The emergence of highly pathogenic avian influenza H5N1 viruses in Asia and their spread globally have delivered a timely reminder of the public health and clinical challenges an influenza pandemic would pose. It is remarkable how little patient-oriented clinical research has been conducted over the past 30 years on a disease that could cause such extensive loss of life. There is currently a single oral drug for the treatment of influenza (the neuraminidase inhibitor oseltamivir) and no licensed parenteral drugs, although these are being developed. A key therapeutic question recently addressed by Zheng et al.1 in an animal model is whether adjunctive interventions would improve the outcome of infection.
Transmission of H5N1 to humans is an inefficient process, but once infection is established, the virus replicates rapidly, resulting in high viral burdens in the respiratory tract, and mortality is high.2 Virally infected cells release cytokines and chemokines designed to recruit and shape the innate and adaptive immune responses. In humans, the magnitude of these cytokine and chemokine responses is proportional to the viral burden, which is itself associated with outcome.2 Inflammatory cells infiltrate pulmonary tissues and, together with direct viral-mediated cytopathic effects, create airway congestion, impair gas exchange, and precipitate the acute respiratory distress syndrome.
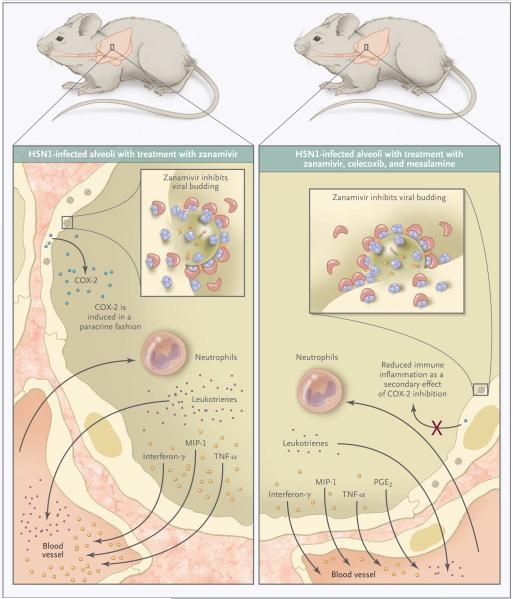
Recent work suggests that epithelial cells in the respiratory tract contribute to the inflammatory milieu during H5N1 infection. In vitro studies and immunohistologic analyses of lung-tissue specimens from autopsies of patients who died from H5N1 infection indicate that these activated epithelial cells produce tumor necrosis factor α and cyclooxygenase-2 (COX-2).3 The induction of COX-2 expression contributes to the biosynthesis of prostanoids that help modulate, in a very local fashion, the immune response. Zheng et al. described the specific inhibition of COX-2 expression in mice infected with the H5N1 influenza virus and then treated with COX inhibitors (Fig. 1). They found a concomitant repression of various cytokines and chemokines in epithelial cells. Thus, COX-2 expression by epithelial cells appears to perpetuate the inflammatory cascade in a manner that is independent of direct viral infection.
Figure 1. Staunching the Inflammatory Response to Influenza Infection.
H5N1 influenza infection in BALB/c mice results in clinically significant pathologic characteristics in pulmonary tissues that are associated with elevated levels of inflammatory cytokines and chemokines and high mortality. Zheng et al.1 found that the coadministration of cyclooxygenase (COX) inhibitors (mesalamine and celecoxib) with intraperitoneal antiviral therapy (zanamivir) significantly improved survival in mice with established H5N1 infections as compared with survival in infected mice treated with antiviral therapy alone. Improved survival was associated with reduced levels of the inflammatory markers macrophage inflammatory protein 1 (MIP-1), interferon-γ, tumor necrosis factor α (TNF-α), and leukotrienes in pulmonary-lavage fluid and diminished cellular infiltrate in the lung, as well as less severe lymphopenia, possibly owing to the antiapoptotic effects of COX inhibitors. Prostaglandin E2 (PGE2) levels were elevated in pulmonary-lavage fluid from mice treated with zanamivir, mesalamine, and celecoxib as compared with that from mice treated with zanamivir alone.
The key finding of Zheng et al. is that coadministration of COX inhibitors (mesalamine and celecoxib) and intraperitoneal antiviral therapy (zanamivir) significantly improves survival in mice with established H5N1 infection as compared with the survival of infected mice treated with antiviral therapy alone. Mice receiving the adjunctive therapy showed diminished pathologic characteristics of the lung, lower concentrations of inflammatory cytokines in pulmonary-lavage fluid, and less-pronounced T-cell lymphopenia—findings that are consistent with the fact that improved survival is a function of immunomodulation. Intriguingly, adjunctive therapy with COX inhibitors also impaired the clearance of H5N1 virus from pulmonary tissues in some mice, highlighting a balance that must be struck between pathogen-clearing and deleterious pathologic immune inflammation. This study should be extended to other species and include more extensive virologic, pharmacologic, and immunologic assessments.
Zheng et al. used a combination of the COX inhibitors mesalamine and celecoxib, both of which are used in clinical practice to treat noninfectious, inflammatory disorders and whose recognized side effects are dwarfed by the urgency of an acute, life-threatening H5N1 infection. The challenge, as ever, is to provide clinical evidence of a benefit for modulating the immune system in the rare and sporadic disease caused by influenza H5N1 that is often treated several days after onset. The clinical outcome in affected patients is likely to be determined by a delicate balance between excessive viral replication and a potentially double-edged host immune response. This complex relationship probably changes over the course of such infections, and hence the timing of administration of the immunomodulatory drugs and their use in combination with antiviral drugs may be critical in determining clinical outcome.
Treatment of H5N1 infections in humans with the use of a single neuraminidase inhibitor, oseltamivir, has been associated with the development of drug resistance during therapy, increasing viral loads, and a poor outcome.4 Combinations of antiviral drugs or therapeutic antibodies to combat the possibility of the development of resistance against single agents would seem to be a rational approach to reduce mortality.5 The work of Zheng et al. supports adjunctive immunomodulation, but it has been difficult to provide clear evidence in support of a role for immunomodulatory drugs in a range of acute severe infectious diseases in humans, many of which are apparently accompanied by a robust immune response called a cytokine storm. In addition, extrapolation from animal models has not been straightforward. These uncertainties require more clinical research on severe influenza of both the avian (H5N1) and seasonal (H3N2 and H1N1) varieties.
Randomized, controlled clinical trials in patients with influenza are urgently needed, because currently, there is little evidence on which to base treatment strategies and international policy. A “business as usual” approach to clinical research on influenza cannot deliver what is needed in a reasonable time frame; the approach for influenza should be like that used for research on the acquired immunodeficiency syndrome that led to the development of antiretroviral drugs. Because of much more powerful advocacy groups and the occurrence of deaths in wealthy countries, ways were found to set pragmatic end points and hence speed up clinical trials and approval of drugs. No one can predict when the next influenza pandemic will occur, and we have been fortunate with H5N1 so far. Approaches such as that of Zheng et al. should be tested in clinical trials of severe respiratory diseases as soon as possible.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A. 2008;105:8091–6. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SM, Cheung CY, Nicholls JM, et al. Hyperinduction of cyclooxygenase-2-mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J Infect Dis. 2008;198:525–35. doi: 10.1086/590499. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 5.Simmons CP, Bernasconi NL, Suguitan AL, et al. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4(5):e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]