Abstract
Objectives
In this investigation, we compare two-dimensional (2D) fluid-attenuated inversion recovery (FLAIR) imaging of the brain to an isotropic three-dimensional (3D) FLAIR technique that uses a modulated refocusing flip angle echo train and parallel imaging with 2D acceleration.
Materials and Methods
Two-dimensional and 3D FLAIR sequences were obtained in 16 patients. All examinations were performed on a 3 Tesla (T) magnetic resonance (MR) system. Flow artifacts within the subarachnoid space and ventricles were scored using a 4-point scale. For 2D and 3D FLAIR, the signal-to-noise ratios and contrast-to-noise ratios were calculated.
Results
Compared to 2D FLAIR, the 3D FLAIR images were less degraded by flow artifacts in the subarachnoid space and ventricle (P < 0.03) based on the qualitative imaging scores. Signal-to-noise ratios and contrast-to-noise ratios were higher for 3D FLAIR (P < 0.02) for all variables when compared with 2D FLAIR sequence.
Conclusions
The acquisition time for whole brain isotropic fast spin echo 3D FLAIR can be dramatically reduced by using an extended echo train with flip angle modulation and parallel imaging. The adiabatic, nonselective inversion pulse encompasses the entire volume and provides uniform suppression of the cerebrospinal fluid signal eliminating cerebrospinal fluid pulsation artifacts. Other advantages include reformatting in any desired plane, volume measurements, displays of surface anatomy, and coregistration.
Keywords: isotropic three-dimensional fluid-attenuated inversion recovery, extended echo train, fast spin echo
Three-dimensional (3D) acquisitions have become increasingly important in clinical application because of the inherent signal-to-noise ratio (SNR) advantages, high resolution, and isotropic voxels. The advantages of 3D imaging have been well documented and include volumetric measurements, coregistration, displays of surface anatomy, and others.1 Volumetric isotropic imaging data can be reformatted to generate high quality, high spatial resolution images in any plane.2
For 3D T2-weighted fast spin echo imaging, varying the flip angles of the refocusing radiofrequency (RF) pulses allows the echo train length to be significantly increased without incurring excessive blurring.3,4 Initial reports of 3D fluid-attenuated inversion recovery (FLAIR) brain imaging using such a technique noted reduced pulsation artifacts and excellent SNR compared with two-dimensional (2D) FLAIR imaging but the imaging time for these examinations was long (8 minutes) even with relatively thick slices (3 mm).5 More recent reports demonstrate thinner slices (1 mm)6 and shorter scan times (7.1 minute).7
In this report, we compare a commonly used 2D FLAIR technique to a recently developed 3D FLAIR technique.
MATERIALS AND METHODS
Three-dimensional FLAIR (also referred to in the literature as 3D-FSE-XETA) is a single-slab 3D fast spin echo (FSE) imaging sequence that applies modulated flip angle refocusing RF pulses to enable very long echo trains,8 an effective echo time calculation that corrects for reduced relaxation rates of stimulated echo contributions to the signal,4 auto-calibrating 2D-accelerated parallel imaging,9 a new 3D FSE view ordering method, and adiabatic broad-bandwidth inversion for homogeneous fluid suppression.
Using a protocol approved by the Institutional Review Board and Health Insurance Portability and Accountability Act Compliance Committee, 2D and 3D FLAIR examinations were obtained during the same scanning session in 16 patients. All examinations were performed on a 3 T MR system using an 8-channel head coil (Signa HDx; GE Healthcare, London, UK) and obtained after contrast administration (Omniscan 0.1 mm/kg). The imaging parameters are summarized in Table 1.
TABLE 1.
Imaging Parameters 3D XETA FLAIR and 2D FLAIR
| 3D FLAIR | 2D FLAIR | |
|---|---|---|
| Imaging options | IR, Zip2, Zip512 | Fast, no zip |
| psd name | Cube | FLAIR |
| TE | 136 ms 170 echoes | 136 ms |
| TI | 1882 ms | 2250 ms |
| TR | 6200 ms | 8500 ms |
| Bandwidth | ±31.25 kHz | ±31.25 kHz |
| FOV | 25 cm | 20 cm |
| Slice-thickness | 1.2 mm | 5.0 mm |
| Frequency resolution | 256 | 352 |
| Phase resolution | 256 | 192 |
| Locs/slab or no. slices | 128 | 28 |
| Nex | 1 | 1 |
| Phase FOV | 1 | 1 |
| Acceleration factor | 3.07 | 1.0 |
| Fat suppression | Yes | No |
| Scan time | 6:03 | 2:16 |
TE indicates echo time; FOV, field of view.
Image analysis was performed on a McKesson Horizon PACS workstation equipped with Vitrea software. For the qualitative assessment of pulsation artifacts, the examinations were randomly presented and scored on a 4-point scale. Each evaluation session contained 2D and 3D datasets. The right or left lateral ventricle, fourth ventricle, suprasellar cistern, and prepontine cistern were scored on a four-point scale (1 = no pulsation artifacts, 2 = minimal pulsation artifacts, 3 = moderate pulsation artifacts, and 4 = severe pulsation artifacts that obscure adjacent structures).
SNR and Contrast-to-Noise Ratio
The 3D data were reformatted into 5 mm thick coronal slices to match the slice thickness of the 2D examination. Quantitative analysis included measurements of the SNR for cerebrospinal fluid (CSF), temporal lobe cortex (gray matter), and corpus callosum (CC) (white matter), and CNR for cortex to CC, cortex to CSF, and CC to CSF for both techniques. Signal intensities from the cortex, CC, and the ventricle were measured using a circular region of interest 4 mm in diameter. SNR for cortex, CC, and ventricle were calculated by dividing the signal level by the standard deviation in both 2D and 3D images.10 Contrast-to-noise ratio (CNR) was calculated by subtracting the ventricle SNR from the cortex, CC SNR from the cortex, and ventricle from the CC in these 2 sets of images. Images were compared using a paired sample Student t test. A P value of less than 0.05 was considered significant.
RESULTS
The qualitative analysis indicated that the 3D FLAIR images were less degraded by pulsation artifacts based on the imaging scores (P < 0.03).
Wilcoxon rank sum test was used to test the difference for 4 signal intensity variables independently. The result shows that the P values were <0.01 for lateral ventricle, fourth ventricle, prepontine cistern, and suprasellar cistern, respectively. This indicates that pulsation artifacts for 3D were significantly less than 2D for all 4 variables (Fig. 1). As shown in Figure 1B, the image signal intensity drops off from cranial to caudal; this can be corrected by normalization of the data.
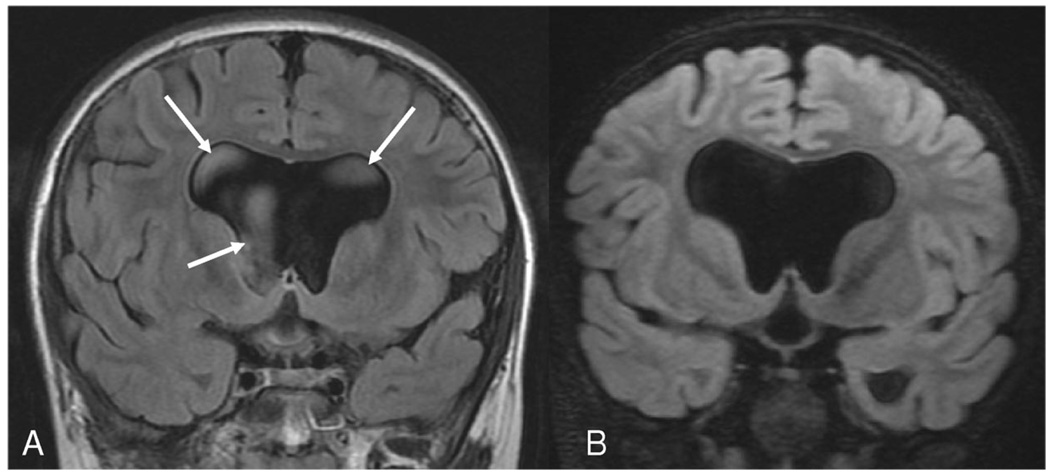
Figure 1.
Hydrocephalous secondary to aqueductal stenosis: (A) Coronal 2D FLAIR and (B) reformatted coronal 3D FLAIR images at similar locations. Note the CSF pulsation artifacts in the 2D FLAIR image (arrows) that are reduced in 3D FLAIR by the nonselective inversion pulse.
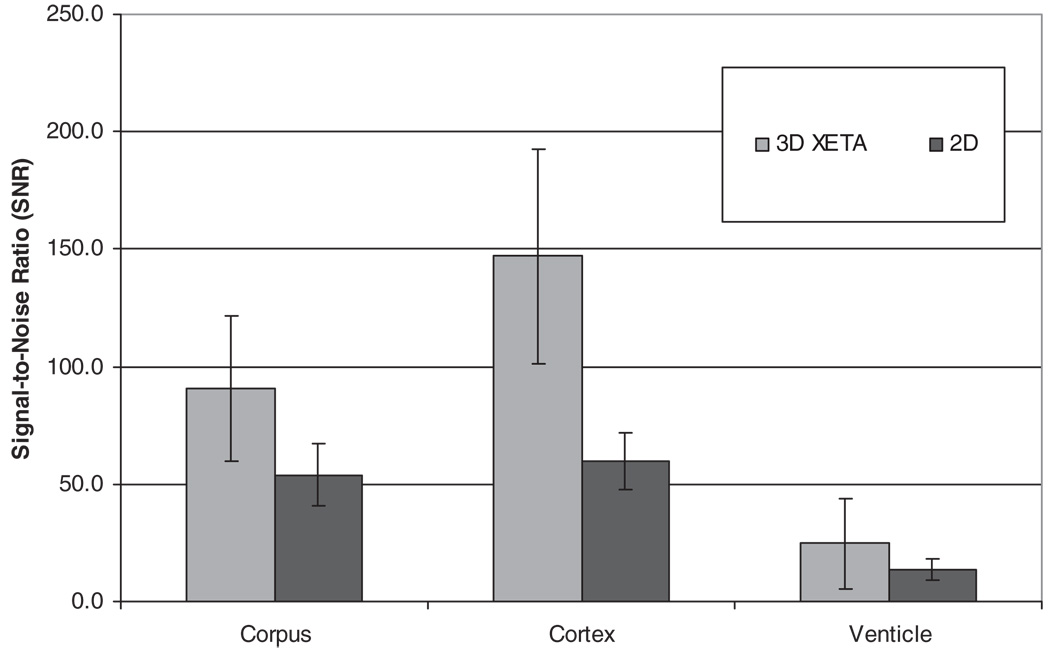
Cortex SNR was statistically higher with 3D FLAIR [147.1 ± 45.6 (SD)]) than with 2D FLAIR (59.4 ± 12.1, P < 0.001). Ventricular SNR was significantly higher with 3D FLAIR (24.7 ± 19.1) than with 2D FLAIR (13.7 ± 4.3, P < 0.003). CC SNR was also higher with 3D FLAIR (90.4 ± 30.9) than with 2D FLAIR (53.8 ± 13.1, P < 0.0016) (Fig. 2).
Figure 2.
Bar graphs showing SNR for 3D FLAIR and 2D FLAIR. Differences in SNR were all significant with P < 0.03.
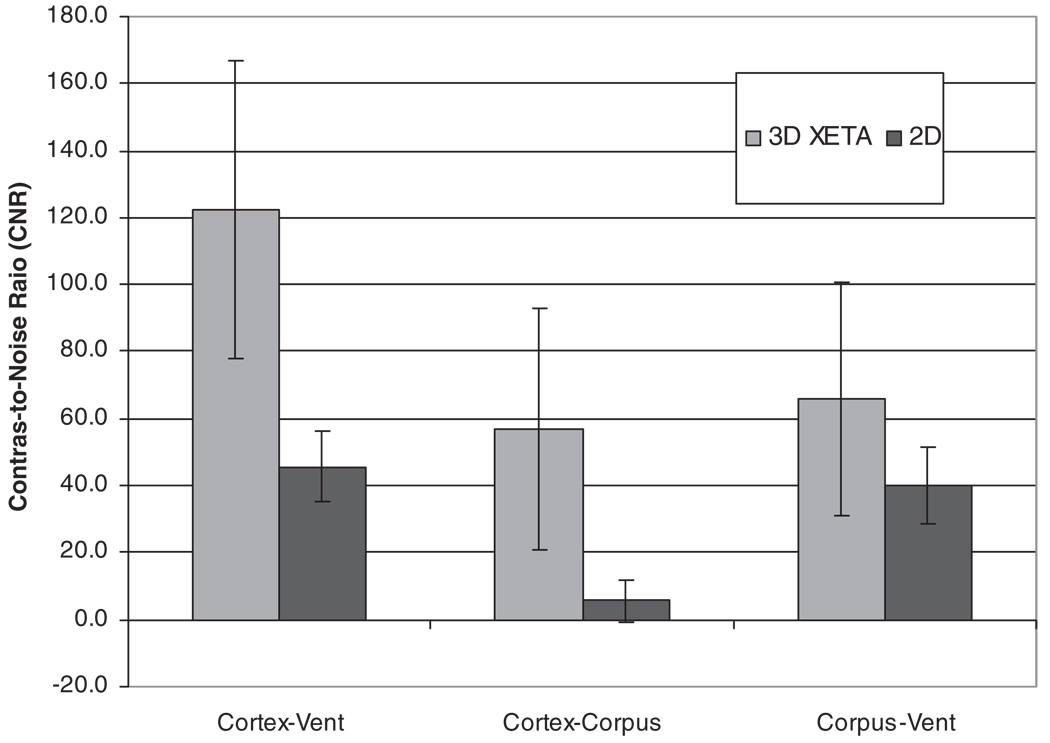
Cortex-ventricle CNR was significantly higher with 3D FLAIR (122.4 ± 44.4) than with 2D FLAIR (45.7 ± 10.6, P < 000.1). CC-cortex CNR was also higher with 3D FLAIR (56.7 ± 36.0) than with 2D FLAIR (5.6 ± 6.3, P < 0.0001). The CNR between the CC and ventricle was higher with 3D FLAIR (65.7 ± 34.9) than for 2D FLAIR (40.1 ± 11.2, P < 0.025) (Fig. 3).
Figure 3.
Bar graphs showing CNR for 3D FLAIR and 2D FLAIR. All differences in CNRs were significant with P < 0.025.
The 3D FLAIR technique yielded significantly higher SNR and CNR values when compared with 2D FLAIR, but the small sample size and relatively large standard deviations make this difference less statistically robust. It should be noted that the acquisition time for 3D FLAIR is approximately 6 minutes versus 2 minutes 16 seconds for the 2D FLAIR and that this difference would partially account for the increased SNR of 3D FLAIR.
DISCUSSION
Because long repetition times (TRs) are required for T2-weighted FLAIR imaging, single-slab 3D techniques would be prohibitively long if not for a number of recent advances. First, modulating the flip angle of the refocusing RF pulses enables greatly increased echo train length while constraining signal modulation. Second, parallel imaging with acceleration in multiple dimensions reduces the number of echoes required to encode a 3D dataset. Third, a new 3D-FSE view ordering technique allows the corners of k-space to be skipped, because they are unneeded for isotropic resolution, further reducing the number of echoes. Together, the scan time is reduced by over 10-fold, compared with conventional, unaccelerated 3D-FSE. By constraining signal modulation and ordering views such that equal numbers of echoes are sampled before and after the center of k-space (echo train length is tied to the prescribed echo time), blurring is comparable to 2D FSE with much shorter echo trains.
A significant advantage of the adiabatic nonselective inversion pulse is uniform CSF signal suppression.11 Artifacts in 2D FLAIR examinations related to CSF pulsation, inflow enhancement, and susceptibility have been described previously.12–14 Kallmes et al5 reported that high signal intensity CSF-related flow artifacts in the posterior fossa within the fourth ventricle were significantly reduced when a single-slab 3D pulse sequence was used. This is consistent with our study that shows marked reduction in CSF flow artifacts calculated in the region of lateral ventricle, fourth ventricle, subarachnoid space, and suprasellar cistern. Naganawa et al15 emphasized that CSF flow artifacts were significantly more common on 2D FLAIR images than on 3D FLAIR images in all regions except the sylvian fissures. Previous studies of 3D FLAIR reported CSF to be not completely dark, attributed to shorter TRs and inversion time (TIs).6 With the 3D FLAIR sequence used in this study, CSF was completely dark because the automatically calculated TI time accounted for the shorter TR and long echo train length.
Another significant feature of the 3D FLAIR sequence used in this study is a very uniform fat suppression that produces an image dataset in which the brain has the highest signal intensity. Because fat suppression is only applied once per TR in a single-slab 3D acquisition during the otherwise “dead” TI recovery period, an adiabatic spectrally selective inversion pulse is used with a recovery period timed to null fat. This greatly improves segmentation and 3D surface rendering (Fig. 4). Three-dimensional models are useful for surgical planning and diagnostic evaluation of large MR imaging and computed tomography image datasets. The models permit the creation of 2D and 3D views of brain anatomy and to interactively navigate within these images to better visualize and understand the internal structures and pathologic processes.16 In Figure 5, a reformatted 3D FLAIR image is compared with the reformatted 2D FLAIR image demonstrating the advantages of isotropic voxels. Three-dimensional surface rendering can display the distribution of cortical gray matter loss that allows the differentiation of Alzheimer disease form dementia with Lewy bodies.17 It also helps in the assessment of volume of gliotic change related to ischemia and radiation.18
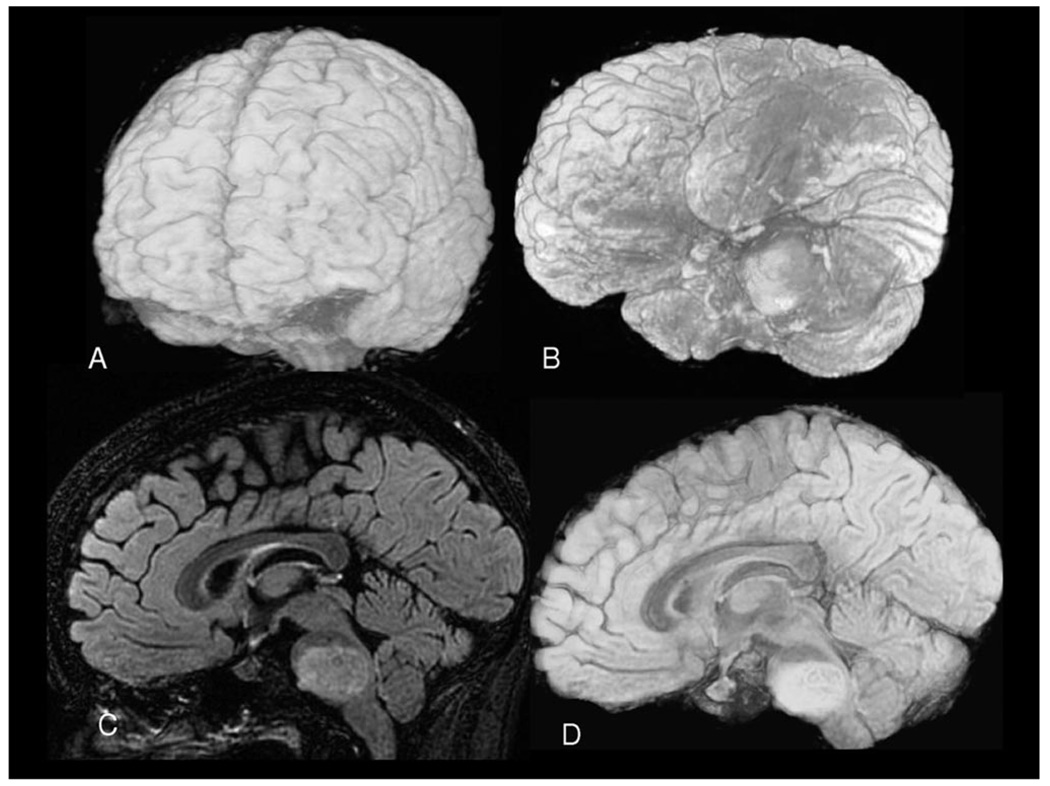
Figure 4.
Three-dimensional FLAIR images of the brain. Three-dimensional surface anatomy (A and B). Sagittal slice (C) and midsagittal cut (D) of a volume rendered image revealing the pontine gliomas. The volume of the pontine gliomas was 14.8 cm3.
Figure 5.
Axial reformatted images from (A) 2D FLAIR and (B) 3D FLAIR acquisition.
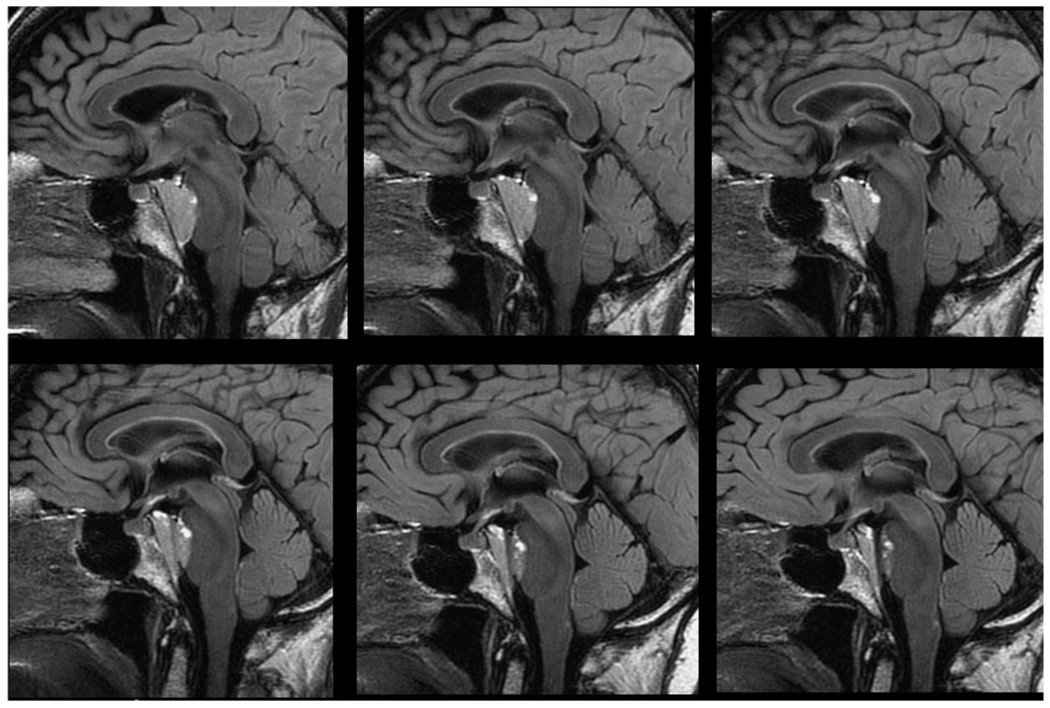
The 3D volume measurements of brain neoplasms can be serially measured to assess the response to therapy. Follow-up studies can be coregistered to facilitate the comparison of examinations. This approach allows the calculation of tumor volume, even for highly irregular tumors with significant necrosis and discontinuous shapes. It can also calculate the ratio between solid and necrotic components of the tumor. In a study done by Esposito et al,19 pre- and postoperative volume analyses of the skull base were considered essential preoperative planning and postoperative radiation therapy. Volumetric analyses have been reported to have value in pediatric brain neoplasms.20 Warren et al21 compared 1D, 2D, and 3D measurements of childhood brain tumors pre- and postchemotherapy to evaluate the treatment response. The 3D calculation of tumor volume was the most accurate method to measure the tumor size and monitor tumor growth. They proposed that the volume of irregular-shaped tumors could be more accurately measured without assuming a spherical shape. In some instances, hemorrhagic and cystic components of tumor were excluded form volumetric analyses to improve the assessment of tumor burden (Fig. 6).
Figure 6.
Six images from a 120 image sagittal 3D FLAIR acquisition. Note the exceptional delineation of the clival meningioma and pituitary stalk allowing for precise radiation treatment planning.
In general, FLAIR imaging provides outstanding lesion conspicuity in numerous central nervous system disorders with high CNR for long T2 lesions;22 3D further adds to its accuracy because of its high SNR and CNR compared with 2D sequences.23 In a recent report,24 the detection of multiple sclerosis (MS) lesions by 2D versus 3D single-slab FLAIR sequences at 3 T was compared. The authors reported that significantly higher CNR was achieved and more MS lesions were detected with the 3D FLAIR sequence when compared with 2D FLAIR sequence. Thus, 3D FLAIR can be considered as a promising technique for quantifying the extent of cerebral pathology in MS. Three-dimensional FLAIR data are nearly ideal for image guided radiotherapy and surgery.25
In conclusion, 3D FLAIR using a modulated refocusing flip angle echo train and parallel imaging with 2D acceleration provides improved CSF suppression with increase in the SNR and CNR when compared with 2D FLAIR techniques and produces isotropic data that may be visualized in any reformatted plane and used for surgical and radiation treatment planning.
REFERENCES
- 1.Mugler JP, Bao S, Mulkern RV, et al. Optimized single-slab three-dimensional spin echo MR imaging of the brain. Radiology. 2001;216:891–899. doi: 10.1148/radiology.216.3.r00au46891. [DOI] [PubMed] [Google Scholar]
- 2.Ghugre NR, Martin M, Scadeng M, et al. Superiority of 3D wavelet-packet denoising in MR microscopy. Magn Reson Imaging. 2003;21:913–921. doi: 10.1016/s0730-725x(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 3.Mugler JP, Kiefer B, Brookeman JR. Three-dimensional T2-weighted imaging of the brain using very long spin-echo trans. Proceedings of International Society for Magnetic Resonance in Medicine 8th Annual Meeting; Denver, CO. 2000. Apr 1–7, [Google Scholar]
- 4.Busse RF, Hariharan H, Vu A, et al. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55:1030–1037. doi: 10.1002/mrm.20863. [DOI] [PubMed] [Google Scholar]
- 5.Kallmes DF, Hui FK, Mugler JP., III Suppression of cerebrospinal fluid and blood flow artifacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: initial experience. Radiology. 2001;221:251–255. doi: 10.1148/radiol.2211001712. [DOI] [PubMed] [Google Scholar]
- 6.Naganawa S, Kawai H, Fukatsu H, et al. High-speed imaging at 3 Tesla: a technical and clinical review with an emphasis on whole-brain 3D imaging. Magn Reson Med Sci. 2004;3:177–187. doi: 10.2463/mrms.3.177. [DOI] [PubMed] [Google Scholar]
- 7.Mugler JP, Menzel MI, Horger W, et al. High-resolution, multi-contrast 3D imaging of the brain in 15 minutes. Proceedings of International Society for Magnetic Resonance in Medicine 13th Annual Meeting; Miami Beach, FL. 2005. May 7–13, [Google Scholar]
- 8.Busse RF, Brau ACS, Beatty PJ, et al. Design of refocusing flip angle modulation for volumetric 3D-FSE imaging of the brain, spine, knee, kidney, and uterus. Proceedings of International Society for Magnetic Resonance in Medicine 15th Annual Meeting; Berlin, Germany. 2007. May 19–25, [Google Scholar]
- 9.Beatty PJ, Brau ACS, Chang S, et al. A method for autocalibrating 2-D accelerated volumetric parallel imaging with clinically practical reconstruction times. Proceedings of International Society for Magnetic Resonance in Medicine 15th Annual Meeting; Berlin, Germany. 2007. May 19–25, [Google Scholar]
- 10.Gold GE, Busse RF, Beehler C, et al. Isotropic MRI of the knee with 3D fast spin-echo extended echo-train acquisition (XETA): initial experience. AJR Am J Roentgenol. 2007;188:1287–1293. doi: 10.2214/AJR.06.1208. [DOI] [PubMed] [Google Scholar]
- 11.Rydberg JN, Hammond CA, Grimm RC, et al. Initial clinical experience in MR imaging of the brain with a fast fluid attenuated inversion-recovery pulse sequence. Radiology. 1994;193:173–180. doi: 10.1148/radiology.193.1.8090888. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Rydberg CH, Krecke KN, et al. Mesial temporal sclerosis: diagnosis with fluid attenuated inversion recovery versus spin echo MR imaging. Radiology. 1996;199:367–373. doi: 10.1148/radiology.199.2.8668780. [DOI] [PubMed] [Google Scholar]
- 13.Bakshi R, Caruthers SD, Janardhan V, et al. Intraventricular CSF pulsation artifact on fast fluid-attenuation inversion-recovery MR images: analyses of 100 consecutive normal studies. AJNR Am J Neuroradiol. 2000;21:503–508. [PMC free article] [PubMed] [Google Scholar]
- 14.Bakshi R, Kamran S, Kinkel PR, et al. Fluid-attenuated inversion-recovery MR imaging in acute and subacute cerebral intraventricular hemorrhage. AJNR Am J Neuroradiol. 1999;20:629–636. [PMC free article] [PubMed] [Google Scholar]
- 15.Naganawa S, Koshikawa T, Nakamura T, et al. Comparison of flow artifacts between 2D-FLAIR and 3D-FLAIR sequences at 3T. Eur Radiol. 2004;14:1901–1908. doi: 10.1007/s00330-004-2372-7. [DOI] [PubMed] [Google Scholar]
- 16.Tubridy N, Barker GJ, Macmanus DG, et al. Three-dimensional fast fluid attenuated inversion recovery (3D fast FLAIR): a new MRI sequence which increases the detectable cerebral lesion load in multiple sclerosis. Br J Radiol. 1998;71:840–845. doi: 10.1259/bjr.71.848.9828796. [DOI] [PubMed] [Google Scholar]
- 17.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essig M, Wenz F, Schoenberg S, et al. Arteriovenous malformation: assessment of gliotic and ischaemic changes with fluid-attenuated inversion recovery MRI. Invest Radiol. 2000;35:689–694. doi: 10.1097/00004424-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Esposito F, Becker DP, Villablanca JP, et al. Endonasal transsphenoidal transclival removal of prepontine epidermoid tumors: technical note. Neurosurgery. 2005;56(2 suppl):E443. doi: 10.1227/01.neu.0000157023.12468.6a. discussion, E443. [DOI] [PubMed] [Google Scholar]
- 20.Poussaint TY, Phillips PC, Vajapeyam S, et al. The Neuroimaging Center of the Pediatric Brain Tumor Consortium—collaborative neuroimaging in pediatric brain tumor research: a work in progress. AJNR Am J Neuroradiol. 2007;28:603–607. [PMC free article] [PubMed] [Google Scholar]
- 21.Warren KE, Patronas N, Aikin AA, et al. Comparison of one-, two-, and three-dimensional measurement of childhood brain tumors. J Natl Cancer Inst. 2001;93:1401–1405. doi: 10.1093/jnci/93.18.1401. [DOI] [PubMed] [Google Scholar]
- 22.Weischmann UC, Barker GJ, Symms MR, et al. Fast fluid-attenuation inversion recovery imaging: first experience with a 3D version in epilepsy. Neuroradiology. 1998;40:483–489. doi: 10.1007/s002340050630. [DOI] [PubMed] [Google Scholar]
- 23.Tan IL, Pouwels PJ, van Schijndel RA, et al. Isotropic 3D fast FLAIR imaging of the brain in multiple sclerosis patients: initial experience. Eur Radiol. 2002;12:559–567. doi: 10.1007/s00330-001-1170-8. [DOI] [PubMed] [Google Scholar]
- 24.Bink A, Schmitt M, Gaa J, et al. Detection of lesions in multiple sclerosis by 2D FLAIR and single-slab 3D FLAIR sequence at 3.0 Tesla: initial results. Eur Radiol. 2006;16:1104–1110. doi: 10.1007/s00330-005-0107-z. [DOI] [PubMed] [Google Scholar]
- 25.Haller JW, Ryken TC, Vannier MW. Image-guided therapy: infrastructure for practical applications. Acad Radiol. 2001;8:888–897. doi: 10.1016/s1076-6332(03)80768-7. [DOI] [PubMed] [Google Scholar]