Abstract
Overexpression of Angiopoietin (Ang) 1 in the brain results in increased vascularization and altered neuronal dendrite configuration. We hypothesized that Ang1 acts directly on neurons inducing neurite outgrowth. We stimulated PC12 cells with Ang1 and observed outgrowth levels comparable to nerve growth factor (NGF). Western blotting and RT-PCR demonstrated the absence of the Ang1 receptor, Tie2 and the presence of β1-integrin. Downstream of β1-integrin, Ang1 stimulation led to a ~2.6 fold increase in focal adhesion kinase (FAK) phosphorylation and no change in activation of mitogen-activated protein kinase (MAPK) nor c-Jun N-terminal kinase (JNK). Conversely, NGF stimulation had no effect on FAK phosphorylation but lead to a ~3.1 and ~2 fold increase in phosphorylation of MAPK and JNK. Ang1, but not NGF-mediated outgrowth was attenuated following functional inhibition of β1-integrin and FAK, and Wortmannin inhibited neurite outgrowth mediated by both. Our results suggest that Ang1 induces neurite outgrowth in PC12 cells in a Tie2-independent, β1-integrin-FAK-PI3K-Akt dependent manner and that NGF and Ang1 mediate neurite outgrowth via two independent signaling mechanisms.
Keywords: Neurovascular, neurite outgrowth, nerve growth factor, angiopoietin, integrin, signaling
The nervous and vascular systems contain many common organizational features and develop similarly in terms of anatomical patterning. These similarities in patterning and proximity may reflect coordinated development based on responsiveness to similar growth factors and their receptors, including VEGF/VEGFR2, netrins/Unc5, Ephs/ephrins; and semaphorins/neuropilin (Jones and Li, 2007, Zacchigna et al., 2008). Angiopoietins represent another family of growth factors that may mediate effects in both vascular and nervous systems (Ward and LaManna, 2004).
Ang1 is a growth factor that binds to its endothelial cell specific receptor Tie2, recruiting pericytes to developing vessels and stabilizing the vasculature (Eklund and Olsen, 2006, Morisada et al., 2006). Ex vivo work done by others (Kosacka et al., 2005, Kosacka et al., 2006) has suggested that Ang1 can elicit neurite outgrowth of explanted dorsal root ganglion (DRG) cells in a Tie2 dependent manner. Moreover, previous work done by our lab suggests that Ang1 overexpression in the mouse nervous system leads to increases in vascularization and altered neuronal dendrite patterning (Ward et al., 2004). The mechanisms underlying the dendritic effects remain unknown as we failed to find Tie2 expression on neurons in these studies. Based on work by others indicating that Ang1 could bind to β1 integrin (Carlson et al., 2001), we hypothesized that Ang1 binding to neuronal β1 integrin could induce neurite outgrowth. We used PC12 cells, a well established experimental model of sympathetic neurons (Greene and Tischler, 1976), and determined that Ang1 could promote cell survival and neurite outgrowth in a Tie2-independent, β1 integrin dependent manner, using signaling mechanisms separate and distinct from those used by NGF. Our findings suggest that Ang1 can directly bind to PC12 cells and elicit neurite outgrowth and confirms previous observations suggesting that angiopoietins can mediate effects in both nervous and vascular systems (Acker et al., 2001, Ward et al., 2004, Kosacka et al., 2005, Bai et al., 2009).
Materials and Methods
Cell culture, differentiation and stimulation
PC12 cells were purchased from American Type Tissue Culture (CRL-1721; ATCC, Virginia, USA) and were cultured in F-12K medium with 15% horse serum and 2.5% fetal bovine serum supplemented with penicillin and streptomycin and grown at 37°C with 5% carbon dioxide. 40,000 cells/mL were plated on collagen, allowed to attach overnight and then pretreated with NGF (50ng/mL; R&D) for 2 days. To determine whether Ang1 could induce differentiation, undifferentiated cells were also stimulated using recombinant Ang1 (100ng/mL; multimerized using an anti-His cross linking antibody; R&D).
Neuronal survival and neurite outgrowth
Following NGF pretreatment, media was aspirated, cells washed with PBS, and media replaced with serum-free media, or serum-free media containing NGF (50ng/ml) or Ang1 (100ng/ml). To determine the effects on neuronal survival, cells were treated for 7 days, with the media being changed on days 2 and 4. Cell survival was quantified using a haemocytometer-based trypan blue exclusion assay described previously (Koh et al., 2003).
To study neurite outgrowth, NGF pretreated PC12 cells were stimulated with NGF, Ang1, or left in serum-free media for 0, 2, 4, or 7 days as described above. Cells were photographed using a 20X objective under phase contrast and 60 images per condition were taken, with each field containing ~10-20 cells/field of view. The neurite lengths of all cells within the field were measured using the interactive image analyses program, Image Pro (MediaCybernetics, Bethesda, Maryland). At least 50 cells were measured within each condition. All experiments were performed in triplicate at least three times.
Function blocking experiments
To study the mechanisms underlying neurite outgrowth, neurite outgrowth assays were performed as described above with the addition of function blocking antibodies against β1 integrin (5ug/ml; Chemicon, Clone JB1A), Tie2 (50ug/ml; R&D) or control IgG (50ug/ml); or with the PI3K inhibitor Wortmannin (100nM, EMD Biosciences) or the FAK inhibitor PF573228 (100nM, Tocris) for the duration of the assay. These reagents have all previously been reported to block biological activity of their respective targets (Emsley and Hagg, 2003, Kim et al., 2004, Radel and Rizzo, 2005, Ohab et al., 2006, Slack-Davis et al., 2007, Cui, 2009). For function blocking experiments in which Ang1 served as the matrix material itself, Ang1 (10μg) was used to coat individual plates prior to seeding the cells. Neurite outgrowth was measured following stimulation with serum containing media in the presence and absence of β1-integrin function blocking antibodies. All experiments were performed in triplicate at least two times.
SiRNA experiments
We utilized an SiRNA approach to inhibit β1 integrin function as an alternative method to using function blocking antibodies. Following 2 days of NGF pretreatment, predesigned Silencer® SiRNA against β1 integrin (sense: CGA UAG GUC CAA CGG CUU Att; antisense UAA GCC GUU GGA CCU AUC Gca) or a random sequence serving as a negative control (Silencer® Negative Control #1 siRNA; Applied Biosystems) was transfected into PC12 cells (50nM) using Lipofectamine 2000 (Invitrogen). Stimulating ligands were added 5 hours following transfection and neurite outgrowth was quantified 2 days following.
RT-PCR
In order to determine the presence or absence of gene expression in PC12 cells, RNA was isolated from undifferentiated and differentiated PC12 cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. First strand cDNA synthesis was achieved using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Samples were run in duplicate with one set undergoing RT and a second set containing no RT to control for possible genomic DNA contamination. PCR was completed on cDNA using primers specific for Tie2, Ang1, NGF, TrkA and β-Actin as described in Table 1. Mouse universal reference total RNA was used as the positive control and water was used as a negative control.
Table1.
Primer sequences used for RT-PCR
| Primer Target | Primer sequence | Product size |
|---|---|---|
| Tie2 | 5'-GAT TTT GGA TTG TCC CGA GGT CAA G-3' | 430bp |
| 5'-CAC CAA TAT CTG GGC AAA TGA TGG-3' | ||
| Ang1 | 5'-GCC TGG ATT TCC AGA GGG GCT GG-3' | 500bp |
| 5'-GGG CCG GAT CAT CAT GGT GGT GG-3' | ||
| NGF | 5'-TTT CAA CAG GAC TCA CCG G-3' | 220bp |
| 5'-TCT CAA CAG GAT TGG AGG C-3' | ||
| TrkA | 5'-GGT ACC AGC TCT CCA ACA CTG AGG-3' | 200bp |
| 5'-CCA GAA CGT CCA GGT AAC TCG GTG-3' | ||
| βActin | 5'-GTG GGC CGC TCT AGG CAC CAA-3' | 540bp |
| 5'-CTC TTT GAT GTC ACG CAC GAT TTC-3' | ||
Western blotting
Western blotting of treated and untreated PC12 cells was performed as described previously (Master et al., 2001, Derksen et al., 2007). Antibodies used included polyclonal anti-β1 integrin (R&D), anti-phospho-FAK(Tyr397 and Tyr925), anti-FAK, anti-phospho Akt, anti-Akt; anti-phospho-JNK/SAPK, anti-JNK/SAPK, anti-phospho p44/42 MAPK, anti-MAPK, anti-phospho NFκB, anti-NFκB, anti-phospho-Crk, and anti-Crk (all Cell Signaling, Danvers, MA). Equal loading of samples was confirmed by performing Western blotting using a monoclonal antibody against β-Actin (Clone AC-15, Sigma). Blots were reproduced using independent samples at least three times. Densitometry was performed using a densitometer (BIORAD) and Quantity One Software (BIORAD).
Results
Ang1 promotes PC12 cell survival and induces neurite outgrowth
To examine and define the potential role for Ang1 on PC12 cell differentiation, survival and neurite outgrowth we stimulated undifferentiated cells and differentiated cells for a period of up to 7 days with either media alone, or media containing Ang1 or NGF. Ang1 was unable to induce differentiation (data not shown) but was capable of promoting cell survival of already differentiated PC12 cells but significantly less than that induced by NGF (Ang1, 6102 ± 1283 cells vs. NGF, 19092 ± 1464 cells; p < 0.05 compared to unstimulated and compared with NGF stimulated). Ang1 induced significant outgrowth on the surviving cells compared to no sera controls and at similar levels as NGF at 2, 4 and 7 days following stimulation (p<0.05; Figure 1A, D). Under serum free conditions, total neurite outgrowth did not significantly change between Day 0 and Day 7 (Figure 1A). Analogous results were found for average neurite length under similar stimulation conditions (Figure 1B). Interestingly, we failed to observe significant NGF- or Ang1-induced increases in the average number of neurites per cell until 7 days post-stimulation (Figure 1C). At this time, both NGF and Ang1 conditions had significantly more neurites per cell than no sera controls (p<0.05), however at this timepoint decreases in the number of neurites/cell in the no sera control were found compared to Day 0 levels (p=0.047; Figure 1C).
Figure 1. NGF and Ang1 induce PC12 cell neurite outgrowth.
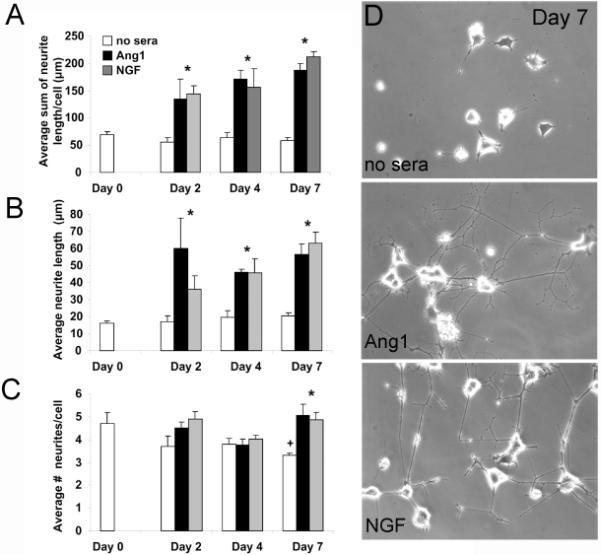
PC12 cells were pretreated with NGF for two days (day 0) and then stimulated for an additional_2, 4, or 7 days with either serum-free media, serum-free media containing NGF (50ng/ml) or Ang1 (100ng/ml). NGF and Ang1 induced neurite outgrowth at significantly greater levels than serum free media alone as early as two days following stimulation, and at similar levels to each other (A, D). Similar findings were observed for average neurite length (B) and average number of neurites per cell (C). *p<0.05 compared to no sera controls; +p=0.047 compared to Day 0 no sera controls.
Tie2 is not expressed in PC12 cells
We previously failed to find Tie2 expression on neurons in vivo but were able to show that neurons expressed the putative angiopoietin receptor β1 integrin (Ward et al., 2004), therefore we used RT-PCR and Western blotting to determine whether undifferentiated and differentiated PC12 cells expressed Tie2 and β1 integrin respectively. Regardless of stimulation condition or differentiation state, PC12 cells did not express Tie2 mRNA (Figure 2A) but did express β1 integrin protein (Figure 2B). In order to rule out Ang1-mediated upregulation of NGF as a potential mechanism for eliciting neurite outgrowth, NGF RT-PCR was also completed. No differences were found between stimulation conditions. As an additional positive control to β-actin, we confirmed expression of the NGF receptor, TrkA under all stimulation conditions. These findings suggest that Ang1-mediated outgrowth occurs in a Tie-2 and NGF independent manner.
Figure 2. β1 integrin and not Tie2 is found in PC12 cells.
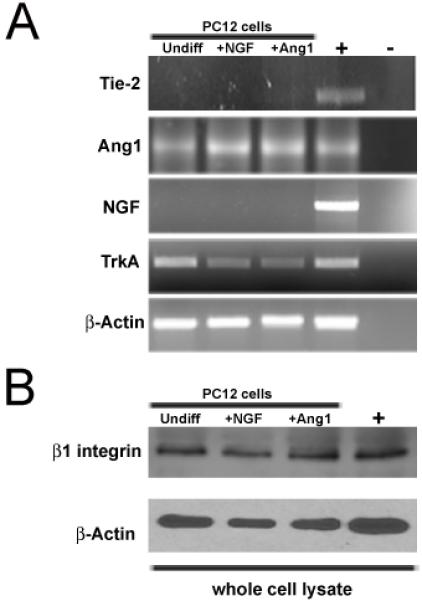
(A) RT-PCR analyses of undifferentiated, differentiated, and Ang1 stimulated PC12 cells for the presence of Tie2, Ang1, NGF, TrkA and β-actin. PC12 cells express Ang1 and TrkA, but not Tie2 or NGF. (B) Western blotting completed on whole cell lysate harvested from undifferentiated, differentiated, and Ang1 stimulated PC12 cells demonstrates the presence of β1 integrin. The positive control (+) in A represents mouse universal reference total RNA and in B is brain whole cell lysate; the negative control in A is water.
Ang1-mediated neurite outgrowth is Tie2 independent, β1-integrin dependent
In order to identify the mechanisms underlying Ang1-mediated neurite outgrowth, we performed neurite outgrowth assays in the presence and absence of function blocking antibodies against β1 integrin and Tie2 (as an additional negative control). As before, we found a ~3 fold increase in neurite outgrowth following stimulation with Ang1 ligand. This observation was maintained even in the presence of Tie2 function blocking antibodies (Figure 3A), consistent with the RT-PCR results demonstrating the absence of Tie2 receptor in these cells (Figure 2A). In contrast, following the addition of function blocking β1 integrin antibodies, neurite outgrowth was attenuated. The addition of blocking β1 integrin antibodies to NGF stimulated conditions had no effect, consistent with NGF-mediated outgrowth occurring in a β1 integrin-independent manner.
Figure 3. Ang1-mediate neurite outgrowth occurs in a Tie2-independent, β1 integrin dependent manner.
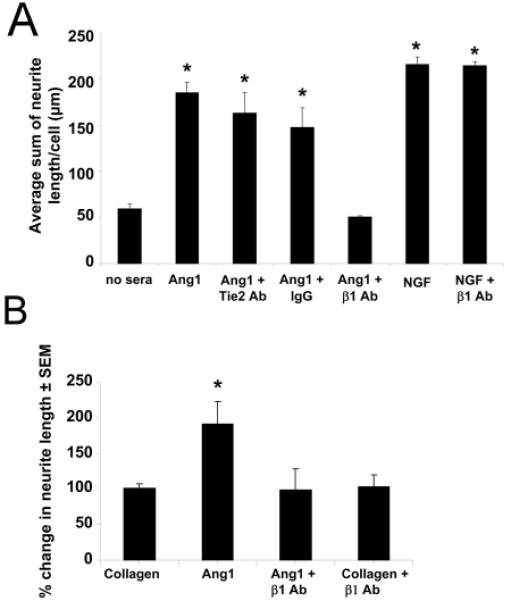
PC12 cells were pretreated with NGF for two days and then stimulated with Ang1 or NGF for an additional 7 days in the presence or absence of function blocking antibodies. (A) Ang1-mediated neurite outgrowth remained unaffected following the addition of Tie2 function blocking antibodies and isotype IgG, but was reduced to basal levels following the addition of function blocking antibodies against β1 integrin. NGF-mediated outgrowth remained unaffected following the addition of β1 integrin antibodies. (B) Neurite outgrowth assays were performed on plates coated with Ang1 or collagen in the presence and absence of β1 integrin function blocking antibodies using normal growth media over a period of 7 days. Neurite outgrowth was not changed between collagen and collagen and β1-integrin treated conditions, whereas Ang1 coating increased neurite length which was prevented in the presence of β1-integrin antibodies. The β1 integrin blocking antibody was not able to elicit neurite outgrowth on its own. *p<0.01 compared to no sera controls.
β1 integrin can serve as receptor for collagen; therefore to eliminate the possibility that the attenuation of neurite outgrowth observed in the Ang1-β1 integrin function blocking experiments did not simply reflect loss of neuronal β1 integrin interactions with the collagen matrix, rather than an inhibition of Ang1-β1 integrin interactions, we coated plates with Ang1 ligand or collagen alone, plated cells, and examined outgrowth in the presence and absence of β1 integrin function blocking antibodies using normal growth media. This allowed us to determine the significance of interrupting β1 integrin-collagen interactions compared with β1 integrin-Ang1 interactions on neurite outgrowth. No change in neurite outgrowth was found between collagen and collagen and β1-integrin treated conditions, confirming loss of β1 integrin-collagen interactions were not responsible for the reduction in neurite outgrowth we observed following Ang1 stimulation; however we again found Ang1-mediated increases in neurite length were prevented in the presence of β1 integrin function blocking antibodies (Figure 3B). Together these findings suggest that Ang1-β1 integrin interactions lead to PC12 cell neurite outgrowth, that functional inhibition of β1 integrin attenuates this effect, and that this effect is not due to impaired neuronal β1 integrin-collagen interactions.
Ang 1 and NGF utilize different signaling mechanisms to induce PC12 cell neurite outgrowth
To characterize and differentiate Ang1-β1 integrin signaling pathways, we used western blotting to analyze expression and activation of FAK, MAPK, Akt, NFκB, and Crk, all known downstream signaling partners of β1 integrin (Giancotti and Tarone, 2003, Rüegg and Mariotti, 2003). Ang1 had no effect on MAPK activation/phosphorylation, whereas NGF elicited a ~3.1 fold increase (p<0.05) which was not attenuated following the addition of β1 integrin function blocking antibodies (Figure 4A). Conversely, Ang1 induced a ~2.6 fold increase in FAK phosphorylation at tyrosine residue 397 (p< 0.05) whereas NGF had no effect (Figure 4C). This effect was specific for FAK (Tyr397) as no differences in phosphorylation of FAK (Tyr925) were observed (data not shown). Addition of β1 integrin-specific function-blocking antibodies either in the presence of Ang1 or NGF, or alone also lead to ~3-4 fold increase in FAK (Tyr397)-activation, indicative perhaps that this soluble β1 integrin blocking antibody can activate outside in signaling, although the β1 integrin blocking antibody was not able to elicit neurite outgrowth on its own (Figure 3B). Ang1 was also capable of eliciting statistically significant increases in activation of Akt (Figure 4D; p<0.05). With the addition of β1 integrin antibodies, Akt phosphorylation levels decreased, although they did not differ significantly from either control or Ang1 stimulated conditions. NGF, but not Ang1 induced increases (~2 fold) in JNK-phosphorylation (Figure 4B), which was diminished following addition of β1 integrin function blocking antibodies, despite the lack of effect this combination had on neurite outgrowth (Figure 3A). For either NGF or Ang1, no changes in activation or expression were observed for Crk or NFκB (data not shown).
Figure 4. Ang1 and NGF mediate neurite outgrowth using different signaling pathways.
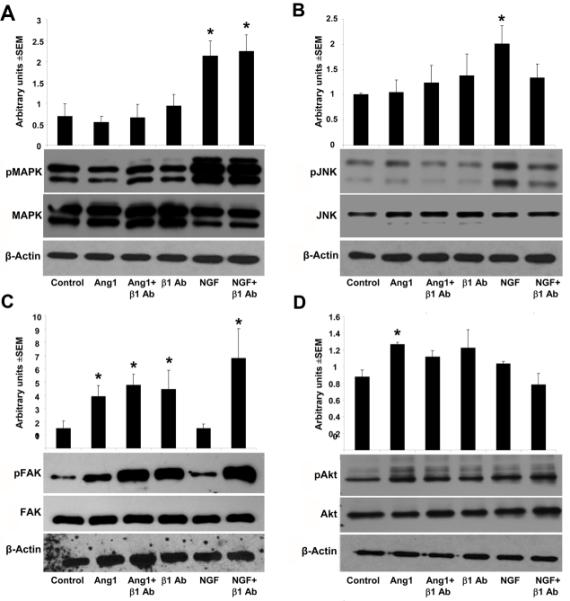
Western blot analyses and densitometry quantification of downstream β1 integrin signaling partners. NGF and not Ang1 induces a ~3.1 and ~2 fold increase in MAPK (A) and JNK phosphorylation (B) respectively; the addition of β1 integrin function blocking antibodies attenuates NGF-induced JNK activation only. Ang1, but not NGF, induced a ~2.6 fold increase in FAK phosphorylation at tyrosine residue 397 (C). Addition of β1 integrin-specific function-blocking antibodies either in the presence of Ang1 or NGF, or alone also lead to ~3-4 fold increase in FAK (Tyr397)-activation. Ang1 elicited small statistically significant increases in activation of Akt (D) which were decreased following addition of β1 integrin antibodies. *p<0.05.
Inhibition of β1 integrin, FAK and PI3K attenuate Ang1-mediated neurite outgrowth
To confirm Ang-1-β1 integrin signaling, we transfected NGF pretreated PC12 cells with siRNA targeting β1 integrin or a random sequence and performed neurite outgrowth analyses. The efficacy of the siRNA approach was confirmed by measuring β1 integrin protein expression with Western blots. β1 integrin was decreased at 2, 5 and 7 days following transfection (Figure 5A). As predicted, we found a concomitant decrease in Ang1-mediated, but not NGF-mediated neurite outgrowth in cells transfected with β1 integrin siRNA (Figure 5B) confirming results from our function blocking antibody experiments (Figure 3A).
Figure 5. SiRNA targeting β1 integrin or functional inhibition of FAK or PI3K signaling prevents Ang1 mediated neurite outgrowth of PC12 cells.
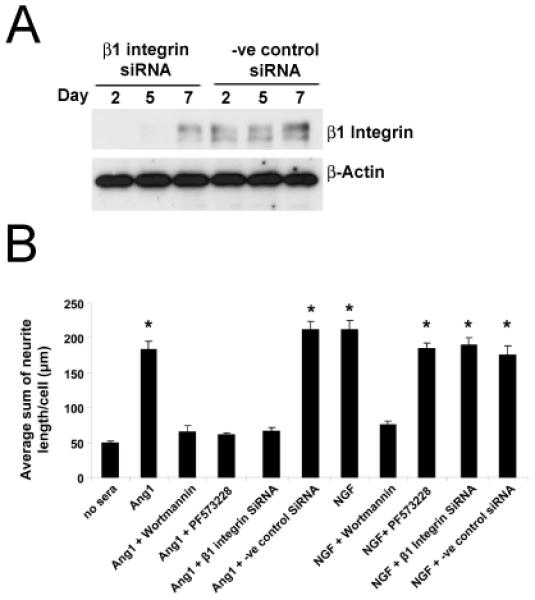
PC12 cells pretreated with NGF for two days were transfected either with Predesigned Silencer® SiRNA against β1 integrin or a negative control random sequence and western blot analyses were completed to confirm decreased β1 integrinexpression at 2, 5, and 7 days post SiRNA transfection (A). Ang1- and NGF-mediated neurite outgrowth assays were then performed in the presence and absence of either the PI3K inhibitor, Wortmannin, the FAK inhibitor PF573288 or following transfection with the SiRNA targeting β1 integrin or a negative control random sequence (B). After two days of stimulation, Ang1-mediated neurite outgrowth was reduced to no sera levels in the presence of Wortmannin, PF573288, and β1 integrin SiRNA (B). NGF-mediated neurite outgrowth was only reduced in the presence of Wortmannin. *p<0.01 compared to no sera controls.
We observed significant increases in FAK and Akt phosphorylation following Ang1 stimulation (Figure 4C-D); therefore neurite outgrowth experiments were completed in the presence or absence of the FAK (Tyr397 specific) inhibitor, PF573228 (Slack-Davis et al., 2007) or the PI3K inhibitor, Wortmannin. As before (Figures 1 and 3), we confirmed a ~3 fold increase in neurite outgrowth following stimulation with either Ang1 or NGF (Figure 5B). In the presence of the Fak inhibitor, PF573228, Ang1-mediated outgrowth was reduced to no sera control levels; whereas NGF mediated outgrowth remained unaffected. In the presence of Wortmannin, both NGF and Ang1-mediated outgrowth were reduced to basal levels (Figure 5B).
Discussion
Ang1-stimulation lead to PC12 cell survival and neurite outgrowth in a Tie2-independent, β1 integrin dependent manner, providing new insight into the role of Ang1 as a neurotrophic growth factor. Consistent with our previous report analyzing Tie2 expression in neurons (Ward et al., 2004), expression of Tie2 was not detectable in PC12 cells at any differentiated or stimulated state. We proposed in our previous work the hypothesis that Ang1-β1 integrin interactions could be a plausible mechanism in which Ang1 could exert its neurotrophic effects. This theory was based on previous observations by others that angiopoietins could bind to β1 integrin (Carlson et al., 2001), and that biological effects could be mediated by angiopoietin:β1 integrin interactions in either a Tie2-independent manner, such as in glioma cells (Hu et al., 2006), breast cancer cells (Imanishi et al., 2007) and skeletal and cardiac myocytes (Dallabrida et al., 2005) or in a Tie-2:β1 integrin codependent manner as observed in neoplastic glial cells (Lee et al., 2006) and ECs (Cascone et al., 2005). Therefore, we confirmed β1 integrin expression in PC12 cells, and following function blocking experiments with antibodies targeting β1 integrin or β1 integrin SiRNA experiments, were able to show that Ang1-mediated neurite outgrowth occurred in a β1 integrin dependent, Tie2 independent manner. Interestingly, NGF-mediated neurite outgrowth remained unaffected following the addition of β1 integrin function blocking antibodies or silencing β1 integrin expression, suggesting that NGF-β1 integrin interactions were not critical for NGF-induced neurite outgrowth.
Ang1 and NGF utilized different signaling pathways to induce their biological outcomes, such that Ang1 stimulation lead to increases in Akt and FAK phosphorylation, whereas NGF stimulation lead to increased MAPK and JNK. Interestingly, the addition of the FAK inhibitor, PF573228 or Wortmannin were both capable of inhibiting Ang1 mediated neurite outgrowth (Figure 5B), whereas Wortmannin only reduced NGF-mediated outgrowth.
Our downstream identification of PI3K-Akt as players in Ang-β1 integrin mediated signaling events is consistent with what others have shown in breast cancer cells (Imanishi et al., 2007) and skeletal and cardiac myocytes (Dallabrida et al., 2005). Similarly FAK activation has also been reported downstream of Ang1-β1 integrin interactions in glioma cells and ECs (Cascone et al., 2005, Hu et al., 2006) but not in myocytes (Dallabrida et al., 2005). In contrast, we failed to find induction of either MAPK or JNK activation, although others have found phosphorylation of these proteins following Ang1 stimulation in myocytes and ECs (MAPK; (Dallabrida et al., 2005, Hu et al., 2006)(JNK; (Hu et al., 2006). One plausible explanation may be that Ang1-β1 integrin signaling events occur in a cell specific manner, such that JNK is activated in response to Ang1-β1 integrin interactions in glioma cells and not ECs or myocytes; whereas MAPK is phosphorylated in ECs and myocytes, but not in glioma cells. NGF signaling in PC12 cells is known to occur via multiple signaling mechanisms, including MAPK and Akt (Lad et al., 2003, Kim et al., 2004), NFκB (Bui et al., 2001) and JNK (Gelderblom et al., 2004, Kim et al., 2004). In our experiments, we observed increases in activation of MAPK and JNK following NGF stimulation, with JNK phosphorylation decreasing following β1 integrin inhibition, although no effects on neurite outgrowth were found. The decrease in JNK activation coupled with the lack of effect on neurite outgrowth may reflect compensatory MAPK signaling, such that the decreases in JNK may not be sufficient enough to overcome MAPK-induced and maintained neurite outgrowth (Gelderblom et al., 2004). Although in these studies we failed to show NGF-induced Akt phosphorylation, Wortmannin treatment inhibited NGF-induced neurite outgrowth validating the importance of this pathway in underlying this biological event and confirming work done by others (Lad et al., 2003, Kim et al., 2004). Our inability to demonstrate Akt phosphorylation in the current experiments most likely reflects differences in timing, such that others have shown PI3K and Akt activation occurs within 10 minutes of NGF stimulation.
Ang1-mediated neurite outgrowth has been reported previously in DRGs by us and others (Ward and LaManna, 2004, Kosacka et al., 2005, Kosacka et al., 2006). Kosacka and colleagues demonstrated Ang1-mediated outgrowth in their system involved Tie2 mediated signaling and transactivation of the Trk receptor (Kosacka et al., 2005, Kosacka et al., 2006). Most recently Ang1-mediated neuronal differentiation of neural stem cells has been demonstrated and found to occur in a Tie2, PI3K dependent manner (Bai et al., 2009). Taken together with the current findings, this suggests that other neuronal populations, including neural progenitor cells, are also responsive to Ang1; and that this responsiveness occurs either through Tie2 or β1 integrin, depending on the presence or absence of each receptor within a cell. Which neurons contain Tie2 remains unclear, as is whether all neurons are capable of responding to Ang1, whether it be through Tie2 or β1 integrin. Ang1 may also be capable of exerting singals through both receptors, as β1 integrin can sensitize and modulate Tie2 receptor activation and signaling in ECs, Tie2 and β1 (as α5β1) can interact constitutively; and Ang1 recruits FAK to this complex, suggesting that Ang1, in ECs can induce cross-talk between Tie2 and α5β1. Whether this occurs in neurons is not yet known. Further study using purified in vivo cell populations under a variety of different conditions may provide more insight into the expression and regulation of Tie2 and the mechanisms underlying how and whether different neuron populations respond to Ang1.
In conclusion, we have shown that Ang1 is capable of eliciting direct effects on PC12 cells via binding and signaling through β1 integrin, and downstream signaling through FAK, PI3K, and Akt, providing support for the idea that Ang1 is neurotrophic within the nervous system. In addition our findings provide further confirmation that Ang1 exerts a wide range of biological, physiological and pathological functions in not only the vascular system but also the nervous system.
Acknowledgements
This work was supported by a Crile fellowship, an American Academy of Neurology award, and an American Foundation of Aging Research scholarship to CET and grants to NLW from the National American Heart Association, Juvenile Diabetes Research Foundation and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mechanisms of development. 2001;108(12):45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Bai Y, Cui M, Meng Z, Shen L, He Q, Zhang X, Chen F, Xiao J. Ectopic expression of angiopoietin-1 promotes neuronal differentiation in neural progenitor cells through the Akt pathway. Biochem Biophys Res Commun. 2009;378:296–301. doi: 10.1016/j.bbrc.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Bui NT, Livolsi A, Peyron JF, Prehn JH. Activation of nuclear factor kappaB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IkappaBalpha. J Cell Biol. 2001;152:753–764. doi: 10.1083/jcb.152.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chen Jieli, Zacharek Alex, Roberts Cynthia, Yang Yuping, Chopp Michael. Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. Journal of Neuroscience Research. 2009;87:86–95. doi: 10.1002/jnr.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- Derksen MJ, Ward NL, Hartle KD, Ivanco TL. MAP2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis. Neurobiol Learn Mem. 2007;87:404–415. doi: 10.1016/j.nlm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Experimental Cell Research. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Eminel S, Herdegen T, Waetzig V. c-Jun N-terminal kinases (JNKs) and the cytoskeleton--functions beyond neurodegeneration. Int J Dev Neurosci. 2004;22:559–564. doi: 10.1016/j.ijdevneu.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jarzynka MJ, Guo P, Imanishi Y, Schlaepfer DD, Cheng S-Y. Angiopoietin 2 Induces Glioma Cell Invasion by Stimulating Matrix Metalloprotease 2 Expression through the {alpha}v{beta}1 Integrin and Focal Adhesion Kinase Signaling Pathway. Cancer Res. 2006;66:775–783. doi: 10.1158/0008-5472.CAN-05-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, Hu B, Jarzynka MJ, Guo P, Elishaev E, Bar-Joseph I, Cheng SY. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67:4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Li DY. Common cues regulate neural and vascular patterning. Curr Opin Genet Dev. 2007;17:332–336. doi: 10.1016/j.gde.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS. A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells. 2004;18:353–359. [PubMed] [Google Scholar]
- Koh S-H, Kim SH, Kwon H, Park Y, Kim KS, Song CW, Kim J, Kim M-H, Yu H-J, Henkel JS, Jung HK. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Molecular Brain Research. 2003;118:72–81. doi: 10.1016/j.molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–19. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Nowicki M, Kacza J, Borlak J, Engele J, Spanel-Borowski K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J Neurosci Res. 2006;83:1160–1169. doi: 10.1002/jnr.20811. [DOI] [PubMed] [Google Scholar]
- Lad SP, Peterson DA, Bradshaw RA, Neet KE. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J Biol Chem. 2003;278:24808–24817. doi: 10.1074/jbc.M212270200. [DOI] [PubMed] [Google Scholar]
- Lee OH, Xu J, Fueyo J, Fuller GN, Aldape KD, Alonso MM, Piao Y, Liu TJ, Lang FF, Bekele BN, Gomez-Manzano C. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated with integrin beta1-dependent adhesion to the extracellular matrix. Mol Cancer Res. 2006;4:915–926. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. The EMBO journal. 2001;20(21):5919–5928. doi: 10.1093/emboj/20.21.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Angiopoietins and Angiopoietin-Like Proteins in Angiogenesis. Endothelium. 2006;13:71–79. doi: 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288:H936–945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- Rüegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cellular and Molecular Life Sciences (CMLS) 2003;60:1135–1157. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- Ward N, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurological Research. 2004;26:879–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Ward NL, Putoczki T, Mearow K, Ivanco TL, Dumont DJ. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol. 2004;482:244–256. doi: 10.1002/cne.20422. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, de Almodovar CR, Carmeliet P. Similarities between angiogenesis and neural development: what small animal models can tell us. Curr Top Dev Biol. 2008;80:1–55. doi: 10.1016/S0070-2153(07)80001-9. [DOI] [PubMed] [Google Scholar]


