Abstract
Purpose
To prospectively compare 0.1 mmol/kg doses of gadobenate dimeglumine and gadopentetate dimeglumine for contrast-enhanced MRI of brain lesions at 3 Tesla (T).
Materials and Methods
Forty-six randomized patients underwent a first examination with gadobenate dimeglumine (n = 23) or gadopentetate dimeglumine (n = 23) and then, after 2–7 days, a second examination with the other agent. Contrast administration (volume, rate), sequence parameters (T1wSE; T1wGRE), and interval between injection and image acquisition were identical for examinations in each patient. Three blinded neuroradiologists evaluated images qualitatively (lesion delineation, lesion enhancement, global preference) and quantitatively (lesion-to-brain ratio [LBR], contrast-to-noise ratio [CNR],%lesion enhancement). Differences were assessed using Wilcoxon’s signed-rank test. Reader agreement was determined using kappa (κ) statistics.
Results
There were no demographic differences between groups. The three readers preferred gadobenate dimeglumine globally in 22 (53.7%), 21 (51.2%), and 27 (65.9%) patients, respectively, compared with 0, 1, and 0 patients for gadopentetate dimeglumine. Similar significant (P < 0.001) preference was expressed for lesion border delineation and enhancement. Reader agreement was consistently good (κ = 0.48–0.64). Significantly (P < 0.05) higher LBR (+43.5–61.2%), CNR (+51.3–147.6%), and % lesion enhancement (+45.9–49.5%) was noted with gadobenate dimeglumine.
Conclusion
Brain lesion depiction at 3T is significantly improved with 0.1 mmol/kg gadobenate dimeglumine.
Keywords: brain tumor imaging, gadobenate dimeglumine, comparative studies, high field MRI
GADOBENATE DIMEGLUMINE (GD-BOPTA, Multi-Hance®; Bracco Diagnostics, Inc., Princeton, NJ) is a gadolinium-based MR contrast agent approved by the United States (US) Food and Drug Administration (FDA) for MR imaging of the central nervous system (CNS) and related tissues (1). Numerous studies have shown that a dose of 0.1 mmol/kg body weight of gadobenate dimeglumine is superior to an equivalent dose of comparator gadolinium agent for MR imaging of lesions of the CNS (2– 8). Specifically, the lesion contrast enhancement and diagnostic information available are superior in significantly larger numbers of patients after gadobenate dimeglumine than after comparator agent under identical imaging conditions.
To date, however, all of the studies that have compared gadobenate dimeglumine with comparator agents have been performed on MR imaging systems operating at 1.0 (3) or 1.5 Tesla (T) ( 3– 6, 8). At these magnetic field strengths, gadobenate dimeglumine has an r1 relaxivity value of 6.3–7.9 Lṡmmol−1ṡs−1 ( 9, 10), while the FDA-approved conventional agents gadopentetate dimeglumine and gadodiamide have r1 relaxivity values of 3.9–4.1 and 4.3 Lṡmmol−1ṡs−1, respectively ( 9, 10). At higher magnetic field strength, the r1 relaxivity values of the various contrast agents are reduced. Thus, at 3T, the r1 relaxivity of gadobenate dimeglumine is reported to be 5.5–5.9 Lṡmmol−1ṡs−1 ( 9, 10), while those of gadopentetate dimeglumine and gadodiamide are 3.7–3.9 and 4.0 Lṡmmol−1ṡs−1, respectively ( 9, 10). Given the twofold higher inherent signal-to-noise ratio (SNR) of 3T systems compared with 1.5T systems and the resulting improvement in achievable spatial resolution, it is unclear to what extent higher magnetic field strength will impact on the proven diagnostic benefits imparted by contrast agents with increased r1 relaxivity.
Recently, Runge et al demonstrated in a rat brain tumor model that 0.1 mmol/kg gadobenate dimeglumine provides significantly higher contrast-to-noise ratio (CNR) at 3T compared with 1.5T and that the CNR achieved at 3T with 0.1 mmol/kg gadobenate dimeglumine is significantly higher than that achieved with gadopentetate dimeglumine at equivalent dose ( 11).
The aim of the present study was to compare 0.1 mmol/kg doses of gadobenate dimeglumine and gadopentetate dimeglumine at 3T in patients with known or suspected lesions of the brain who had been referred for MR imaging of the CNS, with the hypothesis that gadobenate dimeglumine will provide superior lesion contrast enhancement and diagnostic information. In common with previous comparisons at 1.5T, an intra-individual crossover study design was adopted in which each patient received both contrast agents in two otherwise identical MR examinations.
MATERIALS AND METHODS
This study was HIPAA-compliant and was conducted at four centers in the United States according to Good Clinical Practice standards. The study received Institutional Review Board approval, and all patients signed an approved informed consent form before enrollment. The lead author had complete access to the results of the study, and all authors had control of the data and statistical results included in this report.
Patients
A total of 47 patients (22 men, 25 women) signed the informed consent form. Of these patients, one male subject withdrew for personal reasons before receiving either contrast agent. The remaining 46 patients (21 men, 25 women; mean age ± standard deviation [SD], 49.4 ± 15.7 years; range, 19–79 years) who were known or suspected to have enhancing lesions of the brain and who had been referred for MR imaging of the CNS, were enrolled in a consecutive manner at each of the four participating centers between March 2007 and February 2008. The distribution of enrolled patients among the four investigating centers was 20, 11, 10, and 5.
Patients were excluded if they had received an investigational drug within 30 days before admission to the study or were scheduled to receive such a drug during the course of the study or within 24 h of the administration of the second study agent, or if they had had received any other contrast agent within 24 h before administration of the first study agent or were expected to receive any other contrast agent within 24 h of the administration of the second study agent. Patients were also excluded if they had class III or IV congestive heart failure according to the New York Heart Association classification ( 12) or other medical conditions or circumstances (e.g., claustrophobia, hypersensitivity to gadolinium or other metals, pacemaker), which would substantially decrease the chances of obtaining reliable data. Finally, nursing or pregnant female patients were ineligible, as were patients who had received or were going to receive any treatment, which could have changed the visualization of lesions between the two examinations (e.g., whole-brain fractionated radiotherapy, steroid therapy, or stable chemotherapy regimen).
Eligible patients were randomized prospectively into two contrast agent sequence administration orders (study groups A and B). Patients randomized to study group A (n = 23; 12 men, 11 women, 50.8 ± 15.5 years) received gadobenate dimeglumine for the first MR imaging examination and gadopentetate dimeglumine for the second examination, while patients randomized to study group B (n = 23; 9 men, 14 women, 48.1 ± 16.1 years) received gadopentetate dimeglumine for the first examination and gadobenate dimeglumine for the second examination.
MR Imaging
MR imaging was performed on 3T systems (Philips Intera 3T, Philips Medical Systems, Eindhoven, The Netherlands [n = 20]; General Electric [GE] Signa Excite 3T [n = 26], GE Medical Systems, Milwaukee, Wi) using a standard head coil.
A controlled imaging protocol comprising T1-weighted spin-echo (T1SE), T1-weighted gradient-echo (T1GRE), and T2-weighted fast spin-echo (T2FSE) acquisitions before contrast injection (predose) and T1SE and T1GRE acquisitions after injection (postdose) ensured protocol uniformity across sites and within individual patients. The parameters for the imaging sequences varied between investigational centers because of the different imaging systems in use at these centers; however, the same MR scanner, imaging planes, slice prescriptions, and sequence parameters were used for both examinations in each patient. The range of parameters for the T1SE sequence was as follows: TR, 267–708 ms; TE, 9–12 ms; number of excitations (NEX), 1–2; slice thickness, 3 mm; interslice gap, 0–1 mm; number of slices, 40–60; field-of-view (FOV), 22–23 cm; acquisition matrix, 178 × 256–256 × 512. The parameters for the T1GRE sequence ranged as follows: TR, 9–400 ms; TE, 2–5 ms; FA, 10–90°; NEX, 1; slice thickness, 3 mm; interslice gap, 0–1 mm; number of slices, 40–60; FOV, 22–24 cm; acquisition matrix, 205 × 256–256 × 512. Finally, the parameters for the T2FSE sequence ranged as follows: TR, 4000–8000 ms; TE, 68.5–386 ms; echo train length, 8–24; NEX, 1; slice thickness, 3–4 mm; interslice gap, 0–1 mm; number of slices, 32–50; FOV, 22–23 cm; acquisition matrix, 256 × 256–224 × 1024.
Contrast agent administration in both examinations was performed intravenously in an identical manner using a power injector at a rate of 2 mL/s using a 20-Ga needle unless patient-related factors (e.g., small veins) necessitated use of a different sized needle. Each contrast agent was administered in the order determined by a randomization list at a dose of 0.1 mmol/kg body weight (corresponding to 0.2 mL/kg body weight of a 0.5 M formulation). The same exact volume of contrast agent was injected in both examinations. Acquisition of the postcontrast T1-weighted images began at a fixed time point, which was mandated to occur between 3 and 10 min after injection, but could vary within this range depending on the site-specific protocol. However, the postcontrast scans in each patient were strictly controlled to be identical in terms of timing and sequence order for both exams. To ensure precise timing and strict adherence to the study protocol, no other imaging sequences (e.g., perfusion imaging) were performed. The interval between the two MR examinations was greater than 48 h in all patients to avoid any carryover effect, but less than 7 days in all patients to minimize the chance of measurable disease progression or lesion evolution that could have changed the imaging appearance.
Image Evaluation
All images were evaluated by three independent, experienced (between 12 and 25 years) neuroradiologists who were unaffiliated with the study centers and fully blinded to the contrast agent used in each examination, to all patient clinical and radiological information, and to all interpretations by on-site investigators. Each blinded reader evaluated the patient images separately and independently.
All images from each patient were evaluated in a global matched-pairs manner. Images were presented for review on a multi-monitor imaging workstation. For each randomized patient number, all images from the first examination (“Exam 1”) were displayed simultaneously with the corresponding images from the second examination (“Exam 2”). Each reader was able to perform all routine interactive image manipulation functions (e.g., window/level, zoom, pan) on both image sets. If the postinjection images from either examination were considered technically inadequate by any of the three readers (e.g., if artifacts compromised interpretability), no further assessment was performed for that patient by that reader. Once the readers’ assessments were recorded and signed off on an electronic Case Report Form (e-CRF), the database for that reading was automatically locked.
Qualitative Assessment of Diagnostic Information
Technically adequate images were evaluated qualitatively for diagnostic information and scored in terms of lesion border delineation, lesion contrast enhancement compared with surrounding normal tissue, and global diagnostic preference. All assessments were performed using 3-point scales from −1 (Exam 1 superior) through 0 (both exams equal) to +1 (Exam 2 superior). Criteria used to assign superiority for one of the exams over the other included better separation of one or more lesions from surrounding tissue, structures or edema, better definition of lesion extent, clearer depiction of intralesion features, and better difference in signal intensity between lesion(s) and surrounding normal tissue.
Quantitative Assesssment
Quantitative evaluation of up to three enhancing lesions per patient was performed by each reader independently using a simultaneous matched-pairs approach. Measurements of signal intensity (SI) were made at regions-of-interest (ROIs) positioned on areas of normal brain parenchyma, and on up to three lesions identified on postcontrast images from both examinations. Additional SI measurements were made at ROIs placed in selected areas external to the brain to determine the background noise. To ensure that ROIs of equal size were positioned at identical coordinates on all corresponding image sets, each ROI placed on the selected postinjection image from one examination appeared simultaneously on the corresponding image from the other examination, always taking care to avoid inclusion of vessels. If multiple lesions were present in a given patient, ROIs were placed on up to three of the largest, most conspicuous lesions. All SI measurements were made using ANALYZE™ software version 4.0 (Mayo Foundation, Rochester, MN) and were subsequently used to calculate lesion enhancement (% enhancement) from pre-to postinjection as well as the lesion-to-brain and contrast-to-noise ratios (LBR and CNR, respectively) for both T1SE and T1GRE acquisitions. The values for % enhancement, LBR and CNR were calculated using standard formulas described previously (7).
Safety Assessments
Monitoring for adverse events for all patients was performed from the moment the patient signed the informed consent form until 24 h after administration of the first study agent, and then again from the moment the second study agent was administered until 24 h after administration of the second agent. Adverse events were classified by the principal investigator at each center as either serious (i.e., death, life-threatening, requiring or prolonging hospitalization) or not serious (rated as mild, moderate, or severe), and any perceived relationship to the agent was recorded (classified as probable, possible, not related, or unknown).
Statistical Analysis
Comparison of demographic parameters (sex, age, weight, height) between patients in study sequence groups A and B was performed using Student’s t-test for continuous variables and the chi-squared test for categorical variables. Differences in diagnostic information findings for each of the three blinded readers were compared using the Wilcoxon signed rank test. Interreader agreement for diagnostic findings was assessed using generalized kappa (κ) statistics and measured as percentage agreement.
Evaluation of quantitative data was performed using paired t-tests for pre-to postinjection changes in SI. Differences between gadobenate dimeglumine and gadopentetate dimeglumine in terms of study agent effect were analyzed using a mixed effect model. The change from predose was the response variable and factors included in the model were patient, period, sequence, study agent and predose score, where patient nested within sequence was the random effect. All statistical tests were conducted at a significance level of P < 0.05 using the statistical software package SAS version 8.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patients
No significant difference was apparent among patients in study groups A and B regarding sex distribution (P = 0.375), age (P = 0.562), weight (P = 0.552), or height (P = 0.953). Of the 46 patients randomized and evaluated for safety, 5 (10.9%) prematurely terminated the study after the first contrast agent administration (2 after gadobenate dimeglumine; 3 after gadopentetate dimeglumine) and were excluded from subsequent efficacy evaluations. The 5 excluded patients included 3 who failed to return for the scheduled second examination, 1 who was scheduled for surgery after the first examination, and 1 whose intravenous line became dislodged during the first examination making it impossible to discern whether the full 0.1 mmol/kg dose of contrast agent had been administered. A total of 41 patients (21 in group A, 20 in group B) were, therefore, evaluated for diagnostic efficacy by the three off-site blinded readers.
The diagnoses of these 41 patients were primary glial tumor in 23 (56.1%) cases (glioma [n = 4], glioblastoma multiforme [n = 9], oligodendroglioma [n = 5], astrocytoma [n = 3], mixed oligodendroglioma/astrocytoma [n = 2]); secondary metastases in 4 (9.7%) cases (from primary lung cancer [n = 1], breast cancer [n = 1], melanoma [n = 1], unknown cancer [n = 1]); extra-axial lesions in 4 (9.7%) cases (meningioma [n = 4], and “other” or unspecified diagnosis in 10 (24.4%) cases (postoperative scar/fibrosis [n = 3], orbital venous malformation [n = 1], lymphohistiocystic lesion [n = 1], glomus jugulare [n = 1], pineal cyst [n = 1], unspecified/unknown mass lesion [n = 3]).
Qualitative Image Assessment
All of the image sets from each of the 41 evaluated patients were technically adequate for assessment. The findings of the three blinded readers for the three diagnostic information end-points are shown in Table 1. Readers 1, 2, and 3 demonstrated global diagnostic preference for gadobenate dimeglumine in 22/41 (53.7%), 21/41 (51.2%), and 27/41 (65.9%) patients, respectively, compared with 0, 1, and 0 patients for gadopentetate dimeglumine, respectively (Fig. 1–Fig. 3).
Table 1.
Qualitative Assessments of Three Independent Blinded Readers for All Patients
| Reader preference for N = 41 patients |
|||||
|---|---|---|---|---|---|
| Qualitative endpoint | Reader | Gadobenate dimeglumine preferred |
Contrast agents equal |
Gadopentetate dimeglumine preferred |
P Value* |
| Global diagnostic preference | 1 | 22 (53.7) | 19 (46.3) | 0 | < 0.0001 |
| 2 | 21 (51.2) | 19 (46.3) | 1 (2.4) | < 0.0001 | |
| 3 | 27 (65.9) | 14 (34.1) | 0 | < 0.0001 | |
| Lesion border delineation | 1 | 14 (34.1) | 27 (65.9) | 0 | 0.0001 |
| 2 | 11 (26.8) | 30 (73.2) | 0 | 0.001 | |
| 3 | 13 (31.7) | 28 (68.3) | 0 | 0.0002 | |
| Lesion contrast enhancement | 1 | 22 (53.7) | 19 (46.3) | 0 | < 0.0001 |
| 2 | 20 (48.8) | 20 (48.8) | 1 (2.4) | < 0.0001 | |
| 3 | 22 (53.7) | 18 (43.9) | 1 (2.4) | < 0.0001 | |
Wilcoxon signed rank test.
Figure 1.
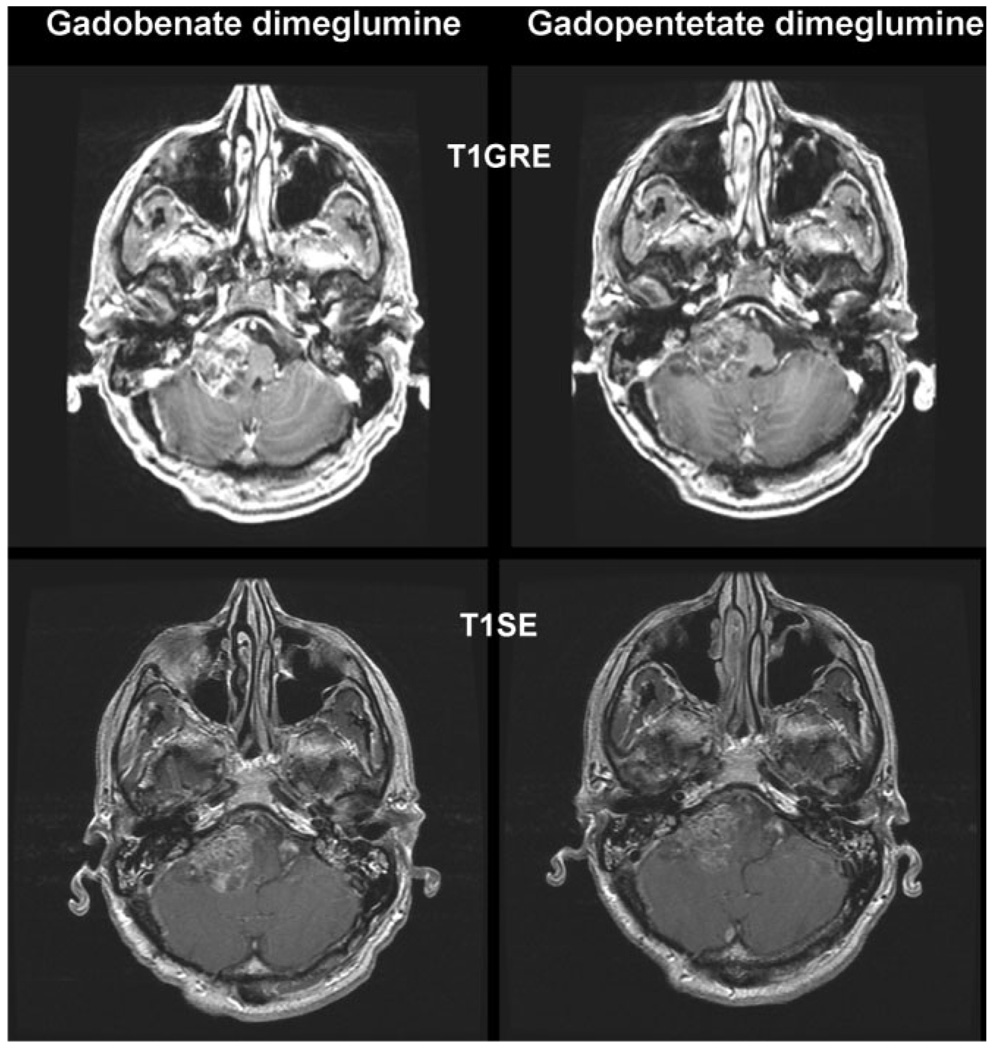
A 55-year-old woman with a grade IV posterior fossa astrocytoma infiltrating the brainstem. T1SE and T1GRE images acquired from 8 min after administration of 0.1 mmol/kg gadobenate dimeglumine and 0.1 mmol/kg gadopentetate dimeglumine. The tumor enhancement is considerably greater after the injection of gadobenate dimeglumine and as a consequence the demarcation from surrounding tissues is better.
Figure 3.
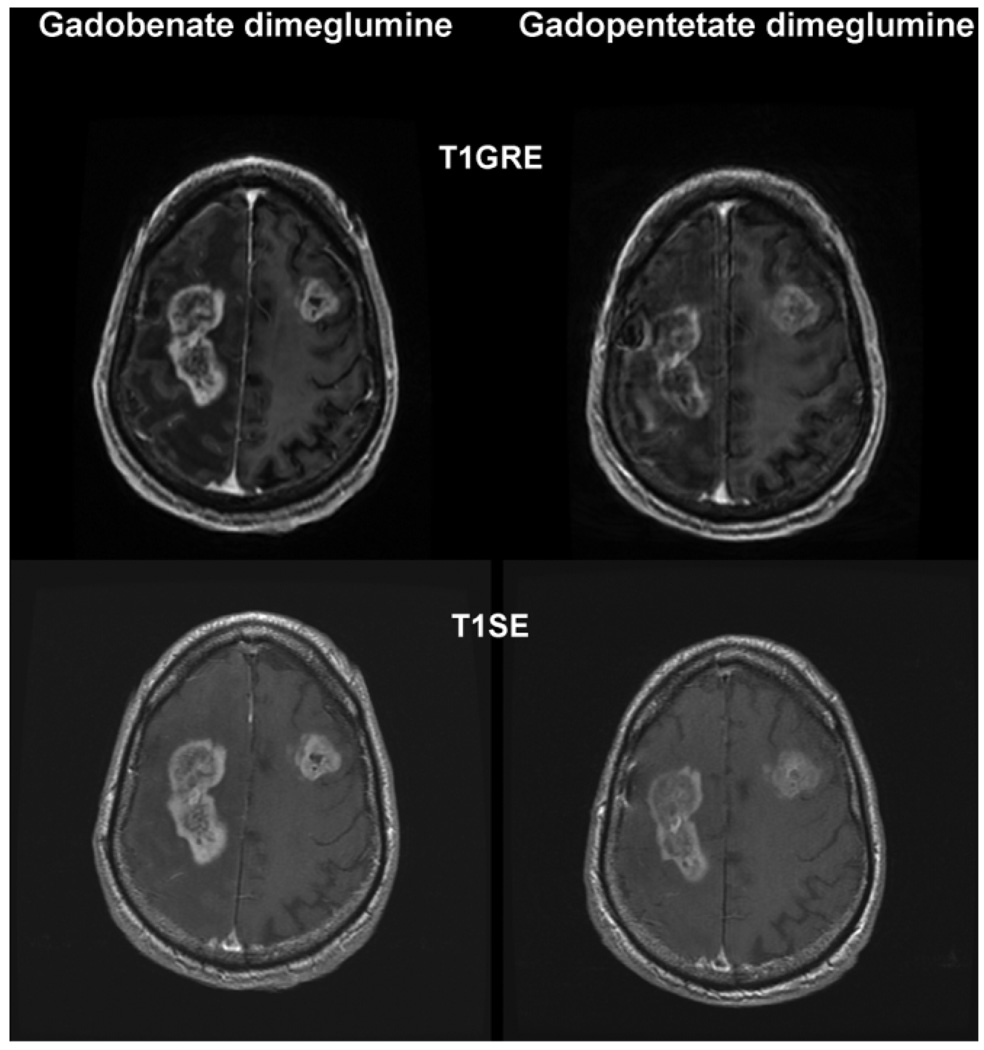
A 77-year-old man with bilateral frontoparietal extension of a glioblastoma. T1SE and T1GRE images acquired from 6 min after after administration of 0.1 mmol/kg gadobenate dimeglumine and 0.1 mmol/kg gadopentetate dimeglumine. Considerably greater contrast enhancement and better depiction of internal lesion morphology is apparent after injection of gadobenate dimeglumine.
Analysis of the level of agreement between the three blinded readers resulted in generalized weighted κ values of κ = 0.48 for global diagnostic preference, κ = 0.58 for lesion border delineation, and κ = 0.64 for lesion contrast enhancement. All three blinded readers agreed completely in their assessments for 61.0%–73.2% of the patients depending on the diagnostic information endpoint under consideration.
Quantitative Evaluation
Readers 1, 2, and 3 reported a total of 39, 32, and 48 lesions, respectively, among the 41 evaluated patients. In 3, 12, and 5 patients, respectively, no lesions were reported for either examination while solitary lesions were reported in 37, 26, and 31 patients, respectively. The remaining 1, 3, and 5 patients, respectively, had multiple (typically two) lesions. Differences between readers concerning the numbers of lesions reflected the individual subjective opinions of the readers. Thus, the relatively low number of lesions reported by reader 2 reflects the fact that this reader did not consider lesions such as pineal cyst, orbital venous malformation, glomus jugulare, postoperative scar/fibrosis, or nonspecific mass lesions, while the relatively high number of lesions reported by reader 3 reflects the fact that this reader alone reported 3 lesions in one patient diagnosed with glioma and 8 lesions in another patient diagnosed with glioblastoma. Readers 1 and 2 each considered both of these to be solitary lesions. There were no differences between examinations in any patient concerning the number of lesions detected, possibly reflecting the small number of patients with secondary metastases.
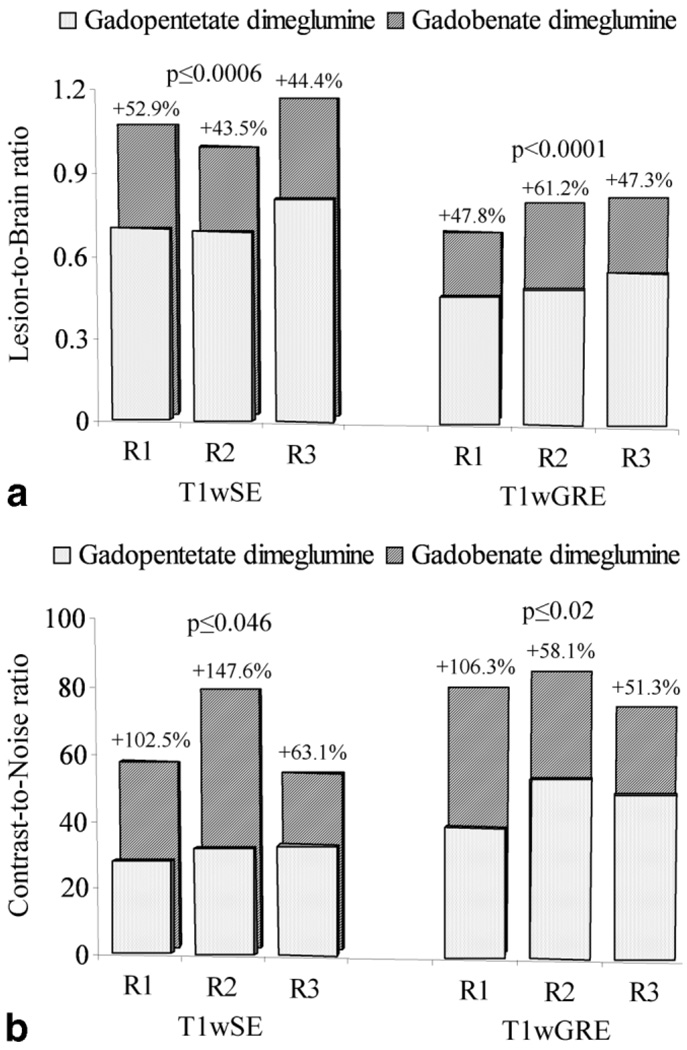
Lesion SI measurements relative to normal brain parenchyma and background noise were recorded by readers 1, 2, and 3 in 26, 24, and 26 patients, respectively, for 26, 24, and 25 lesions, respectively, on T1SE images, and for 27, 25, and 26 lesions, respectively, on T1GRE images. Subsequent determinations of LBR and CNR (Fig. 4) revealed highly significant increases in quantitative lesion enhancement with gadobenate dimeglumine relative to gadopentetate dimeglumine for both parameters. Specifically, readers 1, 2, and 3 recorded significant increases of 43.5%–52.9% (T1wSE sequences; P ≤ 0.0006, all readers) and 47.3%–61.2% (T1wGRE sequences; P < 0.0001, all readers) for LBR (Fig. 4A), and 63.1%–147.6% (T1wSE sequences; P ≤ 0.046, all readers) and 51.3%–106.3% (T1wGRE sequences; P ≤ 0.02, all readers) for CNR (Fig. 4B). Similar increases were noted for the % lesion enhancement obtained with gadobenate dimeglumine relative to gadopentetate dimeglumine. Readers 1, 2, and 3 noted increases with gadobenate dimeglumine of 48.1% (P < 0.0001), 45.9% (P = 0.0004), and 46.8% (P = 0.0009), respectively, on T1wSE images, and 48.6% (P = 0.0019), 46.2% (P = 0.0008), and 49.5% (P = 0.0026), respectively, on T1wGRE images.
Figure 4.
Bar graphs showing significantly increased mean lesion-to-brain ratios (a) and contrast-to-noise ratios (b) with 0.1 mmol/kg gadobenate dimeglumine compared with 0.1 mmol/kg gadopentetate dimeglumine at 3T using both T1wSE and T1wGRE sequences. Data obtained from quantitative measurements of signal intensity enhancement for all patients at ROIs positioned by each of three blinded readers independently.
Safety
No clinically meaningful differences between gadobenate dimeglumine and gadopentetate dimeglumine were noted in terms of the incidence of adverse events among these patients: just 3 patients reported nonserious adverse events that were considered of potential relationship to the administration of contrast agent. The events comprised one case of ear discomfort that was considered moderate in intensity and possibly related to the administration of gadobenate dimeglumine, one case of head-ache that was mild in intensity of unknown relationship, and one case of nausea/vomiting that was again mild in intensity and probably related to the administration of gadobenate dimeglumine. All adverse events were rapidly self-resolving without treatment.
DISCUSSION
For MR imaging applications in the brain, high-resolution depiction of anatomical and pathological processes is critical. Compared with 1.5T imaging systems, 3T systems provide inherently increased SNR, which can be used to increase the spatial resolution and thus improve the visualization of even very subtle disruptions of the blood–brain barrier (13). Nevertheless, the need for exogenous paramagnetic contrast agents to shorten the T1 relaxation times of relevant tissues remains fundamental to most MR imaging examinations of the brain, particularly when the aim is to detect and/or diagnose brain tumors or postoperatively follow-up resected tumors. Given the longer baseline tissue T1 relaxation times at 3T the same r1 relaxivity will yield stronger T1 reduction resulting in greater tissue contrast enhancement at 3T than at 1.5T (13). Gadobenate dimeglumine demonstrates similar pharmacokinetic (14) and safety profiles (15,16) to gadopentetate dimeglumine and other conventional gadolinium agents (17) and similarly shortens the T1 relaxation times of tissues. However, the r1 relaxivity of this agent is roughly twice that of gadopentetate dimeglumine due to weak, transient interaction of the Gd-BOPTA contrast-effective chelate with serum albumin (18,19). This has been shown to result in much stronger contrast enhancement at 1.5T compared with that occuring with gadopentetate dimeglumine when these two agents are administered at equivalent dose (4–6).
For conventional gadolinium contrast agents such as gadopentetate dimeglumine, the difference in r1 relaxivity between 1.5T and 3T is minimal; Pintaske et al (9) reported no difference in relaxivity at 3T compared with 1.5T (3.9 Lṡmmol−1ṡs−1) while Rohrer et al (10) reported similar values of 4.1 Lṡmmol−1ṡs−1 at 1.5T and 3.7 Lṡmmol−1ṡs−1 at 3T. Although the markedly higher r1 relaxivity seen for gadobenate dimeglumine compared with gadopentetate dimeglumine at 1.5T is maintained at 3T, the absolute difference in r1 relaxivity between 1.5T and 3T is more marked for gadobenate dimeglumine (7.9 versus 5.9 Lṡmmol−1ṡs−1 according to Pintaske et al [9] and 6.3 versus 5.5 Lṡmmol−1ṡs−1 according to Rohrer et al) (10). Conceivably, the greater absolute reduction in r1 relaxivity seen for gadobenate dimeglumine at 3T compared with 1.5T may negate the diagnostic benefits of the higher r1 relaxivity of this agent at higher field strength.
The results of this study demonstrate that this is not the case. Blinded assessments of both qualitative and quantitative enhancement criteria revealed significantly greater preference for gadobenate dimeglumine compared with gadopentetate dimeglumine whenever a preference was expressed. Concerning qualitative enhancement criteria, all three blinded readers preferred gadobenate dimeglumine to gadopentetate dimeglumine in half or more of the evaluated patients for assessments of overall diagnostic preference and level of contrast enhancement and for roughly one third of the evaluated patients for assessments of lesion border delineation. Highly significant benefit for gadobenate dimeglumine was also noted by all three readers for quantitative assessments of LBR and CNR both for T1wSE and T1wGRE sequences. Specifically, increases in LBR and CNR of 43.5%–52.9% (P ≤ 0.0006) and 63.1% – 147.6% (P ≤ 0.046), respectively, were noted on T1wSE sequences with gadobenate dimeglumine compared with gadopentetate dimeglumine, while similar increases of 47.3%–61.2% (P < 0.0001) and 51.3% – 106.3% (P ≤ 0.02), respectively, were noted on T1wGRE sequences. Notably, the magnitude of the increased CNR with 0.1 mmol/kg gadobenate dimeglumine was greater than the 25%–30% noted for T1wSE sequences at 1.5T for intra-individual crossover comparisons with gadopentetate dimeglumine at equivalent dose (5) and greater also than the 23%–35% for T1wSE sequences and 42%–49% for T1wGRE sequences observed in comparable intra-individual crossover comparisons with gadodiamide at 1.5T (8). As noted previously (5,6,8,20), the increase in CNR with gadobenate dimeglumine can be considered analogous to the increase expected with a double dose of a conventional gadolinium agent compared with a single dose.
The influence of increased field strength on CNR in patients with contrast-enhancing brain lesions has previously been investigated by Krautmacher et al (21) and Ba-Ssalamah et al (22). In the former study, a gadopentetate dimeglumine dose of 0.1 mmol/kg body weight resulted in a gain in CNR that was 2.8 times higher than that obtained at 1.5T with an identical dose, and contemporaneously, led to improved lesion conspicuity (21). The increase in CNR was greater than the twofold increase anticipated on moving from 1.5T to 3T was ascribed in part to the longer baseline T1 relaxation times at 3T and thus the stronger T1 shortening effect per unit of gadopentetate dimeglumine at 3T compared with 1.5T, and in part to the fact that an identical TR value (500 ms) was used at both field strengths which resulted in differences between nonenhancing and enhancing tissues in terms of the SNR obtained (i.e., a lower than twofold increase for nonenhancing tissues combined with a twofold increase for enhancing tissues leading to an overall greater than twofold increase in CNR at 3T). In our study, the TR values used for the two examinations in each patient were also identical indicating that the greater CNR and LBR with gadobenate dimeglumine can be ascribed solely to the increased r1 relaxivity in blood of this agent.
Concerning the sequence parameters used for the two examinations in each patient, recent studies have shown that improved SNR with gadobenate dimeglumine can be achieved by using shorter TR and TE values than those typically used with gadopentetate dimeglumine and other conventional, non–protein-interacting gadolinium contrast agents (23,24). For this study, the T1w sequence parameters used at each center were those routinely used with gadopentetate dimeglumine and thus can be considered optimized for use with conventional agents of this type. This being the case, it is highly likely that both the LBR and CNR might have been greater still with gadobenate dimeglumine had the TR and TE values been optimized specifically for this agent. Although the strict blinding conditions of the study precluded modification of the sequence parameters and demanded that the two examinations in each patient be identical in all respects, further work should certainly be performed to compare gadobenate dimeglumine with conventional standard-relaxivity agents using sequence parameters optimized specifically for each agent. Similarly, further work should also be performed to intra-individually compare gadobenate dimeglumine with conventional standard-relaxivity agents at 3T in conjunction with parallel imaging techniques, because these techniques are increasingly used routinely even in conventional neuroimaging protocols (25,26). Given that SNR is sacrificed with parallel imaging to improve the spatial and/or temporal resolution of the acquisition, the greater signal intensity enhancement achievable with gadobenate dimeglumine might be of particular value in this setting.
Concerning the work of Ba-Ssalamah et al (22), they intra-individually compared cumulative triple dose (an initial 0.1 mmol/kg body weight dose followed after 10 min by an additional 0.2 mmol/kg body weight dose) gadodiamide at 1.5T and 3T in 22 patients with brain metastases and showed that a cumulative triple dose at 3T was superior to all other combinations in terms of qualitative enhancement criteria, the number of metastases detected (83–84 compared with 79–81 at cumulative triple dose and 1.5T, 71–74 at single dose and 3T and 69–71 at single dose and 1.5T) and both SNR and CNR. Similarly increased tumor-to-brain contrast at 3T compared with 1.5T had previously been noted in 16 patients with primary brain tumors and metastases who received just a single dose of gadodiamide (27). Although our study was not aimed at comparing gadobenate dimeglumine and gadopentetate dimeglumine for the detection of metastases at 3T, the higher r1 relaxivity at all magnetic field strengths (9,10) and the resulting similar contrast enhancement with a single 0.1-mmol/kg dose of gadobenate dimeglumine to that obtained with a double dose of conventional agent (20,28) suggests that improved detection of small or poorly enhancing metastatic lesions may be obtained with just a single dose of gadobenate dimeglumine rather than a higher dose of conventional agent.
Apart from the potential to more accurately define the precise number, size, and location of brain metastases to select the most appropriate treatment option, the higher r1 relaxivity of gadobenate dimeglumine and improved imaging performance at 3T as well as at 1.5T has implications also for the improved presurgical planning and postsurgical follow-up of patients with glial tumors. For patients with primary malignant tumors, macroscopically complete surgical removal is associated with improved prognosis and longer patient survival (29,30). Because glial tumors often extend beyond the contrast-enhancing and T2 signal margins (31), frequently only partial tumor resection is achieved at surgery, resulting in residual tumor on follow-up imaging. As suggested in this and previous studies (3–8) for these lesions the superiority of gadobenate dimeglumine may lie in better defining the extent and internal morphology of lesions to better optimize surgical planning and patient management. In addition, improved detection of residual tumor on early postoperative MR imaging may also improve patient prognosis (30,32). Unfortunately three-dimensional volumetric assessments (33) which might have confirmed the superiority of gadobenate dimeglumine over gadopentetate dimeglumine in defining the true extent of tumors, as well as eliminating potential bias due to slight variations in image angulation between exams, were not performed.
Concerning the different numbers of lesions detected by the different readers in our study, this may be explained by the fact that the readers did not jointly discuss the assessment criteria before image evaluation. Thus, one of them did not include certain extra-axial lesions and post-treatment changes which were included by the others; while on the other hand, each focus of enhancement in two patients with glial tumors was considered a separate lesion by one reader but as part of one lesion only by the other two readers.
A limitation of this study is that the relatively small number of patients enrolled precluded assessment of the potential clinical impact of the greater r1 relaxivity of gadobenate dimeglumine on patient management. However, as noted above and elsewhere (5,6,8), it is clear that the improved contrast enhancement achievable with an identical 0.1 mmol/kg dose would be beneficial in terms of better defining tumor resection margins or better depicting the location and number of small and otherwise poorly enhancing metastatic lesions. Alternatively, the greater contrast enhancement achievable with gadobenate dimeglumine may be of value if the clinical need is to reduce gadolinium dose. In this setting, the possibility to obtain comparable enhancement with a lower dose of gadobenate dimeglumine to that obtained with a standard dose of conventional gadolinium agent may be of particular interest for certain populations of patient for whom high and/or cumulative gadolinium doses are ill-advised (34,35).
In conclusion, this study demonstrates that the contrast efficacy achieved with a dose of 0.1 mmol/kg gadobenate dimeglumine at 1.5T (3–8) is maintained at 3T. Our findings confirm those of an earlier study in an animal model of implanted gliomas (11) in showing that qualitative and quantitative depiction of brain lesions at 3T is significantly improved with 0.1 mmol/kg body weight gadobenate dimeglumine compared with gadopentetate dimeglumine at equivalent dose.
Figure 2.
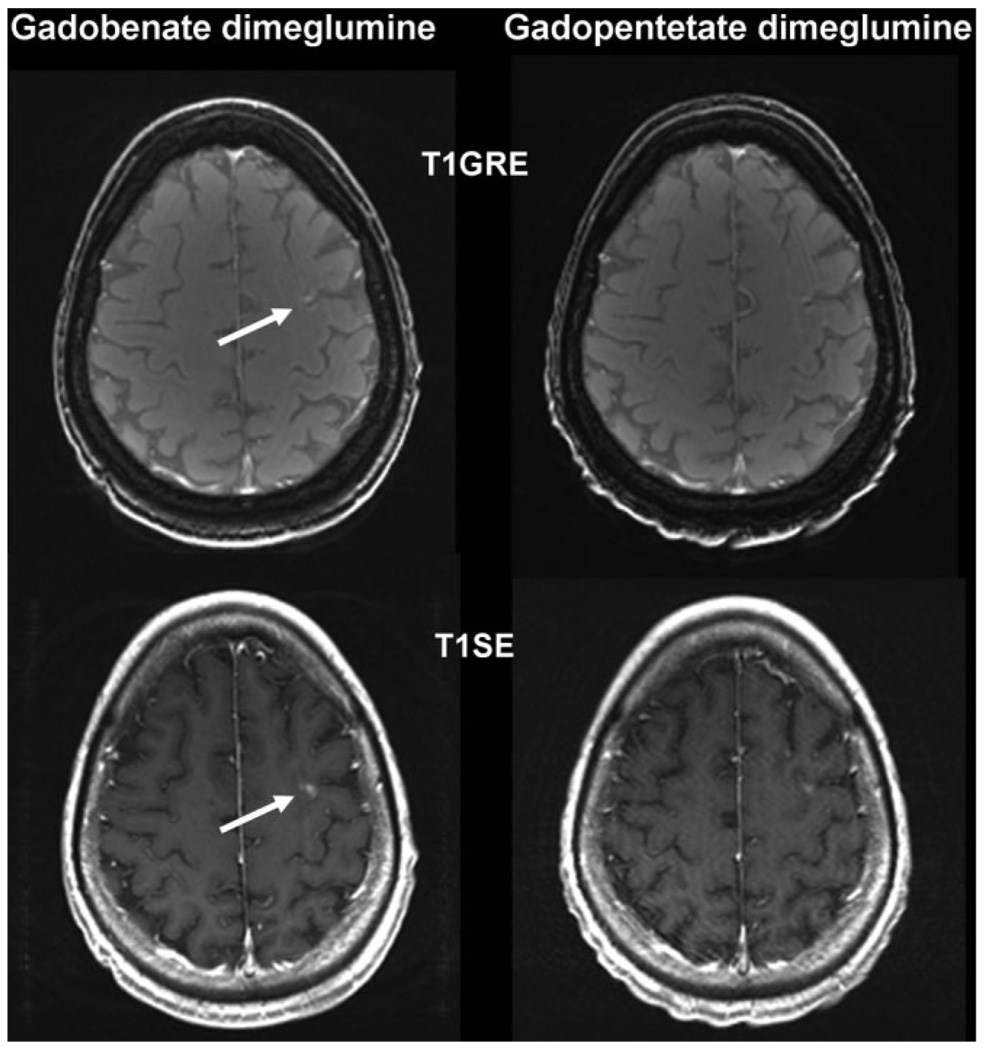
A 59-year-old woman with metastatic disease from primary lung cancer. T1SE and T1GRE images acquired from 6 min after administration of 0.1 mmol/kg gadobenate dimeglumine and 0.1 mmol/kg gadopentetate dimeglumine. The single subcortical metastasis (arrow) is readily diagnosed with gadobenate dimeglumine but is barely visible after the injection of gadopentetate dimeglumine.
References
- 1.Package Insert. Princeton, NJ: Bracco Diagnostics; MultiHance® (gadobenate dimeglumine injection) [Google Scholar]
- 2.Colosimo C, Ruscalleda J, Korves M, et al. Detection of intracranial metastases: a multi-center, intra-patient comparison of gadobenate dimeglumine-enhanced MRI with routinely used contrast agents at equal dose. Invest Radiol. 2001;36:72–81. doi: 10.1097/00004424-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Colosimo C, Knopp MV, Barreau X, et al. A comparison of Gd-BOPTA and Gd-DOTA for contrast-enhanced MRI of intracranial tumours. Neuroradiology. 2004;46:655–665. doi: 10.1007/s00234-003-1128-4. [DOI] [PubMed] [Google Scholar]
- 4.Knopp MV, Runge VM, Essig M, et al. Primary and secondary brain tumors at MR imaging: bicentric intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology. 2004;230:55–64. doi: 10.1148/radiol.2301021085. [DOI] [PubMed] [Google Scholar]
- 5.Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240:389–400. doi: 10.1148/radiol.2402051266. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn MJ, Picozzi P, Maldjian JA, et al. Evaluation of intraaxial enhancing brain tumors on magnetic resonance imaging: intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for visualization and assessment, and implications for surgical intervention. J Neurosurg. 2007;106:557–566. doi: 10.3171/jns.2007.106.4.557. [DOI] [PubMed] [Google Scholar]
- 7.Essig M, Tartaro A, Tartaglione T, Pirovano G, Kirchin MA, Spinazzi A. Enhancing lesions of the brain: intra-individual crossover comparison of contrast enhancement after gadobenate dimeglumine versus established gadolinium comparators. Acad Radiol. 2006;13:744–751. doi: 10.1016/j.acra.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 8.Rowley HA, Scialfa G, Gao P-Y, et al. Contrast-enhanced MR imaging of brain lesions: a large scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29:1684–1691. doi: 10.3174/ajnr.A1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pintaske J, Martirosian P, Graf H, et al. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol 2006;41:213–221. Erratum in Invest Radiol. 2006;41:859. doi: 10.1097/01.rli.0000197668.44926.f7. [DOI] [PubMed] [Google Scholar]
- 10.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 11.Runge VM, Biswas J, Wintersperger BJ, et al. The efficacy of gadobenate dimeglumine (Gd-BOPTA) at 3 Tesla in brain magnetic resonance imaging: comparison to 1.5 Tesla and a standard gadolinium chelate using a rat brain tumor model. Invest Radiol. 2006;41:244–248. doi: 10.1097/01.rli.0000191332.24773.e7. [DOI] [PubMed] [Google Scholar]
- 12.Criteria Committee of the American Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. New York: Little, Brown; 1994. [Google Scholar]
- 13.Kuhl CK, Träber F, Schild HH. Whole-body high-field-strength (3.0-T) MR Imaging in Clinical Practice. Part I. Technical considerations and clinical applications (review) Radiology. 2008;246:675–696. doi: 10.1148/radiol.2463060881. [DOI] [PubMed] [Google Scholar]
- 14.Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerance, biodistribution and MR imaging enhancement of the liver with gadobenate dimeglumine. Acad Radiol. 1999;6:282–291. doi: 10.1016/s1076-6332(99)80451-6. [DOI] [PubMed] [Google Scholar]
- 15.Shellock FG, Parker JR, Venetianer C, Pirovano G, Spinazzi A. Safety of gadobenate dimeglumine: summary of findings from clinical studies and post-marketing surveillance. Invest Radiol. 2006;41:500–509. doi: 10.1097/01.rli.0000209661.99225.c2. [DOI] [PubMed] [Google Scholar]
- 16.Shellock FG, Parker JR, Pirovano G, et al. Safety characteristics of gadobenate dimeglumine: clinical experience from intra- and inter-individual comparison studies with gadopentetate dimeglumine. J Magn Reson Imaging. 2006;24:1378–1385. doi: 10.1002/jmri.20764. [DOI] [PubMed] [Google Scholar]
- 17.Kirchin MA, Runge VM. Contrast agents for magnetic resonance imaging: safety update. Top Magn Reson Imaging. 2003;14:426–435. doi: 10.1097/00002142-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Cavagna FM, Maggioni F, Castelli PM, et al. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997;32:780–796. doi: 10.1097/00004424-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Giesel FL, von Tengg-Kobligk H, Wilkinson ID, et al. Influence of human serum albumin on longitudinal and transverse relaxation rates (R1 and R2) of magnetic resonance contrast agents. Invest Radiol. 2006;41:222–228. doi: 10.1097/01.rli.0000192421.81037.d5. [DOI] [PubMed] [Google Scholar]
- 20.Trattnig S, Pinker K, Ba-Ssalamah A, N0öbauer-Huhmann IM. The optimal use of contrast agents at high field MRI (review) Eur Radiol. 2006;16:1280–1287. doi: 10.1007/s00330-006-0154-0. [DOI] [PubMed] [Google Scholar]
- 21.Krautmacher C, Willinek WA, Tschampa HJ, et al. Brain tumors: full-and half-dose contrast-enhanced MR imaging at 3.0 T compared with 1.5 T—Initial Experience. Radiology. 2005;237:1014–1019. doi: 10.1148/radiol.2373041672. [DOI] [PubMed] [Google Scholar]
- 22.Ba-Ssalamah A, Nöbauer-Huhmann IM, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol. 2003;38:415–422. doi: 10.1097/01.RLI.0000067488.57101.bd. [DOI] [PubMed] [Google Scholar]
- 23.Bleicher AG, Kanal E. A serial dilution study of gadolinium-based MR imaging contrast agents. AJNR Am J Neuroradiol. 2008;29:668–673. doi: 10.3174/ajnr.A0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yrjänä SK, Vaara T, Karttunen A, Koivukangas J. Pulse repetition time and contrast enhancement: simulation study of Gd-BOPTA and conventional contrast agent at different field strengths. Invest Radiol. 2008;43:267–275. doi: 10.1097/RLI.0b013e3181636eb8. [DOI] [PubMed] [Google Scholar]
- 25.Lupo JM, Lee MC, Han ET, et al. Feasibility of dynamic susceptibility contrast perfusion MR imaging at 3T using a standard quadrature head coil and eight-channel phased-array coil with and without SENSE reconstruction. J Magn Reson Imaging. 2006;24:520–529. doi: 10.1002/jmri.20673. [DOI] [PubMed] [Google Scholar]
- 26.Mikulis DJ, Roberts TP. Neuro MR: protocols. J Magn Reson Imaging. 2007;26:838–847. doi: 10.1002/jmri.21041. [DOI] [PubMed] [Google Scholar]
- 27.Nöbauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al. Magnetic resonance imaging contrast enhancement of brain tumors at 3 Tesla versus 1.5 Tesla. Invest Radiol. 2002;37:114–119. doi: 10.1097/00004424-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Yuh WT, Fisher DJ, Engelken JD, et al. MR evaluation of CNS tumors: dose comparison study with gadopentetate dimeglumine and gadoteridol. Radiology. 1991;180:485–491. doi: 10.1148/radiology.180.2.2068317. [DOI] [PubMed] [Google Scholar]
- 29.Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–60. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Warmuth-Metz M. Postoperative imaging after brain tumor resection. Acta Neurochir Suppl. 2003;88:13–20. doi: 10.1007/978-3-7091-6090-9_4. [DOI] [PubMed] [Google Scholar]
- 31.Ciric I, Vick NA, Mikhael MA, et al. Aggressive surgery for malignant supratentorial gliomas. Clin Neurosurg. 1990;36:375–383. [PubMed] [Google Scholar]
- 32.Ekinci G, Akpinar IN, Baltacioglu F, et al. Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor re-growth and recurrence. Eur J Radiol. 2003;45:99–107. doi: 10.1016/s0720-048x(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 33.Keles GE, Chang EF, Lamborn KR, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34–40. doi: 10.3171/jns.2006.105.1.34. [DOI] [PubMed] [Google Scholar]
- 34.Penfield JG, Reilly RF., Jr What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol. 2007;3:654–668. doi: 10.1038/ncpneph0660. [DOI] [PubMed] [Google Scholar]
- 35.Marckmann P, Skov L, Rossen K, et al. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant. 2007;22:3174–3178. doi: 10.1093/ndt/gfm261. [DOI] [PubMed] [Google Scholar]