Abstract
We review work in the major model systems for habituation in Drosophila melanogaster, encompassing several sensory modalities and behavioral contexts: visual (giant fiber escape response, landing response); chemical (proboscis extension reflex, olfactory jump response, locomotory startle response, odor-induced leg response, experience-dependent courtship modification); electric (shock avoidance); and mechanical (leg resistance reflex, cleaning reflex). Each model system shows several of Thompson and Spencer’s (1966) parametric criteria for habituation: spontaneous recovery and dishabituation have been described in almost all of them, and dependence of habituation upon stimulus frequency and stimulus intensity in the majority. Stimulus generalization (and conversely, the delineation of stimulus specificity) has given insights into the localization of habituation or the neural architecture underlying sensory processing.
A strength of Drosophila for studying habituation is the range of genetic approaches available. Mutations have been used to modify specific neuroanatomical structures, ion channels, elements of synaptic transmission, and second messenger pathways. rutabaga and dunce, genes of the cAMP signal pathway that have been studied most often in the reviewed experiments, have also been implicated in synaptic plasticity and associative conditioning in Drosophila and other species including mammals. The use of the Gal4/UAS system for targeting gene expression has enabled genetic perturbation of defined sets of neurons. One clear lesson is that a gene may affect habituation differently in different behaviors, depending on the expression, processing, and localization of the gene product in specific circuits. Mutations of specific genes not only provide links between physiology and behavior in the same circuit, but also reveal common mechanisms in different paradigms of behavioral plasticity. The rich repertoire of models for habituation in the fly is an asset for combining a genetic approach with behavioral, anatomical and physiological methods with the promise of a more complete understanding.
Keywords: Habituation paradigms, Dishabituation, Drosophila, Genetic analysis, Mutations
I. Introduction
Drosophila melanogaster has unique advantages for the study of habituation. As a small invertebrate, it has a relatively simple nervous system with many identifiable neurons. About a dozen behavioral models for habituation have been established, and for several of them, the underlying neural circuits and neurons are at least partially known. Most importantly, a variety of genetic techniques can be combined with standard physiological, pharmacological and behavioral approaches. Much of this work has used mutations that modify the function or expression of proteins that may play a role in habituation, including components of second-messenger signal transduction pathways, ion channels, and mediators of synaptic transmission (Table 1). Some of those mutations were first identified in screens for learning defects (forward genetics), while others were studied because their products seemed suited to play a role in synaptic plasticity (reverse genetics) (Davis, 1996; Dubnau & Tully, 1998; Wu, Renger & Engel, 1998; Waddell & Quinn, 2001). Because many of these mutations have also been examined for effects upon associative learning, they may provide a potential bridge for linking the physiological mechanisms of different conditioning paradigms. Habituation studies in the fly have taken advantage of the fact that sensory and motor elements in several models are well-defined and accessible to electrophysiology, which can be carried out in a semi-intact preparation so that a behavioral component can be preserved. Some workers have also sought to define the anatomical substrates of habituation by using mutations that cause well-defined neuroanatomical defects (Table 1) or transgenic methods such as the enhancer trap Gal4-UAS responder binary system (Brand & Perrimon, 1993) to drive the expression of modified genes in specific regions of the nervous system.
Table 1.
Mutational analysis of habituation in Drosophila
| Gene (Full Name), Product | Effects Upon Habituation (Model System) | References |
|---|---|---|
| Synaptic transmission | ||
| amn (amnesiac), PACAP-like peptide |
Does not clearly alter habituation (PER) | Duerr & Quinn 1982 |
| Reduces rate of habituation (courtship modification) | Gailey et al. 1982 | |
|
iav (inactive), TRPV receptor channel subunit |
Reduces rate of habituation (courtship modification) | O’Dell 1994 |
| Syn (Synapsin), Synapsin | Increases rate of habituation (olfactory jump) | Godenschwege et al. 2004 |
| Second-messenger signaling | ||
|
Btk29a (Btk family kinase at 29A) 1, Bruton’s tyrosine kinase |
Increases rate of habituation (olfactory jump) | Asztalos et al. 2007b |
|
caki (calcium/calmodulin- dependent protein kinase), CaM kinase homologue |
Increases habituation (giant fiber response) | Zordan et al. 2005 |
| dnc (dunce), cAMP | Increases rate of habituation (giant fiber response) | Engel & Wu 1996 |
| phosphodiesterase | Increases rate of habituation (landing response) | Wittekind 1988; Rees 1989 |
| Reduces habituation (PER) | Duerr & Quinn 1982 | |
| Reduces habituation slightly (olfactory jump) | Asztalos et al. 2007a | |
| Slows or eliminates habituation (courtship) | Gailey et al. 1982, O’Dell 1994 | |
| Increases rate of recovery (cleaning reflex) | Corfas & Dudai 1990 | |
|
for (foraging), cGMP-dependent protein kinase |
Habituation slower with greater PKG expression (giant fiber response) |
Engel et al. 2000 |
| Habituation slower with greater PKG expression (PER) | Scheiner et al. 2004 | |
|
Pp1-87B (Protein phosphatase 1 at 87B) 2, protein phosphatase 1 |
Increases rate of habituation (landing response) | Asztalos et al. 1993 |
| rut (rutabaga), adenylyl cyclase | Reduces rate of habituation (giant fiber response) | Engel & Wu 1996 |
| Increases rate of habituation (landing response) | Wittekind 1988; Rees 1989 | |
| Reduces habituation (PER) | Duerr & Quinn 1982 | |
| Reduces habituation (olfactory jump) | Asztalos et al. 2007a | |
| Reduces habituation (locomotory startle) | Cho et al. 2004 | |
| Slows habituation (courtship) | Gailey et al. 1982 | |
| Increases rate of recovery (cleaning reflex) | Corfas & Dudai 1989 | |
|
sgg (shaggy), glycogen synthase kinase 3 homolog |
Habituation faster with greater sgg expression | Wolf et al. 2007 |
| tur (turnip), ?? | Reduces habituation (proboscis extension) | Duerr & Quinn 1982 |
| Increases rate of habituation (?) (olfactory jump) | Mihalek et al. 1997 | |
| Protein Synthesis | ||
|
per (period), transcription factor (circadian) |
Increases habituation in continuous light (giant fiber) | Megighian et al. 2001 |
| Ion channels | ||
| eag (ether-à-go-go), V-gated K+ | Increases rate of habituation (giant fiber response) | Engel & Wu 1998 |
| channel α subunit | Increases rate of habituation (olfactory jump) | Joiner et al. 2007 |
| Hk (Hyperkinetic), V-gated K+ | Reduces rate of habituation (giant fiber response) | Engel & Wu 1998 |
| channel β subunit | Reduces rate of habituation (olfactory jump) | Joiner et al. 2007 |
| Sh (Shaker), V-gated K+ channel α | Increases rate of habituation (giant fiber response) | Engel & Wu 1998 |
| subunit | Reduces rate of habituation (olfactory jump) | Joiner et al. 2007 |
| slo (slowpoke), Ca2+-activated K+ | Reduces rate of habituation (giant fiber response) | Engel & Wu 1998 |
| channel subunit | Reduces rate of habituation (olfactory jump) | Joiner et al. 2007 |
| Neuroanatomy/Development | ||
|
mbm (mushroom body miniature), zinc-finger protein |
Increases rate of habituation (shock avoidance) | Acevedo et al. 2007 |
| nob (no bridge), ?? | Reduces habituation (proboscis extension) | Bouhouche et al. 1993 |
also called fickle
also called Su-var(3)6
In addition to the references cited in this review, information about these genes can be found at www.flybase.org.
Here we will review the major model systems for habituation in the fly, framed in the context of Thompson and Spencer’s (1966) parametric criteria. Along the way, we will highlight the genetic approaches that make the fly particularly valuable.
II. Model Systems
A. Visually evoked behaviors
1. Giant Fiber Response
We begin with the jump-and-flight giant fiber response, mediated by a descending pathway for which many of the anatomical details and physiological properties have been documented (Tanouye & Wyman, 1980; Wyman, Thomas, Salkoff, & King, 1984). A stereotyped sequence of leg extension and flight initiation can be triggered by sudden darkness (most reliably in white-eyed mutants) (Wyman et al., 1984) or the combination of visual stimulation and wind produced by an approaching object (Hammond & O’Shea, 2007). Afferents to the descending giant neurons include visual interneurons and antennal mechanoreceptors (Bacon & Strausfeld, 1986). The specific afferent pathway that mediates the visual response has not been identified. However, the efferent pathway from the descending giant neurons to the thoracic muscles is well-described (Wyman et al., 1984; Trimarchi & Schneiderman, 1995).
Properties of Habituation
The visually evoked giant fiber response is all-or-none, and the likelihood of a failed response increases with repeated stimulation (Kelly, 1983; Engel & Wu, 1996; Hammond & O’Shea, 2007), fitting habituation criteria of frequency dependence, spontaneous recovery, and dishabituation by a novel stimulus such as an air puff (Engel & Wu, 1996). However, in most experiments we have bypassed the earliest visual stages by triggering giant fiber afferents with electric pulses across the brain (Engel & Wu, 1996). This simplifies the circuit and avoids any possibility of sensory adaptation. The jump-and-flight response is recorded as action potentials in leg extensor and wing depressor muscles of the tethered intact fly. The electrically triggered response starts to fail with stimulus frequencies of 2–10 Hz (Engel & Wu, 1996). Response attenuation in this semi-reduced preparation satisfies eight of Thompson and Spencer’s (1966) parametric criteria for habituation (Table 2); the ninth criterion, stimulus generalization (or stimulus specificity) of habituation, has not been tested.
Table 2.
Habituation criteria studied in Drosophila
| Response Decrement |
Spontaneous Recovery |
Potentiation; Below Zero a |
Stimulus Frequency |
Stimulus Intensity |
Stimulus Generalization |
Dishabituation | Habituation of Dishabituation |
|
|---|---|---|---|---|---|---|---|---|
| Giant Fiber Response |
1, 2, 3, 4, 5, 6 |
2, 3, 4 | 2, 3, 4 | 2, 3, 5, 6 | 3 | 2, 3, 4, 6 | 2 | |
| Landing Response |
7, 8, 9, 10, 11, 12, 13 |
7, 9, 11 | 7, 8, 10, 12 | 7, 8 | 7, 9 | 7 | ||
| Proboscis Extension |
14, 15, 16, 17, 18, 19 |
18 | 14, 17, 19 | 15, 16 | ||||
| Olfactory Jump |
20, 21, 22, 23, 24, 25 |
21, 23, 24, 25 |
23, 24 | 23, 24, 25 | 21, 23, 24, 25 |
|||
| Locomotory Startle |
26, 27 | 26 | 26 | 26 | 26 | 26, 27 | ||
| Odor-Induced Leg Response |
28 | 28 | 28 | 28 | ||||
| Shock Avoidance |
29 | 29 | 29 | 29 | ||||
| Courtship Conditioning |
30, 31, 32 | 30, 31 | ||||||
| Leg Resistance |
33 | 33 | 33 | 33 | ||||
| Cleaning Reflex |
34 | 34 | 34 | 34 | 34 | 34 | 34 |
“Potentiation of Habituation” and “Below-Zero Habituation” are combined to save space.
References for Table 2:
1 Kelly 1983; 2 Engel & Wu 1996; 3 Engel & Wu 1998; 4 Engel et al. 2000; 5 Meghigian et al. 2001; 6 Zordan et al. 2005; 7 Fischbach 1981; 8 Fischbach & Bausenwein 1988; 9 Wittekind & Spatz 1988; 10 Rees & Spatz 1989; 11 Waldvogel & Fischbach 1991; 12 Asztalos et al. 1993; 13 Friedrich 1994; 14 Duerr & Quinn 1982; 15 Le Bourg 1983; 16 Fois & Le Bourg 1991; 17 Bouhouch et al. 1993; 18 Minois & Le Bourg 1997; 19 Scheiner et al. 2004; 20 McKenna et al. 1989; 21 Mihalek et al. 1997; 22 Godenschwege et al. 2004; 23 Asztalos et al. 2007a; 24 Asztalos et al. 2007b; 25 Joiner et al. 2007; 26 Cho et al. 2004; 27 Wolf et al. 2007; 28 Chandra & Singh 2005; 29 Acevedo et al. 2007; 30 Gailey et al. 1982; 31 O’Dell 1994; 32 Neckameyer et al. 2000; 33 Jin et al. 1998; 34 Corfas & Dudai 1989
Because of the strong fit with the classic parametric definition, we have been able to take advantage of this semi-reduced preparation (stimulating the brain and recording muscle activity in a restrained animal) to study a habituation process that is localized to the CNS. The diverging efferents of the descending giant fibers are not involved in habituation of the electrically induced response because the jump and flight muscles always respond or fail together during habituation (Engel & Wu, 1996). Early visual stages are not involved because the visually evoked response has a longer latency and habituates at stimulus frequencies of 0.2 Hz (Kelly, 1983; Engel & Wu, 1996), an order of magnitude lower than the direct recruitment of giant fiber afferents by electrical stimulation (Engel & Wu, 1996). This difference in habituating frequency may simply mean that electrical pulses recruit the labile giant fiber afferents more effectively than visual stimuli (which may include parallel input through several steps of processing from the photoreceptors to higher visual centers), and that habituation in both cases occurs by the same mechanism. In keeping with this, dnc (dunce) and rut (rutabaga) mutations (Table 1, and see below) alter the habituation rate of the visually induced response (Xiao-Tien Zhong and Chun-Fang Wu, unpublished) in ways that parallel their effects upon the electrically induced response (Engel & Wu, 1996). Conversely, early stages in the circuit prior to the point of electrical stimulation may have additional plastic elements that might contribute to behavioral habituation, that could be isolated in future work. For instance, Kelly (1983) found that the off-transient of the electroretinogram (an indication of synaptic transmission in early visual centers) diminished as the visually evoked muscle response habituated.
In many well-established models for habituation, such as the Aplysia gill-siphon withdrawal, habituation is seen as a gradual decline in synaptic potential amplitude and behavioral response magnitude (Kupfermann, Castellucci, Pinsker, & Kandel, 1970; Hawkins, Kandel, & Bailey, 2006b). In contrast, the Drosophila giant fiber response is all-or-none because the circuit downstream of the descending giant fibers is not labile under the stimulus parameters that produce habituation (Engel & Wu, 1996). Habituation reduces the signal below threshold at least two neurons earlier than the jump and flight muscles, where most often a single action potential is recorded in a successful response (Engel & Wu, 1996). Response latency has been used as a continuous variable of habituation in the landing response (described below), but the latency of the giant fiber response does not show habituation (Engel & Wu, 1996).
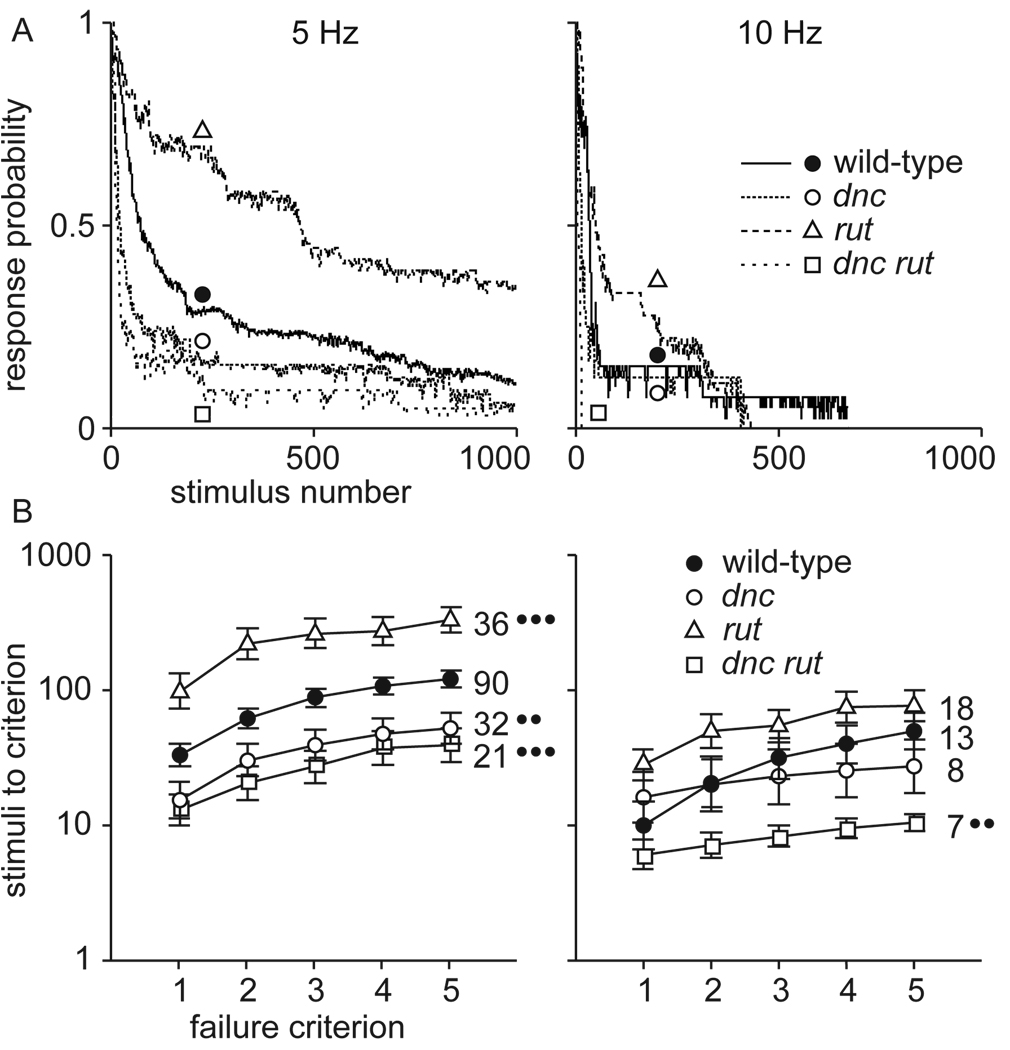
Because there are no intermediate response gradations between “all” and “none”, the degree of attenuation cannot be assessed from a single response of a single animal. One approach, calculating the declining response probability in a pool of flies, gives a clear graphic representation of habituation that can be compared between experimental groups (Figure 1 A). However, it does not allow the degree of habituation to be assessed during a single trial. In a second approach, the number of consecutive failures to respond indicates the degree of habituation, and the rate of habituation is the number of stimuli required to reach a given number of consecutive failures (Figure 1 B). This provides a criterion for habituation that can be assessed during a trial, something that is necessary to assure a consistent level of habituation before testing spontaneous recovery and dishabituation. The string of failures set as the criterion should be long enough to indicated definite habituation, but short enough to avoid significant “below-zero habituation” (habituation that continues to develop after the response has reached its minimum level; Thompson & Spencer, 1966), especially in very rapidly habituating genotypes. After comparing various alternatives, we settled on a criterion of 5 consecutive failures for habituation of the electrically induced giant fiber response (Engel & Wu, 1996).
Figure 1.
Habituation of the electrically induced giant fiber response in wild-type, dnc, rut, and dnc rut mutant Drosophila stimulated at 5 or 10 Hz. The same data are displayed two ways. (a) Frequency-dependent decrement of response probability. Three-point running averages of pooled responses (numbers of flies indicated in b). (b) Number of stimuli to attain criteria of one to five consecutive failures. Mean ± SEM of log-transformed values for the number of flies indicated (1000 was used if a fly did not attain a given criterion within 1000 stimuli). •• p < 0.01; ••• p < 0.001. Habituation is more rapid at the higher stimulus frequency, and rut mutants are resistant to habituation, whereas dnc and dnc rut mutants are abnormally susceptible. From Engel and Wu (1996).
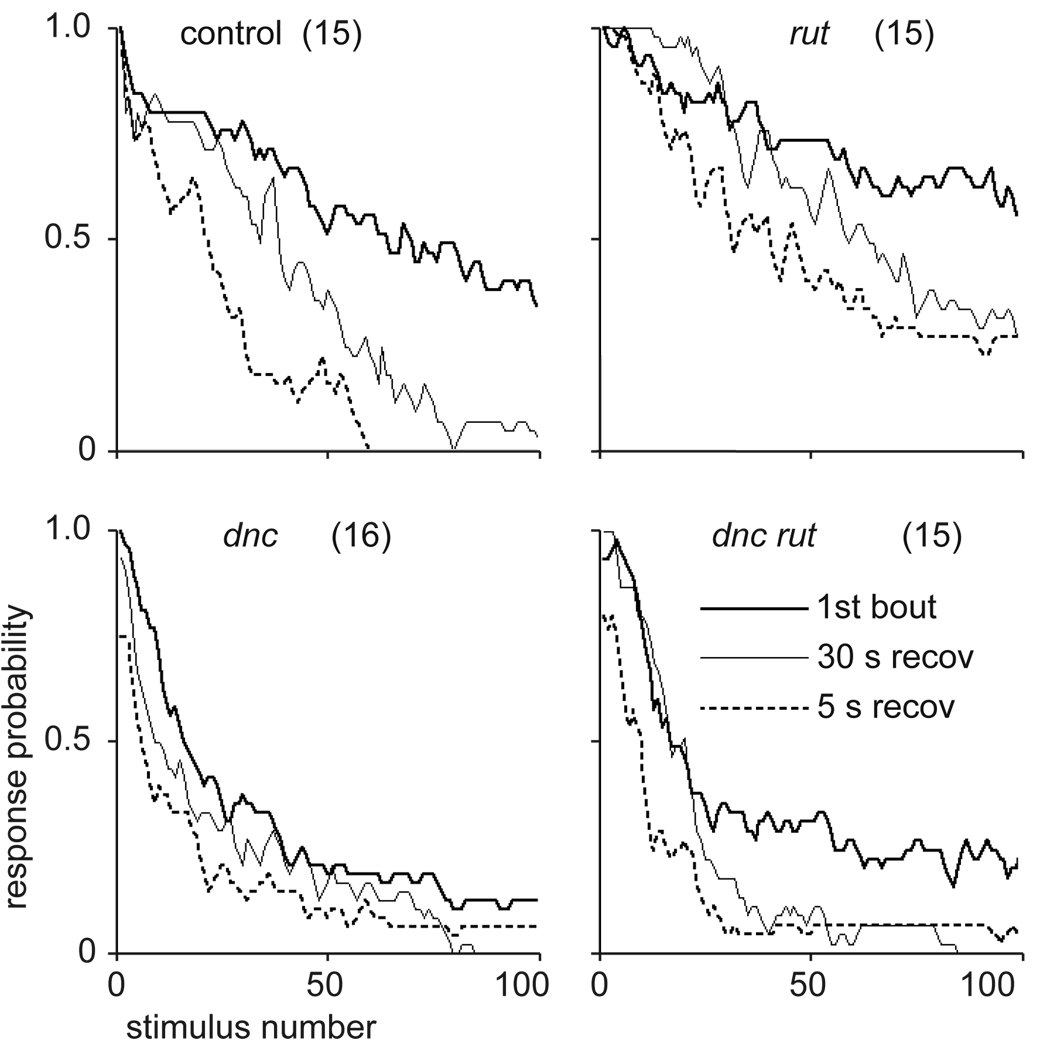
The electrically induced giant fiber response shows robust spontaneous recovery to a probability of about 1 within 5 s (Figure 2). However, habituation is faster after recovery (Figure 2). This potentiation of habituation (Thompson & Spencer, 1966) shows that recovery from habituation is not complete. Dishabituation can be induced by a light flash or an air puff. The degree of dishabituation varies between flies and in repeated tests of the same fly (Engel & Wu, 1996; Engel, Xie, Sokolowski, & Wu, 2000), and effects are confounded by spontaneous increases in response probability that sometimes occur after habituation criterion. As shown in Figure 3, dishabituation can be shown convincingly by comparing dishabituation trials alternated with “sham” trials in which the same fly is habituated to the 5-failure criterion but no dishabituating stimulus is given. Repetitive electrical stimulation of the giant fiber response itself may also induce dishabituation or sensitization; evidence for this includes spontaneous increases in response probability with extended stimulation (Engel & Wu, 1996), a “priming effect” of prior stimulation (within 2 s) upon the refractory period (Engel, 1995), and the fact that flies with a paired-pulse refractory period greater than 100 ms can sometimes respond to stimulus trains of 10 pulses per second (Engel, 1995). Operational and physiological distinctions between dishabituation and sensitization will be discussed further in Conclusions.
Figure 2.
Spontaneous recovery of the electrically induced giant fiber response in wild-type, dnc, rut, and dnc rut mutant Drosophila stimulated at 5 Hz. Flies were habituated to five-failure criterion (heavy line), allowed to recover for 30 sec and habituated again (light line), then allowed to recover for 5 sec and habituated a third time (dashed line). Sample sizes indicated; three-point running averages. Note that dnc and dnc rut did not recover to initial response levels in 5 sec. Potentiation of habituation is most evident for wild-type flies. From Engel and Wu (1996).
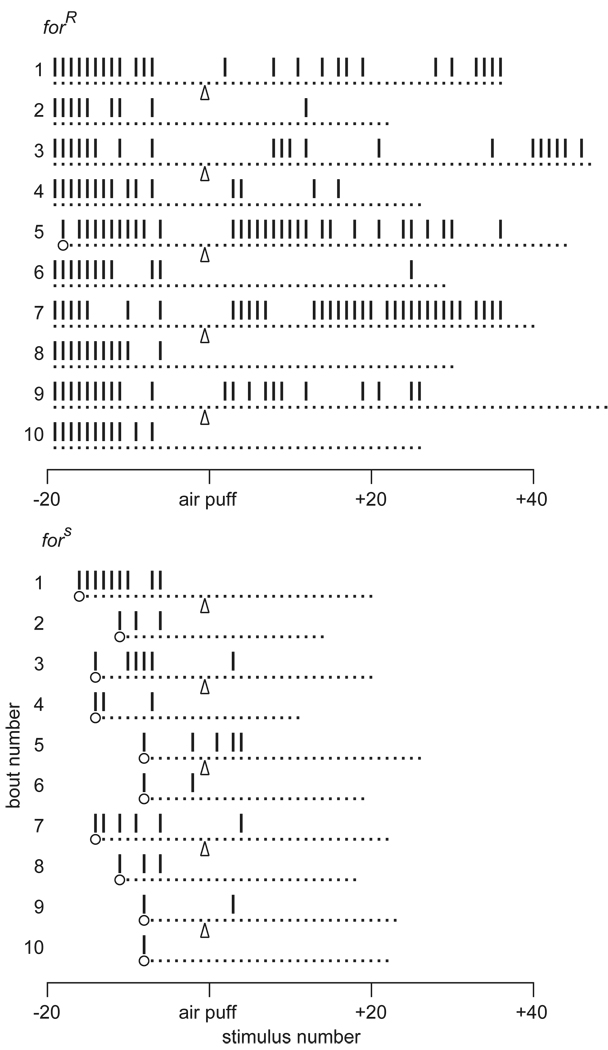
Figure 3.
Dishabituation of the electrically induced giant fiber response in Drosophila by natural variants of the foraging for) locus. forR and fors are naturally-occurring rover and sitter alleles with high and low levels of PKG activity, respectively. Each example shows 10 bouts of 5 hz stimulus trains for a single fly, with 30 sec between bouts. Dots indicate stimuli and ticks indicate responses recorded in jump and flight muscles. In odd numbered bouts, air puffs (arrows) were given after the fly attained the habituation criterion of five failures; even numbered bouts serve as sham controls. For comparison, each sham bout is aligned with the preceding test bout according to the last response before habituation criterion. After habituating to criterion, each bout was continued for 20–50 more stimuli to allow any evoked recovery to be seen. Circles indicate the first stimulus of the bout; in the more slowly habituating forR fly, all but one of the bouts began to the left of these plots. Sitter flies (fors) habituate more rapidly than rovers (forR), and dishabituation is seen in both genotypes. From Engel et al. (2000).
The rate of habituation, whether expressed as the number of stimuli or the amount of time until habituation, obviously depends upon the criterion for habituation (degree of response attenuation or number of consecutive failed responses). It also depends upon stimulus intensity and stimulus frequency (Thompson & Spencer, 1966) and genotype (including identified mutations and the genetic backgrounds of different fly stocks; see below). The rate of habituation also depends on properties of a particular neural circuit. As a case in point, the jumping startle response shows markedly different rates of habituation in three contexts: the giant fiber-mediated response typically habituates after around a hundred trials of electrical stimulation given at 2–5 Hz, but after just ten or so trials of 0.2–0.5 Hz visual stimulation (which activates sensory pathways that are bypassed by electrical stimulation) (Engel & Wu, 1996), while the olfactory jump response (mediated by a different descending pathway; Trimarchi & Schneiderman, 1995) typically habituates within ten trials of odorant stimulation at ~0.02 Hz (60 s intervals) (Joiner, Asztalos, Jones, Tully, & Wu, 2007).
Genetic Analysis of Habituation
By using electrical stimulation to bypass early sensory stages of the giant fiber pathway, and taking advantage of the failsafe nature of the escape circuit from the descending giant fibers onward, this protocol enables us to focus on central synaptic input to the giant fiber neurons, to ensure precise genetic analysis. Synaptic plasticity has often been related to second-messenger signal transduction pathways such as cAMP signaling (Wu et al., 1998; Hawkins et al., 2006). The genes rut and dnc encode adenylyl cyclase and cAMP phosphodiesterase, for cAMP synthesis and degradation, respectively (Davis, 1996), and both genes were first identified in mutagenesis screens for deficits in associative conditioning (Dudai, Jan, Byers, Quinn, & Benzer, 1976; Aceves-Pina, Booker, Duerr, Livingstone, Quinn, Smith, Sziber, Tempel, & Tully, 1983). Habituation of the giant fiber response is slowed by rut and accelerated by dnc mutations, consistent with their opposing effects upon cAMP metabolism, yet dnc rut double mutants habituate even faster (Engel & Wu, 1996) (Figure 1 & Figure 2; Table 1), which suggests that the localization and kinetics of cAMP regulatory processes may be more significant to habituation than the overall level of cAMP.
Cyclic nucleotides can activate protein kinases such as cAMP-dependant protein kinase (PKA), a major mediator of cAMP effects. Another example is a cGMP-dependent protein kinase (PKG) that is encoded by the gene for (foraging) (Osborne, Robichon, Burgess, Butland, Shaw, Coulthard, Pereira, Greenspan, & Sokolowski, 1997), which was first identified as a basis of natural variation in food-searching behavior (Sokolowski, 1980). Flies with “rover” alleles of for have higher PKG activity, move more and sample a wider area while feeding than do “sitters”. Rover genotypes also show slower habituation of the giant fiber response (Engel et al. 2000) (Figure 3) and the proboscis extension reflex (Scheiner, Sokolowski, & Erber, 2004) (Table 1). Habituation may therefore be involved with variations in foraging behavior.
Molecular components of synaptic transmission or membrane excitability could mediate habituation, and they may be targets of second-messenger pathways. Deletion of caki, which encodes a presynaptic membrane-associated protein with domains homologous to calcium-calmodulin (CaM) dependent kinase and guanylate kinase, reduces the effectiveness of synaptic transmission at multiple points in the giant fiber pathway, apparently by increasing spontaneous vesicle release (Zordan, Massironi, Ducato, te Kronnie, Costa, Reggiani, Chagneau, Martin, & Megighian, 2005). Response failures occur at unusually low stimulus frequencies in flies with deletion of the caki gene. This response attenuation can be dishabituated by an air puff (Zordan et al. 2005). It will be interesting to record simultaneously from DLM and TTM muscles to determine whether the rapid habituation in the caki mutant is mediated at or before the giant fiber neuron (as it is in other genotypes; Engel & Wu, 1996) or later, after the motor pathways diverge.
A genetic approach is well-suited to dissecting the functions of a diverse array of K+ channel subunits that are encoded by different genes but often have overlapping pharmacological sensitivities (Wu et al., 1998). Habituation in the giant fiber pathway is enhanced somewhat by mutations of the Sh (Shaker) A-type channel (homologous to vertebrate KV1) and reduced substantially by mutations of the slo (slowpoke) Ca2+-activated channel (homologous to BK). Habituation is most greatly accelerated by mutations of eag (ether-à-go-go), which encodes an α channel subunit (homologous to vertebrate Eag) that is subject to regulation by cyclic nucleotides and protein kinases. However, habituation is most strongly reduced by mutations of Hk (Hyperkinetic), which encodes a β subunit of the Shaker channel (homologous to KVβ1) (Engel & Wu, 1998) (Table 1). It is notable that these K+-channel subunit mutations had effects upon habituation at least as great as the classic "memory" mutants rut and dnc (Engel & Wu, 1996; Engel & Wu, 1998).
Another genetic analysis of habituation in the giant fiber pathway examined the influence of null mutations of per (period) that inactivate the circadian clock (Megighian, Zordan, & Costa, 2001). Habituation occurs at unusually low stimulus frequencies in per mutants, but interestingly, only when the flies have been maintained in continuous light.
2. Landing Response
If flies must take off, they must also land, and the fictive (mid-air) landing response of a tethered intact animal in flight, a stereotyped motor pattern of the legs and wings, has also been exploited as a model for habituation (Fischbach, 1981). The stimulus, a dark bar sweeping across the visual field as if to indicate an approaching substrate upon which the fly must land, allows exquisite control of variables including brightness and contrast (Rees & Spatz, 1989; Asztalos, von Wegerer, Wustmann, Dombrádi, Gausz, Spatz, & Friedrich, 1993), orientation and location in the visual field, and sweep direction and velocity (Fischbach & Bausenwein, 1988; Waldvogel & Fischbach, 1991; Friedrich, Spatz, & Bausenwein, 1994). The motor output, a forward extension of the front legs (as if to touch an approaching substrate), is graded but has usually been treated as all-or-none. Latency of leg extension (Wittekind & Spatz, 1988; Rees & Spatz, 1989; Asztalos et al., 1993) and an increase in wing beat frequency during the landing response (Friedrich et al., 1994) are continuous variables that also habituate. Habituation is typically induced with interstimulus intervals of 2 s. Standard criteria for habituation (Thompson & Spencer, 1966) that have been demonstrated include spontaneous recovery, dependence on stimulus intensity, stimulus generalization (within limits), dishabituation by a vibrational stimulus or the act of landing, and habituation of dishabituation (Table 2).
Genetic studies have shown more rapid habituation in mutants with altered cAMP metabolism (Wittekind & Spatz, 1988; Rees & Spatz, 1989), but unlike the giant fiber response, both dnc and rut mutants increase habituation in spite of opposite effects on cAMP metabolic pathways. A mutation that reduces a protein phosphatase also increases the rate of habituation (Asztalos et al., 1993). Natural variation in habituation is highlighted by the fact that two commonly-used “wild-type” laboratory stocks, first collected on different continents, habituate at different rates (Rees & Spatz, 1989). Genetic differences as well as pharmacological treatments affect habituation of landing response probability but not latency (Wittekind & Spatz, 1988; Asztalos et al., 1993), implying that these two parameters may habituate by different processes. In contrast, Friedrich et al. (1994) found cohabituation of landing response probability and the increase in wingbeat frequency.
A characteristic of work on the landing response is the extent to which workers have made inferences about circuit physiology based upon carefully analyzing the stimulus control of the behavior (Fischbach and Bausenwein, 1988; Wittekind & Spatz, 1988) and developing cybernetic models that account for the kinetics of the acquisition and decay of habituation (Rees & Spatz, 1989; Waldvogel & Fischbach, 1991; Ögmen & Moussa, 1993). Fischbach and Bausenwein (1988) showed that generalization of habituation is restricted in the visual field, especially horizontally, which indicates that habituation occurs before extensive convergence of visual pathways. Habituation is also specific to the direction of movement of the dark bar (Wittekind & Spatz, 1988), so it must occur after the convergence of neurons with adjacent receptive fields. Contralateral sensitization of the landing response by visual stimulation has also been shown (Fischbach, 1981), and kinetics of plasticity have been modeled in a two-process system with terms for habituation and sensitization (Waldvogel & Fischbach, 1991; Ögmen & Moussa, 1993). These well-defined analytical approaches should facilitate future genetic investigations into habituation of the landing response.
B. Chemically evoked behaviors: Gustatory
1. Proboscis Extension Reflex (PER)
Flies have taste receptors on their front leg tarsae, and if sugar water is applied to the tarsus, a hungry Drosophila will extend its proboscis (Duerr & Quinn, 1982; Le Bourg, 1983). It is very convenient that the fly can taste the stimulus with its feet without ingesting it, thus avoiding reinforcement or satiation during habituation experiments. Habituation typically develops with interstimulus intervals of a minute or more (Duerr & Quinn, 1982; Le Bourg, 1983). Parameters of habituation (Thompson & Spencer, 1966) that have been demonstrated explicitly for the PER (Table 2) include dependence on stimulus intensity (sucrose concentration) (Minois & Le Bourg, 1997); stimulus generalization to the contralateral leg (Duerr & Quinn, 1982; Bouhouch, Vaysse, & Corbière, 1993) or to a sucrose concentration greater than the habituating stimulus (Scheiner et al., 2004); and dishabituation induced by yeast odor (Le Bourg, 1983; Fois, Médioni, & Le Bourg, 1991). As with the landing response, stimulus generalization has been used to make inferences that help to localize the site of habituation. Presenting the stimulus to one front leg incompletely habituates the response to stimulating the contralateral leg, and this implies that habituation occurs (at least partly) after inputs from the two legs converge (Duerr & Quinn, 1982; Bouhouche et al., 1993).
Habituation of the PER is reduced by several mutations that reduce the efficacy of second-messenger pathways, including cAMP (dnc and rut) and PKC (tur) (turnip) (Duerr & Quinn, 1982), but enhanced in for variants with reduced cGMP-dependent protein kinase activity (Scheiner et al., 2004) (Table 1). A mutation of amn (amnesiac), which encodes a neuropeptide that may lead to activation of the rut adenylyl cyclase (Feany & Quinn, 1995), reduces the responsiveness to sucrose, making habituation results difficult to interpret (Duerr & Quinn, 1982). The anatomical mutation nob (no-bridge), which disrupts commissural tracts in the brain, reduces habituation but appears to preserve contralateral generalization of habituation, although the effects are complex (Bouhouche et al., 1993). The PER habituates more slowly in aged flies or flies exposed for some time to hypergravity (Fois et al., 1991; Minois & Le Bourg, 1997); the latter authors suggested that sucrose may be more valuable to an older fly because of metabolic changes, so that a given sucrose concentration effectively becomes a more intense stimulus. Because of the technical simplicity of manipulating its stimulus parameters coupled with the complexity of the response to different qualities and localization of tastants (e.g., Duerr & Quinn, 1982; Bouhouche et al., 1993; Bouhouche, Elkhessaimi, Vaysse, & Choulli, 1995), the PER seems especially suitable for a systematic genetic dissection of the underlying neural substrates of the behavioral plasticity.
C. Chemically evoked behaviors: Olfactory
1. The olfactory jump response
A noxious odorant can trigger a jump-and-flight response (McKenna, Monte, Helfand, Woodard, & Carlson, 1989) that is functionally similar to the giant fiber-mediated responses, but appears to involve a different descending pathway (Trimarchi & Schneiderman, 1995). In sensory modality and the odorants used, it is closely related to widely used protocols for the study of associative conditioning in Drosophila (Quinn, Harris, & Benzer, 1974; Tully & Quinn, 1985). Flies are free-standing in a small chamber through which air is flowing. The odorant (usually benzaldehyde) is introduced briefly into the air stream. The jump response is scored as all-or-none. Interstimulus intervals are typically 1 minute. The habituated response shows habituation parameters (Table 2) of spontaneous recovery, dependence on stimulus frequency and intensity, and dishabituation by mechanical stimulation (vortexing the test chamber) (Mihalek, Jones, & Tully, 1997; Asztalos, Arora, & Tully, 2007a; Asztalos et al., 2007b; Joiner et al. 2007).
Mutations of second messenger signaling pathways again strongly influence the habituation of the olfactory jump. Mutations of dnc reduce habituation slightly, instead of enhancing it slightly as in the giant fiber response, but a mutation of rut slows habituation greatly in both systems (Engel & Wu, 1996; Asztalos et al. 2007a). A mutation of tur, suspected to reduce PKC activity, speeds up habituation, but the authors argue that this may be ascribed to reduced vigor in tur flies, in part because the attenuated response is not fully dishabituated in those flies (Mihalek et al., 1997). A mutation of Btk29a (Btk family kinase at 29A, also called fickle), which reduces the activity of Burton’s tyrosine kinase, increases the rate of habituation of the olfactory jump (Asztalos et al., 2007b). The Btk29a mutation also eliminates sensitization produced by vortexing the test chamber, the same stimulus used for dishabituation. Asztalos et al. (2007b) hypothesized that the apparent increase in habituation in Btk29a mutants is due to this defect in sensitization, resulting in a faster-than-normal response decrement in a dual-process model (Groves & Thompson, 1970). Because this difference in the rate of response decrement is seen even when flies have not been vortexed (Asztalos et al., 2007b), this raises the possibility that the olfactory stimulus itself induces sensitization that is suppressed by the Btk29a mutation.
Second messenger pathways can modify ion channels and the machinery of synaptic release, and both have been implicated in habituation of the olfactory jump response. Knock-out mutants of the syn (synapsin) gene lack the synaptic-vesicle-associated protein, synapsin, which is implicated in synapse formation and synaptic transmission in mammals (Godenschwege, Reisch, Diegelmann, Eberle, Funk, Heisenberg, Hoppe, Hoppe, Klagges, Martin, Nikitina, Putz, Reifegerste, Reisch, Rister, Schaupp, Scholz, Schwärzel, Werner, Zars, Buchner, & Buchner, 2004). These mutants are surprisingly normal in many respects, but they habituate rapidly (Godenschwege et al., 2004). It is interesting to compare the effects of mutations on the olfactory jump and the giant fiber response, because these similar startle behaviors are mediated by different descending neural pathways (Trimarchi & Schneiderman, 1995). Mutations that reduce the functionality of individual K+ channel subunits have contrasting effects: habituation is potentiated by eag and slowed by slo in both systems, but habituation of the olfactory jump is only slightly slowed by Hk and slowed most by Sh (Joiner et al., 2007), while the opposite is true for the giant fiber response (Engel & Wu, 1998). This demonstrates that mutations of two different K+ channels can differentially affect two descending pathways.
2. The locomotory startle response
Freestanding flies show a transitory increase in locomotion during the first 30 seconds of exposure to an odorant such as ethanol (Cho, Heberlein, & Wolf, 2004). This response shows habituation with interstimulus intervals on the order of 6 minutes, and can be dishabituated by a mechanical shock (shaking the chamber) (Cho et al., 2004; Wolf, Eddison, Lee, Cho, & Heberlein, 2007). Cho et al. (2004) documented spontaneous recovery and stimulus frequency dependence, but found no relationship between stimulus intensity and habituation kinetics (Table 2). They showed stimulus generalization between three different odorants, two of which are known to be mediated by different primary olfactory neurons, and this indicates that the habituation is central. Consistent with this conclusion, chemical ablation of the mushroom bodies, brain centers implicated in integrating olfactory signals in associative learning (Heisenberg, 2003), reduces habituation without altering the initial response to the odorant (Cho et al., 2004).
A mutation of rut reduces habituation of the response (Wolf et al., 2007), once again implicating cAMP-messenger pathways. A genetic screen for mutations reducing habituation yielded a mutation of sgg (shaggy), which encodes a homolog of glycogen synthase kinase-3 (Wolf et al., 2007). Ubiquitous overexpression of the sgg product increases habituation, but overexpression localized to mushroom bodies does not do so. The authors showed that habituation protocols lead to increased phosphorylation of Shaggy protein in vivo (although expressing a non-phosphorylable construct also increases habituation).
3. The odor-induced leg response
Yet another response to odorants is particularly interesting because of how stimulus generalization has been exploited. A restrained fly exposed to a 4 s odorant pulse responds by moving its front legs after the end of the pulse (Chandra & Singh, 2005). The response habituates with an interstimulus interval of 5 minutes and is dishabituated by feeding the fly a sucrose solution. (The response also declines when the fly is exposed to an air stream without odorant, but greater spontaneous recovery distinguishes this from habituation to the odorant.) The response can be triggered with several different odorants, and stimulus generalization of habituation is most pronounced between chemically similar odorants that are expected to activate overlapping ensembles of olfactory neurons (Chandra & Singh, 2005). In this way, the habituation protocol can also provide information about the basis for odor classification by the fly.
D. Other behaviors in intact flies
1. Shock avoidance
Flies avoid walking on an electrified grid, and this has been used as an aversive unconditioned stimulus for associative olfactory conditioning (Quinn, Harris, & Benzer, 1974; Tully & Quinn, 1985). If the stimulus is reduced to a lower level, avoidance of an electrified grid in a choice test is attenuated by previous conditioning with shocks at a 4 s interstimulus interval (Acevedo, Froudarakis, Kanellopoulos, & Skoulakis, 2007). This habituation of shock avoidance shows spontaneous recovery, dependence on stimulus intensity, and dishabituation by a noxious odorant (Table 2). Long-term habituation, lasting at least 90 min, is induced by multiple training sessions spaced at 5–10 min intervals, and this long-term habituation is also dishabituated by an odorant. The mushroom bodies are known to mediate associative olfactory conditioning with shock as the unconditioned stimulus (Heisenberg, 2003). Functional or anatomical perturbation of the mushroom bodies (Table 1) (Acevedo et al., 2007) enhances habituation of shock avoidance and delays recovery from habituation, with the interesting implication that this brain structure normally provides “protection from premature habituation” (Acevedo et al., 2007). It is noteworthy that shaking the flies, a potent dishabituator of olfactory jump and the locomotory startle response, does not dishabituate shock avoidance.
2. Courtship conditioning (EDCM)
Sexually naïve mature males direct courtship behavior toward newly eclosed males, but this behavior declines quickly (Gailey, Jackson, & Siegel, 1982) in an effect that has been called Experience-Dependent Courtship Modification (EDCM) (O’Dell, 1994). The conditioning stimulus is typically exposure to active young males which reject the experimental male, and this might imply that there is a component of associative conditioning. However, decline of courtship is almost as strong if the young male is inactivated by crushing its head, or if the experimental male is simply held in a chamber previously occupied by a young male (Gailey et al., 1982). Attenuation of the response shows recovery after 1 hour (O’Dell, 1994) or 4 hours (Gailey et al., 1982) of isolation of the experimental male from the conditioning stimulus. EDCM has been called habituation (Gailey et al., 1982; O’Dell, 1994; Neckameyer, Woodrome, Holt, & Mayer, 2000), although additional criteria should be established in this ethologically relevant paradigm (Table 2).
Gailey et al. (1982) found that mutations of dnc eliminate EDCM with 30 minutes of conditioning, and O’Dell (1994) showed that a dnc mutant acquires EDCM more slowly but does habituate after 60 minutes. A mutation of rut also reduces EDCM (Gailey et al., 1982). Mutations of amn (Gailey et al., 1982) and iav (inactive) (O’Dell, 1994) have effects similar to dnc; a tur mutation reduces EDCM similarly to rut, and the learning mutation cab (cabbage), the product of which has not been functionally characterized, has no effect on EDCM. The iav gene encodes a TRPV cation channel subunit, but mutants also have reduced levels of octopamine and tyramine (Gong, Son, Chung, Kim, Shin, McClung, Lee, Lee, Chang, Kaang, Cho, Oh, Hirsh, Kernan, & Kim, 2004). Further evidence for a role of biogenic amines is provided by Neckameyer et al. (2000), who found that the magnitude of EDCM declines between the ages of 5–20 days, ages during which levels of dopamine are also reduced and dopaminergic neurons show signs of degeneration.
E. Mechanically evoked behaviors in decapitated flies
Decapitation facilitates the observation of some evoked behaviors by eliminating incompatible spontaneous behaviors. A decapitated fly is viable for hours to days if desiccation is prevented. This preparation has enabled some ingenious habituation paradigms.
1. Leg resistance reflex
A headless fly resists movement of the tibia by a proprioceptive reflex (Jin, Griffith, & Murphey, 1998). When the mesothoracic tibia is moved in a 2 Hz sinusoidal pattern and excitatory junctional potentials of the tibial extensor muscle are recorded, the frequency of excitatory junctional potentials declines to an asymptote in seconds (Jin et al., 1998). This attenuation shows parametric characteristics of habituation including recovery, dependence upon stimulus frequency and intensity, and dishabituation by touching the abdomen (Jin et al., 1998) (Table 2). This habituation is altered by modifications of CaM kinase II (CaMKII) transgenically expressed in the leg sensory neurons that mediate the reflex (Jin et al., 1998). When a calcium-independent (constitutively activated) CaMKII is expressed, habituation is induced only by decreasing the stimulus intensity (the magnitude of the sinusoidal leg deflection). Conversely, when CaMKII activity is reduced by expressing a non-autophosphorylable CaMKII or a CaMKII-inhibitory peptide, the response to the first deflection is lower than in wild-type, but strong sensitization is apparent with subsequent deflections in the 2 Hz stimulus train, and habituation is either absent or masked by this sensitization.
2. Cleaning reflex
When a thoracic macrochaete (one of the large bristles) of a decapitated fly is moved by puffing air onto it, the fly sweeps a leg up as if to brush away an object (Vandervorst & Ghysen, 1980; Corfas & Dudai, 1989). This response declines with repeated stimulation at 1–5 s intervals (Corfas & Dudai, 1989). This decline shows parametric characteristics of habituation including spontaneous recovery, potentiation of habituation, dependence on stimulus frequency and stimulus intensity, and dishabituation by a train of air puffs directed at a different bristle field (Corfas & Dudai, 1989). Stimulus generalization does not occur, because habituation to stimulation of one bristle does not generalize to a second bristle that triggers a response in the same leg (Corfas & Dudai, 1989). cAMP-pathway mutations alter the response in a very specific way: rut increases the speed of recovery from habituation without altering habituation or dishabituation (Corfas & Dudai, 1989), and dnc is also reported to increase the rate of recovery (Corfas & Dudai, 1990). This habituation appears to be central; recordings of bristle mechanoreceptor action potentials show fatigue (or cumulative adaptation) only with extended (6 sec) bristle deflections, and this fatigue is not reversed by dishabituating stimuli (Corfas & Dudai, 1990). At the same time, this sensory fatigue is reduced by rut and increased by dnc mutations (Corfas & Dudai, 1990), leaving open the possibility that changes in sensory input contribute to differences in central habituation. This interesting model system has untapped potential. The fields of sensory bristles that trigger grooming by each leg are distinct and well-defined (Vandervorst & Ghysen, 1980), their sensory neural projections have been characterized (Ghysen, 1980; Burg, Hanna, Kim, & Wu, 1993), and motor neurons that mediate the reflex have been identified (Green, 1981). The sensory neurons are readily accessible for electrophysiological recording (Corfas & Dudai, 1990; Engel & Wu, 1994), and small-patch mosaic techniques can be used to express a mutation in a single sensory neuron while its targets are wild-type (Burg & Wu, 1989).
III. Conclusions
To localize the neural substrates of habituation, fly workers have access to all of the usual approaches with a few unique twists imparted by genetic tools. Knowledge of the circuit that mediates the behavior is helpful. For example, we know that habituation of the giant fiber response is central in part because our knowledge of the circuit lets us rule out sensory adaptation and motor fatigue (Engel & Wu, 1996). The leg resistance reflex is an excellent example of a system where the circuit was cracked with the help of transgenic expression of tetanus toxin light chain targeted to specific sensory neurons using the Gal4-UAS binary system (Brand & Perrimon, 1993; Reddy, Jin, Trimarchi, Caruccio, Phillis, & Murphey, 1997); the same Gal4 lines were then used to drive expression of CaMKII transgenes in those sensory neurons for the study of habituation (Jin et al., 1998). Targeted gene expression is equally important when it provides negative results, such as the demonstration that overexpression of normal sgg GSK-3 protein in the mushroom bodies does not affect habituation of the locomotory startle response, even though generalized overexpression throughout the nervous system potentiates habituation (Wolf et al., 2007). New non-invasive methods have been developed for imaging neural activity by expressing Ca2+−sensitive fluorescent proteins targeted to specific populations of neurons (Wang, Wong, Flores, Vosshall, & Axel, 2003; Yu, Baird, Tsien, & Davis, 2003; Wang, Guo, Pologruto, Hannan, Hakker, Svoboda, and Zhong, 2004). The high spatial resolution offered by such optical imaging will complement the better temporal resolution of electrophysiology, with the potential to facilitate future genetic analysis of the underlying circuits.
Stimulus generalization has been used to delimit the level of sensory convergence at which habituation occurs (Duerr & Quinn, 1982; Fischbach & Bausenwein, 1988). Similarly, because dishabituation by a novel stimulus depends upon the convergence of two distinct sensory pathways, it is taken to indicate that habituation is a central process. This logic is most convincing if dishabituation can be distinguished from sensitization, because sensitization is an independent process that would not necessarily occur at the same anatomical location as habituation. However, dishabituation and sensitization have been difficult to distinguish beyond the operational level. In this review, “dishabituation” is an operational term for enhancement of a habituated response that does not imply an underlying mechanism. Conceptually, dishabituation has been ascribed to either a reversal of habituation or sensitization superimposed upon habituation (Hawkins, Cohen & Kandel, 2006a; Groves & Thompson, 1970). Distinguishing these two alternatives is not trivial, but has been addressed in other animals through physiological (e.g. Hawkins et al., 2006a) and developmental (Marcus, Nolen, Rankin & Carew, 1988) analyses. Although dishabituation is one of the most commonly demonstrated parameters of habituation in the fly (Table 2), it has not typically been characterized as thoroughly as habituation. Genetic approaches have the potential to distinguish operational dishabituation from sensitization, as exemplified by the report of Asztalos et al. (2007b) that the fickle mutation of Btk29a suppresses sensitization, but not dishabituation, of the olfactory jump response by mechanical shock. Further study of the relationship between sensitization and dishabituation is important to further elucidate the integration of experience-dependent attenuation and potentiation proposed in dual-process models of habituation (Groves & Thompson, 1970).
If a mutation alters habituation, one interpretation is that the gene product mediates the underlying process of neural plasticity, but a mutation could also modify habituation by changing the circuit, either physiologically or developmentally, in such a way that normal mechanisms of neural plasticity operate in an altered context. Therefore, a demonstration of a mutational effect is not a simple answer, but rather an entrée into an ensuing investigation. Mutations also have the potential to reveal links between behavioral habituation and physiological plasticity, if both are altered in a consistent way by the same mutation. In many cases, both non-associative and associative conditioning may be studied in the same behavioral system, and genetic tools can provide a way to determine how much their mechanisms overlap. Prime examples include dnc and rut mutations of the cAMP signaling cascade (Figure 1, Figure 2) which were first identified for their effects upon associative conditioning (Dudai, Jan, Byers, Quinn, & Benzer, 1976; Aceves-Pina, Booker, Duerr, Livingstone, Quinn, Smith, Sziber, Tempel, & Tully, 1983; Davis, 1996; Engel & Wu, 1996).
To exploit the power of genetic analysis, it is important to focus on a well-defined circuit, studied in conjunction with anatomical methods, which may include looking at patterns of gene expression relative to that preparation, so that genetic phenotypes can be interpreted more effectively in terms of the cellular and molecular context. The work reviewed here shows that we should use caution in extrapolating from one system to another, even within the same animal. In addition to differences of sensory stimulus patterns and network architecture, circuits may differ in expression and post-transcriptional modification of the gene of interest and other genes that interact with it. The two mutations that have been studied in the most model systems in the fly, dnc and rut, have different effects upon habituation of different behaviors (Table 1). As another example, even though K+ channel mutations have effects of similar sign (enhancing or reducing habituation) in the giant fiber response and olfactory jump (Table 1), the magnitude and rank order of their effects differs between the two systems (Engel & Wu, 1998; Joiner et al., 2007).
A variety of other genetic tools could be employed to further expand habituation studies in Drosophila. For example, classical ring X mosaic techniques have been used to restrict mutant expression to identified portions of the giant fiber circuit (Lee & Wu, 2002) and individual sensory neurons of the cleaning reflex (Burg & Wu, 1989). These or other more recently developed mosaic techniques such as MARCM (Mosaic Analysis with a Repressible Cell Marker) (Lee & Luo, 1999) could be applied incisively to the study of habituation. RNA interference (RNAi) can be used to “knock down” identified genes (e.g. Clemens, Worby, Simonson-Leff, Muda, Maehama, Hemmings, & Dixon, 2000), and can be targeted to specific spatial or developmental expression patterns by driving with gal4/UAS (e.g. Enerly, Larsson, & Lambertsson, 2002). Targeted expression systems can now be used not only to inactivate identified neurons (e.g. Reddy et al., 1997), but also to activate them non-invasively on command. For example, photostimulation has now been reported for neurons of the giant fiber pathway (Lima & Meisenböck, 2005) and the circuit underlying PER (Zhang, Ge, & Wang, 2007). These and other developments on the horizon ensure that the fly will continue to be a fruitful source of insights about habituation for years to come.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo SF, Froudarakis EI, Kanellopoulos A, Skoulakis EMC. Protection from premature habituation requires functional mushroom bodies in Drosophila. Learning & Memory. 2007;14:376–384. doi: 10.1101/lm.566007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves-Pina EO, Booker R, Duerr JS, Livingstone MS, Quinn WG, Smith RF, Sziber PP, Tempel BL, Tully TP. Learning and memory in Drosophila, studied with mutants. Cold Spring Harbor Symposia on Quantitative Biology. 1983;48:831–840. doi: 10.1101/sqb.1983.048.01.086. [DOI] [PubMed] [Google Scholar]
- Asztalos Z, Arora N, Tully T. Olfactory jump reflex habituation in Drosophila and effects of classical conditioning mutations. Journal of Neurogenetics. 2007a;21:1–18. doi: 10.1080/01677060701247508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos Z, Baba K, Yamamoto D, Tully T. The fickle mutation of a cytoplasmic tyrosine kinase effects sensitization but not dishabituation in Drosophila melanogaster. Journal of Neurogenetics. 2007b;21:59–71. doi: 10.1080/01677060701249488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos Z, von Wegerer J, Wustmann G, Dombrádi V, Gausz J, Spatz H-C, Friedrich P. Protein phosphatase 1-deficient mutant Drosophila is affected in habituation and associative learning. The Journal of Neuroscience. 1993;13:924–930. doi: 10.1523/JNEUROSCI.13-03-00924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon JP, Strausfeld NJ. The Dipteran “giant fibre” pathway: neurons and signals. Journal of Comparative Physiology A. 1986;158:529–548. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bouhouch A, Vaysse G, Corbière M. Immunocytochemical and learning studies of a Drosophila melanogaster neurological mutant, no-bridgeKS49, as an approach to the possible role of the central complex. Journal of Neurogenetics. 1993;9:105–121. doi: 10.3109/01677069309083453. [DOI] [PubMed] [Google Scholar]
- Bouhouche A, Elkhessaimi A, Vaysse G, Choulli MK. Le r#x000F4;le du chlorhydrate de quinine dans l’inhibition conditionnée du réflexe tarsal chez Drosophila melanogaster. Canadian Journal of Experimental Psychology. 1995;49:520–529. doi: 10.1037/1196-1961.49.4.520. [DOI] [PubMed] [Google Scholar]
- Burg MG, Hanna L, Kim Y-T, Wu C-F. Development and maintenance of a simple reflex circuit in small-patch mosaics of Drosophila: effects of altered neuronal function and developmental arrest. Journal of Neurobiology. 1993;24:803–823. doi: 10.1002/neu.480240608. [DOI] [PubMed] [Google Scholar]
- Burg MG, Wu C-F. Central projections of peripheral mechanosensory cells with increased excitability in Drosophila mosaics. Developmental Biology. 1989;131:505–514. doi: 10.1016/s0012-1606(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Chandra SBC, Singh S. Chemosensory processing in the fruit fly, Drosophila melanogaster: Generalization of a feeding response reveals overlapping odour representations. Journal of Biosciences. 2005;30:679–688. doi: 10.1007/BF02703568. [DOI] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW. Habituation of an odorant-induced startle response in Drosophila. Genes, Brain & Behavior. 2004;3:127–137. doi: 10.1111/j.1601-183x.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Dudai Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. The Journal of Neuroscience. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Dudai Y. Adaptation and fatigue of a mechanosensory neuron in wild-type Drosophila and in memory mutants. The Journal of Neuroscience. 1990;10:491–499. doi: 10.1523/JNEUROSCI.10-02-00491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiological Reviews. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Tully T. Gene discovery in Drosophila: new insights for learning and memory. Annual Review of Neuroscience. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Quinn WG. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE. PhD thesis. Iowa City, Iowa: University of Iowa; 1995. Effects of second messenger and excitability mutations upon identified neural circuits underlying activity-dependent plasticity of behavior in Drosophila. [Google Scholar]
- Engel JE, Wu C-F. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. Journal of Comparative Physiology A. 1994;175:267–278. doi: 10.1007/BF00192986. [DOI] [PubMed] [Google Scholar]
- Engel JE, Wu C-F. Altered habituation of an escape circuit in Drosophila memory mutants. The Journal of Neuroscience. 1996;10:3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE, Wu C-F. Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila. The Journal of Neuroscience. 1998;18:2254–2267. doi: 10.1523/JNEUROSCI.18-06-02254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE, Xie X-J, Sokolowski MB, Wu C-F. A cGMP-dependent protein kinase gene, foraging, modifies habituation-like response decrement of the giant fiber escape circuit in Drosophila. Learning & Memory. 2000;7:341–352. doi: 10.1101/lm.31600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerly E, Larsson J, Lambertsson A. Reverse genetics in Drosophila: From sequence to phenotype using UAS-RNAi transgenic flies. Genesis. 2002;34:152–155. doi: 10.1002/gene.10111. [DOI] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Fischbach K-F. Habituation and sensitization of the landing response of Drosophila melanogaster. Naturwissenschaften. 1981;68:332. [Google Scholar]
- Fischbach K-F, Bausenwein B. Habituation and sensitization of the landing response of Drosophila melanogaster II Receptive field size of habituating units. In: Hertting G, Spatz H-C, editors. Modulation of synaptic transmission and plasticity in nervous systems. Berlin Heidelberg: Springer-Verlag; 1988. p. 369.p. 385. [Google Scholar]
- Fois C, Médioni J, Le Bourg E. Habituation of the proboscis extension reflex as a function of age in Drosophila melanogaster. Gerontology. 1991;37:187–192. doi: 10.1159/000213259. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Spatz H-C, Bausenwein B. Visual control of wing beat frequency in Drosophila. Journal of Comparative Physiology A. 1994;175:587–596. doi: 10.1007/BF00199480. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Jackson FR, Siegel RW. Male courtship in Drosophila: The conditioned response to immature males and its genetic control. Genetics. 1982;102:771–782. doi: 10.1093/genetics/102.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A. The projection of sensory neurons in the central nervous system of Drosophila: Choice of the appropriate pathway. Developmental Biology. 1980;78:521–541. doi: 10.1016/0012-1606(80)90351-6. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BRE, Martin J-R, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister R, Schaupp M, Scholz H, Schwärzel M, Werner U, Zars TD, Buchner S, Buchner E. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. European Journal of Neuroscience. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang B-K, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. The Journal of Neuroscience. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SH. Segment-specific organization of leg motoneurones is transformed in bithorax mutants of Drosophila. Nature. 1981;286:65–67. [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hammond S, O’Shea M. Escape flight initiation in the fly. Journal of Comparative Physiology A. 2007;193:471–476. doi: 10.1007/s00359-006-0203-9. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Cohen TE, Kandel ER. Dishabituation in Aplysia can involve either reversal of habituation or superimposed sensitization. Learning & Memory. 2006a;13:397–403. doi: 10.1101/lm.49706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biological Bulletin. 2006b;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nature Reviews Neuroscience. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Jin P, Griffith LC, Murphey RK. Presynaptic calcium/calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. The Journal of Neuroscience. 1998;18:8955–8964. doi: 10.1523/JNEUROSCI.18-21-08955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner MA, Asztalos Z, Jones CJ, Tully T, Wu C-F. Effects of mutant Drosophila K+ channel subunits on habituation of the olfactory jump response. Journal of Neurogenetics. 2007;21:45–58. doi: 10.1080/01677060701247375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LE. An altered electroretinogram transient associated with an unusual jump response in a mutant of Drosophila. Cellular and Molecular Neurobiology. 1983;3:143–149. doi: 10.1007/BF00735278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, Kandel E. Neural correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1743–1745. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Le Bourg E. Aging and habituation of the tarsal reflex in Drosophila melanogaster. Gerontology. 1983;29:388–393. doi: 10.1159/000213149. [DOI] [PubMed] [Google Scholar]
- Lee J, Wu C-F. Electroconvulsive seizure behavior in Drosophila Analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. The Journal of Neuroscience. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22 doi: 10.1016/s0896-6273(00)80701-1. 451–261. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Marcus EA, Nolen TG, Rankin CH, Carew TJ. Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia. Science. 1988;241:210–213. doi: 10.1126/science.3388032. [DOI] [PubMed] [Google Scholar]
- McKenna M, Monte P, Helfand SL, Woodard C, Carlson J. A simple chemosensory response in Drosophila and the isolation of acj mutants in which it is affected. Proceedings of the National Academy of Sciences of the United States of America. 1989;79:8118–8122. doi: 10.1073/pnas.86.20.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megighian A, Zordan M, Costa R. Giant neuron pathway neurophysiological activity in per0 mutants of Drosophila melanogaster. Journal of Neurogenetics. 2001;15:221–231. doi: 10.3109/01677060109167378. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Jones CJ, Tully T. The Drosophila mutation turnip has pleiotropic behavioral effects and does not specifically affect learning. Learning & Memory. 1997;3:425–444. doi: 10.1101/lm.3.5.425. [DOI] [PubMed] [Google Scholar]
- Minois N, Le Bourg E. Hypergravity and aging in Drosophila melanogaster. 9. Conditioned suppression and habituation of the proboscis extension response. Aging (Milan, Italy) 1997;9:281–291. [PubMed] [Google Scholar]
- Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiology of Aging. 2000;21:145–152. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- O’Dell KMC. The inactive mutation leads to abnormal experience-dependent courtship modification in male Drosophila melanogaster. Behavior Genetics. 1994;24:381–388. doi: 10.1007/BF01067539. [DOI] [PubMed] [Google Scholar]
- Ögmen H, Moussa M. A neural model for nonassociative learning in a prototypical sensory-motor scheme: the landing reaction in flies. Biological Cybernetics. 1993;68:351–361. doi: 10.1007/BF00201860. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Jin P, Trimarchi JR, Caruccio P, Phillis RW, Murphey RK. Mutant molecular motors disrupt neural circuits in Drosophila. Journal of Neurobiology. 1997;33:711–723. doi: 10.1002/(sici)1097-4695(19971120)33:6<711::aid-neu1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rees CT, Spatz H-C. Habituation of the landing response of Drosophila wild-type and mutants defective in olfactory learning. Journal of Neurogenetics. 1989;5:105–118. doi: 10.3109/01677068909066201. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Sokolowski MB, Erber J. Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learning & Memory. 2004;11:303–311. doi: 10.1101/lm.71604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski MB. Foraging strategies of Drosophila melanogaster: A chromosomal analysis. Behavior Genetics. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. Journal of Neurophysiology. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Schneiderman AM. Flight initiations in Drosophila melanogaster are mediated by several distinct motor patterns. Journal of Comparative Physiology A. 1995;176:355–364. doi: 10.1007/BF00219061. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Vandervorst P, Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- Waddell S, Quinn WG. Flies, genes, and learning. Annual Reviews in Neuroscience. 2001;24:1283–1309. doi: 10.1146/annurev.neuro.24.1.1283. [DOI] [PubMed] [Google Scholar]
- Waldwogel F-M, Fischbach K-F. Plasticity of the landing response of Drosophila melanogaster. Journal of Comparative Physiology A. 1991;169:323–330. [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo H-F, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y. Stereotyped odor representation in the mushroom body of Drosophila revealed by GFP-based Ca2+ imaging. The Journal of Neuroscience. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind WC, Spatz H-C. Habituation of the landing response in Drosophila. In: Hertting G, Spatz H-C, editors. Modulation of synaptic transmission and plasticity in nervous systems. Berlin Heidelberg: Springer-Verlag; 1988. pp. 351–368. [Google Scholar]
- Wolf FW, Eddison M, Lee S, Cho W, Heberlein U. GSK-3/Shaggy regulates olfactory habituation in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4653–4657. doi: 10.1073/pnas.0700493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-F, Renger JJ, Engel JE. Activity-dependent functional and developmental plasticity of Drosophila neurons. In: Evans PD, editor. Advances in insect physiology. New York: Academic Press; 1998. pp. 385–440. [Google Scholar]
- Wyman RJ, Thomas JB, Salkoff L, King DG. The Drosophila giant fiber system. In: Eaton RC, editor. Neural mechanisms of startle behavior. New York: Plenum; 1984. pp. 133–161. [Google Scholar]
- Yu D, Baird GS, Tsien RY, Davis RL. Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. The Journal of Neuroscience. 2003;23:64–72. doi: 10.1523/JNEUROSCI.23-01-00064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. The European Journal of Neuroscience. 2007;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
- Zordan MA, Massironi M, Ducato MG, te Kronnie G, Costa R, Reggiani C, Chagneau C, Martin J-R, Megighian A. Drosophila CAKI/CMG protein, a homolog of human CASK, is essential for regulation of neurotransmitter vesicle release. Journal of Neurophysiology. 2005;94:1074–1083. doi: 10.1152/jn.00954.2004. [DOI] [PubMed] [Google Scholar]