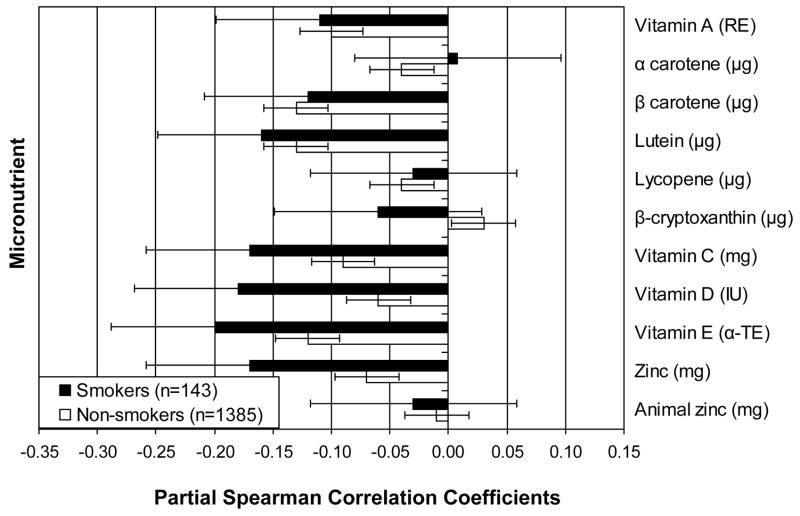
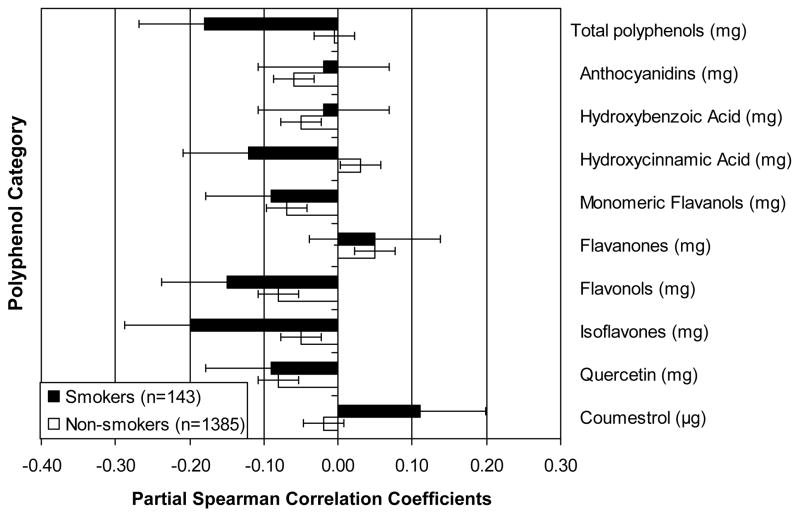
Among both smokers and non-smokers, there were significant positive partial Spearman correlations between the urine F2a-isoprostane concentration and trans fatty acid intake [ρx|y = 0.19 (P =0.03) and 0.13 (P <0.0001), respectively]. These partial correlations reflect the direction and strength of the association of interest, after accounting for the variation from age, race, BMI, physical activity and clinical site. We observed a significant partial correlation among non-smokers between saturated fat and the urine F2a-isoprostane concentration (ρx|y = 0.07; P =0.007). Although the partial correlation between saturated fat and the urine F2a-isoprostane concentration among smokers was the same direction and similar magnitude as that for trans fats (ρx|y = 0.14), this value was not statistically significant (P = 0.1). Higher vitamin E combined diet and supplement intake was associated with lower concentration of urine F2a-isoprostane concentration in both smokers and non-smokers [ρx|y =−0.2 (P =0.02) and −0.12 (P <0.0001), respectively].
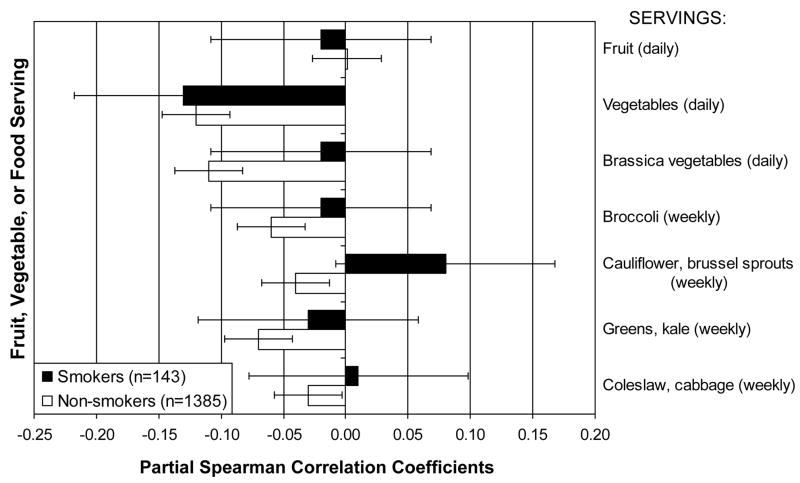
In non-smokers, higher intakes of lutein (ρx|y = −0.13; P <0.0001), β-carotene (ρx|y =−0.13; P <0.0001), along with more daily vegetable servings (ρx|y =−0.12; P <0.0001) and Brassica vegetable servings (ρx|y =−0.11; P =0.0001) were associated with lower concentration of urine F2a-isoprostanes.
Among smokers, we found inverse partial correlations between concentration of urine F2a-isoprostanes and vitamin D (ρx|y =−0.18; P =0.04), total polyphenols (ρx|y =−0.18; P =0.04), and isoflavones (ρx|y =−0.2; P =0.02), indicating lower oxidative stress with higher intakes. There was a borderline concurrent association with zinc (ρx|y = −0.17, P =0.05) in smokers.
Further, in the Japanese women, there were inverse partial correlations with urine F2a-isoprostane concentration for daidzein [ρx|y =−0.15 (P =0.03)] and genistein [ρx|y = −0.15 (P=0.04)], while in Chinese women, there was a borderline association with genistein [ρx|y = −0.15 (P =0.05)].
No relationships were found between coffee or caffeine and urine F2a-isoprostane concentrations.
Nutrient intake change (baseline to Y05) and concentration of urinary F2a-isoprostanes at Y05
Increased trans fat intake between baseline and Y05 follow-up was associated with significantly higher concentration of urine F2a-isoprostanes (Table 3) among non-smokers. Increased lutein and vitamin C consumption between baseline and Y05 follow-up was associated with significantly lower concentration of urine F2a-isoprostanes in non-smokers. Increased zinc consumption was associated with significantly lower concentration of urine F2a-isoprostanes among smokers and non-smokers.
TABLE 3.
Associations between log urine F2a-isoprostanes and change in nutrient intake from baseline to Y05 in regression analyses according to smoking classification
| Independent variable1 | Smokers2 | Non-Smokers3 | ||||
|---|---|---|---|---|---|---|
| β (SE) | p value | Partial r2 (%) | β (SE) | p value | Partial r2 (%) | |
| Change in trans fat intake | 0.186 (0.14) | NS4 | 1.4 | 0.131 (0.04) | 0.0003 | 1.0 |
| Change in saturated fat intake | 0.292 (0.2) | NS | 1.7 | 0.047 (0.06) | NS | 0.1 |
| Change in lutein intake | −0.134 (0.09) | NS | 1.7 | −0.096 (0.03) | 0.0005 | 0.9 |
| Change in lycopene intake | −0.014 (0.1) | NS | 0.0 | −0.003 (0.03) | NS | 0.0 |
| Change in β-carotene intake from diet/supplements | −0.094 (0.11) | NS | 0.6 | −0.055 (0.03) | NS | 0.2 |
| Change in vitamin C intake | −0.166 (0.09) | NS | 2.6 | −0.074 (0.03) | 0.007 | 0.6 |
| Change in Zinc intake | −0.346 (0.14) | 0.01 | 5.0 | −0.117 (0.04) | 0.001 | 0.8 |
Independent variables in regression models were constructed as log (Y05 variable)- log (baseline variable)
Includes African-Americans and Caucasians, n=136; models were adjusted for site, baseline value of independent variable of interest, along with baseline values and change from baseline to Y05 values for fat intake, age, BMI and physical activity score; Japanese and Chinese smokers were excluded due to small cell sizes
Includes all ethnic groups, n=1321; models were adjusted for site, race/ethnicity, baseline value of independent variable of interest, along with baseline values and change from baseline to Y05 values for fat intake, age, BMI and physical activity score
NS=non-significant, P >0.05
Among African-American and Caucasian women who smoked, an increase in total isoflavone intake (not including genistein or daidzein) from baseline to Y05 was associated with significantly lower concentration of urine F2a-isoprostanes (Table 4). High baseline intake of daidzein was significantly associated with lower urine F2a-isoprostane concentration in non-smoking Chinese and Japanese women, respectively, compared to their respective counterparts with the lowest intake. Higher baseline intake of genistein was also significantly associated with lower urine F2a-isoprostane concentrations in non-smoking Chinese women compared to Chinese women with the lowest intake.
TABLE 4.
Associations of log urine F2a-isoprostanes with phytoestrogen intakes in regression models according to smoking classification and race/ethnicity1
| Independent variable | Smokers2 |
Non-Smokers |
||||||
|---|---|---|---|---|---|---|---|---|
| African American & Caucasian | African American & Caucasian3 | Chinese4 | Japanese4 | |||||
| β (SE) | Partial r2 (%) | β (SE) | Partial r2 (%) | β (SE) | Partial r2 (%) | β (SE) | Partial r2 (%) | |
| Change in isoflavones intake5 | −0.285 (0.11)* | 5.3 | −0.008 (0.02) | 0.01 | −0.049 (0.05) | 0.6 | −0.106 (0.07) | 1.2 |
| High baseline daidzein intake6 | −0.334 (0.14)* | 3.2 | −0.319 (0.16)* | 2.3 | ||||
| High baseline genistein intake6 | −0.435 (0.14)* | 5.3 | −0.231 (0.16) | 1.1 | ||||
*P <0.05
n=136. Model adjusted for site, baseline value of independent variable of interest, along with baseline values and change from baseline to Y05 values for fat intake, age, BMI and physical activity score; Japanese and Chinese smokers were excluded due to small cell sizes
n=960. Model adjusted for site, baseline value of independent variable of interest, along with baseline values and change from baseline to Y05 values for fat intake, age, BMI and physical activity score
Chinese n=178, Japanese n=185. Models adjusted for baseline value of independent variable of interest, along with baseline values and change from baseline to Y05 values for fat intake, age, BMI and physical activity score
Independent variables in regression models were: log (Y05 variable)- log (baseline variable)
“High” intake was highest tertile; the reference group was the lowest tertile of intake
Among non-smokers, higher Brassica vegetable consumption at baseline and increased consumption of Brassica vegetables from baseline to Y05 was associated with significantly lower urine F2a-isoprostane concentration. Also among non-smokers, women in the highest category of baseline broccoli consumption (greater than 1.5 servings/wk) had significantly lower urine F2a-isoprostane concentration than women from the lowest category (≤ 0.625 servings/wk).
Physical activity
We examined physical activity as a continuous variable, dichotomized as a categorical variable (vigorous vs. not vigorous), and as an ordinal variable in regression analyses. There were no statistically significant associations with urine F2a-isoprostane concentration.
DISCUSSION
We evaluated whether nutritional factors, physical activity, and smoking behaviors were associated with increased oxidative stress as assessed by urine F2a-isoprostane concentrations in a large multi-race/ethnic sample of midlife women. This has important public health implications because oxidative stress is believed to contribute to risk for cardiovascular disease, diabetes and cancers (1–2), and lifestyle behaviors are potentially modifiable.
Our results show associations between higher intake of trans fats and higher concentration of urine F2a-isoprostanes in smokers and non-smokers concurrently and in non-smokers prospectively. In animal studies, trans fat intake is associated with increased oxidative stress (23). In human studies, higher intakes of trans fatty acids have been associated with elevated coronary heart disease risk (47);
Trans fats, found in some margarines, crackers, salad dressings and other foods, are a result of food processing when hydrogen is added to unsaturated fats in vegetable oils to increase shelf-life. Thus, the link between higher trans fat intake and higher F2a-isoprostanes, a measure of arachidonic acid peroxidation, is logical because trans fats are partially hydrogenated polyunsaturated fats. After ingestion, trans fats can be incorporated into cellular membrane phospholipids, often displacing cis-polyunsaturated fatty acids, leading to decreased cell membrane fluidity (48). This reduced fluidity allows for increased activity of free radicals in the phospholipid bilayer, precipitating oxidative damage. F2a-isoprostanes and several prostaglandins arise from the peroxidation of arachidonic acid (48), a metabolic process believed to contribute to heart disease through altered platelet aggregation and the promotion of insulin resistance (49–51).
We also found an association between urine F2a-isoprostanes and saturated fats among non-smokers. We were unable to resolve whether the association with urine F2a-isoprostanes was only with trans fat, with saturated fats, or both. In our analyses, the types of fat intake were highly correlated, precluding their simultaneous inclusion in a regression model. Further, the two types of fats are not readily distinguished, biologically. Relatively greater proportions of both saturated and trans fatty acids are stored in the phospholipid bilayer leading to increased cell membrane rigidity compared to less saturated fats. Finally, both fat subtypes are found in many of the same foods.
Fairly consistent inverse associations were found with lutein intake; however, less consistent inverse associations were found for β-carotene and lycopene. Block (16) reported that lutein, α and β-carotene and lycopene were inversely related to F2a-isoprostanes. However, in multiple variable analyses adjusting for sex, age, race, smoking status and BMI, only β-carotene remained statistically significant in their analysis.
While lutein, β-carotene and lycopene are found in many of the same foods, there is more commonality in good dietary sources of lutein and β-carotene. Structurally, all three molecules contain long polyene chains; however, β-carotene and lutein have ring structures at either end of the chains, while the lycopene chain contains uncyclized ends. The double bonds on the polyene chains of all three molecules can contribute to oxidation/reduction reactions, thus limiting peroxidation of membrane phospholipids. It is noteworthy that the hydroxyl functional group on lutein’s ε-ring provides an additional target for oxidation/reduction that the others do not possess.
We observed inverse associations between both daidzein and genistein intakes and urine concentration of F2a-isoprostanes in Chinese and Japanese women. The association between soy intake and coronary heart disease has been reported (52–53); however, intervention studies with intermediate, oxidative stress marker endpoints have provided mixed results (54–56). Due to the relative absence of smoking in Chinese and Japanese women, it was not possible to ascertain whether high genistein and daidzein intake would minimize the impact of smoking on the isoprostanes. The inclusion of other broad classes of polyphenols and their evaluation in all women indicated that polyphenols, apart from genistein and daidzein, could be important contributors to the amelioration of oxidative stress.
Several associations between dietary intake and urine F2a-isoprostanes were different among smokers versus non-smokers, supporting the idea that smoking modifies these associations. Because smokers have higher concentration of isoprostanes and consume lower amounts of antioxidants and more trans and saturated fats than non-smokers, they may be more responsive to the beneficial effect of antioxidants (57). Among the strongest inverse concurrent associations observed were among smokers for vitamins C and E. Research indicates that vitamin C has the capacity for regeneration by other antioxidants and subsequent re-enlistment for oxidation/reduction actions (58, 59). Although vitamin E has been reported to disappear in response to oxidative stressors such as cigarette smoke (59), evidence is mixed and a protective effect for cardiovascular disease has not been established (18, 60).
Concurrent relationships between lower zinc and higher urine F2a-isoprostane concentration were noteworthy in both smokers and non-smokers, and increases in zinc intake over time were also related to lower urine F2a-isoprostane concentration in both groups. The mechanisms of action for zinc’s antioxidant effects are an area of active research. Zinc’s antioxidant effects are thought to occur through displacement of pro-oxidant metals, such as iron and copper upon binding with cell membranes, thus decreasing free radical production at the ligand-binding site (61). The zinc-containing enzyme Cu-Zn superoxide dismutase is thought to be a critical enzyme for oxygen free radical defense (62).
Environmental tobacco smoke exposure has been proposed as a risk factor for cardiovascular disease (63). Some evidence shows that it is associated with oxidative stress markers similar to that of active smokers (26). In our investigation, although we observed a graded increase in least squared means of urine F2a-isoprostanes according to increasing levels of total smoke exposure, statistically significant differences based on environmental tobacco smoke exposure disappeared in analyses adjusted for other relevant variables.
In models adjusted for covariates, physical activity was not associated with urine F2a-isoprostanes. Our inability to detect an association could have been due to the small observed range of physical activity scores in our sample and lack of a sizable sub-group of women with regular intense physical activity. The absence of a statistically significant association has also been reported in older persons (32) in whom intense physical activity is less common.
Our investigation had strengths and limitations. Our diverse sample provided a wide range in lifestyle exposures, including intake of soy products and other food sources of polyphenols. A major advantage of this investigation was the availability of exposure data at two points in time. The use of F2a-isoprostanes as a marker of oxidative stress was a strength due to their specificity for lipid peroxidation and chemical stability (64, 65). Little diurnal variation is observed, and therefore, measurement of urine F2a-isoprostanes in a single early morning sample has been described as adequate to represent the daily isoprostane excretion in humans (66). Nonetheless, nutrient values obtained from FFQ inevitably involve some misclassification error due to the difficulty in recalling and quantifying items consumed during the past year and recall of physical activity is susceptible to misclassification error for similar reasons. While the level of Type I error was set at 0.05 for each nutrient or food component, the numerous statistical tests may increase the likelihood of delineating false positive findings; thus, it is important to evaluate the findings also on the basis of the existing literature and biological plausibility. Further, analyses of Chinese and Japanese women who smoked were not possible due to the small numbers of such smokers.
This investigation consistently showed that dietary trans fatty acid intakes were associated with oxidative stress, as measured by urine F2a-isoprostanes, while we observed a protective association for lutein and zinc intakes. Our results suggest that oxidative stress levels may be modifiable by changes in intake of these nutrients over time. Furthermore, the magnitude of concurrent and prospective associations in women who smoke suggest that those with elevated oxidative stress or cancer and cardiac disease risk, may particularly benefit from decreasing trans fat intake and increasing intakes of lutein and other antioxidants (47, 57). Finally, this study provided no evidence to consider physical activity as a primary mediator of oxidative stress.
Footnotes
Sources of financial support: The Study of Women’s Health Across the Nation (SWAN) and the SWAN Repository have grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (Grants AG017719, NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The isoprostane data were the result of a grant for assay of urine samples available from the SWAN Repository (AG017719).
Abbreviations used: IQR, interquartile range; SWAN, Study of Women’s Health Across the Nation; Y05, fifth annual follow-up examination
1 kcal = 4.184 kJ
LITERATURE CITED
- 1.Pratico D, Rokach J, Lawson J, FitzGerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem Phys Lipids. 2004;128:165–71. doi: 10.1016/j.chemphyslip.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions and preliminary results from nutritional supplementation studies. Free Radic Res. 1998;29:469–86. doi: 10.1080/10715769800300531. [DOI] [PubMed] [Google Scholar]
- 3.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., II A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and humans. Free Radic Biol Med. 1999;26:202–26. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 5.Pratico D, Iuliano L, Basili S, Ferro D, Camastra C, Cordova C, FitzGerald GA, Violi F. An Enhanced lipid peroxidation in hepatic cirrhosis. J Invest Med. 1998;46:51–7. [PubMed] [Google Scholar]
- 6.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7:221–35. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 7.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–67. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 8.Fowke JH, Morrow JD, Motley S, Bostick RM, Ness RM. Brassica vegetable consumption reduces urinary F2-isoprostane levels independent of micronutrient intake. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl065. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Hininger I, Chopra M, Thurnham DI, Laporte F, Richard MJ, Favier A, Roussel AM. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr. 1997;51:601–6. doi: 10.1038/sj.ejcn.1600451. [DOI] [PubMed] [Google Scholar]
- 10.van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Adv Exp Med Biol. 1999;472:159–68. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–78. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen H, de Vries A, Nijhoff WA, Schouten A, van Poppel G, Peters WH, van den Berg H. Effect of Brussels sprouts on oxidative DNA-damage in man. Cancer Lett. 1997;114:127–30. doi: 10.1016/s0304-3835(97)04641-7. [DOI] [PubMed] [Google Scholar]
- 13.Pool-Zobel BL, Bub A, Muller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–50. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 14.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81(1 Suppl):243S–55S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Appel LJ, Croft KD, Miller ER, III, Mori TA, Puddey IB. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am J Clin Nutr. 2002;76:549–55. doi: 10.1093/ajcn/76.3.549. [DOI] [PubMed] [Google Scholar]
- 18.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–82. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 19.Elmadfa I, Rust P, Majchrzak D, Wagner KH, Genser D, Lettner R, Pinter M. Effects of beta-carotene supplementation on free radical mechanism in healthy adult subjects. Int J Vitam Nutr Res. 2004;74:147–52. doi: 10.1024/0300-9831.74.2.147. [DOI] [PubMed] [Google Scholar]
- 20.Upritchard JE, Schuurman CR, Wiersma A, Tijburg LB, Coolen SA, Rijken PJ, Wiseman SA. Spread supplemented with moderate doses of vitamin E and carotenoids reduces lipid peroxidation in healthy, nonsmoking adults. Am J Clin Nutr. 2003;78:985–92. doi: 10.1093/ajcn/78.5.985. [DOI] [PubMed] [Google Scholar]
- 21.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, McCann SE, Trevisan M, Cassano PA, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006;60:991–9. doi: 10.1038/sj.ejcn.1602410. [DOI] [PubMed] [Google Scholar]
- 22.Diniz YS, Cicogna AC, Padovani CR, Santana LS, Faine LA, Novelli EL. Diets rich in saturated and polyunsaturated fatty acids: metabolic shifting and cardiac health. Nutrition. 2004;20:230–4. doi: 10.1016/j.nut.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Cassagno N, Palos-Pinto A, Costet P, Breilh D, Darmon M, Berard AM. Low amounts of trans 18:1 fatty acids elevate plasma triacylglycerols but not cholesterol and alter the cellular defence to oxidative stress in mice. Br J Nutr. 2005;94:346–52. doi: 10.1079/bjn20051512. [DOI] [PubMed] [Google Scholar]
- 24.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 25.Sowers MR, Crawford S, McConnell DS, Randolph JF, Jr, Gold EB, Wilkin MK, Lasley B. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136:1588–95. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadzadehfar H, Oguogho A, Efthimiou Y, Kritz H, Sinzinger H. Passive cigarette smoking increases isoprostane formation. Life Sci. 2006;78:894–7. doi: 10.1016/j.lfs.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 27.Bruno RS, Traber MG. Cigarette smoke alters human vitamin E requirements. J Nutr. 2005;135:671–4. doi: 10.1093/jn/135.4.671. [DOI] [PubMed] [Google Scholar]
- 28.Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr. 1996;63:559–65. doi: 10.1093/ajcn/63.4.559. [DOI] [PubMed] [Google Scholar]
- 29.Palaniappan U, Jacobs Starkey L, O’Loughlin J, Gray-Donald K. Fruit and vegetable consumption is lower and saturated fat intake is higher among Canadians reporting smoking. J Nutr. 2001;131:1952–8. doi: 10.1093/jn/131.7.1952. [DOI] [PubMed] [Google Scholar]
- 30.Subar AF, Harlan LC, Mattson ME. Food and nutrient intake differences between smokers and non-smokers in the US. Am J Public Health. 1990;80:1323–9. doi: 10.2105/ajph.80.11.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karolkiewicz J, Szczesniak L, Deskur-Smielecka E, Nowak A, Stemplewski R, Szeklicki R. Oxidative stress and antioxidant defense system in healthy, elderly men: relationship to physical activity. Aging Male. 2003;6:100–5. [PubMed] [Google Scholar]
- 32.Hernandez R, Mahedero G, Caballero MJ, Rodriguez J, Manjon I, Rodriguez I, Maynar M. Effects of physical exercise in pre-and postmenopausal women on lipid peroxidation and antioxidant systems. Endocr Res. 1999;25:153–61. doi: 10.1080/07435809909066137. [DOI] [PubMed] [Google Scholar]
- 33.Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med. 2001;31:911–22. doi: 10.1016/s0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) Racial/ethnic and socioeconomic disparities in multiple risk factors for heart disease and stroke--United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54:113–7. [PubMed] [Google Scholar]
- 35.Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance--United States, 1990–2000. MMWR Surveill Summ. 2004;53:1–108. [PubMed] [Google Scholar]
- 36.Sowers MF, Crawford S, Sternfeld B, Morgenstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, et al. Design, survey sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. [Google Scholar]
- 37.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 38.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 39.Reinli K, Block G. Phytoestrogen content of foods--a compendium of literature values. Nutr Cancer. 1996;26:123–48. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 40.Sternfeld B, Ainsworth BE, Quesenberry CP., Jr Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser Physical Activity Survey in women. Med Sci Sports Exerc. 2000;32:1327–38. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–20. [PubMed] [Google Scholar]
- 43.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130:696–704. doi: 10.1093/oxfordjournals.aje.a115391. [DOI] [PubMed] [Google Scholar]
- 44.Miller ER, 3rd, Appel LJ, Jiang L, Risby TH. Association between cigarette smoking and lipid peroxidation in a controlled feeding study. Circulation. 1997;96:1097–101. doi: 10.1161/01.cir.96.4.1097. [DOI] [PubMed] [Google Scholar]
- 45.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–6. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg FM, Chait A. Antioxidant vitamin supplementation and lipid peroxidation in smokers. Am J Clin Nutr. 1998 Aug;68:319–27. doi: 10.1093/ajcn/68.2.319. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 48.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992;89:10721–5. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovejoy JC. Dietary fatty acids and insulin resistance. Curr Atheroscler Rep. 1999:215–20. doi: 10.1007/s11883-999-0035-5. [DOI] [PubMed] [Google Scholar]
- 50.Hill EG, Johnson SB, Lawson LD, Mahfouz MM, Holman RT. Perturbation of the metabolism of essential fatty acids by dietary partially hydrogenated vegetable oil. Proc Natl Acad Sci U S A. 1982;79:953–7. doi: 10.1073/pnas.79.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinsella JE, Bruckner G, Mai J, Shimp J. Metabolism of trans fatty acids with emphasis on the effects of trans, trans-octadecadienoate on lipid composition, essential fatty acid, and prostaglandins: an overview. Am J Clin Nutr. 1981;34:2307–18. doi: 10.1093/ajcn/34.10.2307. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, Jin F, Zheng W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–8. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 53.Keys A. Seven countries: a multivariate analysis of death and coronary heart disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- 54.Wiseman H, O’Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, Sanders TA. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72:395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 55.Vega-Lopez S, Yeum KJ, Lecker JL, Ausman LM, Johnson EJ, Devaraj S, Jialal I, Lichtenstein AH. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am J Clin Nutr. 2005;81:43–9. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- 56.Nhan S, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr Cancer. 2005;53:73–81. doi: 10.1207/s15327914nc5301_9. [DOI] [PubMed] [Google Scholar]
- 57.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 58.Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, Vera JC. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278:10128–33. doi: 10.1074/jbc.M210686200. [DOI] [PubMed] [Google Scholar]
- 59.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40:689–97. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 60.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Nutrition Committee of the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–41. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 61.Rostan EF, DeBuys HV, Madey DL, Pinnell SR. Evidence supporting zinc as an important antioxidant for skin. Int J Dermatol. 2002;41:606–11. doi: 10.1046/j.1365-4362.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- 62.Duzguner V, Kaya S. Effect of zinc on the lipid peroxidation and the antioxidant defense systems of the alloxan-induced diabetic rabbits. Free Radic Biol Med. 2007;42:1481–6. doi: 10.1016/j.freeradbiomed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 63.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 64.Liu T, Stern A, Roberts LJ, Morrow JD. The isoprostanes: novel prostaglandin-like products of the free radical-catalyzed peroxidation of arachidonic acid. J Biomed Sci. 1999;6:226–35. doi: 10.1007/BF02253564. [DOI] [PubMed] [Google Scholar]
- 65.Basu S. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic Res. 2004;38:105–22. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Ciabattoni G, Creminon C, Lawson J, FitzGerald GA, Patrono C, Maclouf J. Immunological characterization of urinary 8-epi-prostaglandin F2α excretion in man. Journal of Pharmacology and Experimental Therapeutics. 1995;275:94–100. [PubMed] [Google Scholar]