Abstract
We discuss a patient who had poorly differentiated HCC with pyrexia and high CRP in laboratory data, which are not commonly observed in the usual HCC. A 50-year-old man with a history of liver dysfunction was admitted with a chief complaint of a prolonged fever and general fatigue. Preoperative diagnosis was HCC with portal vein tumor thrombus. Posterior segmentectomy of the liver and thrombectomy was performed. Rapid tumor recurrence occurred after surgery, and he died 79 days after the operation. Immunohistochemical stain of HCC in this patient revealed the production of proinflammatory cytokine, interleukin-8 (IL-8). IL-8 production may have contributed to the high fever, high inflammatory reaction, and poor prognosis in this case.
1. Introduction
Hepatocellular carcinoma (HCC) is a common malignancy and is now one of the major causes of death in Asian countries [1]. Generally, HCCs are often asymptomatic, but fatigue, abdominal distention and low-grade fever were sometimes observed because of poor liver function. However, fever as a primary symptom in HCC is relatively rare. Additionally, high CRP in laboratory results is also rare. HCC with pyrexia has been reported only in a few studies [2–4] and the cause of pyrexia has remained unknown.
Recently, increased production of proinflammatory cytokines and chemokines, such as interleukin-1 α (IL-1α), interleukin-1 β (IL-1β), interleukin-6(IL-6), interleukin-8(IL-8), and tumor necrosis factor-α (TNF-α) have been reported to be febriferous in acute and chronic inflammatory disease [5, 6]. These cytokines are produced by immune cells including various populations of lymphocytes macrophages and other cells. Among the proinflammatory cytokines, IL-8 is a neutrophil chemotactic factor and involved in tumor proliferation and migration, angiogenesis in malignant tumors. This is often accompanied by rapid growth and poor prognosis in malignant tumors [7–13].
We herein discuss a case of HCC accompanied by prolonged spiking fevers, which disappeared after tumor resection. Immunohistochemical stain of HCC in this patient revealed the production of IL-8.
2. Case Report
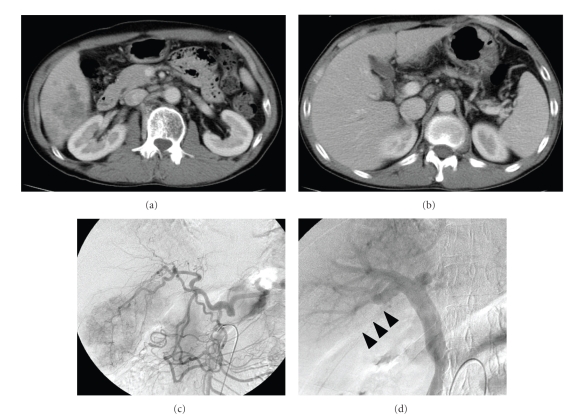
A 50-year-old man with a history of liver dysfunction was admitted to Iizuka Hospital with the chief complaint of a prolonged fever and general fatigue in October, 2005. The temperature was over 38°C. Hematological laboratory data on admission were as follows: WBC 9670/ μl, ALP 552 U/l, CRP 16.7 mg/dl, and AFP 11.1 ng/mL. HBs-antigen was positive (Table 1). A Computed tomography (CT) showed a peripherally enhanced low density mass 7.5 cm in diameter, which located in segment 6 in the right lobe of the liver (Figure 1(a)). The tumor was accompanied with tumor thrombus to the posterior branch of portal vein (Figure 1(b)). Celiac angiography showed this lesion was hypervascular (Figure 1(c)) and portal vein tumor thrombus in posterior segment was observed in the portal phase (Figure 1(d)).
Table 1.
Laboratory data in this patient. (normal range).
| WBC | 9670/ μL | (3500–9500) |
| Neut | 71.8% | (40–74) |
| Lym | 21.5% | (20–74) |
| Hb | 13.2 g/dl | (12.7–17.1) |
| Plt | 34.0 × 104 /μL | (13.8 × 104–33.5 × 104) |
| PT | 77.6% | (86–130) |
| APTT | 41.9 sec | (24.8–36.5) |
| TP | 7.1 g/dl | (6.7–8.3) |
| Alb | 2.6 g/dl | (4.0–5.0) |
| T.Bil | 0.4 mg/dl | (0.3–1.2) |
| D.Bil | 0.1 mg/dl | (0–0.4) |
| AST | 38 U/l | (13–33) |
| ALT | 23 U/l | (6–30) |
| LDH | 330 U/l | (119–229) |
| ALP | 552 U/l | (115–359) |
| γGTP | 79 U/l | (10–47) |
| ChE | 30 U/l | (214–466) |
| TC | 97 mg/dl | (128–219) |
| TG | 48 mg/dl | (30–149) |
| Amy | 111 U/l | (42–132) |
| BUN | 11 mg/dl | (8–22) |
| Cr | 0.6 mg/dl | (0.6–1.1) |
| FBS | 103 mg/dl | (69–109) |
| CRP | 16.7 mg/dl | (<0.2) |
| ICGR15 | 11.5% | (<10) |
| AFP | 11.1 ng/mL | (<20) |
| PIVKA-II | 16 mAU/mL | (<40) |
| CEA | 1.1 ng/mL | (<5) |
| CA19-9 | 13.5 U/mL | (<37) |
| HBs-Ag | HBe-Ag (+) | |
| HBe-Ab | HCV-Ab (−) |
Figure 1.
(a) A Computed tomography (CT) showed a peripherally enhanced low density mass 7.5 cm in diameter, which located in segment 6 in the right lobe of the liver. (b) Tumor was accompanied with tumor thrombus to the posterior branch of portal vein. (c) Celiac angiography showed this lesion to be hypervascular. (d) Portal vein tumor thrombus in posterior segment was observed in portal phase of SMA angiography.
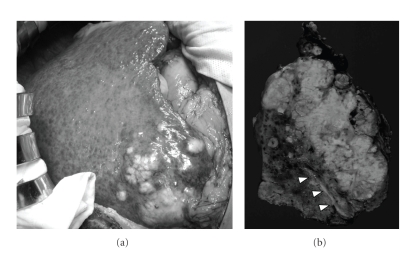
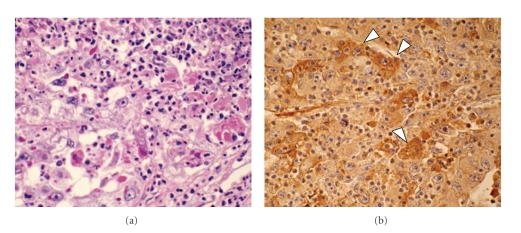
Biopsy was not done in this case because of danger of tumor seeding. Patients have chronic hepatitis B and then tumor was hypervascular and accompanied with portal thrombus, which is the feature of HCC, therefore a preoperative diagnosis was made as HCC with portal vein thrombus and nonsteroidal anti-inflammatory drugs were used to reduce the fever. Enough explanation was performed to the patient and informed consent for operation was obtained. Hepatic resection was conducted in November, 2005. The tumor was white and intrahepatic metastasis was observed (Figure 2(a)). A single nodule of disseminated cancer cells was found near the posterior segment on the abdominal wall during the operation. Posterior segmentectomy of the liver and thrombectomy including the dissemination was performed. On the cut section, the tumor was white and contained coagulation and necrosis (Figure 2(b)). Histological findings revealed poorly differentiated HCC with a trabecular pattern. Multiple tumors of intrahepatic metastases and portal vein invasion were observed. Neutrophil infiltration in the tumor was observed (Figure 3(a)). The noncancerous liver tissue showed a moderate chronic inflammatory infiltrate in the fibrous stroma, diagnosed as liver fibrosis. Immunohistochemical stain of IL-8 was performed with paraffin embedded sections of liver tissue using mouse monoclonal antibody at dilutions of 1 : 100 (Assay designs, Ann Arbor, USA). Immunohistochemical staining was described previously [14]. The subsequent reaction was performed by the peroxidase labeled streptavidin-biotin technique using Histofine SAB-PO kit (Nichirei, Tokyo, Japan). IL-8 was observed in the cytoplasm of cancer cells (Figure 3(b)).
Figure 2.
(a) Intraoperative findings revealed tumor was white and intrahepatic metastasis was observed. (b) On cut section, tumor was white and contained coagulation and necrosis. Portal vein tumor thrombus in segment 6 of the liver was observed (arrow heads).
Figure 3.
(a) Histological findings revealed poorly differentiated HCC with trabecular pattern. Neutrophil infiltration in the tumor was observed. (b) Immunohistochemically, paraffin section of tumor tissue stained positive for IL-8 (arrow heads).
After the tumor resection, fever disappeared and CRP dropped gradually from 16.7 to 2.7, but the patient had recurrence with multiple liver metastasis and pleuritis carcinomatosa and died on February 2, 2006, 79 days after the operation.
3. Discussion
In 1991, Okuda et al. [2] reported five patients with HCC and pyrexia, for an incidence of less than 1%, in Japan. He also mentioned that very poorly differentiated sarcomatoid HCC may frequently appear with pyrexia and leukocytosis mimicking liver abscess. In 1995, Hayashi et al. [3] reported HCC with pyrexia. Histological examination revealed poorly differentiated HCC with sarcomatoid degeneration. Laboratory data revealed high CRP. Nevertheless, in both case reports, the mechanism of a fever was not revealed. In our case, the sarcomatoid change of the cancer cell was not detected, only poorly differentiated HCC.
It is well known that cancer cells produce humoral factors and cause the “paraneoplastic syndrome”. Among the clinical symptoms and laboratory data, fever and high CRP, which is not commonly observed in the patients with HCC, is suspected to be due to humoral factors, especially inflammatory cytokine. IL-8 is a proinflammatory cytokine whose principal role in infection and inflammation appears to be the recruitment and activation of circulating and tissue neutrophils to the site of tissue damage. It has been demonstrated that IL-8 is produced by a wide variety of cell types in vitro, including endothelium, monocytes, eosinophils, astrocytes and keratinocytes. In sepsis patients, for example, IL-8 concentrations have been reported as being markedly elevated at diagnosis and remaining high during the course of the illness [15]. Patients with pyrexia is suffering from a high fever and body weight loss. This symptom is induced by many humoral factors, including IL1β, IL-6, IL8 and TNFα. Zampronio AR reported that IL-8 induced fever by prostaglandin independent mechanism [16]. Additionally, IL-8 was correlated with weight loss in pancreatic cancer [17]. IL-8 resulted in a dose-dependent increase of CRP [18], a decrease in the production of transferring and prealbumin. Therefore, IL-8 is related to pyrexia in parts, not all.
In other cancer, Interleukin-8 Producing Malignant Fibrous Histiocytoma with Prolonged Fever was reported [19]. We examined by immunohistochemical staining of IL-8 and tumor tissue stained positive for IL-8 in this case. Akiba et al. [7] reported that a high IL-8 level in HCC had a significantly higher frequency of portal vein invasion and venous invasion and bile duct invasion. In vitro, IL-8 production accelerated the proliferation of endothelial cells. Tachibana et al. [9] reported IL-8 production was enhanced progressively with escalating severity of hepatitis and the development of HCC and the level of IL-8 were significantly increased in patients with advanced HCC with distant metastasis, and then this leads to poor prognosis. Ren et al. reported serum IL-8 level was correlated with tumor size and tumor stage, and then IL-8 level was a significant prognostic factor in terms of disease-free survival and overall survival [13]. Additionally, elevated IL-8 levels in the drainage vein of colorectal cancer are related to the occurrence of hepatic metastasis [10]. This case presented portal vein tumor thrombus, bile duct invasion, intrahepatic metastasis, peritoneal dissemination, early tumor recurrence, rapid tumor regrowth and finally a poor prognosis. In fact, all patients with IL-8 production do not have a high fever, the serum concentration of IL-8 or the degree of neutrophil infiltration will be concerned with pyrexia.
In many cancers such as esophageal cancer, gastric cancer and colorectal cancer, serum CRP was known as a prognostic indicator [20–23]. In particular, some reported that serum CRP levels indicated a poor prognosis in HCC patients and portal vein invasion was significantly higher in the serum CRP-positive group [24, 25]. According to the in vitro previous studies, cultured HCC can produce CRP that is regulated in part by proinflammatory cytokines, such as IL-6, IL-8 and TNF-α [18, 26, 27]. Among the many proinflammatory cytokines, recent report IL-6 mediates cell cycle arrest in hepatocellular carcinoma through a STAT 3-dependent pathway [28]. Adverse effect may occur according to the type of cytokines. HCC produce proinflammatory cytokines such as IL-8 by autocrine or paracrine, which lead to CRP production and inflammatory cascade or tumor progression.
In this report we discussed a patient who had poorly differentiated HCC with pyrexia and high CRP. The patient had surgical resection, but rapid tumor recurrence occurred. IL-8 production was histologically revealed in this case and may have contributed to the high fever, high inflammatory reaction and poor prognosis.
References
- 1.Munoz N, Bosch X. Epidermiology of hepatocellular carcinoma. In: Okuda K, Ishak KG, editors. Neoplasms of the Liver. Tokyo, Japan: Springer; 1987. pp. 3–19. [Google Scholar]
- 2.Okuda K, Kondo Y, Nakano M, et al. Hepatocellular carcinoma presenting with pyrexia and leukocytosis: report of five cases. Hepatology. 1991;13(4):695–700. [PubMed] [Google Scholar]
- 3.Hayashi T, Honda H, Kaneko K, et al. Hepatocellular carcinoma with pyrexia: report of a case. Radiation Medicin. 1995;13(3):133–136. [PubMed] [Google Scholar]
- 4.Yen T-S, Jan Y-Y, Jeng L-B, Chen T-C, Hwang T-L, Chen M-F. Hepatocellular carcinoma presenting as pyogenic liver abscess: characteristics, diagnosis, and management. Clinical Infectious Diseases. 1998;26(5):1224–1226. doi: 10.1086/520290. [DOI] [PubMed] [Google Scholar]
- 5.Harsh WN. Fever in malignat disease. Annals of Surgery. 1952;18:229–235. [Google Scholar]
- 6.Masuda H, Iwai S, Tanaka T, Hayakawa S. Expression of IL-8, TNF-α and IFN-γ m-RNA in ulcerative colitis, particularly in patients with inactive phase. Journal of Clinical & Laboratory Immunology. 1995;46(3):111–123. [PubMed] [Google Scholar]
- 7.Akiba J, Yano H, Ogasawara S, Higaki K, Kojiro M. Expression and function of interleukin-8 in human hepatocellular carcinoma. International Journal of Oncology. 2001;18(2):257–264. doi: 10.3892/ijo.18.2.257. [DOI] [PubMed] [Google Scholar]
- 8.Kubo F, Ueno S, Hiwatashi K, et al. Interleukin 8 in human hepatocellular carcinoma correlates with cancer cell invasion of vessels but not with tumor angiogenesis. Annals of Surgical Oncology. 2005;12(10):800–807. doi: 10.1245/ASO.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana Y, Nakamoto Y, Mukaida N, Kaneko S. Intrahepatic interleukin-8 production during disease progression of chronic hepatitis C. Cancer Letters. 2007;251(1):36–42. doi: 10.1016/j.canlet.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi M, Komuta K, Akashi A, Matsuzaki S, Furui J, Kanematsu T. Elevated IL-8 levels in the drainage vein of resectable Dukes'C colorectal cancer indicate high risk for developing hepatic metastasis. Oncology Reports. 2002;9(1):159–165. [PubMed] [Google Scholar]
- 11.de Bont ESJM, Vellenga E, Molema G, Van Wering E, De Leij LFMH, Kamps WA. A possible role for spontaneous interleukin-8 production by acute myeloid leukemic cells in angiogenesis related processes: work in progress. Medical and Pediatric Oncology. 2001;37(6):511–517. doi: 10.1002/mpo.1244. [DOI] [PubMed] [Google Scholar]
- 12.Colasante A, Mascetra N, Brunetti M, et al. Transforming growth factor β1, interleukin-8 and interleukin-1, in non- small-cell lung tumors. American Journal of Respiratory and Critical Care Medicine. 1997;156(3):968–973. doi: 10.1164/ajrccm.156.3.9701122. [DOI] [PubMed] [Google Scholar]
- 13.Ren Y, Poon RT-P, Tsui H-T, et al. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clinical Cancer Research. 2003;9(16):5996–6001. [PubMed] [Google Scholar]
- 14.Harimoto N, Shimada M, Aishima S-I, et al. The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver International. 2004;24(4):316–321. doi: 10.1111/j.1478-3231.2004.0927.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Ishikawa K, Nishino M, Shimazu T, Yoshioka T. Changes in granulocyte colony-stimulating factor concentration in patients with trauma and sepsis. Journal of Trauma—Injury, Infection and Critical Care. 1996;40(5):718–725. doi: 10.1097/00005373-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Zampronio AR, Souza GE, Silva CA, et al. Interleukin-8 induces fever by a prostaglandin-independent mechanism. American Journal of Physiology. 1994;266:1670–1674. doi: 10.1152/ajpregu.1994.266.5.R1670. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101(12):2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 18.Wigmore SJ, Fearon KC, Maingay JP, et al. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. American Journal of Physiology. 1997;273:720–726. doi: 10.1152/ajpendo.1997.273.4.E720. [DOI] [PubMed] [Google Scholar]
- 19.Osaka S, Hayakawa S, Yoshida Y, Sakurada E, Ryu J, Sugitani M. Interleukin-8 producing malignant fibrous histiocytoma with prolonged fever. Acta Histochemica et Cytochemica. 2006;39(1):17–21. doi: 10.1267/ahc.05053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Natsugoe S, Ueno S, Baba M, Aikou T. Significant host- and tumor-related factors for predicting prognosis in patients with esophageal carcinoma. Annals of Surgery. 2003;238(2):197–202. doi: 10.1097/01.sla.0000080822.22415.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Mello J, Struthers L, Turner R, et al. Multivariate analyses as aids to diagnosis and assessment of prognosis in gastrointestinal cancer. British Journal of Cancer. 1983;48(3):341–348. doi: 10.1038/bjc.1983.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. American Journal of Surgery. 1998;176(4):335–338. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 23.Chung Y-C, Chang Y-F. Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. European Journal of Gastroenterology and Hepatology. 2003;15(4):369–373. doi: 10.1097/00042737-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 25.Nagaoka S, Yoshida T, Akiyoshi J, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver International. 2007;27(8):1091–1097. doi: 10.1111/j.1478-3231.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldman ND, Liu T-Y. Biosynthesis of human C-reactive protein in cultured hepatoma cells is induced by a monocyte factor(s) other than interleukin-1. Journal of Biological Chemistry. 1987;262(5):2363–2368. [PubMed] [Google Scholar]
- 27.Gabay C, Genin B, Mentha G, Iynedjian PB, Roux-Lombard P. IL-1 receptor antagonist (IL-1Ra) does not inhibit the production of C-reactive protein or serum amyloid A protein by human primary hepatocytes. differential regulation in normal and tumour cells. Clinical and Experimental Immunology. 1995;100(2):306–313. doi: 10.1111/j.1365-2249.1995.tb03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran DM, Mattocks MA, Cahill PA, Koniaris LG, McKillop IH. Interleukin-6 mediates G0/G1 growth arrest in hepatocellular carcinoma through a STAT 3-dependent pathway. Journal of Surgical Research. 2008;147(1):23–33. doi: 10.1016/j.jss.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]