Abstract
The pathogenesis of severe dengue is not well understood. Maternally derived subneutralizing levels of dengue virus-reactive IgG are postulated to be a critical risk factor for severe dengue during infancy. In this study, we found that, in healthy Vietnamese infants, there was a strong temporal association between the Fc-dependent, dengue virus infection-enhancing activity of neat plasma and the age-related epidemiology of severe dengue. We then postulated that disease severity in infants with primary infections would be associated with a robust immune response, possibly as a consequence of higher viral burdens in vivo. Accordingly, in infants hospitalized with acute dengue, the activation phenotype of peripheral-blood NK cells and CD8+ and CD4+ T cells correlated with overall disease severity, but HLA-A*1101-restricted NS3133-142-specific CD8+ T cells were not measurable until early convalescence. Plasma levels of cytokines/chemokines were generally higher in infants with dengue shock syndrome. Collectively, these data support a model of dengue pathogenesis in infants whereby antibody-dependent enhancement of infection explains the age-related case epidemiology and could account for antigen-driven immune activation and its association with disease severity. These results also highlight potential risks in the use of live attenuated dengue vaccines in infants in countries where dengue is endemic.
Dengue is a well-documented public-health burden in many developing countries [1, 2]. Any of the 4 serotypes of dengue virus (DENV)—DENV1-DENV4—can cause a spectrum of outcomes in humans, ranging from asymptomatic infection to clinically significant disease. Severe disease is called “dengue hemorrhagic fever” (DHF) and is characterized by systemic capillary leakage, thrombocytopenia, and, in severe cases, hypovolemic shock.
Substantial epidemiological evidence indicates that DHF in children and adults is typically associated with secondary infection caused by a DENV serotype distinct from that present when an individual is first exposed to DENV [3-6]. In contrast, DHF also occurs in primary DENV infections in infants born to dengue-immune mothers [7, 8]. A unifying hypothesis that explains the age-related epidemiology of DHF is a process called “antibody-dependent enhancement” (ADE) of disease [9].
The ADE model postulates that cross-reactive but subneutralizing levels of DENV-reactive IgG, acquired either passively or because of a previous infection, enhance DENV’s infectivity of Fc receptor-bearing cells. ADE could conceptually result in higher viral burdens in vivo and thereby precipitate some of the clinical events in dengue. Consistent with this model, children with secondary infections and DHF have higher initial plasma viral loads [10-12]. DHF in secondary infections is also associated with both significantly greater plasma concentrations of inflammatory cytokines [13-15] and increased frequencies of activated lymphocytes, compared with those in patients with milder disease [16, 17], presumably in response to higher viral burdens in DHF. Although much less is known about the pathogenesis of dengue in infants, previous studies by our group have suggested that, at least at the time of study enrollment, viral parameters are not associated with disease severity [8].
A critical challenge in dengue pathogenesis and vaccinology is to determine whether the biological character of ADE when measured in vitro can be correlated with disease outcomes and/or epidemiology. Previous prospective studies of children that have attempted to link the ADE phenomenon with disease outcomes in secondary infection but that have employed different methodologies have yielded conflicting results [18, 19]. Kliks et al. have described, in primary infections, a correlation between ADE activity in diluted maternal serum and the age at DHF onset in 13 Thai infants [20]. Against this backdrop, the aim of the present study was to correlate ADE with disease epidemiology in infants and to determine whether innate or acquired immune responses in infants with dengue, responses possibly triggered by ADE-mediated DENV infection, would correlate with disease severity. Our findings are consistent with the notion that infection-enhancing antibody plays a role in the pathogenesis of DHF in infants. Furthermore, we identified aspects of the infant innate and acquired immune response that are associated with disease severity.
PATIENTS, MATERIALS, AND METHODS
Recruitment of Patients
Infants with dengue
Infants suspected to have dengue who were <18 months old were enrolled in a prospective descriptive study at Children’s Hospitals 1 and 2 in Ho Chi Minh City, Vietnam, between November 2004 and March 2006. Recruitment also took place in the outpatient department of Children’s Hospital 1, between July 2005 and December 2005. Daily venous- or capillary-blood samples were collected from infants for 4 days during hospitalization, beginning with the day of entry into the study (study day 1), and again 10-14 days after hospital discharge. The extent of hemoconcentration during symptomatic illness was determined by comparing the maximum hematocrit recorded during hospitalization versus either the value recorded at follow-up (74% of cases) or an age-matched population value (26% of cases). World Health Organization classification criteria [21] were applied to each case after a review of the study notes.
Healthy infants
At Hung Vuong Hospital in Ho Chi Minh City, Vietnam, plasma was collected from healthy Vietnamese infants, at birth (umbilical cord) and at scheduled time points thereafter, as part of an ongoing prospective cohort study of dengue during the first year of life. All plasma samples from healthy infants that were used in the present study were from infants who did not have serological (i.e., IgM) evidence of recent DENV infection. Plasma from healthy infants that was used in ADE experiments was selected in 2 ways. In the first experiments, serial plasma samples (collected at birth and at 6, 9, and 12 months of age) from a group of 42 longitudinally followed infants was used in ADE experiments. Subsequently, a cross-sectional set of independent plasma samples (collected at birth and at 3, 6, 9, and 12 months of age) from 150 infants (30 plasma samples at each time point) were used. All infants in these cohorts were born to mothers with measurable DENV-reactive IgG, as indicated by indirect ELISA assays using recombinant DENV E proteins. Written informed consent was obtained from a parent or guardian of each patient or healthy infant. The study protocols were approved by the scientific and ethical committees at Hung Vuong Hospital, and Children’s Hospitals 1 and 2 and by the Oxford University Tropical Research Ethical Committee.
Flow Cytometry and Tetramers
Flow-cytometric analysis of whole-blood samples stained with fluorochrome-conjugated monoclonal antibodies was performed by use of a FACScalibur flow cytometer (Becton Dickinson). Cell-surface staining was routinely performed on 50 μL of fresh whole blood. All antibodies were purchased from Becton Dickinson. HLA*A-1101 major-histocompatibility-complex class I tetramers containing the dominant NS3133-142 epitope from all 4 DENV serotypes were synthesized as described elsewhere [17]. Tetramers containing each of the DENV1-DENV4 peptides were pooled and used to stain whole blood.
Dengue Diagnostics
A capture IgM and IgG ELISA assay (Venture Technologies) that employed inactivated antigen from DENV1-DENV4 and from Japanese encephalitis virus (JEV) was used to discriminate between DENV-specific and JEV-specific IgM or IgG responses and was performed as described elsewhere [22]. Plasma DENV loads were measured by an internally controlled, serotype-specific, real-time reverse-transcriptase polymerase chain reaction (PCR) assay that has been described elsewhere [23]; results are expressed as cDNA equivalents per milliliter of plasma. A diagnosis of confirmed dengue was made (1) if the results of the PCR assay were positive and/or (2) if, in paired plasma samples, there was evidence of rising levels of IgM against DENV antigen and the response to DENV antigen was greater than that to JEV antigen. A determination of primary dengue was made if IgM levels exceeded IgG levels at the time of discharge from the hospital.
Cytokine Measurements
Plasma levels of cytokines (interleukin [IL]-1β, IL-6, IL-8, IL-10, IL-12p70, and tumor necrosis factor) and of chemokines (interferon-inducible protein [IP]-10, monocyte chemotactic protein [MCP]-1, monokine induced by interferon-γ [MIG], and RANTES) were measured by a cytometric bead-array assay (Becton Dickinson), according to the manufacturer’s instructions except that all samples were fixed in 4% paraformaldehyde before being analyzed.
In Vitro ADE
ADE experiments were performed with K562 cells, essentially as described elsewhere [18]. In brief, neat plasma samples (30 μL) were incubated with DENV2 (250 pfu in 30 μL) for 45 min at 4°C. Virus and plasma were then added to 1 × 104 K562 cells (0.25 MOI) in 100 μL for 2 h at 37°C. Cells were then washed once with 2% fetal calf serum in RPMI, resuspended in complete RPMI (with 10% fetal calf serum, glutamine, and antibiotics), and incubated for 3 days. Cells were then fixed, overnight, in 4% paraformaldehyde containing 0.1% saponin in PBS; the following day, they were stained with fluorescein isothiocyanate-labeled hyperimmune anti-DENV human serum, were washed, and then were analyzed by flow cytometry. Infection enhancement was expressed in 2 ways: (1) when serial plasma samples were used, infection enhancement was represented as the n-fold change in the percentage of infected cells relative to that in the infant’s own cord-plasma sample; (2) when independent plasma samples from unrelated infants were used, infection enhancement was represented as the n-fold change in the percentage of infected cells relative to that in DENV-naive plasma. In addition to being analyzed by flow cytometry, DENV infection also was quantitated by measurement of infectious particles in culture supernatants, by plaque assay of BHK-21 cells.
Statistical Analysis
SPSS software (version 10) was used for statistical analyses. All comparisons were 2-sided, with P < .05 being regarded as significant.
RESULTS
ADE and Maternal Plasma
ADE mediated by maternally acquired DENV-reactive IgG is hypothesized to be central to the pathogenesis of DHF in infants. We postulated that there would be a positive correlation between the age at which Vietnamese infants presented to the hospital with dengue and the dilution at which maternal plasma would best enhance DENV infection of Fc receptor-bearing cells. To assess this hypothesis, plasma samples from 27 mothers of infants with DHF caused by DENV2 infections (described in [8]) were serially diluted before being mixed with DENV2 and K562 cells, an FcγIIa receptor-bearing cell line. At dilutions of 1:100-1:10,000, all maternal plasma samples enhanced DENV2 infection significantly more than did flavivirus-naive serum (figure 1A); unexpectedly, however, the dilution of serum at which maximal enhancement of DENV2 infection occurred was negatively, rather than positively, correlated with the age of the infant at the time of study enrollment (figure 1B) (Spearman’s r = -0.39; P = .04).
Figure 1.
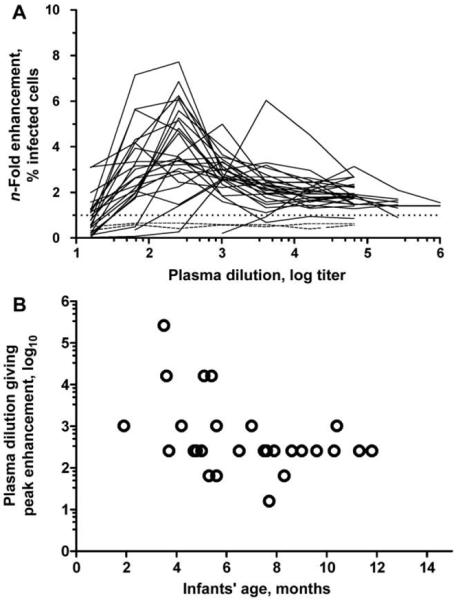
Dengue virus serotype 2 (DENV2) infection enhancement in diluted maternal plasma. A, n-Fold enhancement of infection of K562 cells by DENV2 (unbroken lines) after preincubation with dilutions of maternal plasma (n = 27), compared with plasma control samples (dashed lines) from flavivirus-naive individuals (n = 2), which did not enhance DENV2 infection at any dilution. B, Correlation, for each mother-infant pair, between the titer of maternal plasma that resulted in maximum DENV2-infection enhancement in K562 cells (from panel A) and the age of the infant when the pair presented to hospital with dengue caused by DENV2. There was a negative correlation between antibody-dependent enhancement of infection and the age of the infant (Spearman’s r = -0.39; P = .04).
ADE and Infants’ Plasma
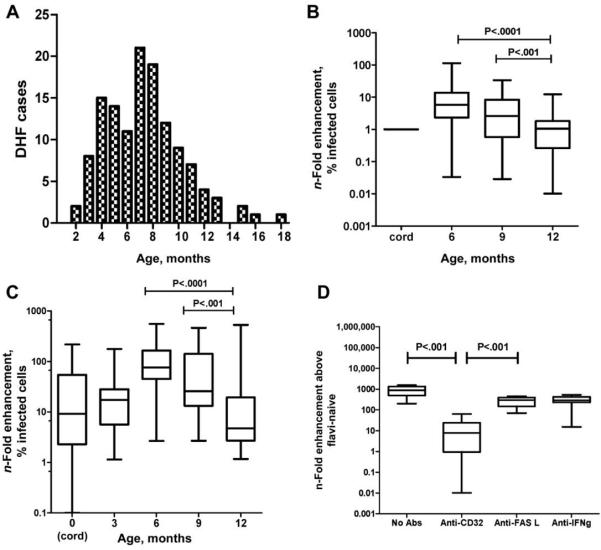
We hypothesized that diluted plasma may not adequately reflect the host-virus interaction in vivo, and we therefore undertook a population-based approach, using neat plasma, to explore the relationship between ADE and dengue in infants. To this end, we collected serial plasma samples from 42 healthy, DENV-naive Vietnamese infants, at birth and at 6, 9, and 12 months of age, and performed DENV2-infection assays with neat plasma and K562 cells. We hypothesized that the DENV infection-enhancing potential of infants’ plasma would be temporally correlated with the distinct age-related epidemiology of DHF in Vietnamese infants ([7] and figure 2A). Consistent with this hypothesis, infection enhancement was observed in K562 cells when DENV2 was premixed with neat plasma samples from healthy infants at 6 or 9 months of age but was not observed when DENV2 was premixed with plasma collected from the same infants at 12 months of age (figure 2B). A similar age-related pattern of infection enhancement was observed when a cross-sectional set of independent plasma samples collected at birth (umbilical cord) and at 3, 6, 9, and 12 months of age from 150 unrelated Vietnamese infants were incubated with DENV2 (figure 2C). Infection enhancement as measured by the number of infected cells was highly correlated with the amount of infectious virus measured in culture supernatants (Spearman’s r = 0.85; P ≤ .001). Infection enhancement was significantly abrogated by preincubation of K562 cells with a CD32 (FcγIIa) blocking monoclonal antibody but not by a control monoclonal antibody (figure 2D), implying that this abrogation was an Fc receptor-dependent process, rather than being due to a deficiency of innate virus inhibitors in infants’ plasma.
Figure 2.
A, Age-related case prevalence of dengue hemorrhagic fever (DHF) in infants (n = 73) enrolled in a prospective study at Children’s Hospitals 1 and 2 in Ho Chi Minh City, Vietnam, between November 2004 and March 2006. The median age of the infants was 7 months. B, Serial neat plasma samples collected, at birth and at 6, 9 and 12 months of age, from 42 healthy Vietnamese infants born to dengue-immune mothers, and then mixed with DENV2 and cultured with K562 cells for 3 days. Shown is the n-fold increase (median, interquartile, and maximum/minimum range) in DENV2-infected K562 cells in infants’ plasma samples at 6, 9 and 12 months, compared with that in the infant’s own cord-plasma sample. Plasma from 6-month-old infants provided significantly more enhancement than did plasma collected at any other time point (P < .001, by paired t test). C, Individual neat plasma samples collected, at birth and at 3, 6, 9 and 12 months of age, from 150 unrelated healthy Vietnamese infants (n = 30 at each time point), and then mixed with DENV2 and cultured with K562 cells for 3 days. Shown is the n-fold increase (median, interquartile, and maximum/minimum range) in DENV2-infected K562 cells in infants’ plasma samples, compared with that in flavivirus-naive control samples. Plasma from 6-month-old infants provided significantly more enhancement than did plasma collected at any other time point (P < .001, by Mann-Whitney test). D, n-Fold infection enhancement obtained when DENV2 and K562 cells were cultured in the presence of both plasma from 10 6-month-old infants and either monoclonal antibody (mAb) to CD32 (FcγIIa) or 1 of 2 different control mAbs (i.e., anti-FASL and anti-interferon [IFN]-γ). Anti-CD32 mAb significantly abrogated infection enhancement, compared with the control mAbs or no mAb (P < .001, by Mann-Whitney test).
Collectively, these age-related patterns of enhanced DENV2 infection of an Fc receptor-bearing cell line by neat plasma from healthy Vietnamese infants are broadly consistent with a disease-pathogenesis model in which infants who have subneutralizing levels of maternally derived anti-DENV IgG are at risk of DHF via ADE-mediated increases in viral burdens in vivo.
Lymphocyte Activation in Infants with Acute Dengue
Cellular immune responses in infants with dengue have not been described elsewhere. We postulated that ADE in infants with dengue might elicit antigen-driven immune activation in a manner that correlated with disease severity. We therefore defined immune responses in consecutive subsets of 75 hospitalized infants with dengue and 29 infants with other febrile illness (OFI) (which was presumed to be viral). The characteristics of the overall patient population are summarized in table 1, and their virological characteristics have been described elsewhere [8]. At the time of study enrollment, infants with primary acute DHF (n = 17) experienced a significant but transient increase in the absolute number of CD19+ B cells, compared with infants with OFI (n = 16) (mean [SD] CD19+ B cells/μL, 1350 [711] vs. 965 [653]; P = .02). The absolute numbers of other leukocyte populations, including myeloid (lin-CD11c+CD123neg) and plasmacytoid (lin-CD11c+CD123+) dendritic cells, were not significantly different between patients with dengue and patients with OFI (data not shown). At the time of study enrollment, T and NK cell activation was prominent in patients with acute DHF (n = 17), who had significant but transient increases in the percentage of activated (CD69+) CD8+ T cells and NK cells and, to a lesser extent, CD4+T lymphocytes, compared with healthy control subjects and patients with OFI (figure 3A-C). Furthermore, at the time of study enrollment, infants with Dengue shock syndrome (DSS) (i.e., DHF grade III) had more CD69+ NK cells and CD8+ and CD4+ T lymphocytes than did infants with non-shock DHF (figure 3A-C). CD69 expression in other leukocyte subsets was not associated with DSS, nor were changes in myeloid or plasmacytoid dendritic cell populations (data not shown). These data suggest that there is a positive association between dengue severity and markers of lymphocyte activation.
Table 1. Summary of clinical features and outcomes in infants with primary dengue and in infants with other febrile illness (OFI).
| Outpatients with dengue | Inpatients with dengue | Inpatients with OFI | |
|---|---|---|---|
| Variable | (n = 9) | (n = 66) | (n = 29) |
| Male sex | 4 (44) | 39 (59) | 18 (62) |
| Age, months | 8 (2-18) | 7 (2-13) | 9 (2-18) |
| Day of illness a | 5 (3-6) | 4 (1-6) | 3 (1-5) |
| World Health Organisation classification | |||
| Dengue fever | 2 | 0 | |
| Dengue hemorrhagic fever | |||
| Grade I | 0 | 0 | |
| Grade II | 3 | 60 | |
| Dengue shock syndrome | 0 | 6 | |
| Indeterminate | 4 | 0 | |
| Mortality | 0 | 2 (3) | 1 (3) |
NOTE. Data are no. (%) or median (range).
At the first day of the study.
Figure 3.
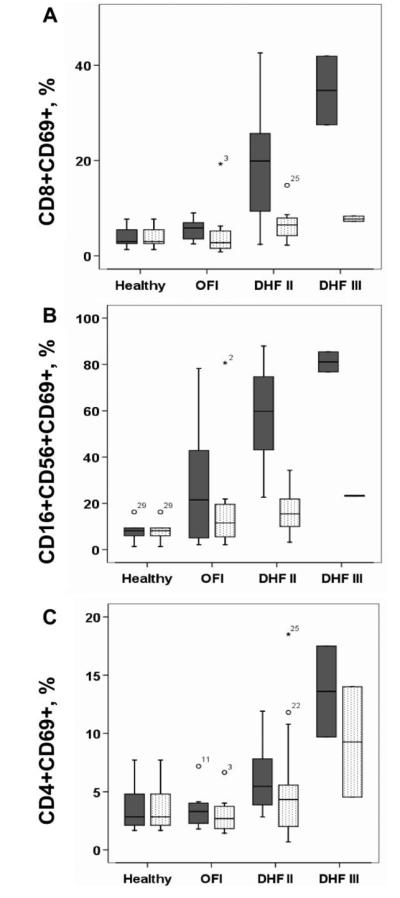
Cellular activation during acute dengue. Shown are the median, interquartile, and 95th-percentile percentage ranges of (A) CD8+CD69+ T cells, (B) CD3-CD16+CD56+CD69+ NK cells, and (C) CD4+CD69+ T cells, as a proportion of lymphocytes in peripheral blood from healthy infants (n = 6) and from infants who had either dengue hemorrhagic fever grade II (DHF II) (n = 15) or III (DHF III) (n = 2) or other febrile illness (OFI) (n = 8), at study enrollment (shaded boxes) and at discharge (unshaded boxes). At the time of study enrollment, infants with acute DHF II had significantly higher percentages of CD8+CD69+ T cells (P = .006, by Mann-Whitney test), CD16+CD56+CD69+ NK cells (P = .001, by Mann-Whitney test), and CD4+CD69+ T cells (P = .04, by Mann-Whitney test) than did infants with OFI.
Dengue-Specific T Cell Responses in Infants
To understand better whether the population of CD69+CD8+ T cells in peripheral blood of patients with acute dengue contained a DENV-specific subset, a major-histocompatibility-complex tetramer carrying NS3133-142, a dominant, DENV-specific HLA-A*1101-restricted T cell epitope [17], was used to stain fresh whole-blood samples from infants with dengue (figure 4A and B). NS3133-142-specific T cells were measurable, but only during convalescence, in 4 (36%) of 11 HLA-A*11-positive infants with dengue (figure 4A); NS3133-142-specific T cells were not detectable during the febrile phase, despite the presence of a population of phenotypically activated CD8+ T cells (figure 3A). The absence of measurable NS3133-142-specific CD8+ T cells in peripheral blood during the febrile phase suggests that these cells have yet to emerge from sites of antigen priming (e.g., lymphoid tissues) and likely contribute only minimally to either resolution of viremia or vasculopathy.
Figure 4.
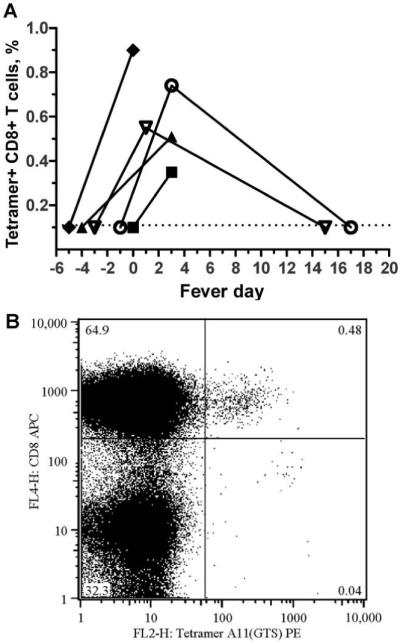
A, Detection of NS3133-142-specific CD8+ T cells in infants with dengue. Shown is the frequency, at different time points, of NS3133-142-specific CD8+ T cells in plasma samples of HLA-A*11-positive infants with acute dengue hemorrhagic fever, compared with that at defervescence (denoted as day 0). B, Flow-cytometry plot showing detection, by pooled tetramer staining, of NS3133-142-specific CD8+ T cells in whole blood.
Cytokine Responses and Relationship to Disease Severity
Antigen-driven inflammatory cytokine release is hypothesized to contribute to dengue capillary-leakage syndrome. To examine this hypothesis, the concentrations of cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70, and tumor necrosis factor) and chemokines (IP-10, MCP-1, MIG, and RANTES) in plasma samples from infants with an acute condition at study enrollment were measured and analyzed in the context of viremia, NS1 antigenemia, liver transaminase levels, hemoconcentration, and thrombocytopenia. Overall, plasma concentrations of cytokines and chemokines (except that of RANTES) were highest in patients with DSS (table 2), a finding that is consistent with a relationship between the magnitude of immune inflammation and capillary leakage. At the time of study enrollment, plasma levels of IP-10 were significantly higher in infants with dengue than in infants with OFI (table 2), and IP-10 concentrations were significantly correlated with plasma DENV loads in the same sample (Spearman’s r = 0.48; P = .001) (figure 5A). Conversely, plasma RANTES levels were significantly lower in infants with acute dengue than in infants with OFI (table 2), and plasma concentrations of RANTES were negatively correlated with plasma levels of aspartate aminotransferase (Spearman’s r = -0.38; P = .002) (figure 5B); in contrast, MIG concentrations were positively correlated with plasma levels of aspartate aminotransferase (Spearman’s r = 0.46; P = .0001) (figure 5C). These data suggest that the plasma level of IP-10 is a correlate of viremia and that RANTES and MIG are correlates of hepatic dysfunction during acute primary dengue in infants. Plasma levels of other cytokines or chemokines in infants were not significantly correlated with either viral loads, NS1 levels, or other measures of disease severity, including thrombocytopenia, hemoconcentration, and dengue disease grade.
Table 2. Cytokine and chemokine concentrations in plasma from healthy infants and from infants with dengue or other febrile illness (OFI), at the time of study enrollment.
| Concentration, mean (SD), pg/mL |
|||||
|---|---|---|---|---|---|
| Cytokine or chemokine | Late convalescenceb (n = 19) | OFIc (n = 29) | DHF II (n = 63) | DSS (n = 6) | P for OFI vs. DHF IIa |
| IL-12p70 | <5d | <5d | 8.1 (15) | 28 (60) | .06e |
| TNF α | <5d | <5d | 6.5 (10) | 17.6 (33) | .08e |
| IL-10 | <5d | 67.5 (173) | 68.1 (58) | 74.5 (59) | .1 |
| IL-6 | <5d | 15.5 (25) | 11.5 (12) | 61.7 (52) | .62 |
| IL-1β | 5.1 (2.6) | 5.3 (3) | 9 (16) | 25.8 (51) | .94 |
| IL-8 | 6.2 (2) | 138 (576) | 25 (16) | 173 (161) | .12 |
| IP-10 | 27118 (44895) | 16775 (13747) | 40881 (38798) | 59649 (63500) | .001 |
| MCP-1 | 256 (242) | 810 (2024) | 269 (190) | 1079 (1897) | .10 |
| MIG | 80439 (119440) | 3556 (2675) | 31009 (95714) | 64603 (124392) | .31 |
| RANTES | 145528 (130121) | 127362 (64800) | 48203 (37642) | 30102 (22990) | <.001 |
NOTE. DHF II, dengue hemorrhagic fever grade II; DSS, dengue shock syndrome; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemotactic protein; MIG, monokine induced by interferon-γ; TNF, tumor-necrosis factor.
By Student’s t test, unless otherwise noted.
Plasma samples were collected from now-healthy infants 2-3 weeks after being discharged from the hospital with dengue or OFI.
The mean length of illness duration before hospitalization was significantly different between patients with DHF II (4.7 days) and patients with OFI (3.5 days) (P = .001, by Student’s t test).
Below the assay’s level of detection.
By χ2 test of proportions.
Figure 5.
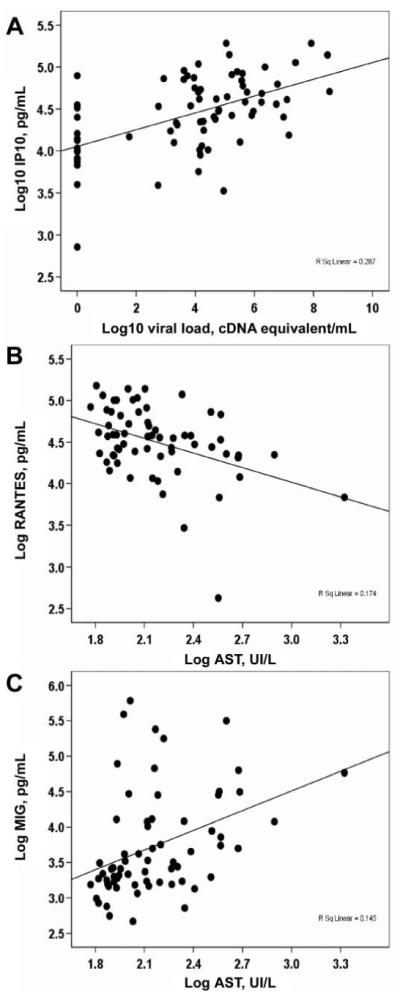
Correlations between virus-host and host-host factors in acute dengue in infants. Shown is the relationship, in the same plasma sample, between (A) plasma viral load and interferon-inducible protein (IP)-10 (n = 72) (Spearman’s r = 0.48; P = .0001), (B) plasma concentrations of RANTES and aspartate aminotransferase (AST) (n = 62) (Spearman’s r = -0.38; P = .002), and (C) plasma concentrations of monokine induced by interferon-γ (MIG) and AST (n = 66) (r = 0.46; P = .0001).
DISCUSSION
To our knowledge, the present study demonstrates for the first time a temporal association between the DENV infection-enhancing activity of neat plasma from healthy Vietnamese infants and the age-related case epidemiology of dengue during the first year of life. In addition, disease severity in infants with dengue was found to be associated with elements of the host immune response, demonstrating for the first time that cellular immune activation is associated with dengue severity during primary infection of infants as well as during secondary infection of older children [13, 16].
One of the hurdles to the development of a dengue vaccine is the lack of precise knowledge about what defines protective or pathogenic polyclonal-antibody repertoires in humans [24]. The capacity of dengue immune serum to enhance DENV’s infectivity of Fc receptor-bearing cells in vitro is a well-described phenomenon—but one that has not been reproducibly linked, at either the individual or the population level, to clinical outcomes of secondary dengue infection [18, 19]. A previous study by Kliks et al. has described the relationship between maternal serum’s DENV infection-enhancing activity in primary monocytes and the age at onset of dengue in 13 Thai infants with DENV2 infections [20]. The present study did not attempt to reproduce these findings, because we have not been able to reliably infect primary monocytes. Instead, we demonstrated a clear relationship, at the population level, between DENV-infection enhancement and the age-related epidemiology of dengue during infancy. These are important findings, because they provide evidence that the results of an in vitro ADE assay can be linked to the epidemiology of DHF. Indeed, this study suggests that it might be possible to use DENV infection-enhancing activity in preinfection plasma to predict DENV-infection outcomes in individual infants. Studies to address this possibility are currently underway. More generally, this study has implications for live attenuated dengue vaccines, by suggesting that vaccination of infants <1 year of age might be accompanied by the risk of antibody-mediated enhancement of vaccine infectivity.
In previous work, we were unable to identify an association between DENV viremia and disease severity in infants, in part because our data were derived from relatively late time points during the infection process (i.e., day 3, 4, or 5 of illness) and probably do not reflect peak viremia levels [8]. Nonetheless, the overall DENV mass in vivo is likely to be a key determinant of the magnitude of the elicited host immune response in infected individuals [10]. At the time of study enrollment in the present study, the proportion of lymphocytes that were phenotypically activated was greater in infants with DSS than in infants with milder disease, potentially reflecting greater antigen-driven immune activation in the former group of patients. The concept of antigen-driven immune activation in infants is consistent with observations in older children with secondary infection, in whom relatively high early viremia levels and robust activation of lymphocytes and inflammatory cytokine responses have been found to be associated with disease of greater severity [11, 13, 16]. It is interesting that, during the febrile phase, HLA-A*1101-restricted NS3133-142-specific CD8+ lymphocytes were not measurable in the peripheral blood of infants with DHF. Presumably, these NS3133-142-specific CD8+ lymphocytes begin proliferating in lymphoid tissue during the viremic phase and only appear in the peripheral circulation during early convalescence. Potentially, this indicates that these epitope-specific CD8+ T cells contribute little to the resolution of viremia or immunopathogenesis in infants with primary dengue, although further data on a larger number of patients are needed before this conclusion can be drawn. However, NS3133-142-specific CD8+cells may play a role in the immunopathogenesis of secondary infection, where responses can be expected to be earlier and dominated by robust serotype-cross-reactive responses [17].
Chemokine expression may be an early event in dengue and might play a role in immunopathogenesis. In infants, the chemokine IP-10 was significantly correlated with plasma DENV loads in the same sample. IP is expressed by hepatocytes in DENV-infected mice and promotes infiltration of NK cells into hepatic tissue [25]. Intriguingly, IP-10 and DENV appear to compete for attachment to heparin sulfate on the cell surface, suggesting that IP-10 might have anti-DENV activity beyond its immunological properties [25]. Plasma levels of RANTES and MIG were associated with liver transaminase levels, possibly suggesting a relationship between hepatic dysfunction and these chemokines whose activities include the recruitment of lymphocytes to sites of infection.
Primary dengue in infants offers a unique setting in which to investigate the complex pathogenesis of DHF [9]. However, results in infants may not be directly applicable to older children with secondary infections, because of the possibility of confounding differences in age-related vascular physiology, immunological maturity, and, in children with secondary infections, the presence of preexisting memory B and T cell responses. Collectively, the findings described herein are consistent with a model of pathogenesis in infants whereby maternally derived DENV-reactive IgG is a determinant of the viral burden in vivo. Conceivably, high viral burdens in vivo would elicit commensurate immune activation—which, in the present study, we have shown to be associated with disease severity—and could perhaps contribute to dengue-related vasculopathy. In this scenario, the burden of viral antigen and the magnitude of the ensuing immune response are important determinants of the clinical phenotype. Efforts to either reduce the viral burden—for example, by using novel antiviral drugs—or modulating immune activation might help to improve clinical outcomes in infants and children with dengue.
Acknowledgments
Financial support: Wellcome Trust (grant 074636/Z/04/Z to C.P.S.). Wellcome Trust had no role in the design, conduct, or analysis of the study.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 2.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. Studies in 1995-1996. Am J Trop Med Hyg. 1999;61:412–9. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 4.Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 5.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 6.Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TH, Lei HY, Nguyen TL, et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–32. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 8.Simmons CP, Chau TN, Thuy TT, et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–24. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstead SB, Lan NT, Myint TT, et al. Dengue hemorrhagic Fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–9. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libraty DH, Endy TP, Houng HS, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–21. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 12.Wang WK, Chen HL, Yang CF, et al. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis. 2006;43:1023–30. doi: 10.1086/507635. [DOI] [PubMed] [Google Scholar]
- 13.Green S, Vaughn DW, Kalayanarooj S, et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–62. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 14.Juffrie M, Meer GM, Hack CE, et al. Inflammatory mediators in dengue virus infection in children: interleukin-6 and its relation to C-reactive protein and secretory phospholipase A2. Am J Trop Med Hyg. 2001;65:70–5. doi: 10.4269/ajtmh.2001.65.70. [DOI] [PubMed] [Google Scholar]
- 15.Azeredo EL, Zagne SM, Santiago MA, et al. Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology. 2001;204:494–507. doi: 10.1078/0171-2985-00058. [DOI] [PubMed] [Google Scholar]
- 16.Green S, Pichyangkul S, Vaughn DW, et al. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis. 1999;180:1429–35. doi: 10.1086/315072. [DOI] [PubMed] [Google Scholar]
- 17.Mongkolsapaya J, Dejnirattisai W, Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–7. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 18.Laoprasopwattana K, Libraty DH, Endy TP, et al. Dengue virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J Infect Dis. 2005;192:510–9. doi: 10.1086/431520. [DOI] [PubMed] [Google Scholar]
- 19.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–51. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 20.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–9. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organisation . Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. World Health Organisation; Geneva: 1997. [Google Scholar]
- 22.Cardosa MJ, Wang SM, Sum MS, Tio PH. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Microbiol. 2002;2:9. doi: 10.1186/1471-2180-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons CP, Popper S, Dolocek C, et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis. 2007;195:1097–107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hombach J, Cardosa MJ, Sabchareon A, Vaughn DW, Barrett AD. Scientific consultation on immunological correlates of protection induced by dengue vaccines report from a meeting held at the World Health Organization 17-18 November 2005. Vaccine. 2007;25:4130–9. doi: 10.1016/j.vaccine.2007.02.079. [DOI] [PubMed] [Google Scholar]
- 25.Chen JP, Lu HL, Lai SL, et al. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J Immunol. 2006;177:3185–92. doi: 10.4049/jimmunol.177.5.3185. [DOI] [PubMed] [Google Scholar]