Abstract
Purpose
We used data from the first large-scale overwhelmingly population-based study to: (1) quantify the risk of adverse pregnancy outcomes in survivors of childhood cancer in relation to cancer type and treatment, and (2) to assess live birth rates relative to the general population.
Methods
A questionnaire including questions enquiring about pregnancy outcomes was completed by 10,483 survivors. A total of 7,300 pregnancies were reported. Odds ratios (ORs) for live birth, miscarriage, termination, stillbirth, premature birth, and low birth weight were calculated for different types of childhood cancer and by whether initial treatment involved chemotherapy, abdominal or brain irradiation. For females, the observed number of live births were compared to that expected based on the general population of England & Wales.
Results
Female survivors exposed to abdominal irradiation had a significantly increased OR of delivering preterm (OR=3.2, 95%CI:2.1-4.7) and producing offspring with a low birth weight (OR=1.9, 95%CI:1.1-3.2). An increased OR of miscarriage was also associated with abdominal radiotherapy (OR=1.4, 95%CI:1.0-1.9). The number of live births observed from all female survivors was two-thirds of that expected (O/E=0.64, 95%CI: 0.62-0.66) and lowest among survivors treated with brain (O/E=0.52, 95%CI:0.48-0.56) and abdominal radiotherapy (O/E=0.55, 95%CI: 0.50-0.61).
Conclusion
Female survivors of childhood cancer treated with abdominal radiotherapy are at three-fold increased risk of delivering preterm, two-fold increased risk of low birth weight, and a small increased risk of miscarriage. Overall, female survivors produce considerably fewer offspring than expected, particularly those treated with abdominal or brain radiotherapy.
Keywords: epidemiology, childhood cancer, survival, pregnancy, offspring
Introduction
Given the major improvements in the treatment of childhood cancer over the last four decades, a large number of children treated for cancer have survived into adulthood. Although some survivors became infertile as a result of treatment with high-dose irradiation or cytotoxic chemotherapy (1-4) , many survivors remain fertile and wish to have children. One of the concerns childhood cancer survivors express is that treatment may adversely affect reproductive function and increase the risk of adverse pregnancy outcomes (5).
Previous studies have shown that radiotherapy to the pelvic area increases the risk of low birth weight and premature birth in the offspring of women who have survived childhood cancer (6-9) primarily among survivors of Wilms’ tumour (10-13). Furthermore, there is some evidence that the risk of miscarriage among women exposed to abdominal irradiation is also increased (7, 9, 14). Exposure to brain irradiation was associated with a slightly increased risk of miscarriage among women in two previous studies (7, 14).
Despite the above observations, one of the principal uncertainties in predicting the risk of adverse pregnancy outcomes arises from the fact that most previous studies lacked sufficient statistical power or were not population-based which may have hindered accurate or valid quantification of risks. Therefore, the main objective of this study was to quantify the risk of adverse pregnancy outcomes among both female survivors and partners of male survivors of childhood cancer in relation to aspects of childhood cancer and its treatment within the first large-scale population-based cohort on this topic. A secondary objective was to assess live-birth rates of female survivors of childhood cancer compared to the general population.
Methods
British Childhood Cancer Survivor Study
We used data from the British Childhood Cancer Survivor Study (BCCSS), a large-scale cohort study that examines the late-effects of treatment among survivors of childhood cancer who were diagnosed with childhood cancer between 1940 and 1991, in Britain, and had survived for at least five years (15). The cohort was ascertained through the National Registry of Childhood Tumours (NRCT) which is maintained by the Childhood Cancer Research Group (CCRG) at the University of Oxford. The NRCT is population-based since 1962 and before then a special attempt was made to locate all treatment centres for which records were available indicating that a complete series of patients could be ascertained. The proportion of 5-year survivors diagnosed before 1962 included in the BCCSS is less than 7%.
Information on type of childhood cancer, site of tumour, initial treatment, and demographics were provided by CCRG. As part of the BCCSS, a questionnaire ascertaining adverse health outcomes was sent to those survivors who were at least 16 years of age and contactable through their general practitioner (n=13,211). In total, 10,483 of the 14,836 survivors who were alive and aged at least 16 years at the time completed a questionnaire yielding a response rate of 71% (15). The questionnaire can be downloaded at www.bccss.bham.ac.uk.
Adverse pregnancy outcomes assessed in the questionnaire and evaluated in this investigation included miscarriage, stillbirth, termination, premature birth, and low birth weight. A pregnancy ending prior to gestational week 24 without the foetus surviving was considered a miscarriage (i.e. spontaneous abortion). Still births were defined as pregnancies ending with the death of the foetus in gestational week 24 or later (16). Medically induced abortions were referred to as terminations. Live births occurring prior to 37 weeks gestation were considered premature. Low birth weight was defined as a live birth weighing fewer than 2500 grams. Pregnancies were excluded from analysis if the pregnancy was of multiple birth, occurred before the onset of the cancer, was achieved by assisted reproductive technology, or if the outcome was unknown.
Information on initial treatment had been obtained by CCRG in a dichotomous format (radiotherapy (yes/no); chemotherapy (yes/no)) by visiting relevant hospitals and abstracting clinical records. Information on the site of the childhood tumour and whether the initial treatment included radiotherapy was used to classify survivors into four mutually exclusive categories: no radiotherapy, radiotherapy other than to the brain or abdomen, radiotherapy to the brain, and radiotherapy to the abdomen. Survivors who were treated with radiation for a brain tumour, retinoblastoma, or nasopharyngeal tumour or who received prophylactic radiotherapy for leukaemia were classified as having received brain irradiation. Radiotherapy to the abdomen was defined as any irradiated tumour below the diaphragm and above the knees. Survivors for whom treatment data was missing were excluded from analyses.
Statistical analysis
Logistic regression models were used to calculate odds ratios (ORs) of the various adverse pregnancy outcomes by type of childhood cancer, treatment with chemotherapy, treatment with brain radiotherapy, abdominal radiotherapy, or other radiotherapy. ORs were also calculated for Wilms’ tumour survivors treated with abdominal irradiation as Wilms’ tumour survivors would have received among some of the highest doses of abdominal irradiation and also compromise a sufficiently large group to fully consider separately.
Each specific natural pregnancy outcome was evaluated relative to all natural pregnancy outcomes, specifically live births, miscarriages and stillbirths. Terminations were excluded from the denominator, as the potential pregnancy outcome for such pregnancies is always unknown. Terminations were expressed as a proportion of all known pregnancy outcomes, specifically the natural outcomes plus all terminations.
To account for potential correlations between pregnancy outcomes of the same survivor a population-averaged generalized estimating equation modification was used (17). Separate analyses were conducted for female survivors and partners of male survivors. Unless otherwise stated, models were adjusted for maternal age, birth order, and exposure to chemotherapy and radiotherapy variables. Given that general population rates for terminations have changed markedly over the last few decades (18) as well as certain treatment modalities (e.g. introduction of chemotherapy), any association between treatment and the outcome termination may be confounded by decade of treatment; hence, models with termination as the outcome were additionally adjusted for decade of treatment.
To assess live birth rates, we compared the observed number of live-born children among survivors to the expected number of live-born, for a specified age, based on birth-cohort fertility rates of the general population of England and Wales (19). A live birth was considered to be a potential recurrent ‘event’ with the observed number of events having a Poisson distribution. Expected numbers of live births could only be calculated for female survivors as there were no general population birth-cohort fertility rates available for males.
survivors were asked to provide the reason for the termination, if applicable. The information given was then assigned to one of the categories: possible health problem foetus, social reason, or health problem mother.
The criterion for statistical significance was a p-value (2-sided) of less than 0.05. Stata statistical software was used for all analyses (20).
Finally, all analyses were executed on the entire data set and separately on the entire population-based sub-cohort which excluded the 7% of survivors diagnosed before 1962. There were no important differences observed, except the confidence intervals were somewhat expanded. As there was no evidence of important bias we included the entire data set in this manuscript.
Results
Pregnancies
Of the 10,483 survivors who completed the questionnaire, 31% reported to have carried or sired at least one pregnancy (n=3,244) resulting in a total of 7,300 pregnancies. Overall, 6,634 singleton pregnancies were reported of which 4,113 were produced by female survivors and 2,521 by partners of male survivors.
Female survivors
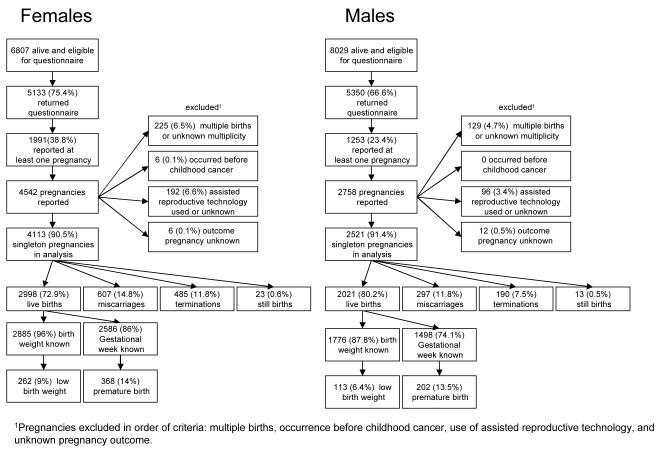
Of the 4,113 singleton pregnancies among female survivors that were eligible for analysis (Figure 1), 72.9% resulted in a live birth, 14.8% in a miscarriage, 11.8% in a termination, and 0.6% in a still birth. Of the 2,885 live born offspring for whom birth weight was known, 9% (n=262) had a low birth weight and of the 2,586 live births for whom the gestational week was known, 14% (n=368) were born prematurely.
Figure 1.
Flow chart of pregnancy outcomes for female and male survivors
Table 1 shows the odds ratios (ORs) of the different pregnancy outcomes by type of childhood cancer, whether treatment included chemotherapy, and for the different levels of radiotherapy treatment. The odds of producing a live birth did not vary significantly in relation to type of childhood cancer, treatment with chemotherapy, brain or abdominal radiotherapy.
Table 1.
Odds ratios of pregnancy outcomes among female survivors of childhood cancer
| natural pregnancy outcome4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| live birth (n=2,998) | miscarriage (n=607) | still birth (n=23)5 | termination (n=485)6,7 | low birth weight (n=262)8 | premature (n=368) | |||||||
|
|
||||||||||||
| n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | |
|
|
||||||||||||
| Type of childhood cancer 1 | ||||||||||||
| leukaemia (reference) |
549 (69.4%) | 1 | 132 (16.7%) | 1 | 5 (0.6%) | 1 | 105 (13.3%) | 1 | 33 (6.2%) | 1 | 62 (12.9%) | 1 |
| Hodgkin’s lymphoma |
251 (72.5%) | 1.2 (0.8,1.8) | 49 (14.2%) | 0.8 (0.5,1.3) | 3 (0.9%) | 1.4 (0.3,5.7) | 43 (12.4%) | 1.1 (0.7,1.8) | 13 (5.5%) | 1.0 (0.4,2.3) | 26 (11.6%) | 0.9 (0.5,1.6) |
| NHL | 156 (74.6%) | 1.4 (0.9,2.4) | 28 (13.4%) | 0.7 (0.4,1.2) | 0 | - | 25 (12.0%) | 1.2 (0.7,2.1) | 17 (11.1%) | 2.1 (1.0,4.8) | 18 (13.4%) | 1.1 (0.6,2.0) |
| CNS tumour | 577 (77.0%) | 1.5 (1.0,2.1) | 90 (12.0%) | 0.7 (0.5,0.9) | 5 (0.7%) | 1.0 (0.3,3.4) | 77 (10.3%) | 1.1 (0.8,1.7) | 27 (4.9%) | 0.8 (0.4,1.5) | 63 (13.4%) | 1.0 (0.6,1.6) |
| neuroblastoma | 139 (73.5%) | 1.4 (0.8,2.3) | 29 (15.3%) | 0.8 (0.5,1.2) | 0 | - | 21 (11.1%) | 1.1 (0.6,2.1) | 20 (15.4%) | 2.2 (1.1,4.6) | 21 (16.5%) | 1.5 (0.8,2.7) |
| NH-retinoblastoma | 213 (77.7%) | 1.5 (0.9,2.3) | 33 (12.0%) | 0.7 (0.4,1.1) | 2 (0.7%) | 1.1 (0.2,6.0) | 26 (9.5%) | 1.2 (0.7,2.1) | 15 (7.3%) | 1.2 (0.5,2.8) | 23 (12.1%) | 1.0 (0.5,1.7) |
| H-retinoblastoma | 83 (73.5%) | 1.5 (0.8,2.7) | 13 (11.5%) | 0.6 (0.3,1.2) | 1 (0.9%) | 1.4 (0.2,11.5) | 16 (14.2%) | 2.1 (1.1,3.7) | 4 (5.1%) | 0.8 (0.2,3.0) | 8 (10.4%) | 0.8 (0.3,1.7) |
| Wilms’ tumour | 248 (65.6%) | 1.0 (0.7,1.5) | 66 (17.5%) | 1.0 (0.6,1.5) | 4 (1.1%) | 1.8 (0.4,7.8) | 60 (15.9%) | 1.6 (1.0,2.4) | 56 (23.1%) | 3.9 (2.0,7.6) | 66 (30.1%) | 2.7 (1.7,4.3) |
| bone tumour | 180 (71.9%) | 1.2 (0.8,1.8) | 37 (14.9%) | 0.9 (0.6,1.4) | 0 | - | 31 (12.5% ) | 1.1 (0.7,1.8) | 18 (10.4%) | 2.3 (1.0,5.3) | 18 (11.5%) | 0.8 (0.4,1.6) |
| soft-tissue sarcoma | 241 (71.9%) | 1.0 (0.7,1.5) | 58 (17.3% ) | 1.0 (0.7,1.6) | 0 | - | 36 (10.8%) | 1.2 (0.8,2.0) | 25 (10.7%) | 2.9 (1.4,5.9) | 25 (12.3%) | 1.0 (0.6,1.7) |
| other | 361 (75.0%) | 1.2 (0.9,1.8) | 72 (15.0%) | 0.8 (0.6,1.2) | 3 (0.6%) | 0.9 (0.2,3.6) | 45 (9.4%) | 0.9 (0.6,1.4) | 34 (9.7%) | 2.2 (1.2,4.3) | 38 (12.5%) | 1.0 (0.6,1.5) |
| p-heterogeneity | 0.492 | 0.45 | - | 0.416 | <0.001 | <0.001 | ||||||
| Treated with chemotherapy 2 | ||||||||||||
| No (reference) | 1515 (75.4%) | 1 | 276 (13.7%) | 1 | 14 (0.7%) | 1 | 205 (10.2%) | 1 | 119 (8.2%) | 1 | 171 (13.6%) | 1 |
| Yes | 1035 (69.3%) | 0.8 (0.6,1.2) | 247 (16.5%) | 1.2 (0.9,1.7) | 7 (0.5%) | * | 204 (13.7%) | 0.8 (0.6,1.3) | 107 (10.7%) | 1.3 (0.8,2.2) | 142 (15.3%) | 1.2 (0.8,1.7) |
| Unknown | 448 (73.4%) | - | 84 (13.8%) | - | 2 (0.3%) | - | 76 (12.5%) | - | 36 (5.9%) | - | 55 (9.0%) | - |
| p-heterogeneity | 0.272 | 0.271 | - | 0.388 | 0.26 | 0.476 | ||||||
| Treated with radiotherapy 3 | ||||||||||||
| No (reference) | 1048 (73.7%) | 1 | 209 (14.7%) | 1 | 7 (0.5%) | 1 | 158 (11.1%) | 1 | 77 (7.6%) | 1 | 95 (10.5%) | 1 |
| Other (non-brain/abdominal) |
619 (75.5%) | 1.0 (0.8,1.4) | 107 (13.0%) | 1.0 (0.7,1.3) | 4 (0.5%) | 1.0 (0.3,3.4) | 90 (11.0%) | 1.1 (0.8,1.5) | 43 (7.4%) | 0.7 (0.4,1.2) | 71 (13.1%) | 1.2 (0.8,1.8) |
| Brain | 662 (72.7%) | 1.1 (0.8,1.4) | 130 (14.3%) | 0.9 (0.7,1.2) | 7 (0.8%) | 2.0 (0.6,6.9) | 112 (12.3%) | 0.8 (0.6,1.2) | 39 (6.2%) | 0.5 (0.3,0.8) | 82 (14.7%) | 1.3 (0.8,1.9) |
| Abdominal | 351 (69.0%) | 0.7 (0.5,1.0) | 96 (18.6%) | 1.4 (1.0,1.9) | 3 (0.6%) | 1.3 (0.2,7.2) | 59 (11.6%) | 1.1 (0.7,1.7) | 75 (22.3%) | 1.9 (1.1,3.2) | 90 (28.7%) | 3.2 (2.1,4.7) |
| Unknown | 318 (72.9%) | - | 65 (14.4%) | - | 2 (0.4%) | - | 66 (14.6%) | - | 28 (6.2%) | - | 30 (6.7%) | - |
| p-heterogeneity | 0.236 | 0.176 | - | 0.454 | <0.001 | <0.001 | ||||||
| Abdominal – Non- Wilms | 159 (71.6%) | 0.7 (0.5,1.1) | 40 (18.0%) | 1.5 (0.9,2.3) | 0 | * | 23 (10.4%) | 0.9 (0.5,1.6) | 30 (20.1%) | 1.6 (0.9,3.0) | 36 (25.0%) | 2.7 (1.6,4.5) |
| Abdominal – Wilms | 192 (66.9)% | 0.7 (0.5,1.1) | 56 (19.5%) | 1.4 (0.9,2.1) | 3 (1.1%) | * | 36 (12.5%) | 1.4 (0.8,2.4) | 45 (24.1%) | 2.3 (1.2,4.6) | 54 (31.8%) | 3.5 (2.1,5.7) |
| p-heterogeneity | 0.185 | 0.151 | .- | 0.457 | 0.028 | <0.001 | ||||||
Adjusted for maternal age and birth order
Adjusted for treatment with radiotherapy, maternal age, and birth order
Adjusted for treatment with chemotherapy , maternal age, and birth order
Outcomes evaluated relative to all natural pregnancy outcomes
Insufficient events to reliably test for heterogeneity
Evaluated relative to all natural pregnancy outcomes plus terminations
adjusted for decade of treatment
adjusted for premature delivery
NHL: Non-Hodgkin’s lymphoma; CNS: Central Nervous System; NH: non-heritable; H:heritable; RT: radiotherapy; CT: chemotherapy
Convergence not achieved
Likewise, the OR of miscarriage did not vary significantly by cancer type, treatment with chemotherapy, or with different levels of radiotherapy treatment. However there was a borderline significantly (p=0.06) increased OR of miscarriage for survivors treated with abdominal radiotherapy (OR=1.4, 95%CI:1.0-1.9). This effect was significant when limiting the analysis to miscarriages occurring in gestational week 12 or later (OR=1.9, 95%CI:1.1-3.2). No increased risk was observed for miscarriages occurring before week 12 of gestation (OR=1.0, 95%CI:0.7-1.5). Treatment with brain radiotherapy was not significantly associated with miscarriages occurring either after or before week 12 (OR=1.0, 95%CI:0.6-1.6; OR=0.9, 95%CI:0.6-1.3, respectively).
The OR of still birth was not significantly related to type of childhood cancer or treatment with radiotherapy. The OR for chemotherapy could not be calculated because of lack of convergence of the regression model (probably due to the rarity of this outcome).
Survivors of heritable retinoblastoma had significantly increased odds of terminating a pregnancy relative to leukaemia survivors (OR=2.1, 95%CI:1.1-3.7). There was no significant effect of any of the treatment variables on the odds of terminations.
Female survivors treated with abdominal radiotherapy exhibited a significantly increased OR of delivering offspring with a low birth weight (OR=1.9, 95%CI:1.1-3.2) adjusted for premature delivery. When those abdominally exposed were separated according to Wilms’/non-Wilms’ then the OR for the Wilms’ group (OR=2.3, 95%CI:1.2-4.6) was significantly (p=0.03) greater than the non-Wilms’ group (OR=1.6, 95%CI:0.9,3.0). The percentage of offspring having a low birth weight for women exposed to abdominal irradiation was 22.3% versus 7.6% for women not exposed to irradiation. The latter percentage is comparable to the 7 to 8% observed in the general population of England and Wales (19).
Overall, 28.7% of survivors treated with abdominal irradiation reported a preterm birth versus 10.5% for survivors not treated with any radiotherapy. In relative terms, this corresponds to a three-fold increased OR (OR=3.2, 95%CI:2.1-4.7). Abdominally irradiated Wilms’ tumour survivors demonstrated a 3.5-fold OR (95%CI:2.1-5.7) of delivering preterm relative to survivors treated without radiotherapy.
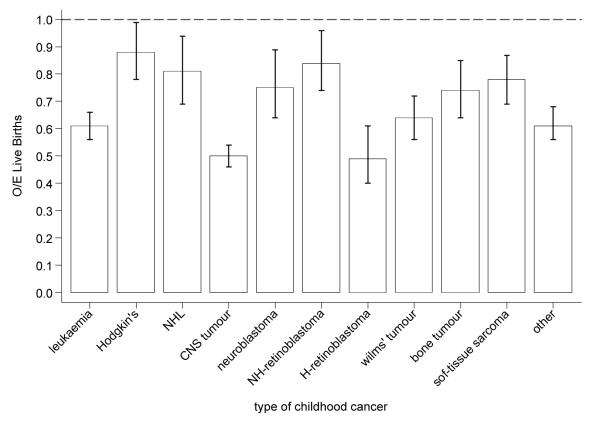
Figure 2a shows the ratio of observed over expected number of live births produced by female survivors by type of childhood cancer. For all female survivors combined, the number of live births observed was two-thirds of that expected (O/E=0.64, 95%CI:0.62-0.66). Survivors of CNS tumours (O/E=0.50, 95%CI: 0.46-0.54) and heritable retinoblastoma (O/E=0.49, 95%CI:0.40-0.61) produced only half the live births expected. The deficit in live births was smallest among survivors of Hodgkin’s lymphoma (O/E=0.88, 95%CI:0.78-0.99) and non-heritable retinoblastoma survivors (O/E=0.81, 95%CI:0.69-0.94). Survivors treated with abdominal or brain radiotherapy each produced almost 50% fewer offspring than expected (O/E=0.55, 95%CI:0.50-0.61; O/E=0.52, 95%CI:0.48-0.56, respectively) (Figure 2b). Figure 2c shows the observed over expected number of live births by maternal age. Although at all ages female survivors produced fewer offspring than expected, the deficit decreased with increasing maternal age (p-trend<0.001).
Figure 2a.
Observed over expected number of live births among female survivors by type of childhood cancer
Figure 2b.
Observed over expected number of live births among female survivors by type of radiotherapy treatment
Figure 2c.
Observed over expected number of live births among female survivors by maternal age
Partners of male survivors
A total of 2,521 singleton pregnancies among partners of male survivors were eligible for analysis (Figure 1). Of these, 80.2% resulted in a live birth, 11.8% in a miscarriage, 7.5% in an termination, and 0.5% in a still birth. Six percent (n=113) of the 1,776 live born offspring for whom birth weight was known had a low birth weight, and 13.5% (n=202) of the 1,498 live born offspring for whom gestational week was known were born prematurely.
Table 2 reveals no significant variation in the ORs of any adverse pregnancy outcome by cancer type, exposure to chemotherapy, brain irradiation, or abdominal irradiation.
Table 2.
Odds ratios of pregnancy outcomes among partners of male survivors of childhood cancer
| natural pregnancy outcome4 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| live birth (n=2,021) | miscarriage (n=297) | still birth (n=13)5 | termination (n=190)6,7 | low birth weight (n=113)8 | premature (n=202) | |||||||
|
|
||||||||||||
| n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95%CI) | |
|
|
||||||||||||
| Type of childhood cancer 1 | ||||||||||||
| leukaemia (reference) |
275 (77.0%) | 1 | 44 (12.3%) | 1 | 2 (0.6%) | 1 | 36 (10.1%) | 1 | 21 (8.4%) | 1 | 25 (12.6%) | 1 |
| Hodgkin’s lymphoma |
227 (78.0%) | 0.9 (0.5,1.6) | 38 (13.1%) | 1.1 (0.6,2.0) | 2 (0.7%) | 0.9 (0.1,10.0) | 24 (8.3%) | 1.2 (0.6,2.4) | 8 (4.0%) | 0.4 (0.1,1.2) | 28 (16.7%) | 1.2 (0.6,2.5) |
| NHL | 164 (80.0%) | 1.1 (0.6,2.0) | 25 (12.2%) | 0.9 (0.5,1.7) | 2 (1.0%) | 1.9 (0.3,13.5) | 14 (6.8%) | 1.0 (0.5,2.1) | 8 (5.5%) | 0.5 (0.2,1.9) | 9 (8.0%) | 0.7 (0.3,1.6) |
| CNS tumour | 397 (83.1%) | 1.3 (0.8,2.2) | 57 (11.9%) | 0.8 (0.5,1.3) | 0 | - | 24 (5.0%) | 0.7 (0.4,1.5) | 33 (9.7%) | 0.7 (0.3,1.9) | 44 (16.1%) | 1.3 (0.7,2.5) |
| Neuroblastoma | 84 (78.5%) | 1.0 (0.5,2.1) | 17 (15.9%) | 1.0 (0.5,2.2) | 0 | - | 6 (5.6%) | 0.4 (0.1,2.1) | 3 (4.0%) | 0.5 (0.1,2.4) | 5 (7.5%) | 0.4 (0.1,1.6) |
| NH-retinoblastoma | 121 (81.8%) | 1.1 (0.5,2.3) | 16 (10.8%) | 0.9 (0.4,1.9) | 1 (0.7%) | 1.6 (0.1,17.5) | 10 (6.8%) | 1.7 (0.7,4.2) | 5 (4.6%) | 0.2 (0.1,0.8) | 23 (24.2%) | 1.9 (0.8,4.4) |
| H-retinoblastoma | 59 (74.7%) | 0.8 (0.4,1.7) | 10 (12.7%) | 1.2 (0.5,2.7) | 1 (1.3%) | 3.0 (0.3,33.9) | 9 (11.4%) | 2.3 (0.8,6.4) | 3 (5.9%) | 0.5 (0.1,3.2) | 7 (13.0%) | 0.9 (0.3,2.9) |
| Wilms’ tumour | 199 (79.9%) | 1.2 (0.6,2.1) | 28 (11.2%) | 0.8 (0.5,1.6) | 2 (0.8%) | 1.1 (0.1,12.2) | 20 (8.0%) | 0.9 (0.4,2.0) | 10 (5.6%) | 0.9 (0.4,2.4) | 13 (9.0%) | 0.7 (0.3,1.6) |
| bone tumour | 118 (78.2%) | 0.9 (0.5,1.8) | 21 (13.9%) | 1.1 (0.6,2.3) | 0 | - | 12 (8.0%) | 1.2 (0.6,2.5) | 6 (5.9%) | 1.1 (0.3,3.3) | 9 (9.5%) | 0.8 (0.3,2.0) |
| soft-tissue sarcoma | 203 (82.9%) | 1.2 (0.6,2.3) | 25 (10.2%) | 0.7 (0.4,1.5) | 3 (1.2%) | 2.3 (0.4,14.5) | 15 (6.1% | 1.2 (0.5,2.6) | 8 (4.8%) | 0.7 (0.2,2.3) | 22 (14.5%) | 1.4 (0.6,2.9) |
| Other | 174 (82.9%) | 2.1 (1.0,4.1) | 16 (7.6%) | 0.5 (0.3,1.0) | 0 | - | 20 (9.5%) | 1.5 (0.8,3.0) | 8 (5.1%) | 0.7 (0.2,2.2) | 17 (12.3%) | 1.0 (0.4,2.2) |
| p-heterogeneity | 0.6 | 0.659 | - | 0.421 | 0.522 | 0.376 | ||||||
| Treated with chemotherapy 2 | ||||||||||||
| No (reference) | 1173 (83.4%) | 1 | 154 (10.0%) | 1 | 6 (0.4%) | 1 | 73 (5.2%) | 1 | 61 (6.1%) | 1 | 131 (15.2%) | 1 |
| Yes | 583 (76.9%) | 0.8 (0.5,1.3) | 92 (12.4%) | 1.2 (0.7,2.0) | 3 (0.4%) | * | 80 (10.6%) | 1.7 (0.9,3.3) | 32 (6.0%) | 1.0 (0.4,2.2) | 44 (10.2%) | 0.7 (0.4,1.3) |
| Unknown | 265 (74.2%) | - | 51 (14.3%) | - | 4 (1.1%) | - | 37 (10.4%) | - | 20 (5.6%) | - | 27 (7.6%) | - |
| p-heterogeneity | 0.364 | 0.451 | 0.131 | 0.971 | 0.254 | |||||||
| Treated with radiotherapy 3 | ||||||||||||
| No (reference) | 639 (81.2%) | 1 | 90 (11.4%) | 1 | 2 (0.3%) | 1 | 56 (7.1%) | 1 | 44 (7.8%) | 1 | 77 (16.2%) | 1 |
| Other (non-brain/abdominal) |
572 (81.0%) | 1.0 (0.7,1.4) | 83 (11.8%) | 1.0 (0.7,1.5) | 6 (0.9%) | 3.4 (0.7,16.4) | 45 (6.4%) | 1.1 (0.7,1.8) | 27 (5.4%) | 0.7 (0.3,1.4) | 55 (12.6%) | 0.8 (0.5,1.2) |
| Brain | 355 (79.2%) | 1.1 (0.7,1.7) | 58 (13.0%) | 1.0 (0.6,1.5) | 0 | - | 35 (7.8%) | 0.8 (0.4,1.4) | 19 (6.3%) | 0.5 (0.2,1.2) | 28 (11.8%) | 0.9 (0.5,1.6) |
| Abdominal | 279 (82.1%) | 1.1 (0.6,1.8) | 38 (11.2%) | 0.9 (0.5,1.5) | 3 (0.9%) | 1.6 (0.2,15.6) | 20 (5.9%) | 0.7 (0.4,1.4) | 11 (4.6%) | 0.5 (0.2,1.3) | 20 (9.3%) | 0.7 (0.3,1.4) |
| Unknown | 176 (73.3%) | - | 28 (11.7%) | - | 2 (0.8%) | - | 34 (14.2%) | - | 12 (5.0%) | - | 22 (9.2%) | - |
| p-heterogeneity | 0.972 | 0.981 | - | 0.486 | 0.311 | 0.58 | ||||||
| Abdominal – Non-Wilms |
111 (85.4%) | 1.2 (0.5,2.7) | 13 (10.0%) | 0.9 (0.4,2.0) | 1 (0.8%) | - | 5 (3.9%) | 0.7 (0.3,1.8) | 4 (4.4%) | 0.5 (0.1,1.9) | 10 (11.4%) | 0.9 (0.4,2.2) |
| Abdominal – Wilms | 168 (80.0%) | 1.0 (0.6,2.0) | 25 (11.9%) | 0.9 (0.5,1.7) | 2 (1.0%) | 1.3 (0.1,12.7) | 15 (7.1%) | 0.6 (0.3,1.4) | 7 (5.7%) | 0.7 (0.2,2.1) | 10 (7.8%) | 0.5 (0.2,1.4) |
| p-heterogeneity | 0.93 | 0.919 | - | 0.481 | 0.446 | 0.409 | ||||||
Adjusted for maternal age and birth order
Adjusted for treatment with radiotherapy, maternal age, and birth order
Adjusted for treatment with chemotherapy , maternal age, and birth order
Outcomes evaluated relative to all natural pregnancy outcomes
Insufficient events to reliably test for heterogeneity
Evaluated relative to all natural pregnancy outcomes plus terminations
adjusted for decade of treatment
adjusted for premature delivery
NHL: Non-Hodgkin’s lymphoma; CNS: Central Nervous System; NH: non-heritable; H:heritable; RT: radiotherapy; CT: chemotherapy
Convergence not achieved
Reasons for termination
Table 3 shows that nearly 70% of all terminations among heritable retinoblastoma survivors were terminated because of reasons relating to the health of the foetus. This percentage was consistent across female and partners of male survivors (data not shown). Among other types of childhood cancer this percentage never exceeded 14%.
Table 3.
Reasons for termination of pregnancy by type of childhood cancer
| Reason for termination |
|||
|---|---|---|---|
| Possible health problem foetus1 |
Social reason2 | Health problem mother3 |
|
|
|
|||
| type of childhood cancer | |||
| leukaemia | 4 (3.3%) | 111 (91.0%) | 7 (5.7%) |
| Hodgkin’s lymphoma | 5 (10.2%) | 37 (75.5%) | 7 (14.3%) |
| NHL | 4 (13.8%) | 25 (86.2%) | 0 (0%) |
| CNS tumour | 6 (6.7%) | 76 (84.4%) | 8 (8.9%) |
| Neuroblastoma | 3 (11.5%) | 22 (84.6%) | 1 (3.9%) |
| NH-retinoblastoma | 4 (12.1%) | 28 (84.6%) | 1 (3.0%) |
| H-retinoblastoma | 13 (68.4%) | 5 (26.3%) | 1 (5.3%) |
| Wilms’ tumour | 4 (5.4%) | 65 (87.8%) | 5 (6.8%) |
| bone tumour | 3 (8.6%) | 28 (80.0%) | 4 (11.4%) |
| soft-tissue sarcoma | 4 (7.5%) | 33 (82.5%) | 4 (10.0%) |
| Other | 4 (9.2%) | 50 (83.6%) | 3 (5.3%) |
NHL: Non-Hodgkin’s lymphoma; CNS: Central Nervous System; NH: non-heritable; H:heritable;
For example: foetus had not developed, possibly affected by retinoblastoma, abnormality
For example: too young, unwanted, unplanned, not in stable relationship, career commitments, financial
For example: ill health, uncertainty future health prognosis
Discussion
Main findings
This study, the largest population-based investigation into pregnancy outcomes among childhood cancer survivors to date, demonstrates that female survivors of childhood cancer treated with abdominal radiotherapy are at a three-fold increased risk of delivering preterm and a two-fold increased risk of delivering low birth weight offspring. Furthermore, there is a risk of miscarriage associated with abdominal radiotherapy, but not with brain radiotherapy. Partners of male survivors of childhood cancer are not at risk of adverse pregnancy outcomes. Overall, female survivors produce two-thirds the offspring expected and almost half that expected for those treated with abdominal or brain radiotherapy.
Low birth weight and premature birth
In the current study, female survivors treated with abdominal radiotherapy experienced a two-fold increased risk of low birth weight. Risks of similar magnitude have previously been reported in non-population based or smaller scale studies (6-8). The three-fold elevated risk we observed for delivering offspring preterm among all female survivors exposed to abdominal radiotherapy is in line with previous findings (8, 11). In our study, Wilms’ tumour survivors had the greatest risk of preterm delivery, which corresponds with previous studies on Wilms’ tumour survivors (10-12). The excess risk of low birth weight and preterm delivery is probably primarily related to the radiation dose to the uterus irrespective of cancer type, but it is difficult to distinguish between treatment effects and cancer type (8). The specific mechanism whereby abdominal irradiation confers an increased risk of delivering preterm and producing offspring with a low birth weight remains elusive. Thus far, a few studies have shown that radiotherapy to the uterus during childhood is associated with reduced adult uterine volume and reduced blood supply of the uterus (21-25). This reduced uterine volume and blood supply may possibly, if a woman is able to conceive at all, restrict foetal growth and the ability to carry the foetus to term.
Miscarriage
The 1.4 increased OR of miscarriage among female survivors treated with abdominal irradiation in our study corroborates the findings by Green et al (7) who reported a significant 1.65-fold increased risk for female survivors whose ovaries were in or near the radiation field. Winther et al (14) found a 2.8-fold increased proportion ratio for miscarriages among female survivors who received high-dose radiotherapy to the ovaries and uterus relative to sisters, but Chiarelli et al (6) could not identify an increased risk. When restricting the analysis to miscarriages occurring at or after week 12 of gestation, the risk we observed was more pronounced, but no significantly increased risk was observed for miscarriages occurring before week 12 of gestation.
It has been suggested that radiation to the brain increases the chance of a miscarriage (7), possibly through impairment of the hypothalamic-pituitary-ovarian-axis function (26). Some support for this hypothesis was found by two previous studies; Winther et al reported a 1.8-fold significantly increased risk of miscarriage among survivors treated with brain irradiation.(14) Green et al found a 1.4-fold significantly increased risk for survivors treated with brain radiotherapy versus those who did not receive radiotherapy, with the highest risks of miscarriage occurring after week 12 of gestation (7). We did not find any evidence that brain irradiation confers an excess risk of miscarriage.
Terminations
Overall, there were no strong indications that the OR of a termination varied by cancer type except for female survivors of heritable retinoblastoma for whom the OR was two-fold relative to leukaemia survivors. This finding suggests that pregnancies among heritable retinoblastoma survivors may be more likely to be terminated as a result of the 50% risk of the foetus inheriting the RB1 gene which identifies retinoblastoma. Evidence for this is that in our data nearly 70% of all pregnancies among heritable retinoblastoma survivors were terminated because of reasons relating to the health of the foetus; a considerably higher percentage than among other survivors.
Live birth rate
Recently, Madanat et al reported a parenthood probability of 0.62 relative to siblings for female survivors of childhood cancer (27). Syse et al demonstrated that the probability of a first live birth among female survivors diagnosed with cancer under 10 years of age was 0.69 (personal communication) (28). These observations are comparable to the live birth ratio of 0.64 which we found.
In our study, brain radiotherapy was associated with a deficit of almost 50% in live births compared to the general population. Nygaard et al found that live birth rates among leukaemia survivors treated with cranial radiotherapy were 0.39 of leukaemia survivors not treated with radiation (29) which is consistent with our findings. Exposure to cranial radiation has been associated with a decrease in fertility. Green et al (4) reported 40% less pregnancies among survivors exposed to 30 Gy or more of hypothalamic/pituitary radiation relative to those treated with less than 10 Gy.
There may be several reasons why survivors produce fewer offspring than the general population such as for example; treatment induced ovarian failure (1-3, 30) or early menopause (31-33), more difficulty finding a partner (34, 35), or concerns about the health of offspring (36, 37). However, a detailed investigation into reasons for the reduced live birth rate among survivors is beyond the scope of this article.
Partners of male survivors
In this study, there was no evidence that partners of male survivors exhibited an excess risk of adverse pregnancy outcomes. This is consistent with findings from the CCSS (38) and thus, based on these two studies, any large excess risk among partners of male survivors can be excluded.
Limitations
A limitation of this study concerns the self-reported nature of pregnancy outcomes which were not independently verified. This may have led to underreporting of pregnancy outcomes, particularly for miscarriages (39). Even though any of such underreporting is likely to be unrelated to the ‘exposure’ factors studied here, it cannot be excluded entirely that our findings might have been affected by recall-bias. Also, birth weight data was not reported for 30% of all live born offspring. How this might affect the odds ratio of the exposure factors on the risk of birth-weight is not entirely clear. However, a sensitivity analysis assuming that all offspring for whom birth weight was missing were of low-birth increased the odds ratio for abdominal irradiation minimally (from 1.9 to 2.2 (1.4, 3.4).). Similarly, assuming that all offspring with missing birth weight had normal birth weight did not alter the odds ratio appreciable (from 1.9 to 1.8 (1.1, 3.1)).
In this study, miscarriage was defined as any pregnancy ending prior to gestational week 24 whereas in most American studies this was 20 weeks. Theoretically, this could impede comparison of the results across studies. However, when we excluded miscarriages occurring after week 20 from the analysis, the odds ratios remained unchanged. Comparison of miscarriages between our and other studies should therefore be possible.
Lack of comprehensive treatment information did not permit investigating the risk of adverse pregnancy outcomes by dose of radiation received to the reproductive organs, hence residual confounding by radiation exposure could have distorted the magnitude of the identified risks.
Survivors included in this investigation were treated between 1940 and 1991 and hence little is known about the potential adverse effects of more recent therapies. Survivors treated more recently are still relatively young and the number of offspring born to these survivors will be relatively small. It is only through continued long-term follow-up of survivors through large-scale prospective cohort studies that it will become clear whether more recent therapies are associated with an excess risk of adverse pregnancy outcomes (40).
Clinical implications
It is reassuring that the majority of survivors who are able to conceive are not at risk of an adverse pregnancy outcome. However, female survivors who received treatment involving radiation to the reproductive organs, and who are still able to become pregnant, are at risk of an adverse pregnancy outcome and such pregnancies should be monitored and managed by a multidisciplinary specialist team (40).
Conclusion
In conclusion, female survivors of childhood cancer treated with abdominal radiotherapy and who are able to become pregnant are at risk of delivering prematurely and producing low birth weight offspring. Furthermore, there is a risk of miscarriage associated with abdominal radiotherapy, but not with brain radiotherapy. Chemotherapy appears not to be associated with any adverse pregnancy outcome. There are no indications of an increased risk of adverse pregnancy outcomes for partners of male survivors. Overall, female survivors produce considerably fewer offspring than the general population, particularly those survivors treated with abdominal or brain radiotherapy.
Acknowledgments
*The British Childhood Cancer Survivor Study (BCCSS) is a national collaborative undertaking guided by a Steering Group that comprises Professor Douglas Easton (chair), Professor Michael Hawkins (secretary), Dr Helen Jenkinson, Dr Meriel Jenney, Dr Emma Lancashire, Professor Kathryn Pritchard-Jones, Professor Michael Stevens, Mr Charles Stiller, Dr Elaine Sugden, Dr Andrew Toogood, and Dr Hamish Wallace. The BCCSS benefits from the contributions of the Officers, Centers, and individual members of the Children’s Cancer and Leukemia Group, the Childhood Cancer Research Group and the Regional Pediatric Cancer Registries. The BCCSS acknowledges the collaboration of the Office for National Statistics, the General Register Office for Scotland, the National Health Service Central Registers, the regional cancer registries, health authorities, and area health boards for providing general practitioner names and addresses and the general practitioners nationwide who facilitated direct contact with survivors. We are particularly thankful to all survivors who completed a 40-page questionnaire. The BCCSS would not have been possible without the support of our two funders: Cancer Research UK and the Kay Kendall Leukaemia Fund to whom we offer our profound thanks. Finally, thanks to all BCCSS staff who have given many years of dedicated work to bring the BCCSS to fruition.
Acknowledgements of research support: Cancer Research UK and the Kay Kendall Leukaemia Fund
References
- 1.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91:1723–8. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 2.Lantinga GM, Simons AH, Kamps WA, Postma A. Imminent ovarian failure in childhood cancer survivors. Eur J Cancer. 2006;42:1415–20. doi: 10.1016/j.ejca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88:5307–14. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 4.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, Donaldson SS, Byrne J, Robison LL. Fertility of Female Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagarajan R, Robison LL. Pregnancy outcomes in survivors of childhood cancer. J Natl Cancer Inst Monogr. 2005;34:72–6. doi: 10.1093/jncimonographs/lgi020. [DOI] [PubMed] [Google Scholar]
- 6.Chiarelli AM, Marrett LD, Darlington GA. Pregnancy outcomes in females after treatment for childhood cancer. Epidemiology. 2000;11:161–6. doi: 10.1097/00001648-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, Pendergrass TW, Robison LL. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 8.Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE, Whitton JA, Green DM, Donaldson SS, Mertens AC, Robison LL, Boice JD., Jr. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst. 2006;98:1453–61. doi: 10.1093/jnci/djj394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: probable effects of abdominal irradiation. Int J Cancer. 1989;43:399–402. doi: 10.1002/ijc.2910430309. [DOI] [PubMed] [Google Scholar]
- 10.Li FP, Gimbrere K, Gelber RD, Sallan SE, Flamant F, Green DM, Heyn RM, Meadows AT. Outcome of pregnancy in survivors of Wilms’ tumor. JAMA. 1987;257:216–9. [PubMed] [Google Scholar]
- 11.Green DM, Peabody EM, Nan B, Peterson S, Kalapurakal JA, Breslow NE. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–13. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 12.Byrne J, Mulvihill JJ, Connelly RR, Austin DA, Holmes GE, Holmes FF, Latourette HB, Meigs JW, Strong LC, Myers MH. Reproductive problems and birth defects in survivors of Wilms’ tumor and their relatives. Med Pediatr Oncol. 1988;16:233–40. doi: 10.1002/mpo.2950160403. [DOI] [PubMed] [Google Scholar]
- 13.Kalapurakal JA, Peterson S, Peabody EM, Thomas PR, Green DM, D’Angio G,J, Breslow NE. Pregnancy outcomes after abdominal irradiation that included or excluded the pelvis in childhood Wilms tumor survivors: a report from the National Wilms Tumor Study. Int J Radiat Oncol Biol Phys. 2004;58:1364–8. doi: 10.1016/j.ijrobp.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Winther JF, Boice JD, Jr., Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol. 2008;26:4340–6. doi: 10.1200/JCO.2007.15.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins MM, Lancashire ER, Winter DL, Frobisher C, Reulen RC, Taylor AJ, Stevens MC, Jenney M. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50:1018–1025. doi: 10.1002/pbc.21335. [DOI] [PubMed] [Google Scholar]
- 16.Still-Birth (Definition) Act 1992. Her Majesty’s Stationery Office; 1992. [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 18.Department of Health . Statistical Bulletin: Abortion Statistics. England and Wales: 2006. [Google Scholar]
- 19.Office of National Statistics . Birth statistics. 2006. (Series FM1 no. 35). [Google Scholar]
- 20.Statacorp . Stata Statistical Software: Release 10. Statacorp LP; College Station, TX: 2009. [Google Scholar]
- 21.Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Muller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83:96–102. doi: 10.1111/j.1600-0412.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum Blask AR, Nicholson HS, Markle BM, Wechsler-Jentzch K, O’Donnell R, Byrne J. Sonographic detection of uterine and ovarian abnormalities in female survivors of Wilms’ tumor treated with radiotherapy. AJR Am J Roentgenol. 1999;172:759–63. doi: 10.2214/ajr.172.3.10063876. [DOI] [PubMed] [Google Scholar]
- 23.Critchley HO, Wallace WH, Shalet SM, Mamtora H, Higginson J, Anderson DC. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol. 1992;99:392–4. doi: 10.1111/j.1471-0528.1992.tb13755.x. [DOI] [PubMed] [Google Scholar]
- 24.Bath LE, Critchley HO, Chambers SE, Anderson RA, Kelnar CJ, Wallace WH. Ovarian and uterine characteristics after total body irradiation in childhood and adolescence: response to sex steroid replacement. Br J Obstet Gynaecol. 1999;106:1265–72. doi: 10.1111/j.1471-0528.1999.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 25.Holm K, Nysom K, Brocks V, Hertz H, Jacobsen N, Muller J. Ultrasound B-mode changes in the uterus and ovaries and Doppler changes in the uterus after total body irradiation and allogeneic bone marrow transplantation in childhood. Bone Marrow Transplant. 1999;23:259–63. doi: 10.1038/sj.bmt.1701569. [DOI] [PubMed] [Google Scholar]
- 26.Bath LE, Anderson RA, Critchley HO, Kelnar CJ, Wallace WH. Hypothalamic-pituitary-ovarian dysfunction after prepubertal chemotherapy and cranial irradiation for acute leukaemia. Hum Reprod. 2001;16:1838–44. doi: 10.1093/humrep/16.9.1838. [DOI] [PubMed] [Google Scholar]
- 27.Madanat LM, Malila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr., Lahteenmaki PM. Probability of parenthood after early onset cancer: a population-based study. Int J Cancer. 2008;123:2891–8. doi: 10.1002/ijc.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syse A, Kravdal O, Tretli S. Parenthood after cancer - a population-based study. Psychooncology. 2007;16:920–7. doi: 10.1002/pon.1154. [DOI] [PubMed] [Google Scholar]
- 29.Nygaard R, Clausen N, Siimes MA, Marky I, Skjeldestad FE, Kristinsson JR, Vuoristo A, Wegelius R, Moe PJ. Reproduction following treatment for childhood leukemia: a population-based prospective cohort study of fertility and offspring. Med Pediatr Oncol. 1991;19:459–66. doi: 10.1002/mpo.2950190603. [DOI] [PubMed] [Google Scholar]
- 30.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 31.Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, Holmes GF, Holmes FF, Latourette HB, Meigs JW, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166:788–93. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 32.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–54. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 33.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y, Robison LL. Premature menopause in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:890–6. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 34.Frobisher C, Lancashire ER, Winter DL, Jenkinson HC, Hawkins MM. Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. Int J Cancer. 2007;121:846–55. doi: 10.1002/ijc.22742. [DOI] [PubMed] [Google Scholar]
- 35.Rauck AM, Green DM, Yasui Y, Mertens A, Robison LL. Marriage in the survivors of childhood cancer: a preliminary description from the Childhood Cancer Survivor Study. Med Pediatr Oncol. 1999;33:60–3. doi: 10.1002/(sici)1096-911x(199907)33:1<60::aid-mpo11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Reinmuth S, Liebeskind AK, Wickmann L, Bockelbrink A, Keil T, Henze G, Borgmann A. Having children after surviving cancer in childhood or adolescence - results of a Berlin survey. Klin Padiatr. 2008;220:159–65. doi: 10.1055/s-2008-1073143. [DOI] [PubMed] [Google Scholar]
- 37.Schover LR. Motivation for parenthood after cancer: a review. J Natl Cancer Inst Monogr. 2005;34:2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- 38.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, Pendergrass TW, Robison LL. Pregnancy outcome of partners of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:716–21. doi: 10.1200/JCO.2003.04.085. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox AJ, Horney LF. Accuracy of spontaneous abortion recall. Am J Epidemiol. 1984;120:727–33. doi: 10.1093/oxfordjournals.aje.a113940. [DOI] [PubMed] [Google Scholar]
- 40.Edgar AB, Wallace WH. Pregnancy in women who had cancer in childhood. Eur J Cancer. 2007;43:1890–4. doi: 10.1016/j.ejca.2007.06.011. [DOI] [PubMed] [Google Scholar]