Abstract
The Sindbis viral expression system enables the rapid production of high levels of recombinant protein in mammalian cells; however, this expression is typically limited to transient production due to the cytotoxicity of the virus. Limiting the lethality inherent in the Sindbis virus vector in order to enable long term, sustained expression of recombinant proteins may be possible. In this study, modifications to virus and host have been combined in order to reduce the cytopathic effects. Non-cytopathic replication competent viruses of two Sindbis viral strains, TE and 633, were developed using a non-structural protein (nsP) P726S point mutation in order to obtain persistent heterologous gene expression in infected Baby Hamster Kidney (BHK) cells and Chinese Hamster Ovary (CHO) cells. Cells infected with the P726S variant viruses were able to recover after infection, while cells infected with normal virus died within 3 days. The P726S mutation did not reduce the susceptibility of 5-day and 14-day old mice to 633 and TE viruses in vivo. In addition, animal survival with the P726S variant viruses was increased and GFP expression was sustained for at least 14 days while the 633 and TE infection resulted in short term GFP expression or an earlier mortality. Modifications to the host BHK and CHO cells themselves were subsequently undertaken by including the anti-apoptotic gene Bcl-2 and a deletion mutant of Bcl-2 (Bcl-2Δ) as another method for limiting the cytopathic effects of the Sindbis virus. The inclusion of anti-apoptotic genes permitted higher production of heterologous GFP protein following Sindbis virus infection, and the combination of the TE-P726S virus and the CHO-Bcl-2Δcell line showed the greatest improvement in cell survival. Sindbis virus infection also induced ER stress in mammalian cells as detected by increased PERK phosphorylation and ATF4 translation. Overexpression of Parkin, an E3 ubiquitin ligase that can protect cells against agents that induce ER stress, suppressed Sindbis virus induced cell death in both BHK cells and in vivo studies in mice. Such findings show that viral and host modifications can improve cell survival and production of heterologous proteins, change viral behavior in vitro and in vivo, and assist in the development of new expression or gene delivery vehicles.
Keywords: Sindbis virus, Bcl-2, mammalian cells, recombinant protein expression, ER stress, Parkin, apoptosis
1. INTRODUCTION
Alphavirus expression vectors have been employed to produce recombinant proteins in mammalian cells (Boorsma et al., 2003; Bredenbeek et al., 1993; Frolov et al., 1996a; Garoff and Li 1998; Huang et al., 1989; Liljestrom and Garoff, 1991; Lundstrom, 1997; Schlesinger and Dubensky, 1999; Xiong et al., 1989). Sindbis virus is a single-stranded positive sense RNA virus belonging to the alphavirus family that can infect a broad range of cells and replicate to high titers (Strauss and Strauss, 1994). Despite the high-level expression of recombinant proteins, Sindbis virus infected cells rapidly undergo apoptosis limiting protein expression to a transient system (Levine et al., 1993; Manstrangelo et al., 2000).
The Sindbis virus RNA contains two open reading frames. The first is located in the 5' terminal two-thirds of the genomic RNA translating the nonstructural proteins (nsP 1-4) required for transcription and replication of the viral RNA. The second is located in the 3' terminal one-third of the genome and codes for structural proteins including capsid, glycoproteins E1 and E2, and a small hydrophobic membrane (6K) protein (Strauss and Strauss, 1994). Several approaches have used the Sindbis virus replication machinery for recombinant protein production. The replicon and defective helper system consists of the replicon RNA encoding the non-structural proteins and the recombinant protein of interest, and the helper RNA encoding the structural proteins (Schlesinger and Dubensky, 1999). The replicons are self-replicating due to the replicase encoded by the non-structural proteins. The helpers are required to package the replicons to create new virus particles. On the other hand, the replication and packaging-competent system contains a duplicated subgenomic promoter to express heterologous proteins. The RNA vector genome encodes the non-structural proteins, the structural proteins, and the recombinant proteins creating infectious particles (Frolov, et al., 1996b).
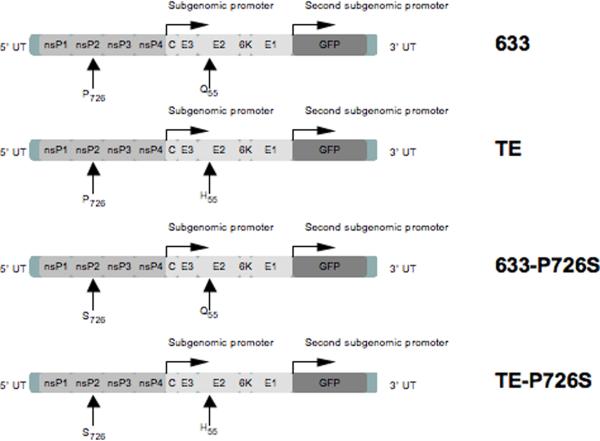
The recombinant Sindbis virus 633 and TE were constructed to study the effects of amino acid changes on neurovirulence (Dropulic et al., 1997). We examined the replication and packaging-competent Sindbis virus vectors 633 and TE engineered to produce green fluorescent protein (GFP) as the heterologous model protein from the additional subgenomic promoter (Fig. 1). In vivo, the neurovirulence of Sindbis virus was influenced by the amino acid change from histidine to glutamine at position 55 of the E2 structural protein. The TE virus (His-55) was fully virulent for 14-day old mice, but the 633 virus (Gln-55) was not (Tucker et al., 1993). The TE virus displayed more efficient infection than the 633 virus in neuronal N18 cells, which was likely due to more efficient virus binding of the TE virus than the 633 virus (Lee et al., 2002). However, in non-neuronal BHK cells, the 633 and TE viruses showed similar level of virus binding and replication (Dropulic et al., 1997; Lee et al., 2002).
Figure 1.
Replication and packaging competent recombinant Sindbis viruses 633, TE, 633-P726S, and TE-P726S. The 633 and TE viruses differ by an amino acid at the position 55 of the E2 protein that is Glutamine (Q) or Histidine (H). The Proline (P) to Serine (S) mutation is at the amino acid position 726 in the nsP2 domain of the nonstructural proteins. The green fluorescence protein (GFP) is expressed from the second subgenomic promoter.
The cytopathic effects of the Sindbis replicons could be reduced by a single amino acid change in the nsP2 non-structural protein domain (Dryga et al., 1997). A small number of BHK cells survived Sindbis virus infection in the presence of defective interfering particles (Weiss et al., 1980). The SIN-1 virus was isolated from the culture and shown to develop persistent infection without the defective interfering particles. The SIN-1 virus was found to contain the P726S mutation (Dryga et al., 1997). Another experiment using Sindbis virus expressing puromycin acetyltransferase as a selectable marker identified an adaptive mutation for noncytopathic infection at the same location in nsP2 protein, P726L (Frolov et al., 1999). This P726L mutation was also used in a Sindbis virus replicon engineered to express heterologous genes (Agapov et al., 1998).
We incorporated the P726S mutation in the 633 and TE viruses and examined cytotoxicity and GFP expression in Baby Hamster Kidney (BHK) and Chinese Hamster Ovary (CHO) cell lines commonly used for recombinant protein expression. Sindbis virus vectors have the potential for investigation of recombinant protein expression in neurobiology due to the ability to express recombinant proteins in neurons both in vitro and in vivo. However, cell death induced by Sindbis virus vectors has limited their application. We also tested the 633-P726S and the TE-P726S variant viruses in a mouse model (5-day and 14-day old) to assess toxicity and duration of expression.
Sindbis virus infection is known to be lethal due to activation of the programmed cell death cascade or apoptosis (Levine et al., 1993; Hardwick et al., 1997; Mastrangelo et al., 1999). Expression of anti-apoptotic genes has been shown to inhibit cell death at least to a limited extent against Sindbis virus infection. AT-3 cells expressing Bcl-2 were protected against apoptosis when infected with the 633 virus. However, the AT-3 Bcl-2 cells were killed following infection with the TE virus (Ubol et al., 1994). Cells over-expressing Bcl-2 or Bcl-xL were protected against cell death following Sindbis virus or Semliki Forest virus infection (Kiiver et al., 2008; Lundstrom et al., 1997; Mastrangelo et al., 2000). Bcl-2 is a widely studied anti-apoptotic gene that includes 4 homology domains (BH1-4) and an unstructured loop region susceptible to post-translational modifications and degradation during apoptosis. The Bcl-2 gene has been modified to remove this loop domain to create a variant called Bcl-2Δ(Chang et al., 1997). The Bcl-2Δprotein was more protective than the wild-type Bcl-2 protein against various apoptosis insults in mammalian cell culture including Sindbis virus infection, IL-3 withdrawal, serum withdrawal, and glucose deprivation (Chang et al., 1997; Figueroa et al., 2001). The Bcl-2Δ variant appeared to be more stable and less susceptible to proteolytic cleavage than the Bcl-2 gene (Figueroa et al., 2001). As another method for altering cytopathic effects of the Sindbis virus, we explored the effect of Bcl-2 and Bcl-2Δ expression in CHO and BHK cells infected with the 633 and TE virus with and without the P726S mutation.
Following Sindbis virus infection, host cell protein synthesis is inhibited within a few hours post infection while Sindbis virus proteins are translated (Frolov and Schlesinger, 1994; Gorchakov et al., 2004). Sindbis virus glycoproteins E1 and E2 are processed in the ER and the Golgi, where the potential exists to exceed ER folding capacity and induce ER stress. Cells respond to increased amount of misfolded ER proteins by initiating an unfolded protein response (UPR) to limit protein translation while increasing the amount of ER chaperones and components for ER-associated degradation (Breckenridge et al., 2003; Schroeder, 2006). PERK, one of the major ER stress sensors, is an ER-resident transmembrane protein activated by the release of the ER chaperone BiP from its ER luminal domain (Liu et al., 2000). PERK activation phosphorylates eIF-2α and represses most of the protein translation to counteract ER protein overload (Harding et al., 1999). However, eIF-2α phosphorylation selectively induces translation of specific mRNAs including ATF4 (Activating Transcription Factor 4) (Harding et al., 2000a). ATF4 further enhances expression of transcription factor ATF3 and CHOP/GADD153, which affects amino acid import, metabolism, and apoptosis (Harding et al., 2003; Jiang et al., 2004). Therefore, we determined if ER stress is activated in Sindbis virus infected cells by PERK activation, eIF-2α phosphorylation, and subsequent ATF4 translation enhancement.
Parkin is an E3 ubiquitin ligase believed to target insoluble and unfolded proteins for proteasomal degradation (Imai et al., 2000). Parkin promoted degradation of the putative G protein-coupled transmembrane polypeptide, named Pael (parkin-associated endothelin receptor-like) receptor, and protected cells against ER stress induced cell death (Takahashi et al., 2003). Parkin represents a potential candidate to suppress cell death from Sindbis virus infection if ER stress is a factor. Therefore, Parkin overexpression was considered as another method to alleviate toxicity from Sindbis virus infection along with modifications to the viral vectors and overexpression of the anti-apoptotic Bcl-2 and Bcl-2Δ genes in the host cell lines.
2. MATERIALS & METHODS
2.1. Plasmid Construction
To construct pds633, the BssH II-Pvu II (9804-11212) fragment of pdsTE12Q (Levine et al., 1996) was replaced with the counterpart from pTE to change the amino acid sequence in E1 region of pdsTE12Q to that of AR339 virus. Next, in order to construct pdsTE, the BssH II-Sac I fragment (9804-13658) segment of pds633 was patched to the corresponding region of pTE.
To insert the second subgenomic promoter and the GFP gene, a shuttling vector with EGFP ORF and the second SV subgenomic promoter was generated by inserting an Apa I-Sac I (11386-13658) fragment of pdsTE12Q into PCR II vector (Invitrogen, Carlsbad, CA) to generate pSINsg. The EcoRI-XbaI fragment of pEGFP-1 (Clontech, Mountainview, CA) was klenowed and ligated into pSINsg linearized with BstEII to generate pSINsg-EGFP. To construct pdsTE-EGFP and pds633-EGFP, the XbaI-SacI (11428-13658) fragment of pdsTE and pds633, respectively, were replaced by the corresponding XbaI-SacI fragment of pSINsg-EGFP.
The construction of pdsTE12Q-p35, Bcl-xL have been previously described (Cheng et al., 1996). pdsTE12Q-Parkin was generated by ligating a klenowed fragment of human Parkin into BstEII site. To construct pds633 P726S-EGFP and pdsTE P726S-EGFP, the ClaI-SpeI fragment of pds633-EGFP and pdsTE-EGFP, respectively, were replaced with a corresponding fragment from pSIN19 (generous gift from Dr. Charles Rice, The Rockefeller University). Creation of Flag-tagged Parkin, Bcl-2, and Bcl-2Δ plasmids have been previously described (Figueroa et al., 2001; Tsai et al., 2003)
2.2. Cell culture and virus plague assays
BHK-21 and CHO cells (ATCC, Manassas, VA) were cultured in Dulbecco's Minimum Essential Medium supplemented with 10% fetal bovine serum, 1% 200 mM L-glutamine, 1% 10 mM MEM-NEAA, and 1% penicillin-streptomycin solution (Invitrogen, Carlsbad, CA). Generation of CHO-Bcl-2 and CHO-Bcl-2Δ cell lines was previously described (Figueroa et al., 2001). The stable cell-lines were maintained in the same media supplemented with 800 μg/mL Geneticin (Invitrogen, Carlsbad, CA). Plague formation was assayed by using BHK-21 monolayers as previously described (Dropulic et al., 1997). For the P726S mutated viruses, plagues were scored at 3 days post-infection.
2.3. Viral RNA transcription, transfection, and cell viability assay
Purified plasmid DNAs were linearized by digestion with XhoI or NotI and transcribed using SP6 polymerase in the presence of cap analog as described earlier (Lewis et al., 1999). The RNA transcripts were immediately used for transfection of BHK-21 cells using Lipofectin (Invitrogen, Carlsbad, CA). The recombinant virus was collected from the supernatant of the transfected BHK-21 cells at 24 hour post transfection. The virus titer was determined from plaque assay described in section 2.2.
BHK-21 cells were plated in the Lab-Tek II Chamber Slide (Nalge Nunc International, Rochester, NY) at a density of 50,000 cells per chamber and infected with the Sindbis virus at a multiplicity of infection (MOI) of 5 on the next day. Cell viability was assayed at the indicated time post-infection. Briefly, cells were washed twice in Hepes buffered saline (HBS) and stained with Propidium Iodide solution (Invitrogen, Carlsbad, CA) for 10 minutes. The cells were observed on a Zeiss Axiovert inverted fluorescence microscope and the number of live cells were counted in 10 random fields at 40X magnification. Only adherent cells expressing GFP and excluding Propidium Iodide were counted as viable; cells showing both GFP and Propidium Iodide fluorescence were scored as dead. At MOI of 5, 100% of cells was infected showing green fluorescence. The final viability was normalized to the number of viable cells at 12 hr post-infection as 100% viable. After the cell count, the cells were washed with HBS and fresh media was added to continue the culture.
2.4. Animal Survival
Three-day old CD-1 mice were inoculated intracerebrally (i.c.) with 5000 plague forming units (PFU) per animal and monitored for 21 days. Survival curves were estimated using the Kaplan-Meier Product Limit method and compared by non-parametric methods in S-Plus (Insightful). To assay viral titer in the brain, three animals from each group were sacrificed at the indicated time points and their brains homogenized for titer assays. To determine whether the P726S mutation affects the age dependent susceptibility of 633 and TE viruses, animals were injected i.c. with 5000 PFU/mouse of the 633, TE, 633-P726S and TE-P726S viruses expressing GFP. The animals were sacrificed at the indicated time and their brains removed. The hippocampus was dissected and sectioned with a vibratome for imaging on a laser scanning confocal microscope (Zeiss LSM 510).
2.5. GFP Fluorescence Analysis
Cells were plated in 6-well plates at a density of 1.5×105 cells per well and infected with the virus (MOI = 5) on the following day. Post infection, all cells were infected showing green fluorescence. At the indicated times post-infection, GFP expression was assessed by measuring fluorescence on a Cytofluor fluorescence plate reader (Applied Biosystems, Foster City, CA). The fluorescence units obtained from the reading were subtracted by the background fluorescence units of the uninfected cells to obtain the arbitrary fluorescence units.
2.6. Immunoblotting
Cells were harvested, washed with PBS, and lysed in Triton-X100 buffer (1% Triton-X-100, 20 mM HEPES, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 10 mM sodium diphosphate, 100 mM NaF, 17.5 mM β-glycerophosphate, 1 mM phenylmethylsulphonyl fluoride, 1 μg ml-1 aprotonin, 1 μg ml-1 pepstatin A, and 1 μg ml-1 leupeptin). Western blots were performed with the following primary antibodies: P-eIF-2α, P-Perk (Cell Signaling Technology), PERK (Santa Cruz Biotechnology), and GFP (Clontech).
2.7. ATF4 translation assay
A firefly luciferase reporter plasmid driven by the thymidine kinase promoter has the reporter genes consist of translational fusions of the ATF4 5' UTR and the luciferase coding region (Harding et al., 2000b, generous gift from Dr. David Ron, New York University). Cells were co-transfected with the firefly luciferase reporter plasmid and the Renilla luciferase pRL-SV40 vector (Promega, Madison, WI) using Lipofectamine2000 (Invitrogen, Carlsbad, CA) to create pools of stably transfected cells. The Renilla luciferase was expressed at comparable level in all the cells and used to determine the cell number. Cells were harvested and analyzed for luciferase levels at 0 h and 16 h post infection with a luminometer using Dual-Glo luciferase assay system (Promega, Madison, WI). The ATF4 translation level was calculated from the firefly luciferase level in each sample normalized against the background Renilla luciferase level.
3. RESULTS
3.1. Persistent infection of Sindbis virus 633-P726S and TE-P726S in BHK and CHO cells
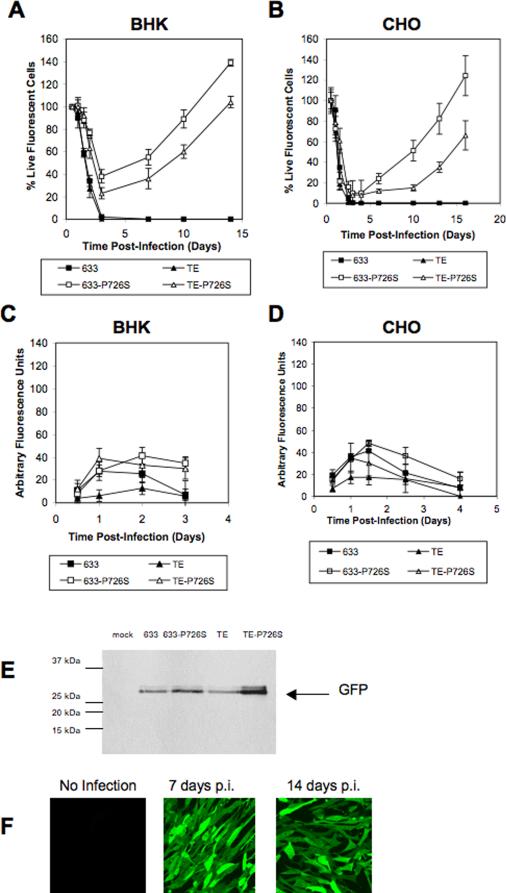
The first aim of this study was to determine if the cytotoxicity of the recombinant Sindbis viruses 633 (Gln-55) and TE (His-55) could be altered by introducing the single point mutation P726S into the nsP2 nonstructural protein (Dryga et al., 1997). All viruses express GFP from the second subgenomic promoter. BHK and CHO cells were infected with the 633, 633-P726S, TE, and TE-P726S viruses (Fig. 1) (MOI = 5), and the viable cell number was examined post infection (p.i.). 100% of the cells expressed GFP at all time points. BHK and CHO cells infected with the 633 and TE viruses were dead by 3 days (Fig. 2a and 2b). In contrast, about 20% of the BHK cells survived the infection by TE-P726S virus and nearly 40% survived the 633-P726S infection at day 3 p.i. By 14 days p.i, the number of viable cells had increased to a level that was 39% and 4% higher than the initial cell number for the 633-726S and TE-726S viruses, respectively (Fig. 2a). Viral titers were assayed at 2 days and 5 days p.i. to indicate that the increase in viable cells with the P726S variants infection was not due to the absence of the virus (Table 1). Also, the supernatant of the culture infected with the 633-P726S and TE-P726S viruses was capable of infecting new BHK cells (data not shown). A similar recovery pattern was observed in the infected CHO cells (Fig. 2b), suggesting that the phenomena is a characteristic of the viral system and not just the particular cell line considered.
Figure 2.
Percentage of live (a) BHK and (b) CHO cells after infection with the Sindbis viruses 633, TE, and its P726S variants. Percentage live cells were calculated as viable cell density at each time point divided by initial viable cell density at 12 h p.i. To exclude uninfected cells, only fluorescent cells (infected cells expressing GFP) were counted (see Materials and Methods). Total recombinant GFP production of the (c) BHK and (d) CHO cells post infection. The arbitrary fluorescence unit maximum (y-axis) is set to140 for all of the fluorescence results in Fig. 2, 4, 5 for comparison purpose. (e) Western blot showing GFP expression in BHK cells at 16 hours p.i. 10 μg of total protein in the cell lysate was loaded into each lane. (f) BHK cells infected with the TE-P726S virus at 7 and 14 days p.i. and no infection control.
Table 1.
Virus titer of the BHK cells infected with the 633-P726S and TE-P726S viruses assayed at 2 days and 5 days p.i.
| Recombinant Sindbis virus | Day 2 p.i. (PFU) | Day 5 p.i. (PFU) |
|---|---|---|
| 633-P726S | 1.1 × 108 | 5.5 × 107 |
| TE-P726S | 3.0 × 107 | 1.1 × 107 |
3.2. The effect of the P726S mutation on GFP production in BHK and CHO cells
We also investigated the effect of the 633 and TE viruses and the P726S variants on producing GFP. BHK and CHO cells were infected with the viruses (MOI = 5) and the GFP fluorescence was measured over the first 3 (BHK) or 4 (CHO) days (Fig. 2c and d). The BHK cells infected with the 633-P726S and TE-P726S viruses showed slightly higher level of GFP compared to the 633 and TE viruses at day 3 (Fig. 2c). Western blot of the cell lysates of the infected BHK cells collected at 16 hours p.i. also indicated higher GFP expression with the TE-P726S and 633-P726S viruses (Fig. 2e). At this time point, cell viability was comparable between the different groups. A different pattern was observed in CHO cells with the 633-P726S virus showing the highest fluorescence (Fig. 2d). Given the presence of viable cells in Fig. 2a for extended periods following the infections with the mutated virus, the BHK cells infected with the TE-P726S virus were also examined under a fluorescence microscope at 7 and 14 days p.i. (Fig. 2f). All cells in the microscope field were found to express GFP suggesting the establishment of long-term infection.
3.3. The in vivo effect of the P726S mutation in Sindbis viruses 633 and TE
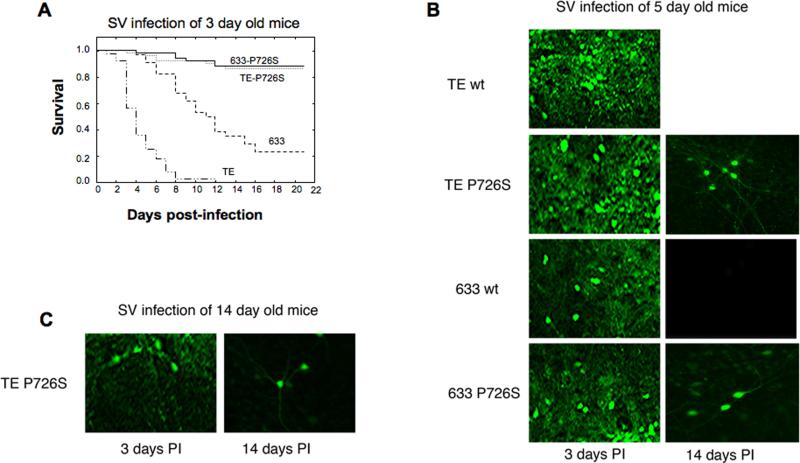
Sindbis virus vectors are also considered for gene therapy and neurobiology applications due to high transduction rates in tumor cell lines and neurons (Lundstrom, 2005). We were interested in reducing the toxicity and increase duration of expression of the virus to enhance the applications of the Sindbis virus vectors for in vivo applications. We examined the toxicity of the 633 and TE viruses with and without the P726S in vivo. Three-day old CD1 mice were injected intracerebrally with the 633, 633-P726S, TE, and TE-P726S viruses (5000 PFU/mouse). The titer of the 633 virus was 3-fold higher than the 633-P726S virus in the mouse brain assayed at 3 days post-injection (Table 2). Similarly, the titer of the TE virus was more than 3 fold higher than the titer of the TE-P726S virus (Table 2). Animal survival was followed post-injection for 3 weeks. The TE virus caused 100% mortality within 12 days, and the 633 virus caused 70% mortality in 21 days (Fig. 3a) In contrast, more than 80% of the mice survived the infection with the 633-P726S and the TE-P726S viruses.
Table 2.
Virus titers in the mouse brain assayed at 3 days post infection.
| Recombinant Sindbis virus | Virus titer (PFU) at day 3 p.i. |
|---|---|
| 633 | 9 × 106 |
| 633-P726S | 3 × 106 |
| TE | 1 × 107 |
| TE-P726S | 3 × 106 |
Figure 3.
(a) Survival of the 3-day old mice after infection of the Sindbis viruses 633, TE, 633-P726S and TE-P726S. (b) Hippocampal sections of the 5-day old mice infected with the 633, 633-P726S, TE, TE-P726S viruses at 3 days and 14 days p.i. (c) Hippocampal sections of the 14-day old mice infected with the TE-P726S virus at 3 days and 14 days p.i.
Previously, recombinant Sindbis virus 633 and TE have been shown to exhibit age-dependent virulence differences in mice (Tucker et al., 1993). The TE virus was virulent when given to 7-day and 14-day old mice. In contrast, the 633 virus caused 18% mortality when inoculated intracerebrally in 7-day old mice but the virus was avirulent and had reduced replication in 14-day old mice (Tucker et al., 1993). In order to investigate whether the P726S mutation reduced the age-dependent virulence of the 633 and the TE viruses while allowing replication and transgene expression, 5-day and 14-day old mice were infected with the viruses. Hippocampal sections of animals that survived the infection were analyzed. The 633, TE, and the P726S variant viruses were able to infect 5-day old mice (Fig. 3b). At 14 days p.i., animal survival was observed only with the P726S variant virus infections showing prolonged GFP expression in the hippocampus (Fig. 3b). In contrast, the TE virus infection was fatal and the 633 virus infection exhibited no detectable GFP expression at 14 days p.i. (Fig. 3b).
When 14-day old mice were subjected to SV infection, the 633 and 633-P726S were avirulent (data not shown). The TE and TE-P726S viruses were able to infect 14-day old mice, and the TE-P726S virus infection showed animal survival with prolonged GFP expression at 14 days p.i. (Fig. 3c). Hence, the P726S mutation did not change the age dependence virulence of the 633 and TE viruses, but did permit non-cytopathic transgene expression for extended periods in mice.
3.4. Viability and GFP production in BHK and CHO cell lines expressing Bcl-2 and Bcl-2Δ
Recombinant protein production is limited by many factors including plasmid integration, transcription, translation, secretion, and cell death (Barnes and Dickson, 2006; Figueroa et al., 2007). Several previous reports have indicated that Sindbis virus infection induces apoptosis and this cell death limits recombinant protein production to a transient process. (Figueroa et al., 2001, 2003, 2004; Levine et al., 1993; Hardwick et al., 1997; Mastrangelo et al., 1999 and 2000; Sauerwald et al., 2002, 2003, 2006). As another approach to limit the cytopathic effects of the Sindbis virus, we investigated the effect of combining the 633-P726S and TE-P726S viruses with BHK and CHO cells expressing the anti-apoptotic genes Bcl-2 and Bcl-2Δ genes on cell survival and GFP expression level.
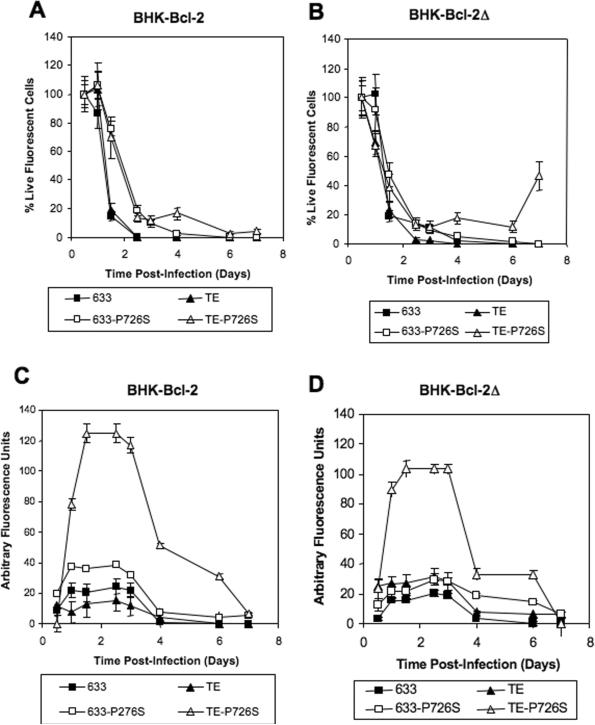
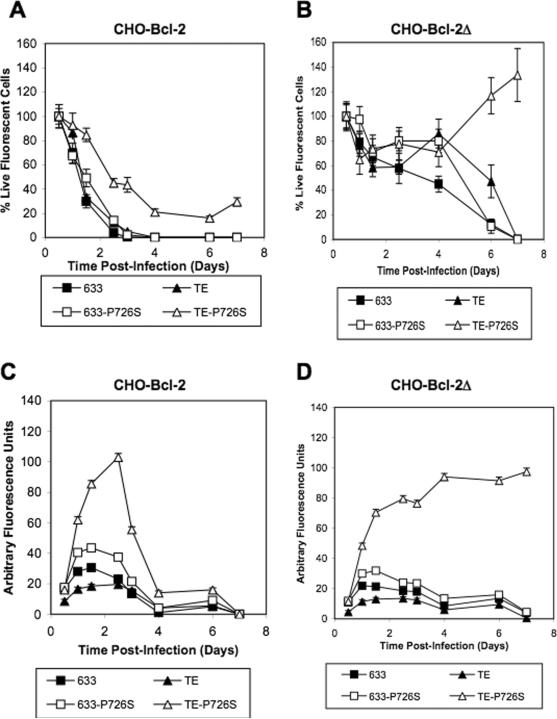
The parental BHK and CHO, Bcl-2, and Bcl-2Δ cell lines were infected with the four recombinant Sindbis viruses: 633, 633-P726S, TE, and TE-P726S. The Bcl-2Δ gene has been modified from Bcl-2 by removing the loop domain which is susceptible to cleavage by caspases during apoptosis (Chang et al., 1997; Figueroa et al., 2001). As shown earlier in Fig. 2a, BHK cells survived infection with the 633-P726S and the TE-P726S viruses but not with the 633 and TE viruses. Surprisingly, the BHK-Bcl-2 and BHK-Bcl-2Δ cell lines did not survive infection with the 633, TE, or 633-P726S viruses (Fig. 4a and 4b). The only virus that showed prolonged cell survival was the TE-P726S virus. At 7 days p.i., 4% of the BHK-Bcl-2 cells survived the TE-P726S infection (Fig. 4a) and this percentage increased to 12% at 12 days p.i. (data not shown). In addition, 47% of the initial BHK-Bcl-2Δ cell number was viable at 7 days p.i. with the TE-P726S virus (Fig. 4b). This cell survival was comparable to the BHK cells infected with the TE-P726S virus (Fig. 2a). Despite only a limited improvement in cell survival p.i. with the TE-P726S virus, the GFP level was 5-fold higher in the BHK-Bcl-2 and the BHK-Bcl-2Δ cell lines infected with the TE-P726S compared to the TE virus (Fig. 4c and 4d).
Figure 4.
Percentage of live (a) BHK-Bcl-2 and (b) BHK-Bcl-2Δ cells after infection with the Sindbis viruses 633, TE, 633-P726S and TE-P726S. Total GFP expression of the (c) BHK-Bcl-2 and (d) BHK-Bcl-2Δ cell lines post infection.
CHO cells infected with the 633 and TE viruses were nonviable at 3 days p.i., but survived the infection with the 633-P726S and the TE-P726S viruses (Fig. 1b). As with the BHK-Bcl2 cells, the CHO-Bcl-2 cell line survived an infection with the TE-P726S virus; however, infection with the 633, 633-P726S, and TE viruses killed all cells at 3 days p.i. (Fig. 5a). The CHO-Bcl-2Δ cell line infected with the 633 and TE viruses showed a delay in cell death from 3 days (CHO and CHO-Bcl-2 cell lines) to 7 days p.i. (Fig. 5b). Furthermore, 71% of the CHO-Bcl-2Δ cell line survived the TE-P726S infection at 4 days p.i., the highest percentage of cells surviving at that point in this study (Fig. 5b). CHO-Bcl-2 and CHO-Bcl-2Δ cells infected with the TE-P726S virus showed up to 5-fold higher GFP levels compared to the TE virus infection (Fig. 5c and 5d). The GFP level of the CHO-Bcl-2 cell line reached a maximum at 2.5 days p.i. and then decreased to the basal level at 4 days p.i. (Fig. 5c). The CHO-Bcl-2Δ cell line showed a rapid increase in GFP level up to 2 days p.i. then a sustained level of high GFP up to 7 days (Fig. 5d) that was observable even by 16 days p.i. (data not shown). Therefore, the combination of theTE-P726S virus and the CHO-Bcl-2Δ cell line showed the ability to maintain cell survival and sustain GFP levels several fold higher than the other viruses for many days after the initial infection.
Figure 5.
Percentage of live (a) CHO-Bcl-2 and (b) CHO-Bcl-2Δ cells after infection with the Sindbis viruses 633, TE, 633-P726S and TE-P726S. GFP expression level of the (c) CHO-Bcl-2 and (d) CHO-Bcl-2Δ cell lines post infection.
3.5. Sindbis virus infection and ER stress
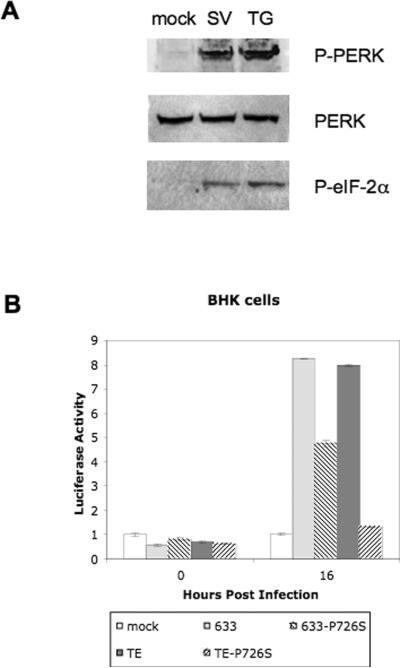
One of the major hallmarks of Sindbis virus infection is the inhibition of the host cell protein synthesis and the translation of the viral proteins (Frolov and Schlesinger, 1994; Gorchakov et al., 2004). Sindbis virus glycoproteins E1 and E2 are processed in the ER and the Golgi which has the potential to exceed ER folding capacity and activate ER stress. To determine if Sindbis virus infection can cause ER stress activation in a mammalian cell, human embryonic kidney (HEK-293) cells were investigated first due to the availability of antibodies to human ER stress proteins and the ability of Sindbis virus to infect this cell line. HEK-293 cells were mock infected, infected with Sindbis virus TE (MOI = 5), or treated with thapsigargin to deplete ER calcium stores and serve as a positive control for ER stress activation (Lytton et al., 1991). PERK, an ER-resident transmembrane protein, is activated by release of the ER chaperone BiP from its ER luminal domain and is the only eIF-2α kinase specific to ER stress activation (Harding et al., 2000a; Liu et al., 2000). At 16 hours p.i., increased phosphorylation of PERK, an ER stress marker, was detected in the TE-infected cells and the thapsigargin treated cells (Fig. 6a).
Figure 6.
(a) Western blots showing PERK phosphorylation, PERK, eIF-2α phosphorylation in 293 cells that were mock infected (mock), infected with the TE virus (SV), or exposed to thapsigargin as a positive control (TG). (b) BHK cells were transfected with the firefly luciferase reporter plasmid consisting of the translational fusions of the ATF4 5' UTR prior to infection with the viruses. Luciferase activity was determined to indicate ATF4 translation level at 0 and 16 hours post-Sindbis virus infection.
In ER stress activated cells, eIF-2α phosphorylation represses protein translation of most transcription factors except those involved in the ER stress response such as Activating Transcription Factor 4 (ATF4) (Harding et al., 2000b). In order to examine for ER stress in cell lines of the current study, a luciferase reporter plasmid with the reporter gene coding region fused to the ATF4 5' UTR was used to examine ATF4 translation (Harding et al., 2000b). BHK cells infected with the 633 and TE viruses showed the highest increase in ATF4 translation followed by the 633-P726S infected cells with TE-P726S infected cells having the lowest ATF4 translation level of the infected cells at 16 hours p.i. (Fig. 6b). Less ATF4 translation in the 633-P726S and TE-P726S infected cells indicated that the P726S mutation may alleviate ER stress activation.
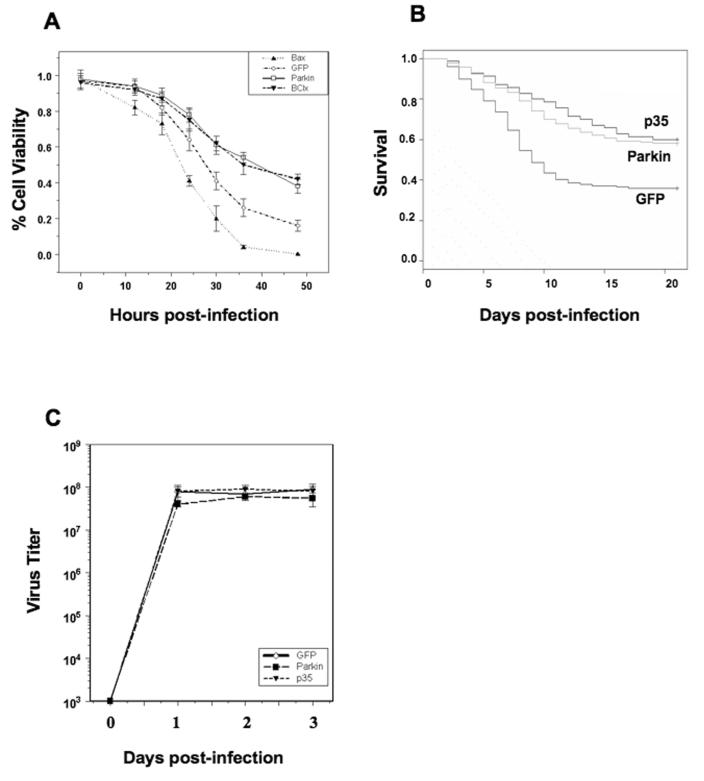
If ER stress plays an important role in Sindbis virus infection, we asked whether reducing ER stress can reduce cytopathogenic effects of Sindbis virus. Parkin is an E3 ubiquitin ligase that has been shown to selectively protect cells against agents that induce ER stress (Takahashi et al., 2003; Tsai et al., 2003). To compare the effect of Parkin to other factors, BHK cells were infected with a recombinant Sindbis virus TE12Q expressing Bcl-xL (a bcl-2 homolog that inhibits apoptosis), Bax (pro-apoptotic homolog), Parkin or GFP. TE12Q virus differs from TE virus by 2 amino acids present in the E1 glycoprotein (positions 72 and 313) (Ubol et al., 1994). At 48 hours p.i., Bax transfected cells were all dead, and less than 20% of GFP transfected cells were viable. In contrast, approximately 40% of Parkin and Bcl-xL transfected cells were viable (Fig. 7a). Consistent with ER stress playing a role in Sindbis virus cytopathogenicity, Parkin overexpression reduced Sindbis virus induced cell death at a level comparable to Bcl-xL overexpression.
Figure 7.
(a) BHK cells infected with the TE12Q virus expressing Bcl-xL (a generic inhibitor of apoptosis), Bax (pro-apoptotic), Parkin or GFP. (b) 3-day old mice infected i.c. with the TE12Q viruses expressing GFP, p35 (caspase inhibitor), and Parkin.
Next, we investigated whether Parkin overexpression can suppress Sindbis virus induced cell death in vivo. Three day old mice were infected i.c. with TE12Q viruses expressing GFP, p35, or Parkin from the second subgenomic promoter. p35 is a caspase inhibitor shown to protect mice after an infection with Sindbis virus TE12Q encoding p35 (Ryan et al., 2002). After 20 days, less than 40% of the mice survived infection with TE-GFP virus while more than 60% of the mice survived infection with TE-Parkin and TE-p35 viruses (Fig. 7b). Virus titer was assayed from the mouse brain at day 2, 3, and 4 p.i., and the titers were comparable (Fig. 7c). Thus Parkin expression suppressed Sindbis virus induced cell death in cell culture and in vivo.
4. DISCUSSION
We examined the applications of the P726S mutation with the replication and packaging-competent Sindbis viral vectors 633 and TE. Cell survival was observed in BHK and CHO cells infected with the P726S virus variants in contrast to the 633 and TE viruses, but it took 10-14 days after the infection until the viable cell number reached 100% of the initial numbers. At 14 days p.i., the GFP level was too low to be detected with the fluorescence plate reader; however, the cells were observed to express GFP with a fluorescence microscope (Fig. 2f) indicating a persistent viral infection of BHK cells.
The GFP fluorescence increased to the highest level in the first few days p.i. and decreased over time except in the case of the CHO-Bcl-2Δ cell line infected with the TE-P726S virus (Figure 5d). The decrease in GFP fluorescence in all other cell lines could be due to several reasons. Additional adaptive mutations of the virus could occur during viral replication. A different Sindbis virus (Toto1101) with the same P726S mutation showed a similar phenomenon in which most of the BHK cells died post infection followed by cell growth (Frolov et al., 1999). The authors believed that these effects occurred due to the additional adaptive mutations of the virus (Frolov et al., 1999). However, in our studies the cultured media from the re-growth phase cells showed similar toxicity rates when used to re-infect new cells (data not shown) suggesting that this phenomenon may be related to the adaptation of the host cells as well. A subset of the host cells may be adapted to low level infection and express low levels of GFP. The cells with the high level of GFP may also express high levels of viral related proteins which could be toxic leading to cell death in a few days post infection. Thus, an adaptation of the host cells and/or a mutation of the viruses led to cell survival and prolonged GFP expression.
Sindbis virus vectors are also able to express recombinant proteins in neurons in vitro and in vivo. Thus, the Sindbis viral vector has been considered as a gene therapy vehicle (Lundstrom, 2005). As a result, additional studies were performed with these viruses in mice. Compared to the 633 and TE viruses, mice injected with the P726S variant viruses survived the infection and also exhibited sustained GFP expression for a number of days. However, the virus virulence is dependent on the age of the mice following exposure and is not affected by the P726S mutation. The current study shows that the modification of the viral genome can also alter the toxicity of the virus in vivo and this reduction in toxicity may potentially enhance the neurobiology applications of the Sindbis virus vectors.
In order to overcome cell death induced by Sindbis virus infection in culture, the host cells were engineered to express the anti-apoptotic genes Bcl-2 or Bcl-2Δprior to infection. Surprisingly, these anti-apoptotic genes did not appear to provide any additional survival in BHK cells and in fact the P726S mutated viruses were more lethal than in wild type cells. However, the production levels of the recombinant protein, especially for the TE-P726S virus, were many times higher in the anti-apoptotic BHK-Bcl2 and BHK-Bcl-2Δcells compared to wild type BHK cells. The exception to viral lethality was observed with the TE-P726S virus in the engineered CHO cells in which the CHO-Bcl2 cells recovered slowly while the infected CHO-Bcl-2Δcell line exhibited high viable cells p.i. and rapid recovery. The improved survival of cells expressing Bcl-2Δ was likely due to its longer half-life, which was previously observed for the Bcl-2Δ variant as compared to the wild type Bcl-2 gene after Sindbis viral infections (Figueroa et al., 2001). For the CHO Bcl-2Δcells infected with the TE-P726S, sustained high GFP levels were obtained in combination with high viability. Thus, these cells may be able to maintain extended infectivity and recombinant protein production without triggering the apoptotic response in the host cell line. In this way, the combination of the Bcl-2Δcell line and the TE-P726S virus represents a potential new expression system for extended production of recombinant proteins in mammalian cell culture. Such an expression system may be advantageous since it can provide for the generation of a continuous production cell line derived from a viral vector in a week. Typically, stable cell lines require many weeks and even months for generating effective recombinant protein production systems (Wurm, 2004). However, the stability of engineered cell lines is not very good. Of course, it will be essential to evaluate the production of a target recombinant protein other than the model protein GFP, and the evaluation should be performed over a longer time period than in the current study.
Sindbis virus infection has been shown to induce apoptosis by activation of caspase-1 and caspase-8, leading to activation of caspase-3 (Nava et al., 1998). We showed that ER stress could be another potential factor leading to cell death in mammalian cells infected with Sindbis virus. We observed ER stress specific PERK activation and subsequent eIF-2αphosphorylation in the model HEK-293 cell line and increased translation of ATF4 in the BHK cells. Mammalian cells infected with Sindbis virus are known to activate PKR (double-stranded RNA (dsRNA)-activated protein kinase) due to the Sindbis virus dsRNA (Gale and Katze, 1998). PKR activation leads to phosphorylation of eIF-2αresulting in inhibition of protein synthesis (Wu and Kaufman, 1997). Nevertheless, this study shows that the ER stress activation is also present from PERK activation as another pathway to phosphorylate eIF-2α. The requirement to produce, glycosylate, and oligomerize high levels of the envelope proteins E1 and E2 in the secretory apparatus may lead to accumulation of significant levels of unfolded proteins that can trigger an unfolded protein response (UPR) in the resulting cell lines. The UPR may ultimately leads to the activation of the apoptosis cascade in some cell lines.
The presence of the P726S mutation may lower the ER stress level as the ATF4 translation was observed to be lower for 633-P726S and the TE-P726S viruses compared to the 633 and TE viruses in infected BHK cells. An earlier study with the Sindbis virus replicon showed that BHK cells infected with the P726S mutated virus SIN-1 produced about twofold less viral RNA in comparison to the virus Toto1101 (Dryga et al., 1997). Less viral RNA may result in smaller amount of viral proteins the host cells must process and therefore alleviate the ER stress activation. However, metabolic labeling of the infected cells in the current study indicated that the amount of viral protein synthesized at 16 hours p.i. was comparable between the 633, TE, and their P726S variants (data not shown). Hence, at least during the initial phase of infection, there was no significant difference in the ability to produce viral proteins of the 633 and TE, and their P726S variant viruses.
In addition, the nsP2 protein has been proposed to play a role in virus-host cell interactions and regulation of host cell transcription (Garmashova et al., 2006). The nsP2 protein expression is cytotoxic and inhibits host cell transcription, and the P726L and P726G mutations were shown to reduce these effects (Garmashova et al., 2006). Hence, the P726S mutation in the nsP2 protein may alleviate the host cell transcription downregulation leading to reduced cytotoxicity. Thus, either the reduction in viral protein processing and/or the increase in host cell transcription due to the P726S mutation that was shown to alleviate ER stress in combination with the expression of the anti-apoptotic gene Bcl-Δmay lead to the elimination of cytotoxicity of the virus and stable expression of recombinant protein.
Significant increases in GFP production were detected in the BHK and CHO Bcl-2 and Bcl-Δcell lines infected with the TE-P726S virus. As observed in Fig. 6b, however, the ATF4 response was lowest in the cells infected with the TE-P726S virus. It is known that ATF4 binds to the promoter of the C/EBP-homologous protein (CHOP), leading to its induction (Ma et al., 2002). CHOP, also known as growth arrest and DNA-damage-inducible gene 153 (GADD153), is a transcription factor that induces growth arrest and apoptosis through the pro-apoptotic Bim protein (Zinszner et al., 1998; Puthalakath et al., 2007). Overexpression of Bcl-2 has been shown to inhibit CHOP induction and suppress apoptosis in cells infected with the Japanese Encephalitis virus (JEV) (Su et al., 2002). If the expression of Bcl-2 or Bcl-Δalso inhibits activation of ER stress response genes in cells infected with Sindbis virus, then its expression could mitigate activation of the PERK gene responsible for inhibiting protein translation. In this way, overexpression of Bcl-2 and Bcl-Δwould allow for continued translation of heterologous protein, leading to the accumulation of higher levels of GFP as observed in the current study. Interestingly, however, the expression of Bcl-2 does not appear to block cell death in BHK cells, perhaps because of alternative pathways for activation of apoptosis or other cell death pathways, which may be triggered by allowing for continued expression of viral and recombinant proteins. In contrast, the shut-down of protein synthesis in wild-type BHK cells may allow for a more rapid recovery of viability observed following the 633-P726S and TE-P726S infections. The increases in both survival and GFP expression in the CHO Bcl-2 and Bcl-Δcell lines when infected with the TE-P726S virus may be due to a combined effect of the modified virus and anti-apoptotic genes reducing ER stress to allow sustained translation while also blocking cell death pathways effectively.
Given the activation of the ER stress pathways as indicated by PERK activation and ATF4 translation, we examined the capacity to use Parkin, a selective inhibitor of ER stress induced cell death, as another method for mitigating ER stress. Parkin's mechanism of action involves targeting misfolded proteins for proteasomal degradation (Imai et al, 2000). Incorporation of the Parkin gene within the Sindbis virus was as effective as BclxL in blocking cell death to provide additional evidence that ER stress is one of the contributing pathways to apoptosis in Sindbis infected cell lines (Fig. 7a). Similarly, Parkin expression improved survival of mice infected with the Sindbis virus at a level comparable to that obtained with expression of a caspase inhibitor p35 (Ryan et al., 2002). These findings suggest that in vivo infection with Sindbis virus also activates an ER stress response pathway leading to cell death but the activation of this pathway can be reduced by blocking apoptosis or by blocking the activation of the ER stress response through Parkin or other potential agents. The ability to manipulate the cell death pathway in vivo may be used to alter particular gene therapy vehicles and protocols in order to allow continued transgene expression while having cell survival in tissues that would otherwise activate the cell death pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agapov EV, Frolov I, Lindenbach BD, Pragai BM, Schlesinger S, Rice CM. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc Natl Acad Sci U S A. 1998;95(22):12989–94. doi: 10.1073/pnas.95.22.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorsma M, Saudan P, Pfruender H, Bailey JE, Schlesinger S, Renner WA, Bachmann MF. Alphavirus cDNA-based expression vectors: effects of RNA transcription and nuclear export. Biotechnol Bioeng. 2003;81(5):553–62. doi: 10.1002/bit.10496. [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22(53):8608–18. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993;67(11):6439–46. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. Embo J. 1997;16(5):968–77. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996;379(6565):554–6. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Dropulic LK, Hardwick JM, Griffin DE. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J Virol. 1997;71(8):6100–5. doi: 10.1128/jvi.71.8.6100-6105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryga SA, Dryga OA, Schlesinger S. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology. 1997;228(1):74–83. doi: 10.1006/viro.1996.8364. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr., Sauerwald TM, Mastrangelo AJ, Hardwick JM, Betenbaugh MJ. Comparison of Bcl-2 to a Bcl-2 deletion mutant for mammalian cells exposed to culture insults. Biotechnol Bioeng. 2001;73(3):211–22. doi: 10.1002/bit.1053. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr., Sauerwald TM, Oyler GA, Hardwick JM, Betenbaugh MJ. A comparison of the properties of a Bcl-xL variant to the wild-type anti-apoptosis inhibitor in mammalian cell cultures. Metab Eng. 2003;5(4):230–45. doi: 10.1016/s1096-7176(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr., Chen S, Oyler GA, Hardwick JM, Betenbaugh MJ. Aven and Bcl-xL enhance protection against apoptosis for mammalian cells exposed to various culture conditions. Biotechnol Bioeng. 2004;85(6):589–600. doi: 10.1002/bit.10913. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Jr., Ailor E, Osbourne D, Hardwick JM, Reff M, Betenbaugh MJ. Enhanced cell culture performance using inducible anti-apoptotic genes E1B-19K and Aven in the production of a monoclonal antibody with Chinese Hamster Ovary cells. Biotechnol Bioeng. 2007;97(4):877–892. doi: 10.1002/bit.21222. [DOI] [PubMed] [Google Scholar]
- Frolov I, Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994;68(3):1721–7. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov I, Hoffman TA, Pragai BM, Dryga SA, Huang HV, Schlesinger S, Rice CM. Alphavirus-based expression vectors: strategies and applications. Proc Natl Acad Sci U S A. 1996a;93(21):11371–7. doi: 10.1073/pnas.93.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov I, Schlesinger S. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J Virol. 1996b;70(2):1182–90. doi: 10.1128/jvi.70.2.1182-1190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov I, Agapov E, Hoffman TA, Jr., Pragai BM, Lippa M, Schlesinger S, Rice CM. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J Virol. 1999;73(5):3854–65. doi: 10.1128/jvi.73.5.3854-3865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Jr., Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78(1):29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- Garmashova N, Gorchakov R, Frolova E, Frolov I. Sindbis virus nonstructural protein nsP2 is cytotoxic and inhibits cellular transcription. J Virol. 2006;80(12):5686–96. doi: 10.1128/JVI.02739-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H, Li KJ. Recent advances in gene expression using alphavirus vectors. Curr Opin Biotechnol. 1998;9(5):464–9. doi: 10.1016/s0958-1669(98)80030-x. [DOI] [PubMed] [Google Scholar]
- Gorchakov R, Frolova E, Williams BR, Rice CM, Frolov I. PKR-dependent and -independent mechanisms are involved in translational shutoff during Sindbis virus infection. J Virol. 2004;78(16):8455–67. doi: 10.1128/JVI.78.16.8455-8467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000a;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000b;6(5):1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hardwick JM. Virus-induced apoptosis. Adv Pharmacol. 1997;41:295–336. doi: 10.1016/s1054-3589(08)61063-7. [DOI] [PubMed] [Google Scholar]
- Huang HV, Rice CM, Xiong C, Schlesinger S. RNA viruses as gene expression vectors. Virus Genes. 1989;3(1):85–91. doi: 10.1007/BF00301989. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275(46):35661–4. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24(3):1365–77. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiver K, Merits A, Sarand I. Novel vectors expressing anti-apoptotic protein Bcl-2 to study cell death in Semliki Forest virus-infected cells. Virus Res. 2008;131(1):54–64. doi: 10.1016/j.virusres.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Knight R, Smit JM, Wilschut J, Griffin DE. A single mutation in the E2 glycoprotein important for neurovirulence influences binding of sindbis virus to neuroblastoma cells. J Virol. 2002;76(12):6302–10. doi: 10.1128/JVI.76.12.6302-6310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Griffin DE. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67(11):6872–5. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Goldman JE, Jiang HH, Griffin DE, Hardwick JM. Bc1-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci U S A. 1996;93(10):4810–5. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Oyler GA, Ueno K, Fannjiang YR, Chau BN, Vornov J, Korsmeyer SJ, Zou S, Hardwick JM. Inhibition of virus-induced neuronal apoptosis by Bax. Nat Med. 1999;5(7):832–5. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991;9(12):1356–61. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275(32):24881–5. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Alphaviruses as expression vectors. Curr Opin Biotechnol. 1997;8(5):578–82. doi: 10.1016/s0958-1669(97)80032-8. [DOI] [PubMed] [Google Scholar]
- Lundstrom K, Pralong W, Martinou JC. Anti-apoptotic effect of Bcl-2 overexpression in RIN cells infected with Semliki Forest virus. Apoptosis. 1997;2(2):189–91. doi: 10.1023/a:1026416515575. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Biology and application of alphaviruses in gene therapy. Gene Ther. 2005;12(Suppl 1):S92–7. doi: 10.1038/sj.gt.3302620. [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266(26):17067–71. [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318(5):1351–65. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Mastrangelo AJ, Zou S, Hardwick JM, Betenbaugh MJ. Antiapoptosis chemicals prolong productive lifetimes of mammalian cells upon Sindbis virus vector infection. Biotechnol Bioeng. 1999;65(3):298–305. [PubMed] [Google Scholar]
- Mastrangelo AJ, Hardwick JM, Bex F, Betenbaugh MJ. Part I. Bcl-2 and Bcl-x(L) limit apoptosis upon infection with alphavirus vectors. Biotechnol Bioeng. 2000;67(5):544–54. [PubMed] [Google Scholar]
- Nava VE, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM. Sindbis virus induces apoptosis through a caspase-dependent CrmA-sensitive pathway. J Virol. 1998;72(1):452–9. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ryan CA, Stennicke HR, Nava VE, Burch JB, Hardwick JM, Salvesen GS. Inhibitor specificity of recombinant and endogenous caspase-9. Biochem J. 2002;366(Pt 2):595–601. doi: 10.1042/BJ20020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwald TM, Betenbaugh MJ, Oyler GA. Inhibiting apoptosis in mammalian cell culture using the caspase inhibitor XIAP and deletion mutants. Biotechnol Bioeng. 2002;77(6):704–16. doi: 10.1002/bit.10154. [DOI] [PubMed] [Google Scholar]
- Sauerwald TM, Oyler GA, Betenbaugh MJ. Study of caspase inhibitors for limiting death in mammalian cell culture. Biotechnol Bioeng. 2003;81(3):329–40. doi: 10.1002/bit.10473. [DOI] [PubMed] [Google Scholar]
- Sauerwald TM, Figueroa B, Jr., Hardwick JM, Oyler GA, Betenbaugh MJ. Combining caspase and mitochondrial dysfunction inhibitors of apoptosis to limit cell death in mammalian cell cultures. Biotechnol Bioeng. 2006;94(2):362–72. doi: 10.1002/bit.20874. [DOI] [PubMed] [Google Scholar]
- Schlesinger S, Dubensky TW. Alphavirus vectors for gene expression and vaccines. Curr Opin Biotechnol. 1999;10(5):434–9. doi: 10.1016/s0958-1669(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Schroder M. The unfolded protein response. Mol Biotechnol. 2006;34(2):279–90. doi: 10.1385/MB:34:2:279. [DOI] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002;76(9):4162–71. doi: 10.1128/JVI.76.9.4162-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Imai Y, Hattori N, Mizuno Y. Parkin and endoplasmic reticulum stress. Ann N Y Acad Sci. 2003;991:101–6. doi: 10.1111/j.1749-6632.2003.tb07467.x. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Fishman PS, Thakor NV, Oyler GA. Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J Biol Chem. 2003;278(24):22044–55. doi: 10.1074/jbc.M212235200. [DOI] [PubMed] [Google Scholar]
- Tucker PC, Strauss EG, Kuhn RJ, Strauss JH, Griffin DE. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67(8):4605–10. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubol S, Tucker PC, Griffin DE, Hardwick JM. Neurovirulent strains of Alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci U S A. 1994;91(11):5202–6. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva L, Merits A, Auvinen P, Kaariainen L. Identification of a novel function of the alphavirus capping apparatus. RNA 5'-triphosphatase activity of Nsp2. J Biol Chem. 2000;275(23):17281–7. doi: 10.1074/jbc.M910340199. [DOI] [PubMed] [Google Scholar]
- Weiss B, Rosenthal R, Schlesinger S. Establishment and maintenance of persistent infection by Sindbis virus in BHK cells. J Virol. 1980;33(1):463–74. doi: 10.1128/jvi.33.1.463-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272(2):1291–6. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–8. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243(4895):1188–91. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]