Abstract
Objective
To show that low luminance visual dysfunction is predictive of subsequent visual acuity (VA) loss in eyes with geographic atrophy (GA) from age-related macular degeneration (AMD)
Design
Cohort Study. Prospective natural history study of GA, from 1992–2000, at the Wilmer Eye Institute.
Participants
Ninety-one participants with GA from AMD without choroidal neovascularization in at least one eye, who completed a two-year study examination.
Methods
Annual examinations included measurement of best-corrected VA, low luminance VA, Pelli-Robson contrast sensitivity, reading speed, examination, and fundus photography. The total GA area was quantified, as was the GA within a 10.2 mm2 circle centered on the fovea.
Main Outcome Measures
Visual acuity loss at two years. Risk factors for visual loss.
Results
Participants with baseline VA>=20/50 had a 40% two-year rate of >=3 line VA loss, compared to 13% for the participants with worse baseline acuities. The baseline low luminance VA deficit (LLD) was a strong predictor of subsequent VA loss for all levels of baseline VA. Within the good baseline VA group, the relative risk of 3-line loss for the worse LLD group compared to the better LLD group was 2.88 [95% confidence interval (CI) 1.13–7.35]. The LLD is a stable and reproducible measure. Other significant visual function predictors of subsequent VA loss in eyes with good baseline VA included foveal dark-adapted sensitivity 4.20 [1.39–12.71], and reduced reading rate 2.43 [1.11–5.31]). The rate of VA loss within the good acuity group was higher when the GA included 25% to75% of the central 10.2 mm2, than in eyes with GA including less than 25%, or more than 75%, of the central 10.2 mm2. The following were not significant predictors of subsequent VA loss among these participants: age, gender, fellow eye diagnosis, fellow eye VA, baseline GA area, GA enlargement rate.
Conclusions
Visual function measures can predict the risk of future VA loss in subjects with GA and good baseline VA. They may allow identification of the highest risk group for VA loss, allowing more efficient design of clinical trials. They also may be appropriate surrogate measures of foveal health in short term treatment trials.
Geographic atrophy (GA) is the advanced ‘dry’, or atrophic, form of age-related macular degeneration (AMD). Large population-based studies report a prevalence of GA of 2% in people age 75–84, and 7.3% in people age 85 and above, 1 and its prevalence rises to over 20% in people age 90 and above. 2 Unlike choroidal neovascularization, the ‘wet’ or exudative form of advanced AMD, GA progresses gradually over time, and it is responsible for moderate as well as severe visual loss. The gradual progression of GA, the tendency to group subjects with GA along with subjects with early atrophic AMD who have little visual loss, and the fact that there currently is no treatment for GA have led to a lack of appreciation of the degree of public health importance of this condition.
GA often first develops surrounding the fovea, but may not directly involve the foveal center until late in the course of the disease. Subjects may have good visual acuity (VA) for single letters, but have difficulty with reading and face recognition, because words or a full face do not ‘fit’ in the spared foveal region surrounded by atrophy. Eventually, the fovea itself becomes atrophic, with VA falling to the legal blindness level. 3, 4 This early preservation of single letter VA despite visual impairment is another factor that has led to a lack of attention to this disorder, a situation that has begun to change only recently.
Subjects with GA from AMD that does not yet involve the foveal center are at high risk for future VA loss. In our NIH-funded prospective natural history study of GA, 40% of study participants with GA and good baseline VA (defined as VA 20/50 and better) experienced at least 3-line VA loss at two years, 4 a rate of VA loss that is greater than that for eyes with clinically significant diabetic macular edema 5 which is widely recognized as a public health issue. In addition, most patients with GA have involvement of both eyes. Consequently, finding treatments to slow GA progression or prevent GA development is a current public health problem. By four years, 27% of these subjects with GA and good baseline VA worsened to 20/200 or worse.4 In contrast, the rate of at least 3-line VA loss in subjects with GA with baseline VA worse than 20/50 was only 13%. In this paper, we focus on risk factors for at moderate or severe VA loss at two years in the good baseline VA group.
A number of studies, as well as general clinical experience, have shown that subjects with AMD in general, and GA in particular, have significant additional impairment in dimly lit environments, and require a great deal of light in order to see and read optimally. 6–8 In our study, we quantified the significant worsening of VA associated with dim lighting. 8 Further analyses have shown that the degree of loss of VA in low luminance conditions at baseline was predictive of the subsequent VA loss at two years. The low luminance measure is stable over time, simple and rapid to perform, and requires only a neutral density filter over and above standard ophthalmologic equipment. These findings suggest that the VA deficit in low luminance for GA subjects can be used to identify subjects at high risk of VA loss in GA. They also suggest that the low luminance VA deficit may be a surrogate outcome that can be used to assess the efficacy of potential treatments for GA. This paper investigates these suggestions. Other visual function measures are also evaluated as risk factors for more rapid VA loss.
METHODS
A National Institutes of Health (NIH)-funded prospective natural history study of GA from AMD was conducted at the Wilmer Eye Institute between 1992 and 2000. The study was approved by the Johns Hopkins University School of Medicine’s institutional review board, and written informed consent was obtained from all study participants. This study adhered to the Declaration of Helsinki. One hundred fifty eight subjects enrolled. The primary entry criterion was having GA from AMD, without evidence of choroidal neovascularization (CNV) on clinical evaluation or baseline fluorescein angiogram, in one or both eyes. Geographic atrophy was defined as one or more discrete areas, measuring 500 microns or more, of loss of retinal pigment epithelium (RPE), with a color and thickness change relative to the surrounding retina, and more prominent visualization of the choroidal vessels. There was no exclusion criterion based on VA or age. Eyes with other concurrent retinal disorders were excluded.
Of the 158 subjects enrolled, 131 completed two or more study examinations, with median follow up of 4.3 years. Ninety one had a 2 year study examination, that is, a follow-up study examination between 1.5 and 2.5 years after the baseline study examination. These 91 participants are the subject of this report. For subjects with bilateral GA without CNV in either eye, the eye with the better VA was chosen as the study eye for this report, so that only one eye per subject is included for the bilateral group as well as the groups with CNV or only drusen in the fellow eye.
Baseline and annual examinations included a protocol refraction, measurement of VA using an Early Treatment Diabetic Retinopathy Study (ETDRS) chart, ophthalmoscopic examination, and 30 and 60 deg color fundus photographs. Fluorescein angiography was performed at the baseline study examination to rule out CNV, and was subsequently repeated only when CNV or a retinal vascular disorder was suspected. In addition, the participants underwent a battery of additional visual function tests at baseline and at each annual examination. These included Pelli-Robson contrast sensitivity, reading rate at nine character sizes for a paragraph of unrelated words, scanning laser ophthalmoscope macular perimetry, foveal dark-adapted sensitivity in selected participants with good central fixation, and low luminance VA. These tests have been described in detail in the past. 8
LogMAR (logarithm of the minimal angle of resolution) VA was used for the analyses, but the VA is presented in equivalent Snellen notation. As is standard, a worsening of VA of 3 or more lines on the ETDRS chart (>=0.3 logMAR) is called at least moderate visual loss, and a worsening of 6 or more lines (>=0.6 logMAR) is considered severe visual loss. Mean loss of VA and mean VA for the different groups also are presented, both at two years and at the final examination for each patient.
Low luminance VA was measured by placing a 2.0 log unit neutral density filter (i.e. a filter that lowers luminance by 100 times) (Kodak Wratten filter, Rochester, NY) over the best correction for that eye, and having the participant read the normally illuminated ETDRS chart. For each eye, a protocol refraction was performed first, followed by the measurement of standard ETDRS VA and then low luminance VA. The baseline low luminance deficit (LLD) was calculated as the difference, in logMAR units, between the low luminance VA measurement and the standard VA measurement at the baseline examination. For example, if the standard ETDRS VA was 0.3 logMAR (20/40), and the low luminance VA was 1.0 logMAR (20/200), the LLD would be 1.0 minus 0.3, or 0.7 logMAR, corresponding to a 7-line difference on the ETDRS chart. As reported previously, for eyes with GA and VA better than 20/50, the median drop of VA under low luminance conditions was 4.5 lines (i.e., median LLD was 0.45 logMAR). In contrast, for elderly participants with drusen and no GA or other advanced AMD, the median LLD was 0.23 logMAR, and no participant had a difference of more than 0.5 logMAR (5 lines on the ETDRS chart). 8 The LLD is a rapid test, and previous work has shown that no additional period of adaptation is required. 8
Foveal dark-adapted sensitivity was performed for participants who had foveal fixation. The pupil was dilated, and the patient was dark-adapted for 45 minutes. Tubingen static perimetry was then performed at the fovea, using a diamond fixation pattern. A 1.8 degree red (650 nm) stimulus was used, and dark-adapted sensitivity at the fovea was measured. 9
Annual fundus photographs were used to define the areas of atrophy, and spared regions within the atrophy, on a fundus drawing. The affected areas were outlined and measured. The total area of atrophy was the sum of all the areas of atrophy present on the fundus photograph (less any spared regions within the atrophic areas). The total GA area data is given both in mm2 on the retina and in Macular Photocoagulation Study Disc Areas (MPS DA), because of the familiarity that many retinal specialists have with this measure. One MPS DA is equivalent to 2.54 mm2 on the retina. The amount of central atrophy was measured as the amount of atrophy within a 4 Macular Photocoagulation Study disc area (4 MPS DA=10.2 mm2) circle centered on the fovea. The methodology used is described in detail elsewhere. 10 Briefly, a 10.2 mm2 circle on an acetate sheet was overlaid on the fundus drawing, and the area of GA within the circle was outlined. The areas of GA outlined were digitized, and the size of the affected area was computed automatically.
Statistical Analysis
JMP expert data analysis software (version 5.0.1 SAS, Inc.,Cary, North Carolina) was used to perform the statistical analyses provided below. The Chi sq and Fisher exact tests, relative risks, and logistic regression, were used to test the significance of differences between groups for categorical variables. Linear regression anad the Student’s t test was used to assess the significance of data with continuous variables.
RESULTS
Table 1 gives the baseline characteristics for the 91 participants with 2-year data.
Table 1.
Baseline characteristics of participants included in this analysis
| 91 participants included in two-year analysis |
||
|---|---|---|
| Baseline characteristics of subjects and study eye | Mean | |
| Age | 78.3 years | |
| Visual acuity | 20/55 | |
| Area of GA | 9.9 sq mm | |
| % of central 10.2 mm2 (4 MPS disc areas) having GA | 50% | |
| Diagnosis of fellow eye (number of subjects (%)) | ||
| GA (i.e. bilateral GA) | 60 (66%) | |
| Choroidal neovascularization | 23 (25%) | |
| Drusen | 6 (7%) | |
| Unknown | 2 (2%) | |
GA = geographic atrophy, MPS = Macular Photocoagulation Study.
Moderate and severe visual acuity loss at two years
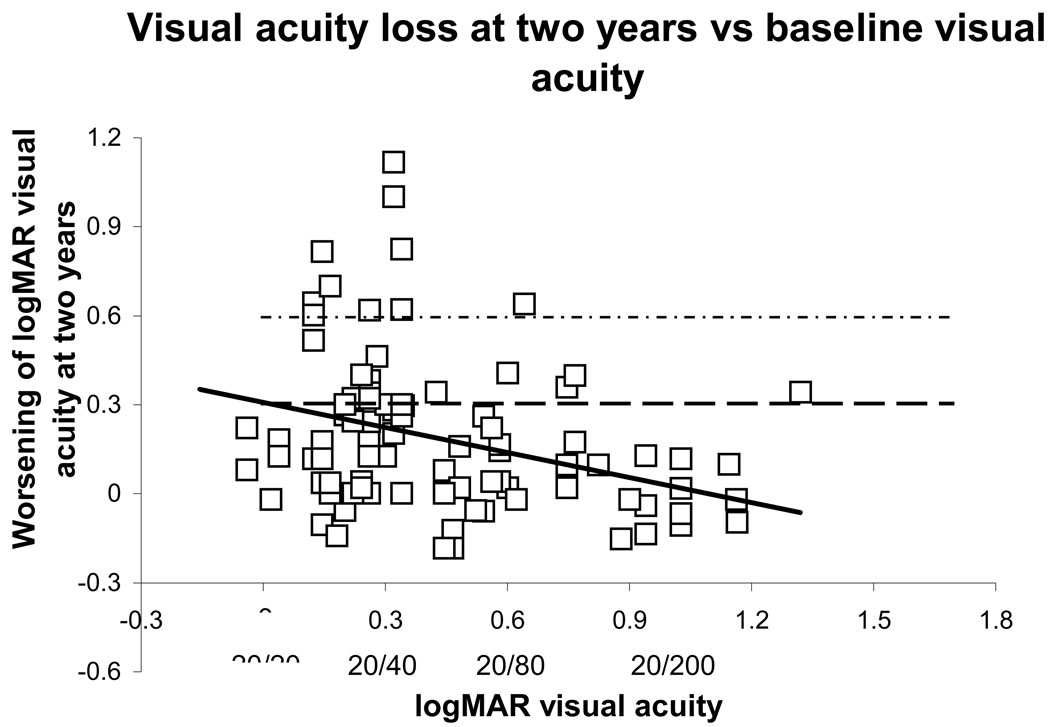
Figure 1 shows the VA loss at 2 years relative to baseline, as a function of baseline VA. The symbols above the dashed horizontal line represent study eyes for which there was at least moderate VA loss. The symbols above the upper dashed horizontal line represent eyes for which there has been severe VA loss. (The solid line represents the univariate linear regression, discussed below.)
Figure 1.
Visual acuity loss at two years as a function of baseline visual acuity for all 91 subjects with a two-year follow-up examination after baseline. Those points above the horizontal dashed line indicate at least moderate visual loss, and those points above the upper horizontal dotted line indicate severe visual loss. The solid line shows the univariate linear regression analysis (slope −0.28, p=0.0015). Visual acuities are graphed in logMAR (logarithm of the minimum angle of resolution) notation, with some Snellen equivalents written below.
The first portion of Table 2 shows the distribution of moderate and severe VA loss, and the mean 2-year and final VA loss, as a function of baseline VA. There was significantly greater VA loss among the participants with baseline VA of 20/50 and better, with 40% of participants in this group experiencing at least moderate VA loss. Since VA was measured at 1 meter when necessary, the largest letters tested were 20/800 (1.6 logMAR), so that a six line drop in VA (0.6 logMAR worsening) could potentially be measured in participants with baseline VA up to 20/200 (1.0 logMAR). Thus, the lesser rates of VA loss in the groups with baseline VA worse than 20/50 were not accounted for by an inability to measure these levels of loss. There also was a significant difference between the baseline VA groups in mean VA loss at two years and at the final examination.
Table 2.
Baseline visual acuity (VA) and low luminance deficit (LLD), and rate of visual acuity loss
| Category at baseline |
# eyes |
# (%) with moderate VA loss at 2 years |
# (%) with severe VA loss at 2 years |
Relative risk of at least moderate VA loss at 2 years |
Relative risk of severe VA loss at 2 years |
Mean VA at baseline (logMAR) |
Mean logMAR VA loss at 2 years [95% CI] |
Mean VA at 2 years (logMAR ) |
Mean final logMA R VA loss* [95% CI] |
Mean final VA (logMAR) |
|---|---|---|---|---|---|---|---|---|---|---|
|
1 VA >=20/50 |
52 | 21 (40%) | 9 (17%) |
1 | 1 | 20/33 (0.22) |
0.28 [0.20– 0.36] |
20/63 (0.50) |
0.65 ([0.55– 0.76]) |
20/148 (0.87) |
| VA 20/51–20/199 | 30 | 4 (13%) p=0.008 |
1 (3%) p=0.04 |
0.33 [0.13– 0.87] |
0.19 [0.03– 1.42] |
20/89 (0.65) |
0.08 [− 0.01to 0.17] p=0.001 |
20/107 (0.73) p=0.001 |
0.37 [0.23– 0.50] p=0.002 |
20/205 (1.01) p=0.14 |
| VA <=20/200 | 9 | 1 (11%) p=0.07 |
0 (0%) p=0.08 |
0.28 [0.04– 1.80] |
undef | 20/264 (1.12) |
−0.04 [− 0.26 to 0.17] p=0.002 |
20/235 (1.07) p=0.0001 |
−0.01 [−.0.26 to 0.24] p=0.000 1 |
20/258 (1.11) p=0.13 |
|
2 VA >=20/50 AND better LLD |
21 | 4 (19%) | 1 (5%) | 1 | 1 | 20/30 (0.18) |
0.16 [0.04– 0.27] |
20/43 (0.33) |
0.46 [0.29– 0.63] |
20/87 (0.64) |
| VA >=20/50 AND worse LLD |
31 | 17 (55%) p=0.008 |
8 (26%) p=0.03 |
2.88 [1.13– 7.35] |
5.42 [0.73– 40.21] |
20/36 (0.25) p=0.02 |
0.36 [0.27– 0.45] p=0.009 |
20/81 (0.61) p=0.002 |
0.78 [0.64– 0.93] p=0.005 |
20/214 (1.03) p=0.002 |
|
3 20/25 < VA <=20.50 AND better LLD |
18 | 4 (22%) | 1 (6%) | 1 | 1 | 20/32 (0.21) |
0.17 [0.04– 0.30] |
20/48 (0.38) |
0.47 [0.28– 0.66] |
20/96 (0.68) |
| 20/25 < VA <=20.50 AND worse LLD |
29 | 17 (59%) p=0.02 |
8 (28%) p=0.06 |
2.64 [1.05– 6.60] |
4.97 [0.68– 36.47] |
20/36 (0.26) p=0.02 |
0.37 [0.27– 0.48 p=0.02 |
20/86 (0.64) p=0.005 |
0.80 [0.65– 0.95] p=0.01 |
20/230 (1.06) p=0.004 |
|
4 VA 20/51-20/199 AND better LLD |
27 | 3 (11%) | 0 (0%) | 1 | 1 | 20/89 (0.65) |
0.05 [− 0.01 to 0.12] |
20/103 (0.71) |
0.32 [0.21– 0.44] |
20/191 (0.98) |
| VA 20/51–20/199 AND worse LLD |
3 | 1 (33%) p=0.34 |
1 (33%) p=0.03 |
3.00 [0.44– 20.53] |
undef | 20/80 (0.60) p=0.58 |
0.34 (0.14– 0.55] p=0.01 |
20/174 (0.94) p=0.14 |
0.74 [0.38– 1.10] p=0.03 |
20/438 (1.34) p=0.09 |
logMAR = logarithm of the minimal angle of resolution, CI = confidence interval, VA = visual acuity, LLD = low luminance deficit.
The p values are from comparisons of the rates of at least moderate and severe VA loss based on the Chi square test. The p values for the continuous variables (mean VA loss, mean VA) are based on Student’s t test.
Mean total follow-up was 4.9 years for the best VA group, 5.2 years for the intermediate VA group, and 4.3 years for the worst VA group (p=0.25)The top grouping of three rows include the data on VA loss for each VA group. The p values reflect the given row relative to the good VA group.The second grouping of two rows presents the data for the good VA group, stratified into better and worse LLD. The third grouping of two rows compares the data for the eyes with acuities worse than 20/25 but equal to or better than 20/50, stratified into better and worse LLD. The fourth grouping of two rows presents the data for the intermediate VA group, stratified into better and worse LLD. All subjects with VA of 20/200 or worse had baseline LLD in the better LLD category.
The low luminance deficit and subsequent VA loss
The baseline low luminance visual acuity deficit (LLD) was calculated for each participant. The median LLD was 0.47 logMAR units (i.e. 4.7 lines of worsening on the ETDRS chart with the filter interposed) for eyes with VA of 20/50 or better, and 0.22 for eyes with VA worse than 20/50. The median LLD for eyes with VA of 20/100 or better was 0.40, but only 3 of the 39 participants with VA worse than 20/50 had an LLD of 0.40 or greater.
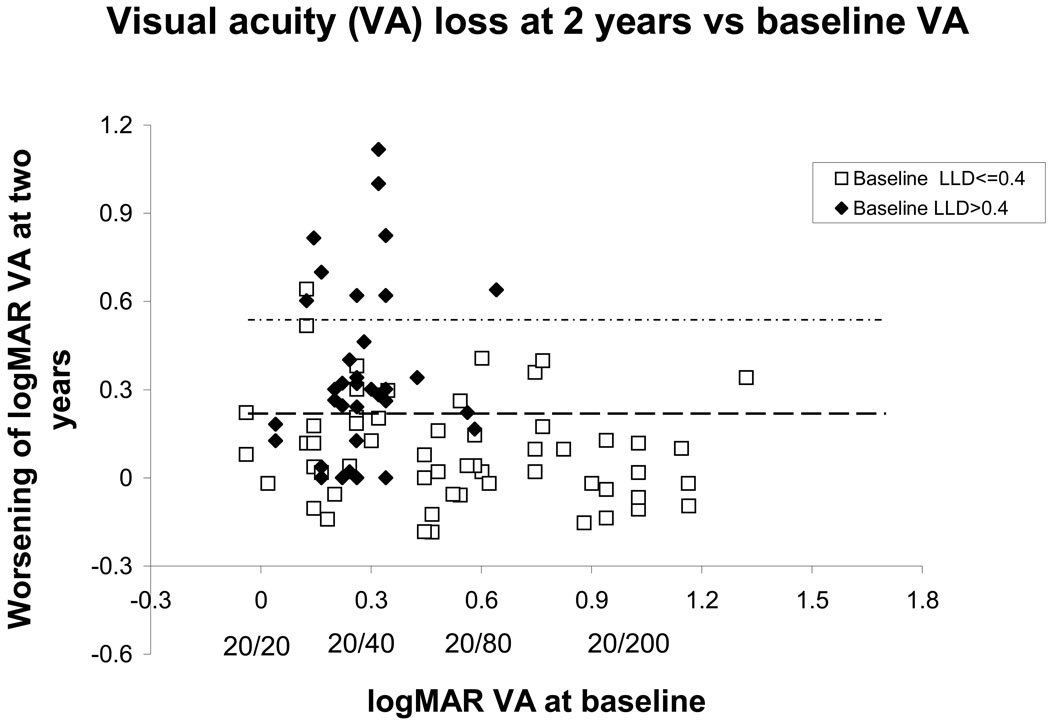
The LLD was stratified by whether it was better than or equal to 0.40 (better LLD, open squares in figure 2), or whether it was worse than 0.40 (worse LLD, closed diamonds in figure 2). The data are tabulated in Table 2. Within the good baseline VA group (Table 2, second panel), 55% of the participants with worse LLD experienced at least moderate visual loss, compared with 19% of the participants with better LLD. (relative risk for the worse LLD group 2.88 [95% confidence interval (CI) 1.13–7.35]. Even when the eyes with VA 20/25 or worse are excluded, (Table 2, third panel) there remains a significant difference in the rate of experiencing at least moderate visual loss (relative risk for the worse LLD group 2.64 [95% confidence interval (CI) 1.05–6.60]). Table 2 also provides the details of rates and relative risks of severe visual loss conferred by worse LLD, as well as the mean baseline VA, mean two-year VA and VA loss and the mean final VA and VA loss. The fourth panel of Table 2 shows the relationship for the group with VA worse than 20/50 but better than 20/200. (All patients with VA 20/200 or worse had LLD in the better category, as shown in figure 2. See discussion section.)
Figure 2.
Visual acuity loss at two years as a function of baseline visual acuity for all 91 subjects with a two-year follow-up visit after baseline, stratified by baseline low luminance deficit (LLD). Those points above the horizontal dashed line indicate at least moderate visual loss, and those points above the upper horizontal dotted line indicate severe visual loss. The open squares indicate eyes with a lesser baseline deficit under reduced luminance conditions (better LLD), while the solid diamonds indicate eyes with a greater deficit under reduced luminance conditions (worse LLD). Visual acuities are graphed in logMAR (logarithm of the minimum angle of resolution) notation, with some Snellen equivalents written below.
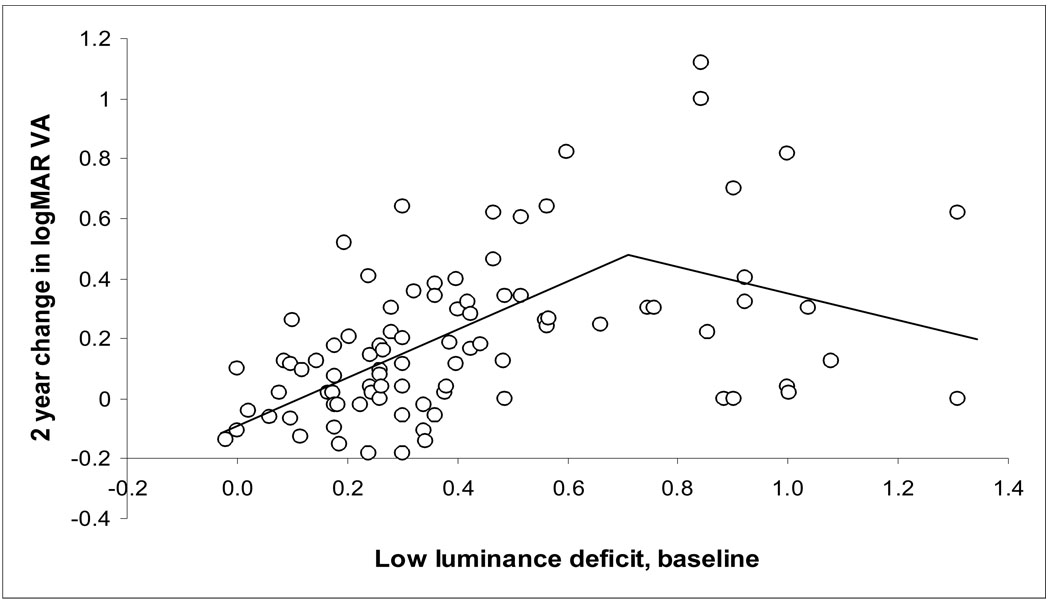
The relationships between the baseline VA, low luminance deficit, and change in 2 year VA was explored as continuous variables through univariate and multivariate linear regression for the 91 patients, and are presented in Table 3 (available at http://aaojournal.org). In univariate analysis, baseline VA and LLD were each associated with 2 year change in VA (figure 1, and figure 3--available at http://aaojournal.org). When baseline VA and LLD were in the model at the same time, LLD had a stronger relationship to change in 2 year VA, and baseline VA was no longer significant. (LLD standardized beta coefficient 0.40, p=0.0003; Baseline VA standardized beta coefficient 0.14, p=0.20; see Table 3 Multivariate linear analysis, available at http://aaojournal.org). However, once the LLD is greater than 0.40, the proportion of participants experiencing at least moderate visual loss does not increase further with increasing LLD. For example, 8 of 16 eyes (50%) with LLD between 0.41 and 0.60 experienced at least moderate visual loss, as did 9 of 18 eyes (50%) with LLD of 0.61 or greater. Therefore, a bilinear (spline) model also was explored, and is presented in the supplement (Figure 3 and Table 3, (available at http://aaojournal.org). Possible reasons why the rate of at least moderate visual loss did not continue to increase with increasing LLD are discussed below.
Table 3.
Relationship of Baseline visual acuity and low luminance deficit to 2 year change in visual acuity in a univariate and multivariate analysis
| Univariate analysis | Multivariate linear analysis |
Multivariate spline (bilinear) regression analysisc |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | SE | p | Slope | SE | p | Slope | SE | p | |
| Baseline Visual Acuity |
−0.28 | 0.09 | 0.0015 | −0.14 | 0.09 | 0.20 | 0.03 | 0.09 | 0.74 |
| Baseline Low Luminance Deficit (LLD) |
0.42 | 0.08 | 0.0001 | 0.40 | 0.10 | 0.0003 | 0.79 | 0.17 | <.0001 |
| Difference in slope for Low Luminance deficit at LLD>=0.7 |
−1.18 | 0.38 | 0.003 | ||||||
SE = standard error
Figure 3.
(online only supplement). Two-year visual acuity (VA) loss in logMAR (logarithm of the minimum angle of resolution) units, as a function of baseline low luminance deficit (LLD) for all 91 subjects. Two-year VA loss increases with worsening LLD (slope 0.42, p=0.0001), but this dependence levels off for very high LLD. A spline (bilinear) model, with a negative term for LLD above 0.7 (bilinear regression lines shown), provides a better fit to the data than does a single linear regression line. Table 3 (online only) provides further details.
None of the 5 eyes with baseline VA of 20/25 or better (logMAR VA<=0.10) lost three or more lines by the two year examination, and all had baseline LLDs in the better group.
Six of the 52 eyes in the good VA group at baseline with two-year follow-up data developed choroidal neovascularization (CNV) at some time during the study. One eye developed CNV by one year and two eyes developed CNV by two years; these 3 participants lost more than 3 lines of ETDRS VA by the two-year examination. The LLD values of these 3 eyes were 0.20, 036, and 1.00. One eye developed CNV in each of years 3, 4, and 5; none of these participants lost more than 3 lines of ETDRS VA by two years. The LLD values for these 3 participants were 0.26, 0.38, and 1.00. Four of the 6 participants already had CNV in their fellow eye at baseline.
Stability of the LLD measure
The participants in this study were examined annually. While more frequent measurements are not available, a comparison of the LLD at baseline with the LLD at the first annual follow-up examination can give an estimate of the stability of the LLD measure. Figure 4 (available at http://aaojournal.org) shows the LLD at baseline and one year for all 78 participants examined at one year. For the 51 participants whose VA changed by less than one line at one year (open symbols), 45 (88%) had the same LLD stratification at one year as at baseline, and the coefficient of repeatability 11 (defined as 1.96 multiplied by the standard deviation of the difference in measurements for each eye) was 0.25. Twenty-six of these eyes had good baseline VA; the median change in low luminance deficit was −0.017 LogMAR, and the coefficient of repeatability was 0.30. Twenty-three of the 26 eyes (88%) agreed in classification as better or worse LLD between the baseline and one year measure (kappa=0.77). Thus, the LLD measure appeared to be reproducible and stable over one year in eyes that had not undergone significant VA change. (See below for further discusssion)
Figure 4.
(online only supplement). Stability of low luminance deficit (LLD) over a one-year period. For eyes that had stable visual acuity over the one-year period, the LLD measure remained stable as well. Open symbols indicate eyes that lost no more than one line of ETDRS (Early Treatment Diabetic Retinopathy Study) visual acuity over one year; closed symbols indicate eyes that lost more than one line of ETDRS visual acuity over one year. The graph is divided into quadrants by lines dividing the LLD measures into worse LLD and better LLD groups.
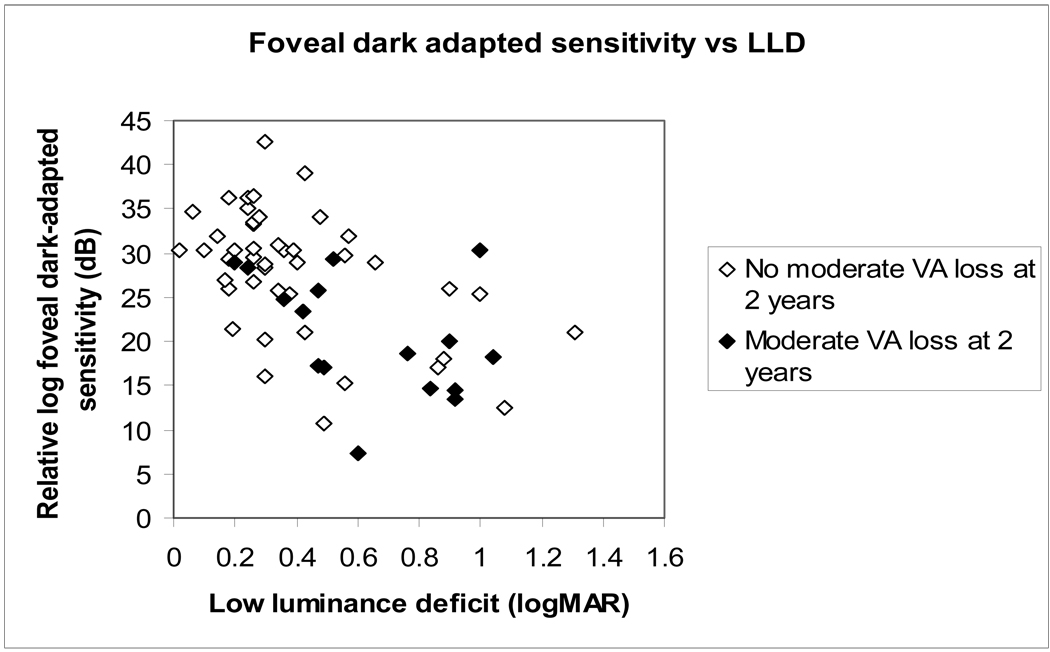
Comparison of LLD with foveal dark-adapted sensitivity
For 60 participants with foveal fixation, foveal dark-adapted sensitivity, a more standard test of dark-adapted visual function, was performed. Figure 5 (available at http://aaojournal.org) shows the relationship of foveal dark-adapted sensitivity, to LLD. The Pearson correlation coefficient between LLD and foveal dark-adapted sensitivity was 0.55 (p=0.0001). The eyes in the worse LLD group had significantly worse foveal dark-adapted sensitivity (mean log sensitivity 32 dB for better LLD group, 24 for worse LLD group, Student t=4.9, p<0.0001). When the foveal dark-adapted sensitivity measurements were stratified by the median (relative log sensitivity 25 dB), the relative risk of 2 year VA loss for the worse sensitivity group relative to the better sensitivity group was 4.20 [95% CI 1.39–12.71].
Figure 5.
(online only supplement). The relationship between baseline foveal dark-adapted sensitivity and low luminance deficit (LLD) at baseline. There is a strong correlation between foveal dark-adapted sensitivity and the LLD measure (Pearson correlation coefficient=0.55, p=0.0001). The solid symbols indicate those eyes with >=3-line of ETDRS (Early Treatment Diabetic Retinopathy Study) visual acuity (VA) loss at two years.
Other risk factors for moderate and severe VA loss at two years
Table 4 presents a number of possible risk factors for visual loss in this group of participants with GA and good VA at baseline. Demographic factors, including age and gender, showed no influence on the risk of VA loss at two years. Likewise, there was no significant difference between participants whose fellow eye had CNV as compared with participants with bilateral GA, in terms of VA loss. There were 3 participants with drusen and no advanced AMD in the fellow eye, and none of these participants developed moderate or severe VA loss in the GA eye at two years. The VA of the fellow eye at baseline was likewise not predictive of subsequent VA loss in the study eye.
Table 4.
Relative risk (and 95% confidence interval [CI]) by patient and eye characteristics, for 52 study eyes with VA 20/50 or better
| #in subgro up |
#with >=3 lines VA change (%) |
p value |
Relative Risk for >=3 line loss [95% CI] |
||
|---|---|---|---|---|---|
| PATIENT AND FELLOW EYE CHARACTERISTICS | |||||
| Age (years) | 63–77 | 28 | 12 (43%) | 0.78 | 1.14 [0.58–2.23] |
| 78–89 | 24 | 9 (38%) | ref | ||
| Gender | Women | 31 | 13 (42%) | 1.00 | 1.10 [0.56–2.18] |
| Men | 21 | 8 (38%) | ref | ||
| Fellow Eye Diagnosis | Bilateral GA |
34 | 14 (41%) | ref | |
| Fellow eye w/CNV |
14 | 7 (50%) | 0.40 | 1.21 [0.63–2.35] | |
| Fellow eye w/ Drusen |
3 | 0 (0%) | 0 | ||
| Other | 1 | 0 (0%) | 0 | ||
| Fellow eye VA | 20/50 or better |
11 | 5 (45%) | 0.82 | 1.30 [0.54,3.13] |
| 20/51– 20/199 |
21 | 9 (43%) | 1.22 [0.56–2.66] | ||
| 20/200 or worse |
20 | 7 (35%) | ref | ||
| VISUAL FUNCTION CHARACTERISTICS OF STUDY EYE | |||||
| Visual acuity (LogMAR) |
<=0.24 | 25 | 7 (28%) | 0.10 | ref |
| >0.24 | 27 | 14 (51%) | 1.85 [0.90–3.83] | ||
| Low Luminance Deficit at baseline (LogMAR) |
−0.02 to 0.4 | 21 | 4 (19%) | 0.01 | ref |
| >0.4 to 1.31 |
31 | 17 (55%) | 2.88 [1.13–7.35] | ||
| Log Contrast Sensitivity (Pelli- Robson) |
>1.20 | 23 | 6 (26%) | ref | |
| <=1.20 | 29 | 15 (52%) | 0.09 | 1.98 [0.92–4.29] | |
|
Foveal Dark adapted sensitivity (dB) |
26–42 | 21 | 3 (14%) | ref | |
| 7–25 | 20 | 12 (60%) | 0.003 | 4.20 [1.39–12.71] | |
| Maximum Reading | 97–141 | 26 | 6 (23%) | ref | |
|
Rate (words per minute) |
|||||
| 0–96 | 25 | 14 (56%) | 0.02 | 2.43 [1.11–5.31] | |
| MEASURES OF GEOGRAPHIC ATROPHY | |||||
| Baseline area of atrophy* |
< 2.39 MPS DA |
27 | 10 (37%) | 0.48 | ref |
| >= 2.39 MPS DA |
24 | 10 (42%) | 1.13 [0.57–2.23] | ||
| Two year enlargement rate of atrophy |
< 2.54 sq mm/year |
23 | 9 (39%) | 0.54 | ref |
| >= 2.54 sq mm/year |
23 | 7 (30%) | 0.78 [0.35–1.73] | ||
| GA within 500 µm of foveal center |
No | 11 | 4 (36%) | 0.74 | ref |
| Yes | 41 | 17 (41%) | 1.14 [0.48–2.70] | ||
|
Amount of central 4 MPS DA with GA* |
<= 25% | 18 | 3 (17%) | 0.02 | ref |
| 26–74% | 26 | 15 (58%) | 3.46 [1.17–10.24] | ||
| >= 75% | 7 | 2 (29%) | 1.71 [0.36–8.17] | ||
VA=visual acuity. GA = geographic atrophy. CNV = choroidal neovascularization. MPS DA = Macular Photocoagulation Study disc area, equivalent to 2.54 mm2 on the retina. Ref = the reference subgroup for the relative risk calculations. 95% CI = the 95% confidence interval. The p values represent Fisher’s exact test for the dichotomous variables, and the Chi sq test for the others.
The measures of the area of GA atrophy include 51 patients, because the photographs for one patient were not adequate for measuring area. Reading rate could not be measured for one patient, so 51 patients are included. Foveal dark adapted sensitivity was measured only in patients who had stable central fixation who could view the fixation diamond, and included 41 patients.
Several parameters describing the GA were analyzed. The baseline total area of GA and the two-year enlargement rate of the atrophy did not modify risk for VA loss. No significant difference in risk was found between study eyes with GA within 500 µm of the foveal center and those with no GA that close to the foveal center, but few eyes were in the group without GA near the center. Finally, those eyes with GA involving from 25% to 75% of the central 4 MPS DA had significantly higher risk for subsequent VA loss than did eyes with 25% or less of the central 4 MPS DA involved. This group with 25 to 75% of the central 4 MPS DA involved also had a higher, but not statistically significant, relative risk than eyes with 75% or more of the central 4 MPS DA involved.
Baseline visual function measures were studied as possible risk factors for subsequent VA loss (Table 4). Low reading rate was a significant risk factor for subsequent VA loss (relative risk 2.43 [1.11–5.31]), as were the low luminance deficit and reduced foveal dark-adapted sensitivity discussed above. Pelli-Robson contrast sensitivity loss showed the same trend, but was not statistically significant. When a logistic regression was performed including LLD and reading rate, the LLD measure was more strongly associated with VA loss (for LLD, odds ratio 5.93 [1.5–30.4], p=0.017; for max reading rate, odds ratio 3.54 [1.003–13.6], p=0.545) (Foveal dark adaptation was not included, because only a selected group of patients underwent this test, and it is not a clinically practical measure.)
The visual function measures are highly correlated with one another (Table 5), with correlation coefficients ranging from 0.35 to 0.68. The highest correlation (0.68) was between Pelli-Robson log contrast sensitivity and the LLD.
Table 5.
Pearson correlation of risk factors for change in 2 year visual acuity (VA), eyes with baseline VA better than 20/50
| Low Luminance Deficit |
Contrast Sensitivity |
Foveal Dark Adapted Sensitivity |
Maximum Reading Rate |
|
|---|---|---|---|---|
| Visual Acuity | 0.35 (p=0.01) |
−0.51 (p=0.0002) |
−0.46 (p=0.002) |
−0.38 (p=0.006) |
|
Low Luminance Deficit |
−0.68 (p<.0001) |
−0.52 (p=0.0005) |
−0.47 (p=0.0004) |
|
|
Contrast Sensitivity |
0.57 (p=0.0003) |
0.39 (p=0.006) |
||
|
Foveal Dark Adapted Sensitivity |
0.41 (p=0.008) |
VA = visual acuity
DISCUSSION
Low luminance visual dysfunction
These prospective natural history data show that the low luminance deficit, a simple, inexpensive, and rapid measure of visual function, was a strong predictor of the subsequent risk for losing VA in eyes with GA. This measure was studied because of the clear difficulties experienced by participants with GA in dim illumination, and the body of research showing reductions in dark-adapted function in patients with AMD. We previously showed, in a small pilot study, that eyes with drusen with reduced dark-adapted foveal sensitivity to a small red stimulus were more likely to develop advanced AMD. 9 In the present study, the foveal dark-adapted sensitivity was the strongest predictor of visual acuity loss. The dark-adapted foveal sensitivity measure, however, was difficult to implement, required 45 minutes of dark adaptation and specialized equipment, and suffered from uncertainty as to where the patient was fixating. The present study shows that a much simpler measure (which only requires a 2 log unit neutral density filter (Kodak Wratten filter, Kodak, Rochester) mounted in a lorgnette occluder) can capture the risk of moderate and severe visual loss in eyes with GA, particularly those with good VA.
Other instruments that measure dark-adapted function include the Goldmann-Weekers adaptometer and the Scotopic Sensitivity Tester (SST, LKC Inc., Gaithersburg Maryland). The Goldmann-Weekers test depends on the subject having stable fixation, so that the same retinal area is bleached and tested at each time point, which may not be the case in GA. The SST tests global dark adaptation, that is, the response of the whole retina to a diffuse light stimulus. This test avoids the problem of fixation stability, but has not been tested or validated in GA to our knowledge.
The LLD is a measure of cone function in reduced illumination, as was our previous foveal dark-adapted sensitivity measure. Cone dark adaptation as well as rod dark adaptation are impaired in advanced AMD. 8, 12, 13 Cone measures can capture foveal function in a way that rod measures cannot, given the paucity of rods in the foveal region. Cone dark-adapted function is affected more than cone function under photopic conditions. Studies of rod versus cone function in AMD often compare dark-adapted rod function to light-adapted cone function, 14 and thus do not capture the impairment of cone function in dim illumination.
However, it is foveal cone function in particular that is the most critical for preservation of VA. A reduction in foveal cone function in dim illumination will dramatically reduce VA, while worsening of peripheral cone function in dim illumination will not cause as dramatic a change in VA. As seen in figure 1, those eyes that no longer had foveal vision at baseline (i.e. the eyes with worst VA) had better baseline LLDs; that is, they had little further reduction in VA in dim illumination. Thus, there is an added complexity in interpreting the LLD. If the LLD improves in the presence of stable VA, it probably indicates an improvement in foveal function. If the VA drops, however, and the patient shifts to eccentric fixation, the LLD may improve because peripheral photoreceptors are not as sensitive to changes in luminance as are foveal photoreceptors, as assessed by VA. So, an improvement in LLD has to be interpreted in the context of whether any changes in standard VA have taken place. When patients worsen in VA and lose foveal vision, their LLD may improve because the VA of extrafoveal sites is less affected by reduction in luminance. Since the LLD reflects the difference between conventional ETDRS VA and low luminance VA, it corrects for the lower conventional VA at peripheral locations. Thus, the stability of the LLD measure will be affected by whether the patient has lost conventional VA. Figure 4 (available at http://aaojournal.org) shows that some of the eyes that lost more than one line of VA at one year had improvements in the LLD over that time (closed symbols in the lower right quadrant), presumably because of the shift to eccentric retina.
The mechanism for reduction in VA with reduced luminance may involve not only a reduction in foveal cone function, but an actual switching of fixation to an eccentric retinal locus. Lei and Schuchard 15 showed that when a fixation cross was made dimmer in the scanning laser ophthalmoscope, subjects sometimes changed from central to eccentric fixation in order to fixate the dim target. The distance of the eccentric fixation site from the fovea (as dictated by the extent of the scotoma) might determine the amount the VA drops under reduced luminance. Thus, the actual magnitude of the LLD (once it is worse than 0.40) might be a function of the eccentricity of the site of the new fixation, and thus may not be directly related to the amount of subsequent VA loss at the fovea. This may be the reason for the flattening of the VA loss at 2 years vs LLD relation at high LLD levels (figure 3 and table 3, available at http://aaojournal.org).
Other visual function measures
Maximum reading rate also was a significant risk factor for subsequent VA loss, although in multivariate logistical analysis, the LLD was a stronger risk factor. The Pelli-Robson score shows the same trend. The reading rate measure is difficult to implement in subjects who have central or macular ring scotomas, and requires more specialized equipment and more time. Table 5 shows that the various visual function risk factors are correlated with one another, so that assessment of the simpler measures should be adequate in determining risk of VA loss. Future papers will look at these visual function measures more closely.
Implications for design and sample sizes in clinical trials
Trials based on VA loss as the primary outcome measure will require the fewest number of participants if the group at high risk of visual loss is selected. For subjects with GA, this would include the group with VA better than or equal to 20/50. Within this group in our study 40% lost >= 3 lines at two years. To detect a reduction in the rate of visual loss by 25% (i.e. from a 40% rate to a 30% rate), a total sample size of 696 participants would be required at alpha=0.05 and power=0.80. The LLD measure allows for the further selection of subjects at highest risk of subsequent VA loss at two years, thus reducing the sample size necessary to detect the same relative effect. If only subjects within the worse LLD category are enrolled, the rate of at least moderate VA loss at two years is expected to be 55%. Detection of a reduction in the rate of visual loss by 25% (i.e. from a 55% rate to a 41% rate) would then require 402 participants.
Perhaps more importantly, changes in the LLD may be able to serve as an early indicator of treatment benefit, and as a surrogate measure for the risk of subsequent two-year VA loss‥The LLD is very reproducible and is stable over time. Detection of a change in the LLD as a result of a treatment requires few participants. In subjects with good VA who were assessed at one year, the mean difference in LLD between baseline and one year was −0.01, with a standard deviation of 0.15. If subjects underwent a given therapy, and the LLD was repeated after three or six months, few participants would be required to detect an effect. For example, to detect an improvement in LLD of 0.1, only 20 participants would be required.
Combining the LLD measure with recruitment suggestions given above and elsewhere 16, 17 will allow for progress in the determination of effective therapies for GA. In addition, unlike CNV, patients with GA and good VA are unlikely to improve in VA, since there is already photoreceptor loss in areas of GA (unlike CNV, in which fluid and hemorrhage can resolve and lead to improvement in VA). However, measures of foveal photoreceptor health such as LLD or contrast sensitivity could conceivably improve, and reflect an improvement in quality of life for these patients.
The relationship of subsequent VA loss with percent involvement of the central area was somewhat surprising. The group of study eyes with atrophy involving between 25% and 75% of the central 4 MPS DA were at significantly higher risk of VA loss than were eyes with lesser amounts of central atrophy, and also tended to have a higher risk of VA loss than eyes with more central atrophy. In addition, it might have been expected that the presence of GA within 500 µm of the foveal center would have conferred significantly more risk. These features are being further studied. It is possible that there is a subgroup of patients that is very resistant to foveal loss, and these are the eyes with large areas of GA and large amounts of atrophy in the central 10.2 mm2. These spared areas can persist for a number of years (Applegate CA, Sunness JS. Reading with a 0.1 MPS disc area island in age-related macular degeneration. Invest Ophthalmol Vis Sci 40:S316, 1999.). We currently are analyzing the role of these and other ophthalmoscopic features in the loss of VA and enlargement of GA.
It should be noted that the eligibility criteria for this study differed from the definition used by the Age-Related Eye Disease Study (AREDS) for GA. In the current study, participants had to have at least a 500 micron diameter area of GA, while in AREDS the size criterion for GA was 175 microns or greater. While AREDS divided GA into central and noncentral groups, the current study looked at all of GA as a continuum. 18 As we have reported previously, those eyes with less than 0.5 MPS DA (1.26 mm2) of GA were very unlikely to have rapid enlargement of the GA or to have rapid VA loss. 4, 17 Therefore, the aim in recruiting participants for a proof of concept trial should be to recruit participants with at least 0.5 MPS DA of atrophy (1.26 mm2). 17 Likewise, no patient with VA better than 20/25 experienced at least moderate visual loss at two years, so including subjects from 20/25 to 20/50 would be most appropriate. 16
Acknowledgments
Supported by NIH grants EY08552 and EY14148
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the American Academy of Ophthalmology Annual Meeting, November 2006, Las Vegas, Nevada.
No conflicting relationship exists for any author
REFERENCES
- 1.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 2.Hirvela H, Luukinen H, Laara E, et al. Risk factors of age-related maculopathy in a population 70 years of age or older. Ophthalmology. 1996;103:871–877. doi: 10.1016/s0161-6420(96)30593-9. [DOI] [PubMed] [Google Scholar]
- 3.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 4.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 6.Brown B, Kitchin JL. Dark adaptation and the acuity/luminance response in senile macular degeneration (SMD) Am J Optom Physiol Opt. 1983;60:645–650. doi: 10.1097/00006324-198308000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sloan LL. Variation of acuity with luminance in ocular diseases and anomalies. Doc Ophthalmol. 1969;26:384–393. doi: 10.1007/BF00943999. [DOI] [PubMed] [Google Scholar]
- 8.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunness JS, Massof RW, Johnson MA, et al. Diminished foveal sensitivity may predict the development of advanced age-related macular degeneration. Ophthalmol. 1989;96:375–381. doi: 10.1016/s0161-6420(89)32883-1. [DOI] [PubMed] [Google Scholar]
- 10.Sunness JS, Bressler NM, Tian Y, et al. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1761–1769. [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical method for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 12.Brown B, Tobin C, Roche N, Wolanowski A. Cone adaptation in age-related maculopathy. Am J Optom Physiol Opt. 1986;63:450–454. doi: 10.1097/00006324-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Eisner A, Klein ML, Zilis JD, Watkins MD. Visual function and the subsequent development of exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1992;33:3091–3102. [PubMed] [Google Scholar]
- 14.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273. [PubMed] [Google Scholar]
- 15.Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Invest Ophthalmol Vis Sci. 1997;38:1812–1818. [PubMed] [Google Scholar]
- 16.Sunness JS, Bressler NM, Hawkins BS, Hawkins Designing clinical trials for age-related geographic atrophy of the macula: Enrollment data from the Geographic Atrophy Natural History Study. Retina. 2007;27:204–210. doi: 10.1097/01.iae.0000248148.56560.b1. [DOI] [PubMed] [Google Scholar]
- 17.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–277. doi: 10.1016/j.ophtha.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]