Abstract
4-Hydroxynonenal (HNE), a major electrophilic product of lipid peroxidation, is regarded as both a marker of oxidative stress and a mediator of oxidative damage. At subtoxic concentrations, however, this compound has been shown to be a signalling molecule that can induce the expression of various antioxidant/detoxification enzymes, including glutamate-cysteine ligase (GCL), the rate-limiting enzyme in the de novo synthesis of glutathione. GCL consists of a catalytic (GCLC) and modulatory (GCLM) subunit, which are encoded by separate genes. Here, we investigated the effect of submicromolar concentrations of HNE on the expression of the GCL genes and the transcription factors involved. We demonstrated that submicromolar concentrations of HNE (as little as 0.3 µM) could increase the expression of both GCLC and GCLM. We also found that the induction of GCL expression was abrogated by siRNA for Nrf2. Our data suggest that a submicromolar concentration of HNE, as found in human plasma under physiological conditions, can induce GCL transcription in cultured cells implying that ‘basal’ expression of GCL is under regulation by lipid peroxidation that occurs under physiological conditions. Moreover, this induction is mediated through the EpRE-Nrf2 signalling pathway thought to be predominantly active only during stress.
Keywords: 4-Hydroxynonenal, glutamate-cysteine ligase, EpRE-Nrf2 pathway
INTRODUCTION
HNE is the major α,β-unsaturated aldehyde produced during the peroxidation of ω-6 polyunsaturated fatty acids. Since its discovery by Esterbauer et al.,1 this compound has been widely used as a biomarker of patho-physiological processes associated with oxidative stress; indeed, its tissue concentration is increased in any investigated conditions of oxidative stress.2 As a reactive electrophile, HNE is toxic at high concentration and has been complicated in various diseases, such as atherosclerosis, neurodegenerative diseases, and chronic obstructive pulmonary disease (COPD). At subtoxic concentration, however, HNE could activate multiple signaling pathways3 and is involved in cell proliferation,4 differentiation, apoptosis5 and gene regulation. A variety of genes have been found to be regulated by HNE, including genes involved in stress adaptation and detoxification, such as glutathione S-transferase (GST),6 heme oxygenase-1 (HO-1),7 γ-glutamyl transpeptidase,8 and glutamate cysteine ligase.9
Glutamate cysteine ligase (GCL) catalyzes the first and rate-limiting step of the de novo synthesis of glutathione (GSH), the most abundant non-protein thiol in the cell. GSH plays key roles in detoxifying peroxides, electrophiles, and maintaining the normal redox status. GCL protein is a heterodimer composed of a catalytic (GCLC) and modulatory (GCLM) subunit, which are encoded by separate genes in humans. GCL can be regulated at kinetic, post-translational and transcriptional levels. An example of the first regulatory mechanism is the feedback inhibition of GCL activity by GSH; indeed, a decrease in GSH will cause a transient elevation of GSH synthesis due to a decrease of this feedback inhibition on the activity of pre-existent GCL. However, the regulation of GCL at the transcription level produces a more persistent effect and thus is more important for the maintenance of GSH homeostasis in response to oxidative stress. It has been well established that both GCLC and GCLM are inducible at the transcription level by various agents;10 interestingly, many of the GCL inducers are redox active and can, therefore, cause generation of reactive oxygen species and thus HNE in vivo, while others cause GSH depletion that could indirectly result in increased HNE.
Several DNA cis-elements including the NF-κB binding site, the AP-1 binding site (TRE element), and electrophile response element (EpRE, also called the antioxidant response element) have been implicated in GCL gene regulation. 11 Previously, we found that both GCLC and GCLM gene expressions were induced by HNE in human bronchial epithelial cells (HBE1) and both TRE and EpRE appeared to be involved.9 Many transcription factors have been reported to bind EpRE, such as Nrf family members (Nrf1/2/3), small Maf proteins (MafG/K/F), Jun (c-Jun, JunB, JunD), and Fos family members (c-Fos, FosB, Fra1, Fra2, etc.) as reviewed by Jaiswal12. Among them, Nrf2 is the most firmly established EpRE binding protein. Nrf2 is located in the cytosol at resting state and, upon stimulation, is translocated into the nucleus, where it heterodimerizes with other leucine zipper proteins such as c-Jun and small Maf proteins, and binds EpRE to transactivate gene transcription. We have previously found the transcription factors binding to the consensus human GCL EpRE might include Nrf2, c-Jun, JunB, and JunD in HBE1 cells.13
HNE is reported to induce different pathways depending on the intracellular concentration. For example, while HNE promotes proliferation at low concentrations, at higher concentration it induces differentiation and apoptosis.5 HNE is present in the free form at a concentration beginning at 0.1 µM up to 1.4 µM in human plasma and its concentration can increase 10 times or more during oxidative stress in vivo.14 It has been shown in previous studies that HNE induces GCLC and GCLM expression at micromolar concentration (5–15 µM).9 However, whether physiological concentrations (submicromolar) of HNE could induce GCL gene expression was unclear. Also, which transcription factors are involved in HNE-mediated GCL induction remained uncertain. Herein we present data to answer these questions and our results demonstrate that HNE could be involved in GCL gene regulation under physiological conditions through activation of EpRE-Nrf2 signalling pathway.
MATERIALS AND METHODS
Chemicals and reagents
4-HNE was purchased from Cayman Chemical (Ann Arbor, MI, USA). TaqMan reverse transcription reagent and SYBR Green PCR Master Mix were from Applied Biosystems (Foster City, CA, USA). pGL3 luciferase reporter vectors, competent cells, luciferase activity assay kit and restriction enzymes were from Promega (Madison, WI, USA). FuGENE 6 transfection reagent was from Roche (Indianapolis, IN, USA). Antibodies and siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). M-PER mammalian protein extraction reagent and NE-PER nuclear extraction reagents were from Pierce (Rockford, IL, USA). All chemicals used were at least analytical grade.
Cell culture and treatments
HBE1 is an immortalized human bronchial epithelial cell line obtained from Dr James Yankaskas at the University of North Carolina. HBE1 cells were cultured in a modified Ham’s F12 medium (Life Technologies, Grand Island, NY, USA) supplemented with six additives (5 µg/ml insulin, 3.7 µg/ml epidermal growth factor, 3 × 10−8 M tri-iodothyrorine, 1 × 10−6 M hydrocortisone, and 5 µg/ml transferrin), 100 U/ml penicillin and 100 µg/ml streptomycin in collagen-coated dishes in 5% CO2 at 37°C. 4-HNE was dissolved in ethanol. HBE1 cells near confluence (about 90% confluent) were treated with vehicle control (0.05% ethanol) or different concentrations of HNE as indicated in the Results.
Real-time PCR assay of mRNA levels
Total RNA was extracted using TRIzol reagent and treated with DNA-free reagent according to the manufacturer’s protocol (Ambion). RNA samples were reverse-transcribed using the TaqMan random hexamers (Applied Biosystems) and the contents of GCLC and GCLM mRNA were measured by real-time polymerase chain reactions (RT-PCR) with a Cepheid 1.2 real-time PCR machine. Briefly, 5 µl of reverse transcription reaction product was added to a reaction tube containing 12.5 µl SYBR Green PCR Master Mix and primers specific for GCLC or GCLM mRNA; the total PCR sample was 25 µl. GAPDH was used as an internal control. The primers are as following: GCLC, sense 5′-ATGGAGGTGCAATTAACAGAC-3′, anti-sense 5′-ACTGCATTGCCACCTTTGCA-3′; GCLM, sense 5′-GCTGTATCAGTGGGCACAG-3′, antisense 5′-CGCTTGAATGTCAGGAATGC-3′; GAPDH, sense 5′-TGGGTGTGAACCATGAGAAG-3′, antisense 5′-CCATCACGACACAGTTTCC-3′.
Plasmids
The plasmids −3802/GCLC 5′-luc and −1927GCLM 5′-luc were kindly provided by Professor Dale A. Dickinson. The −3802/GCLC 5′-luc and −1927GCLM 5′-luc were constructed by cloning 3802 bp or 1927 bp nucleotides upstream of transcription start site of the GCLC or GCLM gene, respectively, into pGL3-basic luciferase vector. For details about the plasmids cloning, see elsewhere. 15,16
Transfection of Nrf2 siRNA
Transfection of siRNA was performed using FuGENE 6 transfection reagent following the procedure provided with the transfection reagent. For Nrf2 protein assay, HBE1 cells at about 60–80% confluence were transfected with 50 nM Nrf2 siRNA or control siRNA (Santa Cruz Biotechnology) in 60 mm plates. Twenty-four hours after transfection, medium was replaced and cells were treated with or without 10 µM HNE for 1 h. The cells were collected and the cytosol and nuclear proteins were extracted using NE-PE0R nuclear extraction reagents for Nrf2 protein assay. For the effect of Nrf2 siRNA on promoter activity, transfection was done in 12-well dishes as described below and 50 nM Nrf2 siRNA or control siRNA was co-transfected with plasmids.
Transfection of plasmids and assay of luciferase and β-galactosidase activity
Cells (60–80% confluence) were transfected with plasmid (0.5 µg/well) or plasmid and 50 nM Nrf2 siRNA or control siRNA using FuGENE 6 transfection reagent in 12-well plates, and β-galactosidase plasmid (1/10 of total amount of plasmid) was co-transfected to normalize for transfection efficiency. Twenty-four hours after transfection, the medium was replaced and cells were treated with/without HNE. After treatment, cells were rinsed with 1x PBS and then lysed with M-PER mammalian protein extraction reagent (Pierce). After centrifugation, the supernatant was used for determination of the activity of luciferase and β-galactosidase with procedures described previously.17
Western blotting
Western blotting was performed as described previously. 9 Briefly, protein was extracted, and 25 µg protein was heated for 15 min at 95°C in a 2x loading buffer containing SDS (Tris base, pH 6.5, glycerol, DTT, and pyronin Y), electrophoresed under denaturing conditions on a 10% Tris-glycine acrylamide gel (Invitrogen, Carlsbad, CA, USA), and then electroblotted onto a poly-vinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA, USA). Membranes were blocked with 5% fat-free milk at room temperature for 1 h, and then incubated overnight at 4°C with appropriate primary antibody in 5% milk in Tris-buffered saline (TBS). After being washed with Tris-buffered saline containing 0.05% Tween 20 (TTBS), the membrane was incubated with appropriate secondary antibody at room temperature for 2 h. After TTBS washing, the membrane was treated with an enhanced chemiluminescence (ECL Plus; Amersham, Arlington Heights, IL, USA) reagent mixture for 5 min. The target bands were imaged on a Kodak Image Station 2000R.
Statistical analysis
We used the comparative ΔΔCT method for the relative mRNA quantification as described previously.17 All data were expressed as the mean ± SE. Sigma Stat software was used for statistical analysis and statistical significance was accepted when P < 0.05. Comparison of variants between experimental groups was performed with ANOVA and the Tukey’s test.
RESULTS
Submicromolar concentration of HNE up-regulates GCLC and GCLM gene expression
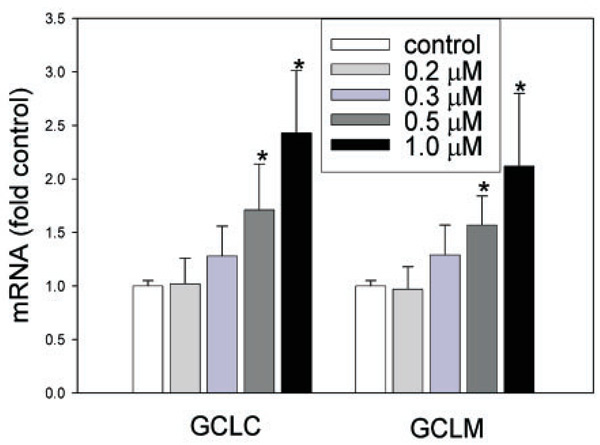
HNE exposure has been shown to cause an increase in both GCLC and GCLM mRNAs in HBE1 cells at micromolar concentration. To determine the effect of the submicromolar concentration of HNE, HBE1 cells were repeatedly treated with different concentrations of HNE every 3 h for 3 times (additions at 0, 6, and 9 h with a total of 12 h of incubation). As shown in Figure 1, exposure of HBE1 cells to submicromolar concentrations of HNE dose-dependently increased the mRNA levels of both GCLC and GCLM, and the inductions by 0.5 µM and 1 µM HNE are statistically significant.
Fig. 1.
Submicromolar concentrations of HNE induce GCL gene expression.(A) mRNA levels of GCLC and GCLM after exposure to HNE. HBE1 cells were exposed to HNE or vehicle alone at the doses indicated every 3 h for 9 h and then harvested at 12 h. GCLC and GCLM mRNA levels were measured with real-time PCR method. n = 4, *P < 0.05.
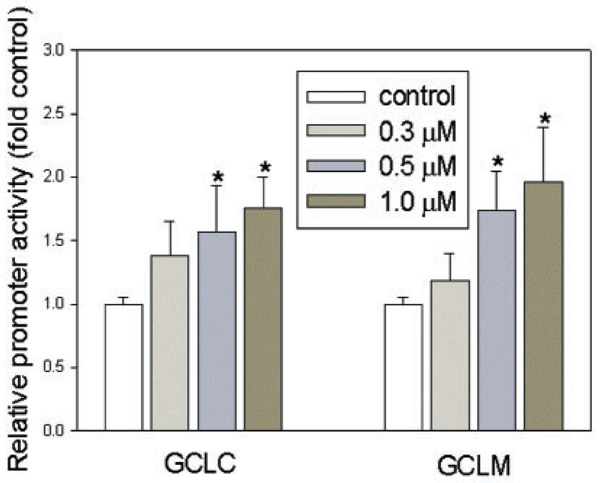
To examine whether the increase in mRNA content could be due to an increase in transcription rather than mRNA stability, HBE1 cells were transfected with reporter plasmids containing the promoter region of GCLC or GCLM and exposed to vehicle control (0.05% ethanol) or submicromolar HNE. A dose-dependent increase in the promoter activities of both GCLC and GCLM (as reflected by luciferase activity) was observed (Fig. 2), with a significant increase at concentration of 0.5 µM and 1 µM. These data suggest that a submicromolar concentration of HNE increases the expression of both GCLC and GCLM genes by inducing the transcription.
Fig. 2.
Effects of HNE on activity of GCL promoters. HBE1 cells were transfected with plasmids −3802/GCLC 5′-luc and −1927/GCLM 5′-luc and 24 h after transfection, cells were treated as described above. Luciferase activity was normalized with the activity of co-transfected β-galactosidase. n = 3, *P < 0.05.
Nrf2 is involved in HNE-mediated induction of GCLC and GCLM gene
Previous studies reported that both EpRE and TRE were potentially involved in the induction of GCL genes, and electrophoretic mobility shift assays with antibodies to transcription factors (known as supershift or immunodepeletion assays) were used to demonstrate that transcription factors binding to these cis-elements could be increased by treatment of HBE1 cells with micromolar concentrations of HNE.9 These are useful assays but do not actually demonstrate the involvement of transcription factors in vivo. In order to check the involvement of Nrf2, the most established EpRE binding protein, in the induction of both GCLC and GCLM genes, we investigated the effect of Nrf2 silencing on GCL induction.
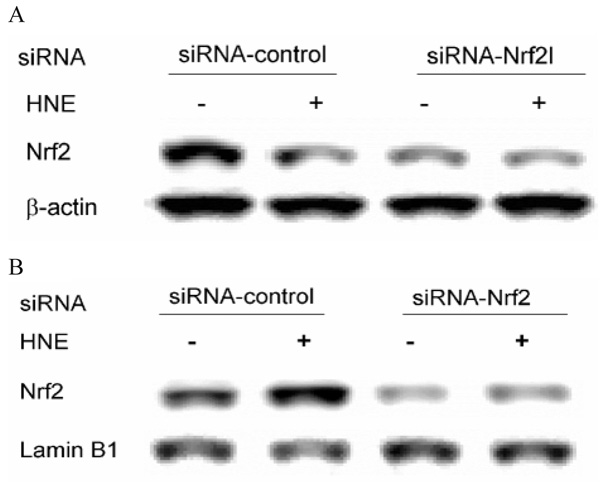
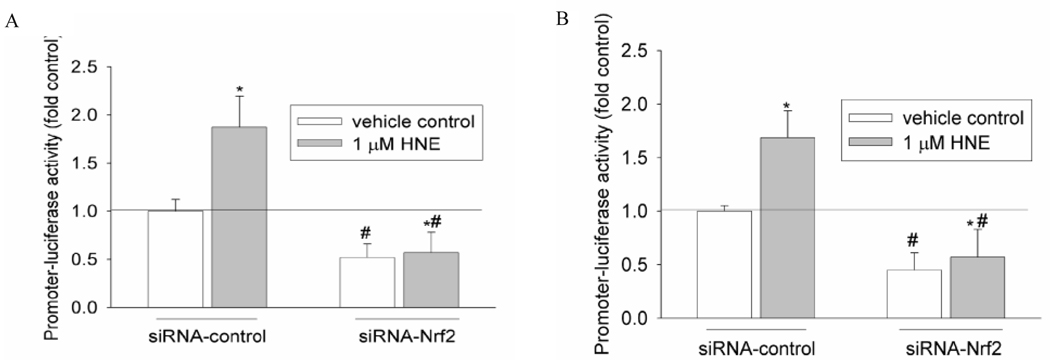
For this purpose, siRNA of Nrf2 was co-transfected with −3802/GCLC 5′-luc and −1927GCLM 5′-luc, respectively, and its effect on GCL promoters’ activation was determined. After transfection with 50 nM Nrf2 siRNA for 24 h, either the expression of Nrf2 protein or its nuclear translocation after 10 µM HNE treatment was significantly decreased (Fig. 3). Meanwhile, Nrf2 siRNA abrogated 1 µM HNE-mediated induction of both GCLC and GCLM (Fig. 4A,B). These data reveal that Nrf2 is involved in HNE-mediated induction of GCL genes.
Fig. 3.
Nrf2 protein in the cytosol (A) and nucleus (B) after siRNA treatment. HBE1 cells were transfected with 50 nM siRNA specific for human Nrf2 mRNA (siRNA-Nrf2) or 50 nM siRNA that does not target any known cellular mRNA (siRNA-control) for 24 h before treatment with 10 µM HNE for 1 h as described in Materials and Methods. The cytosol and nuclear proteins were then extracted and Western blotting was performed.
Fig. 4.
Effects of Nrf2 silencing on HNE-mediated GCL induction. (A) Effect on GCLC induction. (B) Effect on GCLM induction. HBE1 cells were transfected with plasmids −3802/GCLC 5′-luc or −1927/GCLM 5′-luc and 50 nM siRNA-Nrf2 or siRNA-control. Twenty-four hours after transfection, cells were treated with 1 µM HNE every 3 h for 12 h and the luciferase activity was determined. n = 3, *P < 0.05 compared with siRNA-control without HNE treatment, and #P < 0.05, compared with siRNA-control with HNE treatment.
DISCUSSION
GSH is the most abundant non-protein thiol in cells and it plays key roles in multiple biological functions, including scavenging free radicals, conjugation and detoxification of electrophiles, and maintenance of normal cellular redox status. It has been well established that the GSH homeostasis is maintained under various oxidative conditions via the increased expression of GCL genes. We demonstrate here that HNE, at submicromolar levels as present in human tissues at physiological condition, increases GCL gene expression in cultured tracheobronchial epithelial cells. We also showed that an EpRE-Nrf2 signaling pathway is involved in this HNE-mediated GCL induction.
Previous studies have shown that micromolar concentrations of HNE can increase the gene expression of both GCLC and GCLM, and thus leads to an increase in the GSH concentration and protection of cells from higher oxidative/electrophilic stress;18 whether physiological concentrations (less than 1 µM) of HNE have such effect, however, was unknown. This study showed that a submicromolar concentration of HNE could induce GCL gene expression. Given the fact that HNE can interact with molecules present in the serum of the medium19 and that a large portion of HNE added exogenously (90–95% of 100 µM) is degraded very quickly,20 it is likely that the effective dose of HNE was even less than that initially used in this study. These data suggest that a very small concentration of HNE appears to be enough to induce GCL expression and that HNE may play important roles in the regulation of GCL and in the maintenance of GSH homeostasis in vivo even under non-stressed conditions.
We also found that transcription factor Nrf2 was involved in both GCLC and GCLM gene regulation upon the exposure of submicromolar concentrations of HNE. This result is consistent with other studies which have shown the presence of Nrf2 in the EpRE complex by electrophoretic mobility shift assay.17 Since Nrf2 binds only EpRE, these data also suggest that it is EpRE instead of other cis-elements that are involved in HNE-mediated GCL induction. Nrf2 is a redox-sensitive transcription factor and its activation upon exposure to HNE has been well recognized. Once translocated into the nucleus, Nrf2 forms heterodimers with other leucine zipper proteins; these dimmers then bind EpRE and activate gene expression. Many nucleoproteins can dimerize with Nrf2, such as small Maf proteins and Jun family members (c-Jun, JunB, JunD). Further study on which nuclear proteins are involved in Nrf2 dimerization and EpRE (both GCLC and GCLM) activation is underway.
CONCLUSIONS
4-HNE, a major α,β-unsaturated aldehyde present in human plasma under physiological conditions and increasingly produced during stressful conditions such as inflammation or exposure of pollutants, increased GCL gene expression at submicromolar levels through an EpRE-Nrf2 signalling pathway in human bronchial epithelial cells.
ACKNOWLEDGEMENTS
This work was supported by funds from the California Tobacco Related Diseases Research Program (14RT-0059) and the National Institutes of Health (ES05511).
REFERENCES
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 3.Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Cambiaggi C, Dominici S, Comporti M, Pompella A. Modulation of human T lymphocyte proliferation by 4-hydroxynonenal, the bioactive product of neutrophil-dependent lipid peroxidation. Life Sci. 1997;61:777–785. doi: 10.1016/s0024-3205(97)00559-6. [DOI] [PubMed] [Google Scholar]
- 5.Awasthi YC, Yang Y, Tiwari NK, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda A, Nakamura Y, Ohigashi H, Osawa T, Uchida K. Cellular response to the redox active lipid peroxidation products: induction of glutathione S-transferase P by 4-hydroxy-2-nonenal. Biochem Biophys Res Commun. 1997;236:505–509. doi: 10.1006/bbrc.1997.6585. [DOI] [PubMed] [Google Scholar]
- 7.Ishii I, Akahoshi N, Yu XN, et al. Murine cystathionine gammalyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-Hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic Biol Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson DA, Iles KE, Watanabe N, et al. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 10.Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of gamma-glutamylcysteine synthetase. J Cell Physiol. 2000;182:163–170. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 14.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J Lipid Mediat Cell Signal. 1995;11:51–61. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 15.Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 16.Moinova HR, Mulcahy RT. An electrophile responsive element (EpRE) regulates beta-naphthoflavone induction of the human gamma-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J Biol Chem. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Liu H, Iles KE, et al. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated EpRE/Nrf2 signaling. Am J Respir Cell Mol Biol. 2006;34:174–181. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med. 2005;38:547–556. doi: 10.1016/j.freeradbiomed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Zarkovic N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 20.Siems W, Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol Aspects Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]