Abstract
Here we examine how BMP, Wnt, and FGF signaling modulate activin-induced mesendodermal differentiation of mouse ES cells grown under defined conditions in adherent monoculture. We monitor ES cells containing reporter genes for markers of primitive streak (PS) and its progeny and extend previous findings on the ability of increasing concentrations of activin to progressively induce more ES cell progeny to anterior PS and endodermal fates. We find that the number of Sox17- and Gsc-expressing cells increases with increasing activin concentration while the highest number of T-expressing cells is found at the lowest activin concentration. The expression of Gsc and other anterior markers induced by activin is prevented by treatment with BMP4, which induces T expression and subsequent mesodermal development. We show that canonical Wnt-signaling is required only during late stages of activin-induced development of Sox17-expressing endodermal cells. Furthermore, Dkk1 treatment is less effective in reducing development of Sox17+ endodermal cells in adherent culture than in aggregate culture and appears to inhibit nodal-mediated induction of Sox17+ cells more effectively than activin-mediated induction. Notably, activin-induction of Gsc-GFP+ cells appears refractory to inhibition of canonical Wnt signaling but shows a dependence on early as well as late FGF signaling. Additionally, we find a late dependence on FGF signaling during induction of Sox17+ cells by activin while BMP4-induced T expression requires FGF signaling in adherent but not aggregate culture. Lastly, we demonstrate that activin-induced definitive endoderm derived from mouse ES cells can incorporate into the developing foregut endoderm in vivo and adopt a mostly anterior foregut character after further culture in vitro.
Keywords: Embryonic stem cell, gastrulation, endoderm, mesendoderm, anterior-posterior patterning, TGF-β, Wnt, FGF
Introduction
Directed differentiation of embryonic stem (ES) cells into meso- and endodermal derivatives is intensely studied due to their potential clinical applications. Meso- and endoderm is formed by epiblast cells that ingress through the primitive streak (PS) during gastrulation (reviewed in (Tam and Loebel, 2007)). Fate mapping studies have shown that cells that migrate through different anterior-posterior regions of the streak give rise to different mesodermal and endodermal components (Carey et al., 1995; Lawson, 1999; Lawson and Pedersen, 1992). At early stages, mesodermally fated cells ingress alongside endodermally fated cells but it is unclear when and how ingressing cells acquire their ultimate fate. The definitive endoderm (DE) is derived from progenitors migrating through the anterior PS at early and mid-streak stages (Carey et al., 1995; Lawson, 1999; Lawson and Pedersen, 1987; Lawson and Pedersen, 1992). Moreover, recent evidence suggests that a common progenitor population, the mesendoderm, exists in the PS (Kinder et al., 2001; Lawson et al., 1991; Tada et al., 2005) and that the cumulative exposure to nodal signaling determines mesendodermal fates such that increasing exposure to nodal shifts the fate from posterior mesoderm through anterior mesoderm and posterior endoderm to anterior DE at the largest dose (Ben-Haim et al., 2006).
The use of mouse ES (mES) cell lines with the green fluorescence protein (GFP) targeted to the PS-and early mesodermal-specific genes Brachyury (T), Mix1 homeobox-like 1 (Mixl1), and Goosecoid (Gsc) has made it possible to quantify mesendoderm induction and isolate and characterize different mesodermal and endodermal populations (Fehling et al., 2003; Gadue et al., 2006; Kubo et al., 2004; Ng et al., 2005; Tada et al., 2005; Yasunaga et al., 2005). Anterior PS fates and endoderm was induced with high concentrations of activin A (activin hereafter) that activates Smad2/3 signaling through binding to the same receptor as nodal. Recent studies have extended the endoderm-inducing properties of activin to human ES cell differentiation cultures (D’Amour et al., 2005; D’Amour et al., 2006). However, as nodal signaling in the early embryo interacts with other signaling pathways such as the BMP, Wnt, and FGF pathways (reviewed in (Tam and Loebel, 2007)), we address here the role of these signals and their potential to modulate activin-induced mesendodermal differentiation of mES cells grown under standard conditions in feeder- and serum-free adherent monoculture (Ying et al., 2003a; Ying et al., 2003b). We use ES cells containing reporter genes (lacZ or GFP) targeted to the T (Fehling et al., 2003), Mixl1 (Hart et al., 2002), Gsc (Tada et al., 2005), Flk1 (Shalaby et al., 1995), Sox17 (Kim et al., 2007), and Sox2 (Li et al., 1998) loci to monitor, over time, the effects of different growth factors on the expression of markers specific to different anterior and posterior regions of the PS and derivatives thereof. We confirm and extend previous findings on the ability of increasing concentrations of activin to progressively induce more ES cell progeny to an anterior PS fate. Remarkably, while the number of Gsc- and Sox17-expressing cells increases with increasing activin concentration, the highest number of T-expressing cells is found at the lowest activin concentration, similar to the activin response seen in Xenopus animal cap cells. Furthermore, expression of Gsc and other anterior markers induced at high activin doses is prevented by simultaneous treatment with BMP4 which redirects development towards mesodermal fates, also similar to results from Xenopus. Extending previous work, we find that inhibition of canonical Wnt-signaling by treatment with Dkk1 is able to prevent activin-induced development of endodermal cells but only at late stages of differentiation. Dkk1 also inhibits activin-induced Mixl1-expression and consistent with this finding Wnt3a and activin act additively on Mixl1 expression but not on Gsc expression. Wnt3a by itself appears to induce only posterior PS fates depending, however, on endogenous Smad2/3 signaling. Additionally, we demonstrate that induction of anterior and posterior PS fates by activin or BMP4, respectively, is dependent on FGF signaling. Lastly, we demonstrate for the first time that activin-induced DE derived from mES cells can incorporate into the developing foregut endoderm when implanted into chicken embryos but respond only to a limited degree to posteriorising cues in vitro by initiating expression of regional foregut markers.
Materials and Methods
Cell culture and differentiation of ESCs
Mouse ES cells (40,000 cells/cm2) were kept undifferentiated on gelatin-coated cell culture plastic (Nunc) in serum-free medium; KO-DMEM supplemented with N2, B27, 0.1 mM nonessential amino acids, 2 mM L-glutamine, Penicillin/Streptomycin (all from Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), 1500 U/ml leukemia inhibitory factor (LIF, Chemicon) and 10 ng/ml BMP4 (R&D Systems), essentially as described by Ying et al. (Ying et al., 2003a). ES cells were passaged every second day with daily media changes for at least three passages (6 days) prior to initiation of differentiation studies.
For differentiation experiments cells grown as described above were dissociated to single cells and differentiation was induced by seeding 2000 cells/cm2 on gelatin-coated cell culture plastic in KO-DMEM supplemented with N2, B27, 0.1 mM nonessential amino acids, 2 mM L-glutamine, Penicillin/Streptomycin (all from Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich) without LIF and BMP4. The medium was supplemented with one or more of the following growth factors, soluble receptors, and small molecule compounds: activin (3, 10, 30 or 100 ng/ml), Wnt3a (5 or 100 ng/ml), BMP4 (10 ng/ml), Dkk1 (320 ng/ml; all from R&D Systems), FGF2 (100 ng/ml; Invitrogen). Soluble FGF receptors (all from R&D Systems) were first useed to achieve inhibition of ligands specific for both b and c splice forms by mixing sFGFR1IIIc, sFGFR2IIIb, and l sFGFR4 (12, 8 and 24 ng/ml, respectively). To achieve. selective inhibition of the b or c splice form specific FGFs we mixed sFGFR1IIIb and sFGFR2IIIb (both at 250 ng/ml) or sFGFR1IIIc and sFGFR4 (both at 250 ng/ml), respectively. The medium containing FGF2 or sFGFRs was supplemented with 10 µg/ml heparan sulfate (Sigma-Aldrich). 1 µM SB431542 (Inman et al., 2002), 10 µM SU5402 or 100 nM PD173074 (Calbiochem). The cells were cultured for up to 6 days and the medium was changed daily, beginning at the second day of differentiation. It should be noted that our B27 supplement contain retinyl acetate which is a precursor during RA synthesis. However, experiments using B27 supplement without retinyl acetate (Invitrogen) did not affect the number of activin-induced Sox17-GFPHi endodermal cells. Further differentiation of day 5 activin-induced cultures was done by 3 days of additional culture in serum-free medium (KO-DMEM, N2, B27, 0.1 mM nonessential amino acids, 2 mM L-glutamine, Penicillin/Streptomycin, 0.1 mM 2-mercaptoethanol) supplemented with Wnt3a (5 ng/ml), FGF4 (10 ng/ml) and/or 0.1 µM all-trans retinoic acid (Sigma). .
Differentiation in embryoid bodies (EBs) was carried out using the hanging drop method. Cells were dissociated to single cells using non-enzymatic Cell Dissociation Solution (Sigma Aldrich) and diluted in N2B27 medium containing the relevant growth factors to yield 100 cells per 20 µl drop. Approximately 150 drops were applied to the lid of a 14 cm cell culture dish (Nunc), and placed upside down over autoclaved Millipore water. Drops were left over night and EBs were washed down with HBSS w/o Ca2+ and Mg2+ and left to sediment for 3–4 minutes before removing the supernatant and transferring EBs to 50 mm Petri dishes (Sterilin) containing N2B27 medium with the relevant growth factors. The medium was changed daily. We frequently observed that 3–5 individual aggregates would form in the hanging drop yielding aggregates composed of 20–30 cells.
Flow cytometry
The cells were dissociated in 0.05% Trypsin-EDTA (Invitrogen) and percentage GFP+ cells was analysed on a FACSCalibur flow cytometer (BD Biosciences) at day 2–6 in at least three independent experiments. Mean % GFP+ cells ± standard deviation (S.D.) was calculated and statistical analyses were performed using a two-tailed Student’s t-test for paired samples, unless we had a clear expectation of the outcome in which case a one-tailed test was used. Sorting of GFP+ cells for RNA extraction was performed on a FACSAria (BD Biosciences). CXCR4 expression was analysed on a FACSCalibur flow cytometer using a human anti-CXCR4 monoclonal antibody (MAB172; R&D Systems). Cells were dissociated using Collagenase (Sigma-Aldrich) for 5 minutes. The cell suspension was fixed and stained as described below without permeabilisation. Visual inspection of the stained cells by confocal microscopy confirmed surface localization of the antigen.
Immunofluorescence and X-gal staining
The cells were cultured on gelatin-coated chamber slides for 3, 5 or 8 days and fixed at room temperature for 30 minutes in 4% formaldehyde solution (Mallinckrodt Baker) for immunofluorescence or 5 minutes in 0.2% glutaraldehyde for X-gal staining. For immunofluorescence, the cells were permeabilised in graded ethanol followed by blocking in 10% donkey serum for 1 hour and incubation with primary antibody for 1 hour at room temperature or over night at 4°C. The following antibodies were used: Goat anti-Foxa2 (Santa Cruz Biotechnology), goat anti-T (R&D Systems), rat anti-E-cadherin (E-cad; Zymed/Invitrogen), goat anti-Sox17 (R&D Systems), Alexa 448 conjugated rabbit anti-GFP (Molecular Probes/Invitrogen), rabbit anti-β galactosidase (MP Biomedicals), rabbit anti-Lhx1 (Chemicon), goat and rabbit anti-Pdx1 (a kind gift from C. Wright), mouse and rabbit anti-Nkx6-1 (Jensen et al., 1996; Pedersen et al., 2006), rabbit anti-Sox2 (Chemicon) and mouse anti-Cdx2 (BioGenex). The cells were incubated with Cy2-, Cy3-, Texas Red- or Cy5-conjugated species-specific secondary antibodies (Jackson ImmunoResearch Laboratories) and 4’,6-diamidino-2-phenylindole (DAPI, MP Biomedicals). The Lhx1 staining used tyramide signal amplification (PerkinElmer) according to the manufacturer’s recommendations. Negative controls, where the primary antibodies were omitted, were included for all stainings. These controls showed no unspecific staining of the secondary antibodies (data not shown). β-galactosidase activity was visualised by adding X-gal stain solution for 4 hours at 37°C. The slides were analysed using a LSM 510 META laser scanning microscope (Carl Zeiss) or a BX60 epifluorescence microscope equipped with a DP71 camera (both from Olympus).
RT-PCR and qPCR
Cells were dissociated using 0.05% Trypsin-EDTA and collected by centrifugation. Total RNA was isolated using the RNeasy kit with DNAse treatment (Qiagen) following the manufacturer’s protocol. cDNA was prepared from 5 or 100 ng RNA using MMLV Reverse Transcriptase (Invitrogen). PCR reactions were performed using 1 µl cDNA, 1 µl 20 µM primer mix and 23 µl Reddy Mix PCR master mix (Abgene). The PCR was carried out with an initial denaturation step at 96°C for 2 minutes, followed by 32–38 cycles of 96°C for 30 seconds, 55°C for 30 seconds and 72°C for 1 minute. The PCR was finished with a final extension step at 72°C for 5 minutes. QPCR was performed using the standard SYBR® Green program with dissociation curve of the Mx3005P (Stratagene). PCR reaction were run in duplicates using 5 µl Brilliant® SYBR® Green QPCR Master Mix (Stratagene), 1 µl cDNA, 1 µl 10 µM primer mix and 3 µl DEPC-treated water. Quantified values for each gene of interest were normalized against the input determined by the housekeeping genes G6pdh and Tbp. The results are expressed as the relative expression level compared with the vehicle control condition or the scrambled control siRNA in the vehicle condition. Primer sequences are available on request.
siRNA transfection
The sequence effective for mouse β-catenin knock-down was designed using software available at the web site of Invitrogen (http://rnaidesigner.invitrogen.com/rnaiexpress/). The β-catenin (accession number NM_0079614.2) target sequences of the STEALTH siRNAs were 5’-GCCTTCATTATGGACTGCCTGTTGT-3’ (siRNA1) and 5’-GAGCAAGGCTTTTCCCAGTCCTTCA-3’ (siRNA2). The STEALTH negative control siRNA (scrambled; Invitrogen) has been used as negative control and is labelled “scrambled”. The cells were cultured on gelatin-treated 24-well plates as previously described with a starting density of 4000 cells/cm2. After one day of differentiation the cells were transfected with 100 nM STEALTH siRNAs using Lipofectamine2000 according to the manufacturer’s instructions. The transfected cells were grown further and analyzed for β-catenin expression by western blot at day 3 and 5 of differentiation. QPCR and flow cytometry analysis was performed at day 5.
Western Blot
Total cell lysates were obtained on day 3 and 5 of differentiation culture using RIPA lysis buffer. 20 µg of each protein sample were loaded and analysed by western blot using a rabbit anti-β-catenin monoclonal antibody (Lab Vision) and a mouse anti-β-Actin monoclonal antibody (Sigma-Aldrich) as primary antibodies as well as secondary HRP-conjugated antibodies (Santa Cruz Biotechnology).
Generation of stable Wnt-reporter ES cell lines and chimera formation
The SuTOP-CFP construct was generated by cutting the luciferase gene from the Super8XTOPFLASH Wnt reporter (generously provided by Dr. R. Moon, University of Seattle, WA) with Fse and Nco1 and replacing it with Cerulean PCR product from the pmCerulean-C1 vector (Rizzo et al., 2004). To generate stably transfected cell lines, E14 cells (Hooper et al., 1987) of low passage number were co-transfected with SuTOP-CFP and a ploxP-Neo vector, conferring resistance to Neomycin. The relationship between plasmids was 10:1 (reporter:ploxP-Neo). Transfection was carried out using Lipofectamine 2000 (Invitrogen) and 24 hours after transfection, selection was added (200 µg/ml G418, Invitrogen). Medium was changed daily for 9 days and 0,5 µM BIO (GSK3β inhibitor, Calbiochem), which activates canonical Wnt signaling, was added to the medium for the last 2 days of selection to identify Wnt responsive colonies. Fluorescent colonies were then picked and expanded and one clone was selected for chimera formation via blastocyst injection. E3.5 blastocysts were harvested by flushing the uteri of mature, time mated NMRI mice (Taconic). Chimeric embryos were generated by injection of approximately 10 SuTOP-CFP ES cells into the blastocoel using a paraffin-oil driven manual injector (Cell Tram Vario, Eppendorf) and a Narishige micromanipulator. Following 3 hours of culture in M16 medium, embryos were transferred to the uterus of E2.5 pseudopregnant, 7 weeks old NMRI foster mothers. Care of the animals was done according to institutional guidelines. Embryos were harvested at E10.5 and fixed in Lilly’s fixative (4% phosphate buffered formaldehyde) for 30 minutes before being analyzed for native Cerulean fluorescence. All animal experiments were performed in accordance with institutional and national regulations.
Chick embryo grafting
Fertilised eggs from white leghorn chicken were purchased from Triova and incubated at 38°C to Hamburger and Hamilton (HH) stage 8–10 (Hamburger and Hamilton, 1951). The embryos were explanted as previously described (Chapman et al., 2001). E14 ES cell progeny was prepared for grafting by labelling with fluorescent CMTMR CellTracker dye (Molecular Probes/Invitrogen). Clumps of cells were scraped off, washed in PBS and inserted between endoderm and mesoderm of chicken embryos via a small incision in the endoderm. Grafted embryos were incubated for 48 hours in a humidified incubator at 38°C. The embryos were isolated, washed in PBS, fixed in 4% PFA at room temperature for 2 hours and stored in methanol at −20°C until time of analysis. Whole-mount immunofluorescent analyses of grafted chicken embryos were performed as previously described (Ahnfelt-Ronne et al., 2007).
Results
Dose-dependent effects of activin on the expression of PS genes are modulated by BMP and Wnt signaling
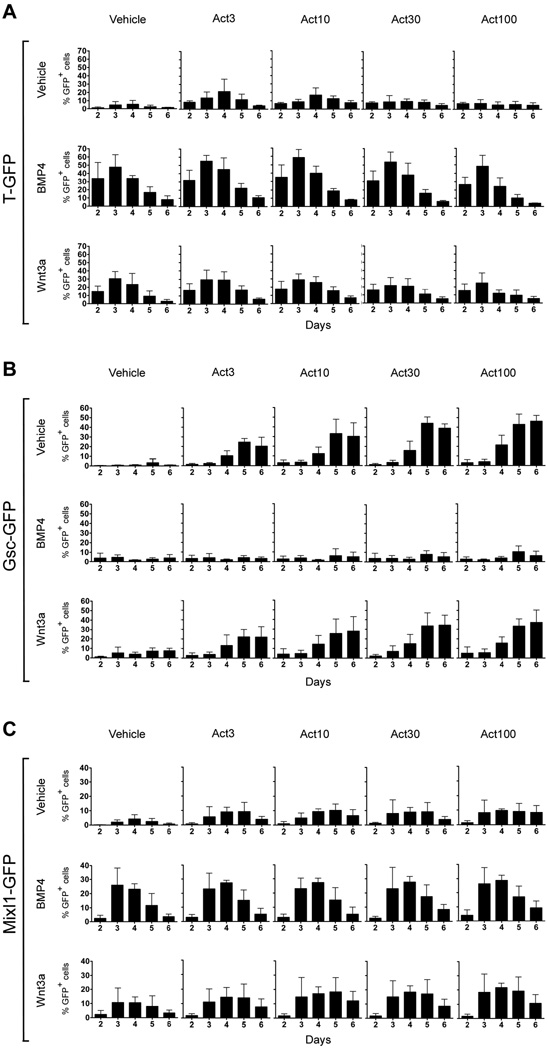
Previous studies have demonstrated induction of Brachyury (T) and Goosecoid (Gsc) by activin in mES cells (Gadue et al., 2006; Kubo et al., 2004; Tada et al., 2005; Yasunaga et al., 2005). However, differences in media compositions and the culture methods used make a direct comparison of the response of these two genes to varying doses of activin difficult. Prior to induction of differentiation by addition of growth factors, we culture the undifferentiated ES cells under defined conditions (Ying et al., 2003a). As activin is known to dose-dependently regulate T- and Gsc expression in Xenopus animal cap cells such that low doses will activate T and high doses will activate Gsc (Dyson and Gurdon, 1998; Green et al., 1992; Gurdon et al., 1994; Gurdon et al., 1999; Latinkic et al., 1997), we initially tested if exposure of mES cells to increasing doses of activin would lead to a shift from T to Gsc expression. ES cell lines carrying T-Gfp (TGfp/+) and Gsc-Gfp (GscGfp/+) knock-in alleles (Fehling et al., 2003; Tada et al., 2005) were cultured with increasing amounts of activin (from 3 to 100 ng/ml) and the number of GFP+ cells was analysed by flow cytometry. Examples of primary flow cytometry diagrams are shown in Fig. S1. When GFP+ cells were quantitated we found that 3 ng/ml activin transiently induced 21±15% T-GFP+ cells (mean % ± S.D., n = 3) peaking at day 4 (Fig. 1A). However, this induction was not statistically significant. At higher activin concentrations the number of T-GFP+ cells declined gradually such that the highest dose (100 ng/ml) resulted in only 5±4% T-GFP+ cells at day 4, comparable to the control samples cultured in the absence of activin (6±5% T-GFP+ cells, Fig. 1A). In contrast, flow cytometric analyses of GscGfp/+ cells showed that the expression of this anterior PS marker (Blum et al., 1992) was induced by activin in a dose-dependent manner with expression peaking at day 5–6 (Fig. 1B). 3 ng/ml activin induced 25±4% Gsc-GFP+ cells at day 5, and this number increased with increasing concentration of activin reaching 43±11% Gsc-GFP+ cells in cultures treated with 100 ng/ml activin (Fig. 1B). Control samples grown in the absence of activin contained 3±4% Gsc-GFP+ cells at this time point. The induction of Gsc expression at day 5 was statistically significant for all activin concentrations tested (p < 0.05). Thus, similar to the case in Xenopus animal cap cells, low doses of activin support T expression while high doses stimulate Gsc expression. Intriguingly, while expression of the PS marker Mixl1 (Pearce and Evans, 1999) was induced by activin, the number of Mixl1-GFP+ cells was independent of the activin concentration (Fig. 1C).
Fig. 1. Activin, BMP and Wnt signaling control the dynamic expression of primitive streak genes in differentiating ES cells.
Flow cytometric analysis of TGfp/+ (A), GscGfp/+ (B), or Mixl1Gfp/+ (C) cells grown in adherent culture for up to 6 days in serum-free media containing 0, 3, 10, 30 or 100 ng/ml activin in the presence or absence of 10 ng/ml BMP4 or 100 ng/ml Wnt3a. The mean % GFP+ cells ± standard deviation of three independent experiments is presented.
In vivo, BMP4 is a ventralising agent, acting during gastrulation to induce T and repress Gsc expression (Fainsod et al., 1994; Jones et al., 1996; Steinbeisser et al., 1995). We found a strong but transient induction of T expression in response to BMP4 (Fig. 1A). Peaking at day 3, we observed 48±15% T-GFP+ cells, which was significantly higher than detected in vehicle-treated cells (p < 0.05). The induction of T expression by BMP4 was observed regardless of the presence or absence of activin. However, the activin-mediated induction of Gsc-GFP+ cells at day 5 was strongly inhibited by BMP4 (p < 0.05), irrespective of the activin concentration (Fig. 1B). Cultures containing BMP4 never contained more than 10±6% Gsc-GFP+ cells, which is comparable to vehicle-treated cells. BMP4 also stimulated Mixl1 expression peaking at day 3 with 26±12% Mixl1-GFP+ cells but further addition of activin did not affect the number of Mixl1-GFP+ cells (Fig. 1C).
In Xenopus, Wnt molecules have both organiser-inducing and posteriorising activities (Niehrs, 2004) and in mice Wnt3 is required for proper axis formation and induction of the primitive streak (Barrow et al., 2007; Liu et al., 1999). We therefore tested the ability of Wnt3a by itself or in combination with different doses of activin to induce PS markers. 5 ng/ml Wnt3a induced 23±4% T-GFP+ cells (data not shown), whereas 100 ng/ml induced 30±9% T-GFP+ cells at day 3, significantly higher than the control cells (Fig. 1A; p < 0.05). Activin did not have a significant effect on Wnt3a–induced T expression although we observed a tendency to reduced numbers of T-GFP+ cells with the highest doses of activin. The prominent induction of T expression seen with both BMP4 and Wnt3a was confirmed by immunofluorescence at day 3 (Fig. S2A). Wnt3a also induced Mixl1 expression. Cultures containing 100 ng/ml Wnt3a contained 11±4% Mixl1-GFP+ cells at day 4. Notably, the combination of 100 ng/ml activin and 100 ng/ml Wnt3a induced 22±3% Mixl1-GFP+ cells at day 4, approximately twice that achieved by either factor alone (Fig. 1C; p < 0.05). When examining co-expression of T and GFP at day 3 by immunofluorescence we found that most, if not all, Mixl1 expressing cells also expressed T, while the converse was not the case (Fig. S2B). While Wnt3a stimulated T and Mixl1 expression, it did not affect the number of Gsc-GFP+ cells (Fig. 1B and Fig. S2). The induction of Gsc expression by activin in the presence or absence of Wnt3a and its inhibition by BMP4 was confirmed by immunofluorescence at day 5 (Fig. S2C). Notably, the few T-expressing cells present in activin-treated GscGfp/+ cells after 5 days did not express GFP. Considering that T is found not only in the PS, but also in the emerging mesoderm at the late gastrula stage (Inman and Downs, 2006), this may indicate that mesoderm is also formed in activin-treated cultures. Collectively, the different ES lines all responded similarly to growth factor treatment (Fig. S2). Overall, our results indicate an anteriorising role of activin during ES cell differentiation that can be modulated by the posteriorising factors BMP4 and Wnt3a, consistent with the roles of these factors before and during gastrulation (reviewed in (Tam and Loebel, 2007)).
BMP4 induces mesoderm and blocks activin-mediated induction of definitive endoderm
To establish if our cultures contained embryonic or extraembryonic cell types we first examined expression of CXCR4 (chemokine (C-X-C motif) receptor-4), which is expressed in embryonic but not in extraembryonic tissues (McGrath et al., 1999; Sherwood et al., 2007). Using FACS analysis we compared surface expression of CXCR4 on cells isolated from dissociated E11 mouse embryo heads with that of differentiated ES cell progeny. Two distinct CXCR4-expression populations could be detected among cells from mouse embryos, a CXCR4lo and a CXCR4hi population (Fig. S3A). When ES cell progeny from either vehicle or activin-treated cultures was analysed it was clear that the vast majority of the cells were CXCR4hi (Fig. S3A), indicating that most cells, in both vehicle and activin-treated cultures, are embryonic rather than extraembryonic in nature. Furthermore, significant levels of Sox7 (SRY-box containing gene 7) transcripts, which is exclusively expressed in the extraembryonic part of the endoderm in the gastrula stage embryo (Kanai-Azuma et al., 2002), could only be detected in Wnt3a–treated cultures but not in vehicle, BMP4-, or activin-treated cultures (Fig. S3B).
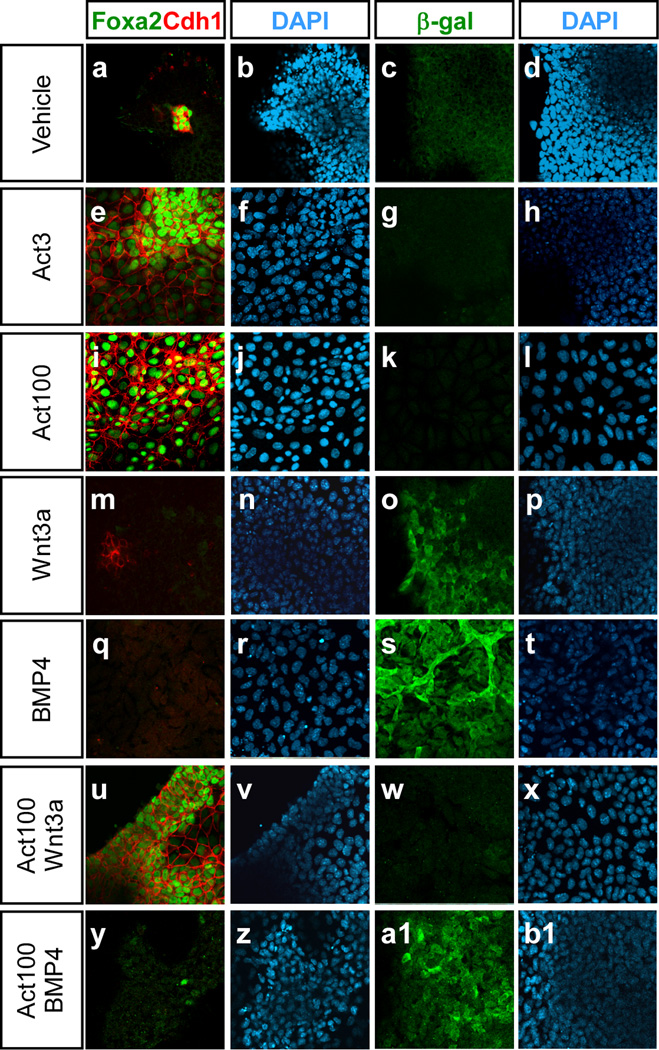
Having established the embryonic nature of the ES cell progeny in our cultures we next examined the expression of a number of germ layer specific markers. We initially analysed expression of the transcription factor gene Foxa2 and the epithelial marker E-cadherin (E-cad; Cdh1), both of which are expressed in developing endoderm (Ang et al., 1993; Sasaki and Hogan, 1993). Immunofluorescent staining of cells grown in 3 or 100 ng/ml activin showed that these cultures contained many Foxa2+Cdh1+ cells compared to vehicle-treated samples (Fig. 2). Notably, addition of BMP4 (10 ng/ml) but not Wnt3a (100 ng/ml) was able to drastically reduce the number of Foxa2+Cdhl+ cells induced by activin (Fig. 2). Analysis of VEGF receptor-2 (Kdr or Flk1) expression using Flk1-LacZ ES cells revealed that both BMP4 (with or without 100 ng/ml activin) and Wnt3a were capable of inducing Flk1-expressing cells (Fig. 2), indicative of mesoderm formation (Ema et al., 2006).
Fig. 2. BMP4 but not Wnt3a inhibits the expression of Foxa2 and E-cadherin, and promotes expression of Flk1 in the presence of activin.
The expression of Foxa2, E-cad (Cdh1), and Flk1 (β-gal) was analysed by immunofluorescence in Flk1LacZ/+ ES cells cultured for 5 days in media containing 0, 3 or 100 ng/ml activin, 100 ng/ml Wnt3a, 10 ng/ml BMP4, 100 ng/ml activin+100 ng/ml Wnt3a, or 100 ng/ml activin+10 ng/ml BMP4.
Wnt signaling augments the development of Sox17-expressing definitive endoderm induced by activin
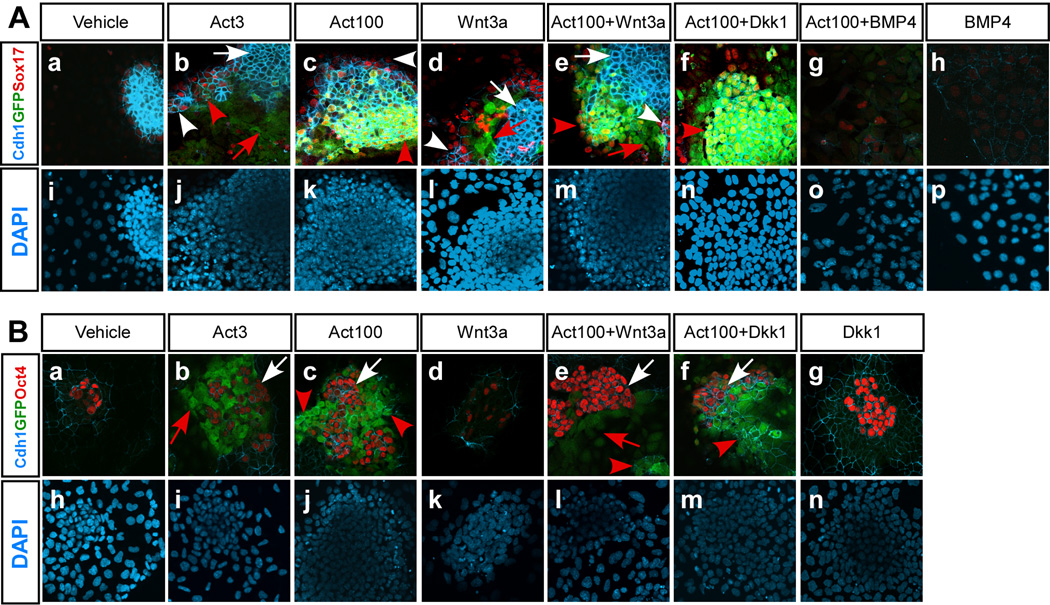
Based on the analysis of Foxa2 and Cdh1 expression it was not clear if the concentration of activin used influenced subsequent differentiation towards DE. Furthermore, analyses of Foxa2 and Cdh1 expression cannot distinguish between DE from different A-P positions. We therefore examined the number of GscGfp/+ cells that co-expressed GFP, Cdh1, and Sox17 by ICC as an indicator of anterior DE (ADE), in response to varying doses of activin with or without additional BMP4, Wnt3a, or Dkk1 treatment. Notably, we found that 100 ng/ml activin resulted in higher numbers of Cdh1+GFP+Sox17+ triple positive cells than seen with 3 ng/ml activin (Fig. 3A, compare panels b and c), supporting that efficient formation of ADE depends on the activin concentration (Yasunaga et al., 2005). Treatment with 3 ng/ml activin resulted in many Cdh1+ cells but the majority of these were not co-expressing GFP or Sox17 and most likely represent undifferentiated ES cells (see below). Most of the GFP+ cells generated in response to 3 ng/ml activin were Cdh1−Sox17−, suggesting that they may represent mesoderm (Fig. 3A, panel b). Similarly, after treatment with Wnt3a alone (100 ng/ml) most GFP+ cells were Cdh1−Sox17− (Fig. 3A, panel d). We tested if Wnt signaling was required for the development of Cdh1+GFP+Sox17+ triple positive cells in response to activin and found that such cells were still generated efficiently in response to 100 ng/ml activin if Dkk1 was included (Fig. 3A, panel f). In contrast, addition of BMP4 completely prevented the development of such cells (Fig. 3A, panel g). We often found clusters of Cdh1+GFP− Sox17− cells in cultures treated with different combinations of activin and Wnt3a (Fig. 3A, panels b – e). Immunocytochemistry revealed that many Cdh1+Gsc− cells were Oct4+ and thus likely represent undifferentiated ES cells (Fig. 3B). Attempts to quantify the relative number of Gsc-GFP+Cdh1+ and Gsc-GFP−Cdh1+ cells by FACS under the various conditions failed due to problems with achieving reliable FACS data using the Chd1 monoclonal antibodies available.
Fig. 3. Activin-induced expression of the anterior primitive streak marker Gsc is inhibited by BMP4 but not by Dkk1.
GscGfp/+ ES cells were grown in serum-free medium supplemented with one or more of the following growth factors or inhibitor; 10 ng/ml BMP4, 100 ng/ml Wnt3a, 320 ng/ml Dkk1, 3 or 100 ng/ml activin as indicated. (A) Triple-label immunofluorescence was performed to analyse the co-expression of E-cad (Cdh1), Gsc (GFP), and Sox17 in cells grown for 5 days under the indicated conditions. Note Cdh1+GFP− Sox17− cells (white arrows), Cdh1−GFP+Sox17− cells (red arrows), Cdh1+GFP− Sox17+ cells (white arrowheads), and Cdh1+GFP+Sox17+ cells (with yellow nuclei, red arrowheads) (B) Triple-label immunofluorescence was also performed to analyse co-expression of Cdh1, Gsc (GFP), and Oct4 on day 5. Note Cdh1+GFP− Oct4+ cells (white arrows), Cdh1− GFP+Oct4− cells (red arrows), and Cdh1+GFP+Oct4− cells (red arrowheads).
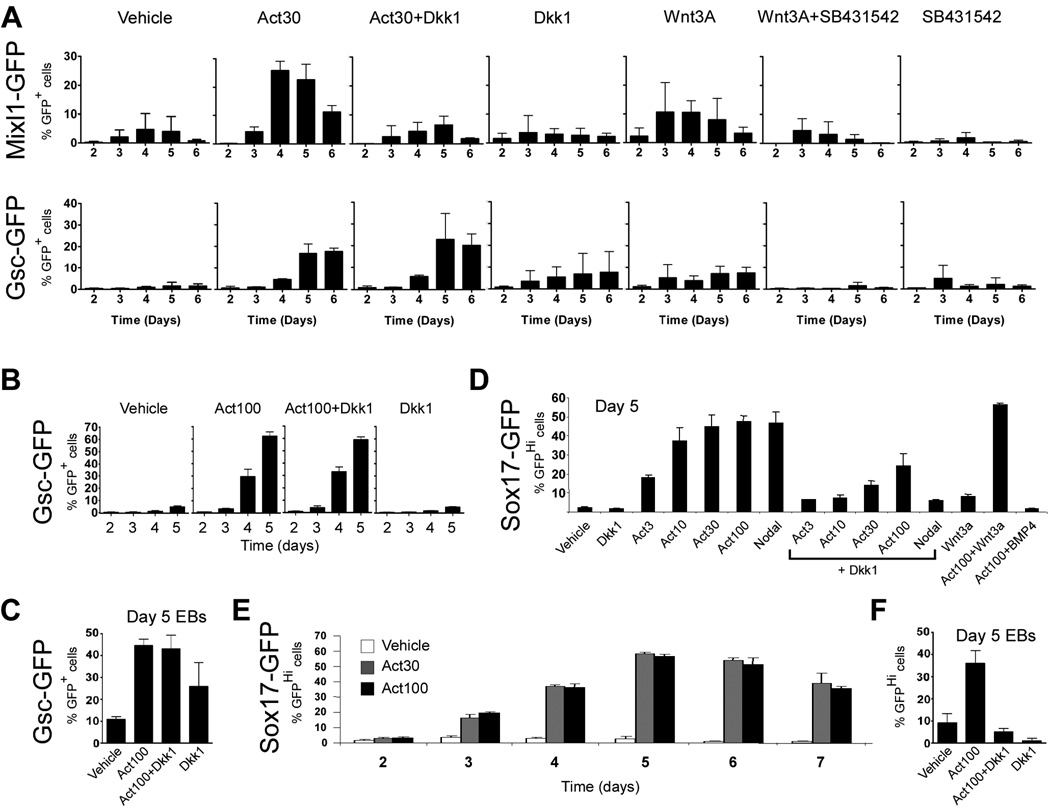
Although it was not evident from the above experiments with the GscGfp/+ cells that canonical Wnt signaling influenced the expression of PS markers or formation of DE in response to activin we wanted to examine closer if Wnt activity was required for expression of other PS markers and DE formation in our ES cell cultures since gastrulation and thereby also endoderm formation requires Wnt3 activity in vivo (Barrow et al., 2007; Liu et al., 1999) and because we detected expression of Wnt3 and Wnt3a in our differentiating ES cell cultures (Fig. S4). To test this notion, we first cultured Mixl1Gfp/+ cells with activin or Wnt3a in the presence or absence of 320 ng/ml Dkk1 or 1 µM of the ALK4/5/7-specific inhibitor SB431542, respectively. The concentration of the inhibitors was titrated by using a Wnt- or activin-responsive luciferase assay (data not shown), choosing the concentration that blocked the response to exogenous Wnt3a or activin, respectively, without causing non-specific toxicity in ES cells. When analyzing the number of Mixl1-GFP+ cells after four days of activin treatment (30 ng/ml) we found that these were significantly reduced when Dkk1 was included (26±3% vs. 4±3%; p < 0.05; Fig. 4A). Similarly, the number of Mixl1-GFP+ cells induced by 100 ng/ml Wnt3a was reduced from 11±4% on day 4 to 3±4% by simultaneous SB431542 treatment (Fig. 4A). Thus, activin and Wnt3a act cooperatively to induce Mixl1 expression and both signaling pathways are required for Mixl1 expression in ES cell progeny. Although Wnt3a only induced low numbers of Gsc expressing cells, these were dependent on endogenous nodal/activin signaling as SB431542 significantly inhibited the development of Gsc-GFP+ cells in response to Wnt3a (Fig. 4A). In contrast, FACS analyses confirmed that activin-induced Gsc expression was not inhibited by Dkk1 treatment. Cultures of GscGfp/+ cells contained 17±4% Gsc-GFP+ cells at day 5 when grown in 30 ng/ml activin and 23±12% when cultured in the presence of activin and Dkk1 (Fig. 4A) consistent with the above mentioned immunofluorescent analysis that demonstrated that Gsc-GFP+Sox17+Cdh1+ triple positive cells were efficiently generated in response to activin treatment, regardless of the presence of Dkk1. Undifferentiated ES cells have a low level of active canonical Wnt signaling despite the lack of exogenously added Wnt factors (Sato et al., 2004). To test if this low level of Wnt signaling has any influence on later activation of Gsc expression we cultured undifferentiated GscGfp/+ cells for three passages in media containing Dkk1 before differentiation was induced by removing LIF and BMP4 and adding activin and Dkk1 for 5 days. We found that inclusion of Dkk1 prior to differentiation did not prevent activin from efficiently inducing Gsc expression (Fig. 4B). Furthermore, Dkk1 failed to prevent activin from inducing Gsc-GFP+ cells in aggregate culture (Fig. 4C).
Fig. 4. The requirement for canonical Wnt signaling during activin-induced Sox17 expression is more pronounced in aggregate culture than in adherent culture, and Dkk1 inhibits nodal-induced Sox17 expression more than activin-induced Sox17 expression.
(A) Nodal/activin and Wnt signaling interactions were analysed in Mixl1Gfp/+ and GscGfp/+ cells at day 2–6 of differentiation using flow cytometry. (B) GscGfp/+ cells cultured in the presence of Dkk1 prior and during activin induction were analysed by flow cytometry. (C) GscGfp/+ cells were induced to form embryoid bodies in the presence of the indicated growth factors and analysed for GFP-expression by flow cytometry after 5 days of culture. (D) Sox17Gfp/+ cells cultured for 5 days were analysed for GFP-expression by flow cytometry. The mean % GFP+ cells ± S.E.M. of three independent experiments is presented. (E) Sox17Gfp/+ cells cultured for 2 – 7 days in presense of activin (30 or 100 ng/ml) were analysed for GFP-expression by flow cytometry at the indicated time points. (F) Sox17Gfp/+ cells were induced to form embryoid bodies in the presence of the indicated growth factors and analysed for GFP-expression by flow cytometry after 5 days of culture. The mean % GFP+ cells ± standard deviation of three independent experiments is presented for all flow cytometric analyses unless otherwise noted.
To obtain quantitative data on the number of DE cells after growth factor treatment we subjected a Sox17Gfp/+ reporter line (Kim et al., 2007) to our differentiation protocol. At mid-streak stage Sox17 expression marks the definitive endoderm forming adjacent to the anterior end of the PS. Simultaneous with the movement of DE to the anterior region of the gastrula, the Sox17 expression domain expands to include the endoderm underlying the neural plate of the early tail-bud-stage embryo (Kanai-Azuma et al., 2002). Thus, Sox17 is an early marker that similar to genes such as Hex, Foxa2, and Cer1, are expressed simultaneously in the anterior visceral endoderm and the DE in the embryonic part of the gastrulating embryo. Sox17Gfp/+ cells express a low level of GFP (Sox17-GFPLo) when kept undifferentiated in the presence of LIF and BMP4 (not shown). Differentiation under neural promoting conditions (Ying et al., 2003a) results in the development of a Sox17-GFP− population and some remaining Sox17-GFPLo cells while treatment with activin for five days induces Sox17-GFPHi and Sox17-GFP− populations in addition to a remaining Sox17Lo population (Fig. S1). Increasing concentrations of activin resulted in development of progressively more Sox17-GFPHi cells with the highest numbers reached with 30 and 100 ng/ml of activin (Fig. 4D). We observed an increase in Sox17-GFPHi cells over time, peaking at day 5, followed by a modest decrease at days 6 and 7 (Fig. 4E). The induction of Sox17-GFPHi cells was at least partly dependent on Wnt signaling as treatment with Dkk1 reduced the number of GFPHi cells by ∼50% at the highest activin concentration (Fig. 4D). Notably, the number of Sox17-GFPHi cells induced by 1 µg/ml nodal appeared more strongly reduced in response to Dkk1 treatment than did a similar number of Sox17-GFPHi cells induced by 30 and 100 ng/ml of activin (Fig. 4D). Furthermore, when differentiation was performed in aggregate culture, which may rely more on endogenous signaling (Sachlos and Auguste, 2008; ten Berge et al., 2008), the number of activin-induced Sox17-GFPHi cells were strongly reduced by Dkk1 treatment (Fig. 4F). As expected, addition of BMP4 prevented activin-induced formation of Sox17-GFPHi cells (p < 0.001), while addition of Wnt3a resulted in a marginal, but significant (p < 0.05) increase in the development of Sox17-GFPHi cells (Fig. 4D).
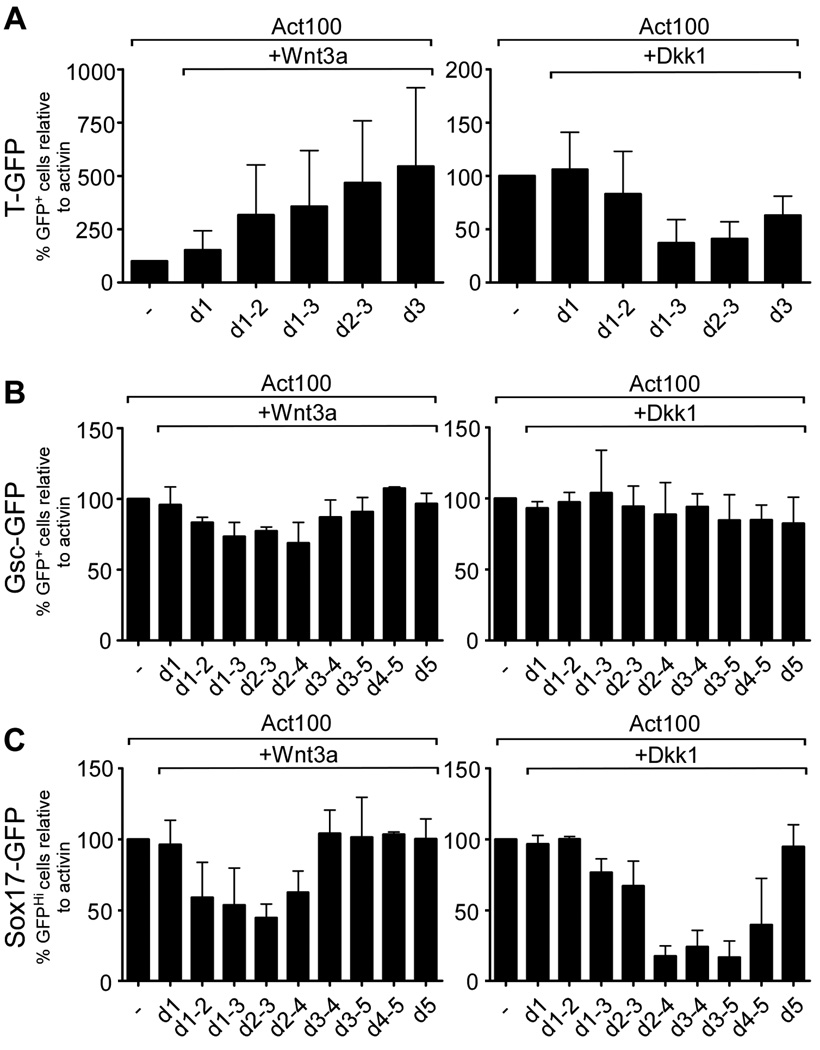
To further define the time at which canonical Wnt signaling was required for the formation of T-, Gsc- and Sox17-GFPHi cells, we cultured the cells with activin for three (TGfp/+) or five (GscGfp/+ and Sox17Gfp/+) days and added either Wnt3a or Dkk1 for shorter periods during the five day activin treatment. To monitor the effectiveness of Dkk1 treatment in reducing canonical Wnt-signaling we first generated an ES cell line stably transfected with a SuperTOP-Cerulean reporter (SuTOP-CFP) and established its ability to report Wnt-signaling in E10.5 chimeric embryos after injection into E3.5 blastocysts and implantation into pseudo-pregnant females. As shown in Fig. S5A, native Cerulean fluorescence can be observed in several sites known to harbour active Wnt signaling at this stage, including the peripheral aspects of the otic vesicles (Maretto et al., 2003). When SuTOP-CFP cells were cultured in activin we found that CFP+ colonies developed at day 3 even in the absence of exogenous Wnt addition and that additional treatment with Dkk1 reduced or abolished reporter activity depending on the duration of treatment (Fig. S5B). Similarly, when SuTOP-CFP cells were assayed at day 5, we found that Dkk1 treatment at day 3–4 or 4–5 inhibited reporter activity (Fig. S5C). Thus, treatment with Dkk1 for as little as 1 to 2 days at the concentration used appeared quite effective in suppressing SuTOP reporter activity.
We then analyzed the number of T-GFP+ cells developing in response to activin when Wnt signaling was experimentally perturbed. T-GFP+ cell numbers were augmented by Wnt3a with the largest effect reached when Wnt3a was added only at day 3. Treatment with Dkk1 on the other hand reduced the number of T-GFP+ cells most effectively when included in that last part of the three day period (Fig. 5A). Neither stimulating nor inhibiting Wnt-signaling in GscGfp/+ cells, by treatment with Wnt3a and Dkk1, respectively, resulted in a significant change in the number of activin-induced GFP+ cells irrespective of the treatment period (Fig. 5B).
Fig. 5. Wnt signaling is required during late stages of activin-induced definitive endoderm formation.
ES cells were cultured in serum free medium with 100 ng/ml activin and supplemented with 100 ng/ml Wnt3a or 320 ng/ml Dkk1 for a variable number of days. TGfp/+ cells (A) were cultured for 3 days and GscGfp/+ (B) and Sox17Gfp/+ (C) cells were cultured for 5 days before being analyzed for GFP-expression by flow cytometry. The mean % GFP+ cells ± standard deviation of three independent experiments is presented.
However, treatment of Sox17Gfp/+ cells with Wnt3a prior to appearance of GFP+ cells reduced the number of Sox17-GFPHi cells, while later Wnt3a treatment had no effect. Conversely, treatment with Dkk1 reduced the number of Sox17-GFPHi cells only if present after the first appearance of these (Fig. 5C).
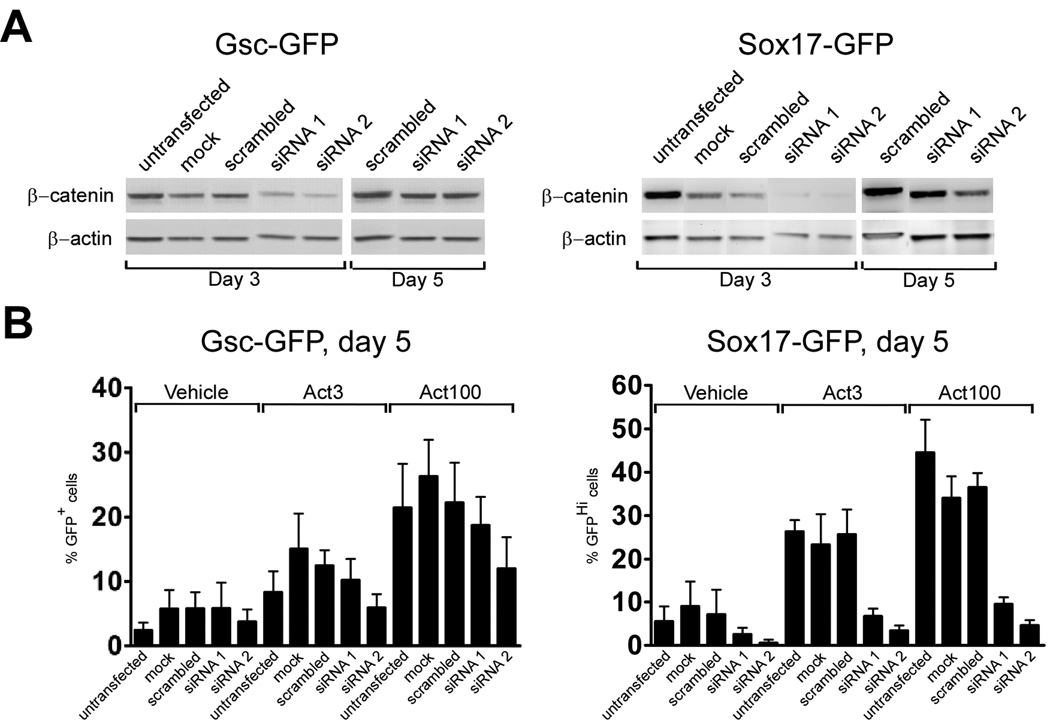
We next used siRNA mediated knock-down of β-catenin (encoded by Ctnnb1) to confirm that inhibition of canonical Wnt signaling at the latter part of the five day activin stimulation period could suppress the appearance of Sox17-GFPHi cells and to further test the GscGfp/+ cell line which appeared refractory to Wnt-inhibition in previous experiments. We transfected GscGfp/+ and Sox17Gfp/+ cells with control and two different Ctnnb1 siRNAs at day 2 of differentiation and assayed β-catenin expression by western blotting at days 3 and 5. In GscGfp/+ cells we found a reduction of β-catenin expression at day 3 which was normalized at day 5. siRNA treatment appeared more effective in Sox17Gfp/+ cells with strong inhibition of β-catenin expression at day 3 which was only partly recovered by day 5 (Fig. 6A). FACS analysis at day 5 showed a reduction in the number of Gsc-GFP+ cells after β-catenin knock-down, although this only reached significance in Ctnnb1 siRNA2 treated samples (p < 0.05, Fig. 6B). Furthermore, Q-RT-PCR analysis of GscGfp/+ cells after β-catenin knockdown did not reveal significant changes in expression of Lhx1 and Chrd (Fig. S6).
Fig. 6. Inhibition of canonical Wnt signaling inhibits activin-induced Sox17-GFPHi definitive endoderm formation, but has little effect on Gsc expression.
(A) Western blot analysis of β-catenin expression after siRNA mediated knock-down. Two different siRNAs targeting β-catenin (siRNA1 and siRNA2) or a scrambled control siRNA were introduced to GscGfp/+ and Sox17Gfp/+ cell lines on day 2 and β-catenin levels assayed at day 3 and 5. An anti-β-actin western blot served as loading control. (B) siRNA treated GscGfp/+ and Sox17Gfp/+cells were analysed for GFP-expression by flow cytometry after 5 days of serum free culture supplemented with 0, 3 or 100 ng/ml activin. The mean % GFP+ cells ± standard deviation of three independent experiments is presented. Untransfected and mock transfected controls are also shown.
However, in agreement with the results obtained with Dkk1 treatment, we found a prominent reduction in the number of Sox17-GFPHi cells at day 5, in both siRNA1 and siRNA2 treated samples (p < 0.05, Fig. 6B).
Activin dose-dependently induces an anterior gene expression pattern
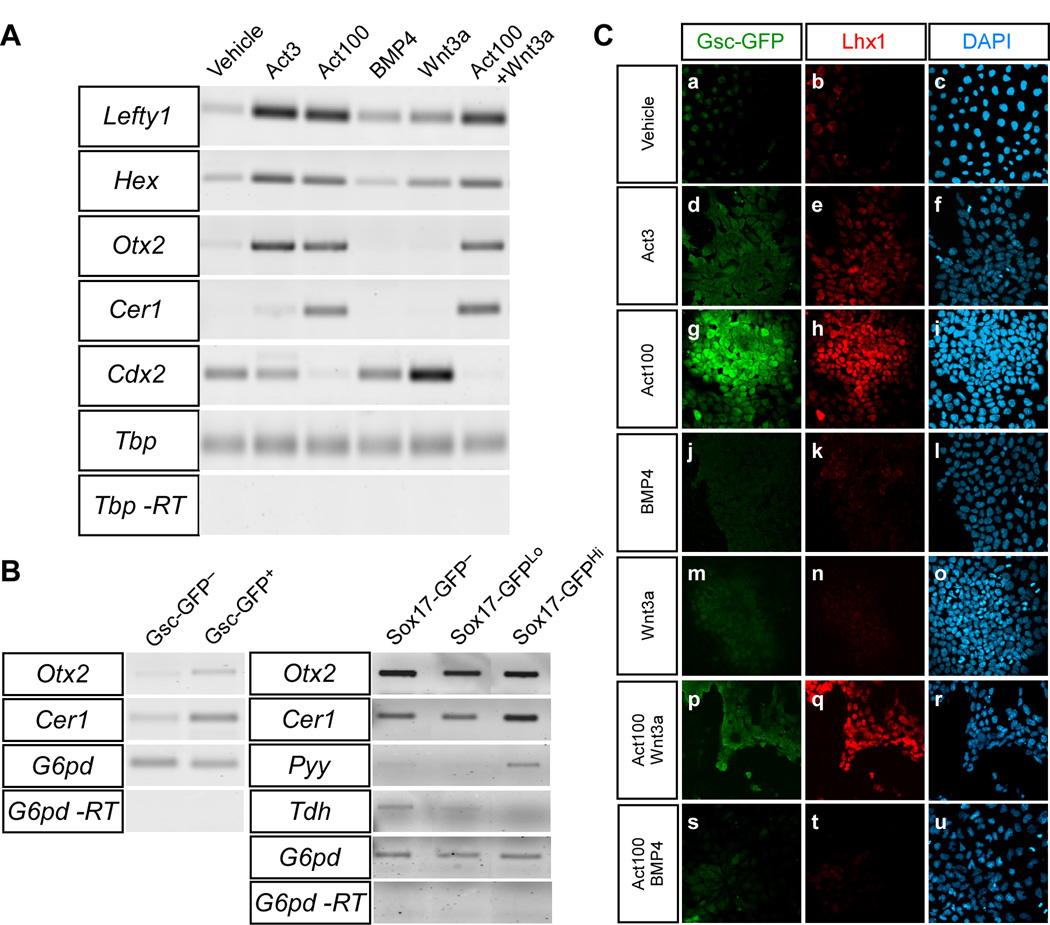
To determine whether the DE formed in the presence of high concentrations of activin was anterior or posterior in character we analysed the expression of a number of markers displaying differential expression depending on the anterior-posterior position of the cells. RT-PCR analyses at day 5 showed that activin, regardless of concentration, could induce expression of genes associated with anterior cell fates, including Lefty1, Hex, (Martinez Barbera et al., 2000) and Otx2, (Rhinn et al., 1998). However, robust expression of Cer1 as well as repression of the posterior marker Cdx2 was only seen in the presence of the high activin concentration (Fig. 7A). In contrast, Cdx2 (Beck et al., 1995) was expressed in samples treated with 10 ng/ml BMP4 or 100 ng/ml Wnt3a, as well as cells treated with the low dose of activin (Fig. 7A). We next isolated Gsc-GFP+ and Gsc-GFP− cells as well as Sox17-GFPHi, Sox17-GFPLo and Sox17-GFP− cells from activin stimulated cultures at day 5 by FACS and prepared RNA for gene expression analysis. Message for Otx2 and Cer1 was detected in both Gsc-GFP+ and Gsc-GFP− fractions, but with an enrichment in the GFP+ fraction (Fig. 7B). Cerl1 was also enriched in Sox17-GFPHi cells compared to Sox17-GFP− and Sox17-GFPLo cells (Fig. 7B). Furthermore, when analyzing the expression of the respective DE and VE markers, Pyy and Tdh (Hou et al., 2007; Sherwood et al., 2007) we only found Pyy in the Sox17-GFPHi cells while Tdh was found in the Sox17-GFP− and, to a lesser degree, Sox17-GFPLo populations (Fig. 7B). However, it should be noted that FACS sorted populations are probably not completely pure. For example, the Sox17Hi and Sox17Lo populations are likely displaying “cross-contamination” to some extent. Finally, as Lhx1 is expressed in the anterior part of the PS in the late-streak embryo and expected to co-localise with Gsc (Shawlot and Behringer, 1995; Tada et al., 2005) we performed GFP-Lhx1 double immunofluorescent stainings on GscGfp/+ cells after 5 days of differentiation. As expected, we found Gsc-GFP+Lhx1+ double positive cells in activin-treated cultures and the formation of these cells was inhibited by the addition of BMP4 but not Wnt3a (Fig. 7C).
Fig. 7. Activin, BMP and Wnt signaling influence anterior-posterior patterning during differentiation of ES cells.
ES cells were grown for 5 days in serum-free medium supplemented with indicated growth factors. (A) The expression of Lefty1, Hex, Otx2, Cer1 and Cdx2 was analysed in TGfp/+ cells by RT-PCR. The expression of TATA binding protein (Tbp) was used as internal standard. (B) ES cells were cultured with 100 ng/ml activin and FACS sorted based on native GFP fluorescence after 5 days of culture. The expression of Otx2 and Cer1 was analysed in the GFP+ and GFP− fraction of GscGfp/+ cells, and Otx2, Cer1, Pyy and Tdh expression was analysed in the GFPHi, GFPLo and GFP− fraction in Sox17 Gfp/+cells. Glucose-6-phosphate dehydrogenase (G6pd) was used as internal standard. (C) The expression of Lhx1 and GFP was examined in GscGfp/+ cells by immunofluorescence after 5 days in the presence of the indicated factors.
FGF receptor signaling is required for mesendoderm and endoderm differentiation
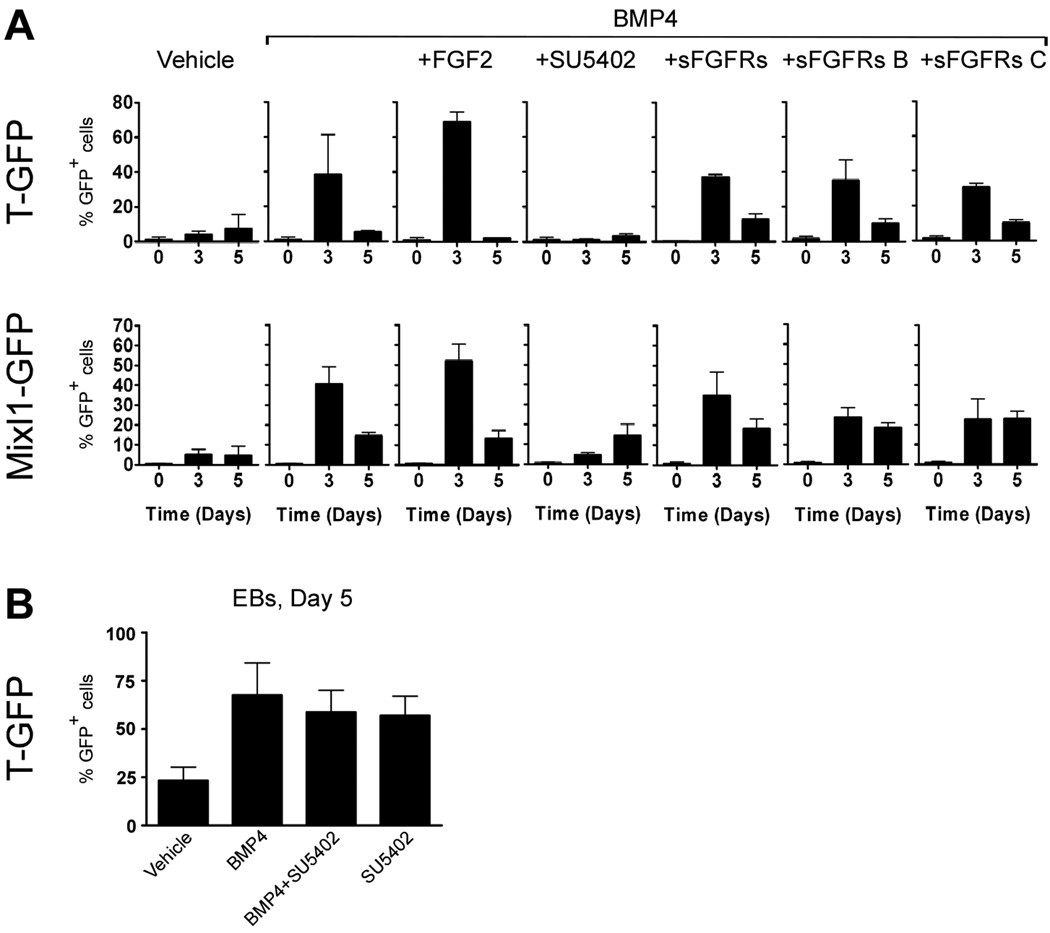
If FGF signaling is compromised during early development as in FGF8, FGFR1, or UDP-glucose dehydrogenase mutants, the embryos arrest at gastrulation and no embryonic mesoderm- or endoderm-derived tissues develop (Ciruna et al., 1997; Deng et al., 1994; Garcia-Garcia and Anderson, 2003; Sun et al., 1999; Yamaguchi et al., 1994). The phenotype is associated with a failure of migration and it is unclear to what degree FGF signaling regulates allocation of mesodermal and endodermal fates. However, studies in zebrafish indicate that FGF signaling promotes mesodermal development at the expense of endodermal development (Mathieu et al., 2004; Mizoguchi et al., 2006; Poulain et al., 2006). We first examined the expression of members of the FGF family in differentiating ES cell cultures treated with activin and BMP4 (alone or in combination). We found that FGF4 and FGF8 were expressed in ES cell cultures treated with activin or BMP4 (Fig. S4). We therefore addressed the role of FGF signaling by culturing the cells in activin (100 ng/ml) or BMP4 (10 ng/ml) with or without the FGF receptor inhibitor SU5402 (Mohammadi et al., 1997) or in the absence or presence of exogenously added FGF2. Consistent with a recent report (Kunath et al., 2007), flow cytometric analyses of undifferentiated cells (day 0) and at day 3 and 5 of differentiation revealed that both BMP4-induced T and Mixl1 expression were strongly inhibited by 10 µM SU5402 (Fig. 8A), a concentration of SU5402 that did not compromise cell viability (data not shown). As BMP4-induced T expression has been shown to be insensitive to SU5402 in EB culture (Willems and Leyns, 2008), we repeated this experiment to determine if choice of culture system could explain this difference. Indeed we also find that BMP4-induced T-expression is unaffected by addition of SU5402, and even by itself SU5402 is able to induce T-expression (Fig. 8B). Nevertheless, addition of soluble FGF receptors (sFGFRs, b and c splice forms) failed to inhibit BMP4-induced T or Mixl1 expression at day 3 of adherent culture (Fig. 8A). Conversely, addition of FGF2 augmented the number of T-GFP+ and Mixl1-GFP+ cells formed in response to BMP4 treatment (from 39±23% to 68±6% and from 41±8% to 53±8%, respectively). However, this induction was only significant for Mixl1-GFP (p <0.01).
Fig. 8. FGF receptor signaling is required for BMP4-induced mesendoderm formation in adherent, but not in aggregate culture.
(A) TGfp/+ and Mixl1Gfp/+ cells were cultured in medium containing 10 ng/ml BMP4, with or without 100 ng/ml FGF2, 10 µM SU5402, or different concentrations of soluble forms of FGF receptors (b or c splice forms, or a combination of both). Cells were analysed for GFP-expression by flow cytometry on day 0, 3 and 5. (B) TGfp/+ were induced to form embryoid bodies with the indicated growth factors and analysed for GFP-expression by flow cytometry on day 3. The mean % GFP+ cells ± standard deviation of three independent experiments is presented.
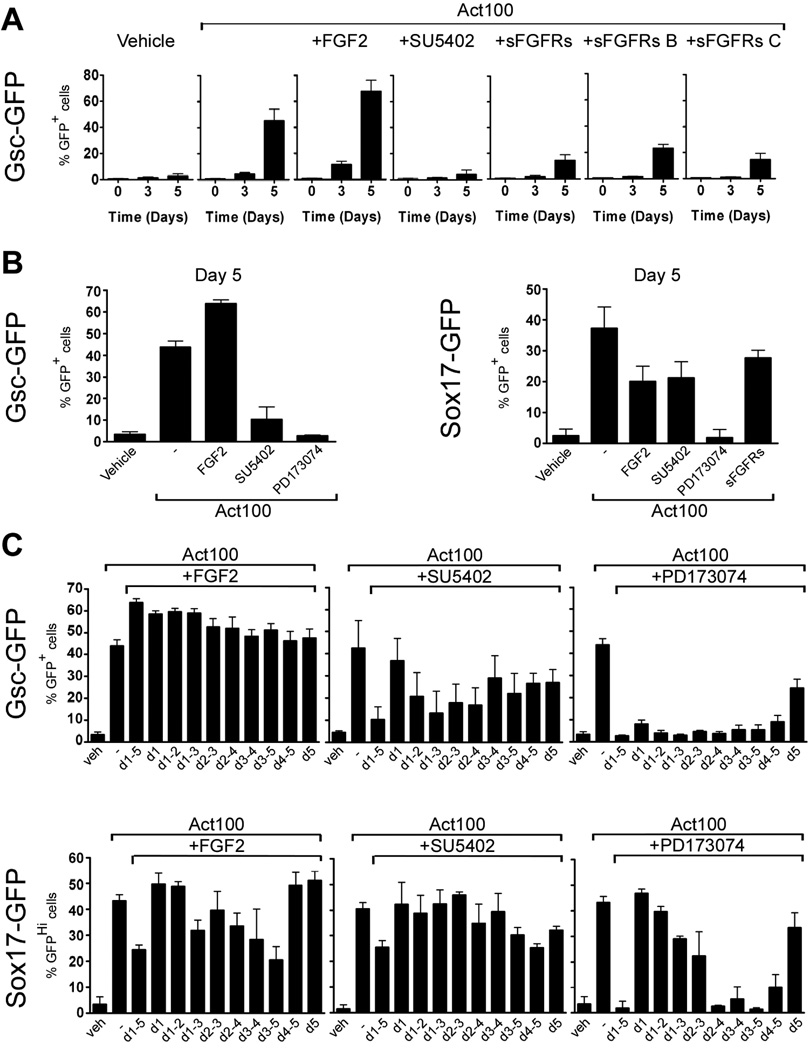
From studies in zebrafish we would expect that FGF signaling would inhibit endoderm development and redirect cells towards mesoderm (Mizoguchi et al., 2006; Poulain et al., 2006). When GscGfp/+ cells were cultured in the presence of activin with addition of FGF2, SU5402, PD173074, or soluble FGF receptors we observed a strong dependence on FGF signaling for development of Gsc-GFP+ cells (Fig. 9A & B). The number of Gsc-GFP+ cells appearing at day 5 in the presence of activin was significantly inhibited by addition of SU5402 (p < 0.05), PD173074 (p < 0.001) or sFGFRs (p < 0.05). Furthermore, both the b and c splice forms of soluble FGF receptors reduced the number of Gsc-GFP+ cells (p < 0.05 using Student’s one-tailed t-test). Moreover, addition of FGF2 significantly enhanced the amount of Gsc-GFP+ cells seen at day 5 in activin-treated cultures (p < 0.01). These results demonstrate that activin-induced Gsc expression depends on FGF signaling and suggest that anterior PS fate is augmented by FGF signaling. However, as Gsc-expressing cells develop further into both meso- and endoderm we next asked whether development of definitive endoderm is also FGF dependent. Hence we examined if activin-induced formation of Sox17-GFPHi cells was influenced by FGF signaling. Culture of Sox17Gfp/+ cells in activin together with SU5402 or PD173074 resulted in a ∼50% and ∼90% reduction in the number of Sox17-GFPHi cells compared to activin alone (p < 0.001; Fig. 9B), demonstrating that FGFR signaling is required for normal formation of definitive endoderm.
Fig. 9. FGF receptor signaling is required throughout the five day period for activin-induced Gsc-expression but only required late for activin-induction of Sox1 7-expressing definitive endoderm.
GscGfp/+ and Sox17Gfp/+ cells were cultured in medium containing 100 ng/ml activin, in combination with 100 ng/ml FGF2, 10 uM SU5402, 100 nM PD173074, or soluble forms of FGF receptors as inficated. (A) Time course analysis of appearance of Gsc-GFP+ cells by flow cytometry at time 0 and after 3 and 5 days in culture. (B) GscGfp/+ and Sox17Gfp/+ cells were anayzed for GFP-expression at day 5 after culture with the indicated factors. (C) GscGfp/+ and Sox17Gfp/+ cells were anayzed for GFP-expression at day 5 after culture with activin and either FGF2, SU5402, or PD173074 for shorted periods as indicated. The mean % GFP+ cells ± standard deviation of three independent experiments is presented.
However, in contrast to results obtained with the Gsc reporter, addition of sFGFR did not affect Sox17 expression. Moreover, where addition of FGF2 boosted the formation of Gsc-GFP+ cells it resulted in an almost 50% reduction in the number of Sox17-GFPHi cells (p < 0.005; Fig. 9B), suggesting that precise control of FGF levels is important for regulation of Sox17 promoter activity.
To better define the time where FGF signaling acted during induction of Gsc- and Sox17 expression we cultured Gsc- and Sox17-GFP cells with activin for five days and added either FGF2, SU5402, or PD173074 for shorter periods. The (modest) stimulatory effect of FGF2 upon Gsc-GFP expression required that FGF2 was present early in the 5 day culture period, prior to the initial appearance of GFP+ cells (Fig. 9C). In contrast, SU5402 and PD173074 reduced the number of Gsc-GFP+ cells both when added early and late in the culture period (Fig. 9C). In contrast, the number of Sox17-GFPHi cells was reduced when FGF2 and FGFR inhibitors were present after the initial appearance of GFPHi cells (Fig. 9C).
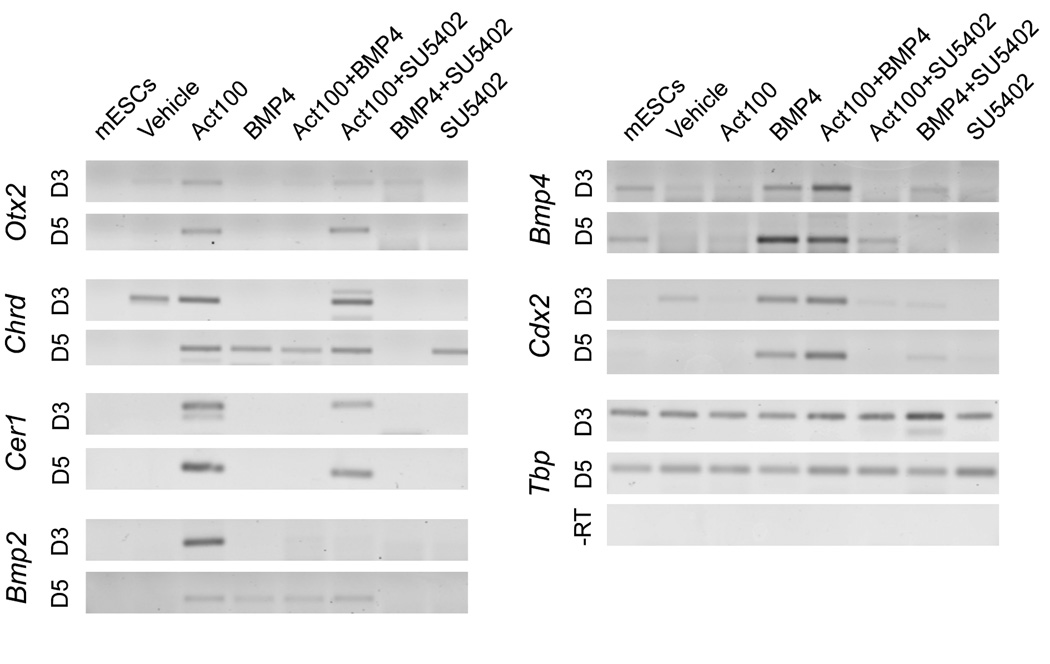
We used RT-PCR to analyse whether expression of additional markers, informative in relation to anterior-posterior positional identity, were affected by blocking FGF receptor signaling in activin-treated cells. Expression of Otx2, Chrd, and Cer1, genes that are repressed by BMP4, is not affected by addition of SU5402 after 3 days (Fig. 10). Among the markers analysed only Bmp2, which is first expressed in the embryo proper at E7.5 just lateral to the anterior neural folds and in precardiac mesoderm and slightly later in foregut endoderm (Winnier et al., 1995), is repressed both by BMP4 and SU5402 at day 3. Chrd expression appears at day 3 in vehicle-treated cultures and at day 5 in BMP4-treated cultures but in both cases it is sensitive to SU5402. Also BMP4-induced expression of mesoderm markers Chrd, and Nog at day 5, as well as the posterior markers Bmp4 and Cdx2, is sensitive to SU5402 (Fig. 10 and Fig. S4).
Fig. 10. TGF-β, Wnt and FGF signaling control the dynamic gene expression in differentiating ES cells.
TGfp/+cells cultured for 3 or 5 days in serum-free medium supplemented with 100 ng/ml activin, 10 ng/ml BMP4 and/or 10 µM SU5402, as indicated, were analysed by RT-PCR. The expression of Otx2, Chrd, Cer1, Bmp2, Bmp4 and Cdx2 was analysed. Tbp was used as internal standard.
Foregut and pancreatic competence of ES cell-derived endoderm
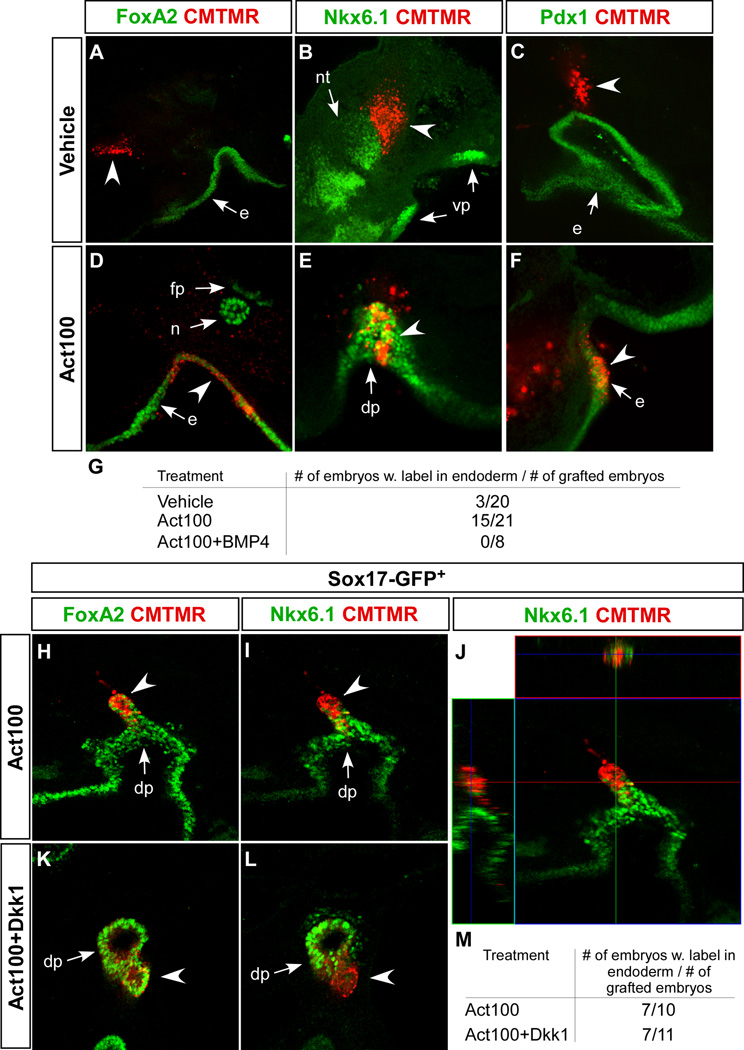
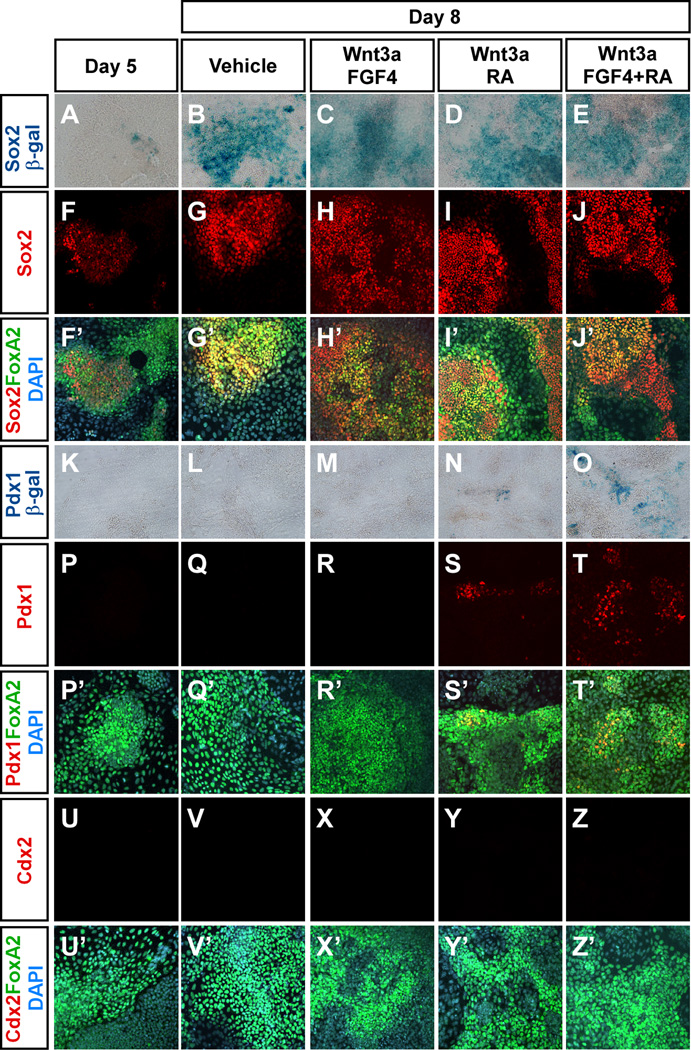
To test if the DE-like cells had potential to functionally integrate into developing embryonic endoderm, we implanted approximately 50 cells labelled with a fluorescent dye into 6 to 10 somite stage chicken embryos at the level of the prospective pancreatic endoderm and incubated for 48 hours. When ES cell progeny from activin-treated cultures were grafted, 15 out of 21 transplanted embryos contained dye-labelled, Foxa2+ cells incorporated into the endoderm. In contrast only 3 out of 20 embryos receiving cells cultured without activin and 0 of the 8 embryos receiving activin- and BMP4-treated cells contained dye-labelled cell in the endoderm (Fig. 11A, D, G). Frequently, the activin-treated cells incorporated in the Nkx6-1+Pdx1+ pancreatic endoderm (Fig. 11E, F) (Pedersen et al., 2005). In a second series of grafting experiments we tested if the Sox17-GFPHi cells obtained after activin treatment for five days with or without additional Dkk1 treatment were capable of incorporating into chick foregut endoderm. Such cells were equally capable of contributing the foregut endoderm (Fig. 11H, I, K – M), although the number of Sox17-GFPHi cells were reduced in cultures containing Dkk1. Orthogonal projections of the confocal stacks obtained from grafted embryos suggested expression of Nkx6-1 in some of the grafted cells (Fig. 11J). Considering the anterior markers expressed by activin-treated cells, and the absence of pancreatic or other regional foregut markers at day 5 (Fig. 12), the grafting experiments suggest that DE formed in our cultures can respond to posteriorising cues from the embryonic environment and progress further in differentiation. We therefore tested if the cells were able to respond to suspected posteriorising cues in vitro. After 5 days of culture in activin, the cells were shifted to conditions where activin was replaced by candidate posteriorising factors Wnt3a, FGF4, and retinoic acid (RA) (Grapin-Botton and Constam, 2007) and analysed for expression of Sox2, Pdx1, and Cdx2, markers of fore-, mid-, and hindgut, respectively (Grapin-Botton and Melton, 2000). Remarkably, we found large numbers of Sox2+Foxa2+ cells in cultures treated with posteriorising factors (Fig. 8). We confirmed Sox2 expression using the OS25 cell line which carries a β-geo reporter gene in the Sox2 locus (Li et al., 1998). β-galactosidase activity was visualised with X-gal staining (Fig. 12). Appearance of Sox2+ cells was depending on neither FGF4 nor RA. Scattered Pdx1+ cells also appeared but in contrast to Sox2+ cells these only appeared in the presence of RA. Cdx2 positive cells were not detected under any of the conditions tested. Together, our data demonstrate that DE formed from mES cells in adherent monoculture is capable of differentiation toward foregut endoderm in vivo and in vitro but that only a limited number of these cells appear to respond to posteriorising cues in vitro.
Fig. 11. mES cell-derived endoderm grafts contribute to the developing chicken endoderm (A–F).
ES cells were cultured for 5 days in serum-free medium containing vehicle, 100 ng/ml activin or 100 ng/ml activin and 10 ng/ml BMP4 as indicated. Small clusters of cells were labelled with the red fluorescent cell tracker dye CMTMR and grafted between the endoderm and mesoderm of explanted chicken embryos and allowed to develop in ovo for two days before being processed for confocal immunohistochemistry. (H–M) Sox17Gfp/+ cells were cultured for 5 days in serum-free medium containing either 100 ng/ml activin or 100 ng/ml activin plus 320 ng/ml Dkk1. Small clumps of GFP+ cells were labelled with the red fluorescent cell tracker dye CMTMR and grafted between the endoderm and mesoderm of explanted chicken embryos and allowed to develop in ovo for two days before being processed for confocal immunohistochemistry. (A–F, H, I, K, L) Optical sections of chicken embryos whole-mount stained with the indicated antibodies and transplanted with ES cell progeny developing after 5 days with the indicated growth factors. (J) Orthogonal view of a Z-stack revealing an Nkx6-1-expressing CMTMR-labelled cell. (G, M) Tabulated results of the grafting experiments. Arrowheads in A–F and H–L indicate implanted cells. dp: dorsal pancreas, e: endoderm, fp: floor plate, n: notochord, nt: neural tube, vp: ventral pancreas.
Fig. 12. ES cell-derived endoderm acquire foregut cell fates.
(A–J’ and P–Z’) OS25 (Sox2βgeo/+) or (K–O) Pdx1LacZ/+ cells were cultured for 5 days in 100 ng/ml activin and subsequently treated with 5 ng/ml Wnt3a, 10 ng/ml FGF4 and/or 0,1 µM RA for 3 days as indicated. The co-expression of Foxa2 and Sox2 (foregut), Pdx1 (midgut) or Cdx2 (hindgut) was analysed by immunofluorescence. The expression of Sox2 (A–E) and Pdx1 (K–O) was confirmed by analysing β-galactosidase activity using X-gal staining.
Discussion
Recent work from Smith and colleagues have demonstrated that mES cells can be kept pluripotent under defined serum- and feeder-free conditions (Ying et al., 2003a), and be efficiently converted to Sox1+ neuroectodermal progenitors when kept in adherent monoculture in the absence of LIF and BMP4 (Ying et al., 2003a; Ying et al., 2003b). In the present work we extend this defined system to demonstrate that mES cells kept in serum- and feeder-free adherent monoculture respond dose-dependently to inducers of primitive streak formation by developing mesendoderm with capacity to differentiate further into cells resembling foregut endoderm, in vivo and in vitro. Although several recent studies have established that the TGF- β family members BMP4 and activin induce mesodermal and endodermal gene expression in differentiating mES cells (Gadue et al., 2006; Kubo et al., 2004; Mossman et al., 2005; Ng et al., 2005; Tada et al., 2005; Yasunaga et al., 2005), the varied conditions under which differentiation was induced; e.g. adherent vs. suspension culture, differences in basal cell culture media and supplements, cell density, absence or presence of serum; as well as the different reporter lines utilised make a direct comparison of these studies difficult. Here we use a series of GFP-based reporters to study the dynamic expression of T, Mixl1, Gsc, and Sox17 after manipulating one or more of the FGF, TGF-β, and Wnt signaling pathways. Remarkably, we find that the lowest concentration of activin used (3 ng/ml) induced the highest number of T-GFP+ cells, whereas higher concentrations (10–100 ng/ml) resulted in progressively fewer T-GFP+ cells. These data apparently conflict with results obtained by Keller and co-workers who observed increasing numbers of T-GFP+ cells with increasing activin concentration until a plateau of about 50–60% T-GFP+ cells was reached at 30 ng/ml of activin (Kubo et al., 2004). It is not entirely clear why a direct correlation between activin concentration and the number of T-GFP+ cells is observed by Kubo et al. while we observe an inverse correlation. One possible explanation relates to the embryoid body formation used by Kubo and colleagues in which only cells located at the periphery, directly exposed to the cell culture medium, may experience the full concentration of added growth factor as recently demonstrated (Sachlos and Auguste, 2008). Cells located in the interior of the embryoid body are most likely experiencing a lower concentration and the overall dose-response curve may therefore appear shifted towards higher concentrations. It is also possible that the environment of the embryoid body is more conducive to secondary signaling events that may influence the number of T-GFP+ cells. A recent study did however report that T mRNA levels in differentiating EBs were inversely correlated with activin concentration within the 5 – 50 ng/ml range (Willems and Leyns, 2008). Moreover, our results are strikingly similar to data from Xenopus animal cap explants, where activin dose-dependently controls cell fate specification. The expression of the Xenopus T homologue Xbra is induced by low concentrations of activin while higher concentrations induce the expression of the Gsc homologue Xgsc (Green et al., 1992; Gurdon et al., 1994; Latinkic et al., 1997). However, even Xgsc-expressing cells induced by high doses of activin have undergone a transient Xbra-expressing phase, but direct repression of Xbra transcription by binding of Xgsc to the Xbra promoter limits the duration of Xbra expression (Latinkic et al., 1997). In this regard, analyses of the duration of T expression by time-lapse microscopy under different conditions would be highly informative. The notion of activin as a dose-dependent inducer of anterior fate in ES cell progeny is indicated by several observations: the differential effect of 3 and 100 ng/ml activin on gene expression such that only the high dose induces the anterior marker Cer1 and represses the posterior marker Cdx2. Also, extensive co-localisation of E-cadherin, Gsc-GFP and Sox17 was only observed after treatment with 100 ng/ml of activin. Additional marker analyses revealed that such cells were also Pyy+, Foxa2+ and CXCR4+ but Sox7− and Tdh−, suggesting embryonic rather than extraembryonic endoderm had formed (Kanai-Azuma et al., 2002; Tada et al., 2005; Yasunaga et al., 2005). Conversely, BMP4- and Wnt3a–treated cells expressed low levels of anterior markers and instead expressed the posterior markers Bmp4 and Cdx2. Furthermore, BMP4 prevented activin-induced gene expression and stimulated expression of T and Mixl1. These observations are consistent with previous in vivo data showing that BMP4 is necessary for T expression (Winnier et al., 1995) and that activin-induced Gsc expression can be counteracted by BMP4 in vivo (Imai et al., 2001; Jones et al., 1996; Sander et al., 2007; Shapira et al., 1999; Steinbeisser et al., 1995). Additionally, Keller and co-workers also noted a posteriorizing effect of BMP4 upon mouse ES cells derived, activin-induced PS populations (Nostro et al., 2008) and Suemori and co-workers found that inhibition of BMP signaling redirected human ES cell derived mesodermal cells (induced by forced expression of stabilized β-catenin) towards an anterior PS/DE lineage, in a process dependent on activin/nodal signaling (Sumi et al., 2008).
Further analysis of BMP4- (and Wnt3a-) treated cells revealed the presence of Flk1+ cells demonstrating that mesodermal differentiation had occurred. Interestingly, Smith and colleagues recently reported that BMP4 treatment of mES cells, under conditions nearly identical to ours, resulted in cells of unknown identity that were unlikely to be mesodermal based on presence of E-cad immunoreactivity (Kunath et al., 2007). While we occasionally detect a faint plasma membrane staining in BMP4-treated cells (see for example Fig. 3), the intensity of the staining is strongly reduced compared to the signal seen in activin-treated cells, and most cells appear E-cad− in our experiments. Furthermore, the presence of Flk1 expressing cells would appear to conclusively demonstrate mesodermal differentiation. This is also consistent with a number of studies where embryoid bodies are stimulated with BMP4 (Czyz and Wobus, 2001; Finley et al., 1999; Johansson and Wiles, 1995; Lengerke et al., 2008; Ng et al., 2005; Willems and Leyns, 2008).
Our examination of the role of Wnt signaling during mesendoderm differentiation seems to indicate a major difference between differentiation in embryoid bodies and in adherent monoculture. Apparently, the extent to which Wnt signaling is required to induce anterior PS formation is different in the two systems. Gadue et al. found that 100 ng/ml Wnt could induce formation of Foxa2-CD4+T-GFP+ anterior PS-like cells, and that this was dependent on endogenous ALK4/5/7 signaling. Moreover, the ability of activin to induce the same fate was dependent on Wnt signaling since Dkk1 could prevent the effect of activin (Gadue et al., 2006). At first glance these findings may appear to contrast with our results, namely that the same concentration of Wnt3a could not induce significant numbers of Gsc-GFP+ in adherent culture and that Dkk1 could not prevent induction of Gsc-GFP+ cells by activin. However, the Gsc-GFP+ cells in this work do not express Brachyury and very likely represent a mixed population and are thus difficult to compare to the Foxa2/T double positive cells studied by Gadue and co-workers. Furthermore, although siRNA-mediated knock-down of β-catenin significantly reduced the number of Gsc-GFP+ cells developing in response to activin the effect never exceeded 50%.. In contrast, the formation of Sox17-GFPHi DE cells was more sensitive to inhibition of canonical Wnt signaling, but only when signaling was inhibited at a relatively late stage of development, suggesting that the requirement for Wnt signaling is not during formation of PS cells but may rather represent a Wnt-mediated lineage choice by a common mesendodermal precursor or alternatively, a requirement for Wnt signaling in the maintenance of Sox17-expressing DE cells. Indeed, our observation that Dkk1 only reduces the number of Sox17Hi cells if present at day 4, i.e. after Sox17 expression is initiated, is consistent with a requirement for Wnt signaling in the maintenance of DE. Also, our observations are consistent with the requirement for β-catenin function in DE to acquire or maintain DE fate (Lickert et al., 2002) as well as the observation of synergy between Sox17 and β-catenin in the activation of DE gene expression (Sinner et al., 2004). We also speculate that the proposed action of Wnt3 during gastrulation might offer at least a partial explanation for the difference in sensitivity towards Dkk1 treatment observed between activin and nodal as well as between adherent and aggregate culture. Wnt3 is required in vivo for nodal to initiate its auto-stimulatory cascade. In the absence of Wnt3, nodal expression is initiated but then regresses rather that expands (Barrow et al., 2007; Liu et al., 1999). Wnt3 is thought to induce expression of Cfc1 which encodes the obligate nodal co-receptor Cripto (Morkel et al., 2003). It was recently reported that the three dimensional environment in embryoid bodies prevent exogenously added growth factors from diffusing more than a limited distance into the embryoid body (Sachlos and Auguste, 2008) possibly making these more reliant on endogenous relay signaling via nodal which subsequently would require Wnt activity to induce Cripto expression. This notion is supported by our observation that nodal-induced Sox17-GFPHi cells appear more sensitive to Dkk1-mediated suppression that activin induced Sox17-GFPHi cells, and by our observation that activin-induced Sox17-GFPHi cells are more sensitive to Dkk1 treatment in aggregate culture compared to adherent culture. The apparent absence of such an autocrine nodal/Wnt signaling loop in adherent culture is not surprising given the remarkable instability of nodal when secreted by cultured cells (Le Good et al., 2005). Nodal may simply be degraded before it can reach other cells in the dish whereas in the confined environment of an embryoid body it may well signal to nearby cells and such signaling might propagate in a relay fashion. Thus, Wnt3a treatment would not be sufficient to induce this loop in adherent culture. Conversely, since all the cells in adherent culture are exposed evenly to exogenous activin there may be no requirement for Wnt signaling to facilitate ALK4 signaling. Nevertheless, Gsc-GFP+ cells appear largely refractory to the inhibitory effects of Dkk1 treatment. Since Wnt induced expression of Xgsc is mediated by two homeodomain proteins (Siamois and Twin;,which have no apparent homologs in the mouse) binding to the PE-element, this finding raises the question of whether the conserved Wnt-responsive element in the mouse Gsc promoter (Watabe et al., 1995) is functional in all contexts. It is possible that Gsc is not always induced by Wnt as Gsc expression is reduced rather than increased in E8.5 Dkk1 mutant embryos (Lewis et al., 2008). Lastly, we cannot rule out a more trivial explanation related to the particular Gsc-GFP cell line, which may potentially harbour a defect in its responsiveness to Dkk1-mediated Wnt inhibition. It is possible that more efficient knock-down of β-catenin than we achieved might further reduce or even prevent formation of Gsc-GFP+ cells.
In contrast, we do observe a requirement for Wnt signaling for induction of Mixl1 expression. Our experiments revealed the simultaneous requirement of nodal/activin and Wnt signaling to induce expression of Mixl1 in ES cell progeny. Stimulation of one pathway while inhibiting the other reduced Mixl1 induction, which corroborates previous findings (Gadue et al., 2006). The Mixl1 promoter has previously been shown to be nodal/activin-responsive via the presence of Foxh1 and Smad binding sites in the promoter (Hart et al., 2005) and inspection of the published sequence reveals the presence of consensus TCF/LEF binding sites in the promoter as well. In vivo, Wnt/β-catenin signaling is upstream of two distinct gene expression programs acting during anteroposterior axis and mesoderm formation, respectively (Morkel et al., 2003). We suspect that we observe a requirement for Wnt signaling in mesoderm formation but did not pursue this aspect further.
The two differentiation systems, adherent and aggregate culture, also differ strikingly in the requirement for FGF signaling. In agreement with our results a recent study using embryoid body formation found that FGF signaling was not required for BMP4-induced T expression (Willems and Leyns, 2008), which is unlike the situation in adherent culture where BMP4-induced T expression is completely prevented by SU5402. Moreover, consistent with our results Kunath and co-workers, also using adherent culture, recently reported that FGF4 deficiency or treatment with PD173074 prevented the switch from BMP4-mediated support of pluripotency to BMP4-induced differentiation (Kunath et al., 2007). The requirement for FGF signaling in induction of mesodermal gene expression is also consistent with the in vivo requirement for FGF signaling in Xenopus and zebrafish mesodermal induction (Cornell et al., 1995; Mathieu et al., 2004). In zebrafish, FGF signaling is required downstream of Nodal for induction of One-eyed-pinhead, the zebrafish homolog of Cripto (Mathieu et al., 2004). However, as discussed above activin does not require Cripto to activate its receptors, thus activin induced Sox17 expression should not necessarily be inhibited by FGFR inhibitors. This is indeed what we find when inhibiting FGF signaling prior to appearance of Sox17-GFPHi cells, the time where one would expect to find a requirement for Cripto function if Nodal was the inducer. It might be illuminating to determine if Nodal induced Sox17 expression would be sensitive to these inhibitors. Nevertheless, we do observe that Sox17 expression depends on FGF signaling after the initial appearance of Sox17-GFPHi cells at the same time where we observe a dependency for Wnt signaling. It thus appears that maintenance of Sox17 expression and/or propagation of Sox17 expressing cells depend on both FGF and Wnt signaling. Consistent with our results, Brickman and co-workers also observed an absolute requirement for FGF signaling at day 3–7 for formation of Hex+CXCR4+ ADE cells when differentiating ES cells in monolayer culture under defined conditions (Morrison et al., 2008).
In some cases we noticed what appeared to be conflicting results when inhibiting FGF and FGFR signaling by soluble FGF receptors and SU5402, respectively. It is possible that this is a reflection of FGF independent FGFR activation. FGF receptors are known to form complexes with N-cadherin and NCAM in neurons and in pancreatic β-cells, resulting in ligand independent receptor activation (Cavallaro et al., 2001; Saffell et al., 1997; Williams et al., 1994), and this would not be expected to be sensitive to the addition of soluble FGF receptors. More trivial explanations are also possible. We cannot rule out that SU5402 may have unknown non-FGFR-mediated effects or be certain that our soluble FGFR preparations are capable of inhibiting all FGF family members. Additional experiments with dominant negative receptors and cell lines mutated in genes coding for FGF signaling components will likely shed more light on these questions.
The expression of anterior markers such as Otx2 and Cer1 in Sox17-GFPHi cells suggest that these could be anterior definitive endoderm. This notion is supported by the selective presence of Pyy transcripts in this population, but the lack of good ADE specific markers prevents us from categorically making this conclusion. However, in vivo ADE forms under conditions of high nodal signaling (Ben-Haim et al., 2006; Lu and Robertson, 2004; Vincent et al., 2003) which we believe we mimic with culture conditions containing 30 – 100 ng/ml activin or 1 µg/ml nodal. Thus, it is likely that the Sox17-GFPHi cells formed in our cultures represent ADE. However, it is likely that also mesoderm is formed to some extent in cultures treated with high doses of activin or nodal. The presence of Otx2 transcripts in Sox17-GFPLo and -GFP− populations suggest that these also contain anterior cell types including perhaps small numbers of AVE cells. The latter is indicated by the presence of the VE marker Tdh and the presence of Otx2 and Cer1 is consistent with this idea. Lastly, we cannot exclude that Sox17-negative endoderm is present in our cultures.
Chick embryo xenografting has previously been used to investigate the developmental potential of embryonic mouse cells (Fontaine-Perus, 2000; Fontaine-Perus et al., 1996; Fontaine-Perus et al., 1997; Fontaine-Perus et al., 1995) as well as mouse and human ES cell derivatives (Lee et al., 2007; Wichterle et al., 2002) but to our knowledge we report the first functional assay for mES cell-derived endoderm. Previous studies have relied almost exclusively on expression of cell-specific markers for the characterization of in vitro generated endoderm. Although the ES cell-derived endoderm did not express transcription factors associated with the regionalisation of the primitive gut tube after 5 days of activin treatment, the cells were capable of turning on these genes when integrating into endodermal epithelium in vivo. Moreover, some embryos contained grafted cells in the Nkx6-1+ and Pdx1+ pancreatic endoderm. Overall, our data suggest that ES cell-derived DE, formed under defined conditions, can contribute to the developing gut tube and further suggests that such cells are, at least partly, capable of responding to patterning cues from the in vivo environment. The latter notion is corroborated by the induction of pancreas markers in a limited number of cells when such cells are cultured further in the presence of known and suspected posteriorizing factors.
It is remarkable how the requirement for certain signaling events are strikingly different when comparing aggregate culture (i.e. embryoid body formation) and adherent culture. However, we do not think that one system is superior to the other but rather that comparison of directed differentiation under adherent and aggregate culture conditions will prove valuable when attempting to decipher the extent of secondary signaling events and their role in lineage selection. In this regard it is also noteworthy that endogenous signaling likely plays a prominent role also in adherent culture. This notion is based on several of our observations, including that many SuTOP-CFP+ cells formed in cultures that received activin as the only exogenous factor, the interdependence between activin and Wnt signaling for generation of Mixl1-GFP+ cells, and on the dependence on Wnt and FGF signaling for efficient activin-induced formation of Sox17-GFP+ cells.
Supplementary Material
Primary flow cytometry histograms of GFP expression in TGfp/+ and Mixl1Gfp/+ cells cultured for 3 days with or without 10 ng/ml BMP4 and GscGfp/+ and Sox17Gfp/+ cells cultured for 5 days in the presence or absence of 100 ng/ml activin.
Immunofluorescent analyses of T expression in TGfp/+ (A), Mixl1Gfp/+ (B) or GscGfp/+ cells (C) grown in adherent culture for 3 or 5 days in the presence of the indicated growth factors. The arrows in (C) indicate areas with high T expression and low/no Gsc (GFP) expression. (D) Immunofluorescent analyses of Foxa2 and E-cad expression in TGfp/+ (a–d), Mixl1Gfp/+(e–h), or GscGfp/+ cells (i–l) after activin treatment.
(A) Flow cytometric analysis of CXCR4 expression in Mixl1Gfp/+ cells grown for 5 days with or without 100 ng/ml activin. Mouse embryonic day (E)11 head tissue was used as control. (B, C) RT-PCR analysis for Sox7 expression in TGfp/+ cells (B) and Sox17, Pyy, Sox7 and Tdh expression in Flk1LacZ/+ cells (C). Cells were cultured in 0, 3 or 100 ng/ml activin, 10 ng/ml BMP4, 100 ng/ml Wnt3a or 100 ng/ml activin+100 ng/ml Wnt3a for 5 days as indicated. E7 mouse embryo cDNA was used as positive control and expression of Tbp and G6pd was analysed as reference genes.
RT-PCR analysis of TGfp/+cells cultured for 3 or 5 days in medium containing growth factors and/or inhibitor, as indicated. The expression of components of the nodal/activin, BMP, Wnt and FGF signaling pathway was analysed. The expression of Tbp was analysed as reference gene.
(A) Chimeric E10.5 embryo recovered after blastocyst injection of SuTOP-CFP ES cells. Note CFP-expression at sites known to harbour active Wnt signaling at this stage including the otic vesicle (white arrow), optic vesicle (white arrowhead), tail bud (red arrow), and somites and dorsal neural tube (red arrowhead). (B) SuTOP-CFP cells were cultured in the presence of 100 ng/ml activin for 3 days and supplemented with 100 ng/ml Wnt3a or 320 ng/ml Dkk1 for a variable number of days as indicated. Scale bar is 80 µm. (C) SuTOP-CFP cells were cultured in the presence of 100 ng/ml activin for 5 days and supplemented with 100 ng/ml Wnt3a or 320 ng/ml Dkk1 for a variable number of days as indicated in the figure. “–“ indicates treatment with activin alone. Scale bar is 200 µm.
GscGfp/+ cells were cultured in 100 ng/ml activin for five days and (A) transfected with β-catenin siRNA as indicated or (B) treated with Dkk1 as indicated. At the end of day 5 of the culture period RNA was prepared and qRT-PCR was performed to measure the relative abundance of Lhx1 and Chrd message.
Acknowledgements
We are indebted to Drs. G. Keller, A.G. Elefanty, S. Nishikawa, A. Smith, S.J. Morrison, J. Rossant, and C. Wright for the T-GFP, Mixl1-GFP, Gsc-GFP, Sox2-LacZ, Sox17-GFP, Flk1-LacZ and Pdx1-LacZ reporter ES cell lines and anti-Pdx1 antibody. We thank Ragna Jørgensen, Søren Refsgaard Lindskog, Gurmeet Kaur Singh, Heidi Ingemann Jensen, and Rodrigo Garcia for excellent technical assistance. This work was made possible by funding from the Juvenile Diabetes Research Foundation, EU Integrated Project No. 512145, PS; NIDDK U19-DK04-017, PS; and the Danish Stem Cell Research Doctoral School (PS and PM-H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahnfelt-Ronne J, Jorgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An Improved Method for 3D Reconstruction of Protein Expression Patterns in Intact Mouse and Chicken Embryos and Organs. J Histochem Cytochem. 2007 doi: 10.1369/jhc.7A7226.2007. [DOI] [PubMed] [Google Scholar]
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Blum M, Gaunt SJ, Cho KW, Steinbeisser H, Blumberg B, Bittner D, De Robertis EM. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- Carey FJ, Linney EA, Pedersen RA. Allocation of epiblast cells to germ layer derivatives during mouse gastrulation as studied with a retroviral vector. Dev Genet. 1995;17:29–37. doi: 10.1002/dvg.1020170105. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Musci TJ, Kimelman D. FGF is a prospective competence factor for early activin-type signals in Xenopus mesoderm induction. Development. 1995;121:2429–2437. doi: 10.1242/dev.121.8.2429. [DOI] [PubMed] [Google Scholar]
- Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Dyson S, Gurdon JB. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell. 1998;93:557–568. doi: 10.1016/s0092-8674(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. Embo J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol. 1999;40:271–287. [PubMed] [Google Scholar]
- Fontaine-Perus J. Interspecific mouse-chick chimeras. Methods Mol Biol. 2000;135:443–446. doi: 10.1385/1-59259-685-1:443. [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J, Cheraud Y, Halgand P. Developmental potentialities of the mouse neural tube grafted in chick embryo. Int J Dev Biol. 1996 Suppl 1:135S. [PubMed] [Google Scholar]
- Fontaine-Perus J, Halgand P, Cheraud Y, Rouaud T, Velasco ME, Cifuentes Diaz C, Rieger F. Mouse-chick chimera: a developmental model of murine neurogenic cells. Development. 1997;124:3025–3036. doi: 10.1242/dev.124.16.3025. [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J, Jarno V, Fournier le Ray C, Li Z, Paulin D. Mouse chick chimera: a new model to study the in ovo developmental potentialities of mammalian somites. Development. 1995;121:1705–1718. doi: 10.1242/dev.121.6.1705. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Anderson KV. Essential role of glycosaminoglycans in Fgf signaling during mouse gastrulation. Cell. 2003;114:727–737. doi: 10.1016/s0092-8674(03)00715-3. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–278. doi: 10.1016/j.mod.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. doi: 10.1016/s0168-9525(99)01957-5. [DOI] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Harger P, Mitchell A, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371:487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Standley H, Dyson S, Butler K, Langon T, Ryan K, Stennard F, Shimizu K, Zorn A. Single cells can sense their position in a morphogen gradient. Development. 1999;126:5309–5317. doi: 10.1242/dev.126.23.5309. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Exp. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PP, Elefanty AG, Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- Hart AH, Willson TA, Wong M, Parker K, Robb L. Transcriptional regulation of the homeobox gene Mixl1 by TGF-beta and FoxH1. Biochem Biophys Res Commun. 2005;333:1361–1369. doi: 10.1016/j.bbrc.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Hou J, Charters AM, Lee SC, Zhao Y, Wu MK, Jones SJ, Marra MA, Hoodless PA. A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE) BMC Dev Biol. 2007;7:92. doi: 10.1186/1471-213X-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development. 2001;128:2407–2420. doi: 10.1242/dev.128.12.2407. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Inman KE, Downs KM. Localization of Brachyury (T) in embryonic and extraembryonic tissues during mouse gastrulation. Gene Expr Patterns. 2006;6:783–793. doi: 10.1016/j.modgep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jensen J, Serup P, Karlsen C, Nielsen TF, Madsen OD. mRNA profiling of rat islet tumors reveals Nkx6.1 as a beta-cell- specific homeodomain transcription factor. Journal of Biological Chemistry. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Dale L, Hogan BL, Wright CV, Smith JC. Bone morphogenetic protein-4 (BMP-4) acts during gastrula stages to cause ventralization of Xenopus embryos. Development. 1996;122:1545–1554. doi: 10.1242/dev.122.5.1545. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PP. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development. 2001;128:3623–3634. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kunath T, Saba-EI-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Umbhauer M, Neal KA, Lerchner W, Smith JC, Cunliffe V. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 1997;11:3265–3276. doi: 10.1101/gad.11.23.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA. Fate mapping the mouse embryo. Int J Dev Biol. 1999;43:773–775. [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Found Symp. 1992;165:3–21. doi: 10.1002/9780470514221.ch2. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Joubin K, Giraldez AJ, Ben-Haim N, Beck S, Chen Y, Schier AF, Constam DB. Nodal stability determines signaling range. Curr Biol. 2005;15:31–36. doi: 10.1016/j.cub.2004.12.062. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JB, Zon LI, Daley GQ. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Lewis SL, Khoo PL, De Young RA, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson RV, Robb L, Tam PP. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135:1791–1801. doi: 10.1242/dev.018853. [DOI] [PubMed] [Google Scholar]
- Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol. 2004;273:149–159. doi: 10.1016/j.ydbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Griffin K, Herbomel P, Dickmeis T, Strahle U, Kimelman D, Rosa FM, Peyrieras N. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development. 2004;131:629–641. doi: 10.1242/dev.00964. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Izawa T, Kuroiwa A, Kikuchi Y. Fgf signaling negatively regulates Nodal-dependent endoderm induction in zebrafish. Dev Biol. 2006;300:612–622. doi: 10.1016/j.ydbio.2006.08.073. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Morrison GM, Oikonomopoulou I, Migueles RP, Soneji S, Livigni A, Enver T, Brickman JM. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Mossman AK, Sourris K, Ng E, Stanley EG, Elefanty AG. Mixl1 and oct4 proteins are transiently co-expressed in differentiating mouse and human embryonic stem cells. Stem Cells Dev. 2005;14:656–663. doi: 10.1089/scd.2005.14.656. [DOI] [PubMed] [Google Scholar]
- Ng ES, Azzola L, Sourris K, Robb L, Stanley EG, Elefanty AG. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4-induced ventral mesoderm patterning in differentiating ES cells. Development. 2005;132:873–884. doi: 10.1242/dev.01657. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JJ, Evans MJ. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech Dev. 1999;87:189–192. doi: 10.1016/s0925-4773(99)00135-5. [DOI] [PubMed] [Google Scholar]
- Pedersen IL, Klinck R, Hecksher-Sorensen J, Zahn S, Madsen OD, Serup P, Jorgensen MC. Generation and characterization of monoclonal antibodies against the transcription factor Nkx6.1. J Histochem Cytochem. 2006;54:567–574. doi: 10.1369/jhc.5A6827.2006. [DOI] [PubMed] [Google Scholar]
- Pedersen JK, Nelson SB, Jorgensen MC, Henseleit KD, Fujitani Y, Wright CV, Sander M, Serup P. Endodermal expression of Nkx6 genes depends differentially on Pdx1. Dev Biol. 2005;288:487–501. doi: 10.1016/j.ydbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Poulain M, Furthauer M, Thisse B, Thisse C, Lepage T. Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling. Development. 2006;133:2189–2200. doi: 10.1242/dev.02387. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Shawlot W, Behringer RR, Le Meur M, Ang SL. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development. 1998;125:845–856. doi: 10.1242/dev.125.5.845. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29:4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Sander V, Reversade B, De Robertis EM. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. Embo J. 2007;26:2955–2965. doi: 10.1038/sj.emboj.7601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shapira E, Marom K, Yelin R, Levy A, Fainsod A. A role for the homeobox gene Xvex-1 as part of the BMP-4 ventral signaling pathway. Mech Dev. 1999;86:99–111. doi: 10.1016/s0925-4773(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Jitianu C, Cleaver O, Shaywitz DA, Lamenzo JO, Chen AE, Golub TR, Melton DA. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. doi: 10.1016/j.ydbio.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Steinbeisser H, Fainsod A, Niehrs C, Sasai Y, De Robertis EM. The role of gsc and BMP-4 in dorsal-ventral patterning of the marginal zone in Xenopus: a loss-of-function study using antisense RNA. Embo J. 1995;14:5230–5243. doi: 10.1002/j.1460-2075.1995.tb00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Kim S, Candia A, Rothbacher U, Hashimoto C, Inoue K, Cho KW. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Willems E, Leyns L. Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires Fibroblast Growth Factor activity. Differentiation. 2008;2:2. doi: 10.1111/j.1432-0436.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003a;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003b;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary flow cytometry histograms of GFP expression in TGfp/+ and Mixl1Gfp/+ cells cultured for 3 days with or without 10 ng/ml BMP4 and GscGfp/+ and Sox17Gfp/+ cells cultured for 5 days in the presence or absence of 100 ng/ml activin.
Immunofluorescent analyses of T expression in TGfp/+ (A), Mixl1Gfp/+ (B) or GscGfp/+ cells (C) grown in adherent culture for 3 or 5 days in the presence of the indicated growth factors. The arrows in (C) indicate areas with high T expression and low/no Gsc (GFP) expression. (D) Immunofluorescent analyses of Foxa2 and E-cad expression in TGfp/+ (a–d), Mixl1Gfp/+(e–h), or GscGfp/+ cells (i–l) after activin treatment.
(A) Flow cytometric analysis of CXCR4 expression in Mixl1Gfp/+ cells grown for 5 days with or without 100 ng/ml activin. Mouse embryonic day (E)11 head tissue was used as control. (B, C) RT-PCR analysis for Sox7 expression in TGfp/+ cells (B) and Sox17, Pyy, Sox7 and Tdh expression in Flk1LacZ/+ cells (C). Cells were cultured in 0, 3 or 100 ng/ml activin, 10 ng/ml BMP4, 100 ng/ml Wnt3a or 100 ng/ml activin+100 ng/ml Wnt3a for 5 days as indicated. E7 mouse embryo cDNA was used as positive control and expression of Tbp and G6pd was analysed as reference genes.
RT-PCR analysis of TGfp/+cells cultured for 3 or 5 days in medium containing growth factors and/or inhibitor, as indicated. The expression of components of the nodal/activin, BMP, Wnt and FGF signaling pathway was analysed. The expression of Tbp was analysed as reference gene.
(A) Chimeric E10.5 embryo recovered after blastocyst injection of SuTOP-CFP ES cells. Note CFP-expression at sites known to harbour active Wnt signaling at this stage including the otic vesicle (white arrow), optic vesicle (white arrowhead), tail bud (red arrow), and somites and dorsal neural tube (red arrowhead). (B) SuTOP-CFP cells were cultured in the presence of 100 ng/ml activin for 3 days and supplemented with 100 ng/ml Wnt3a or 320 ng/ml Dkk1 for a variable number of days as indicated. Scale bar is 80 µm. (C) SuTOP-CFP cells were cultured in the presence of 100 ng/ml activin for 5 days and supplemented with 100 ng/ml Wnt3a or 320 ng/ml Dkk1 for a variable number of days as indicated in the figure. “–“ indicates treatment with activin alone. Scale bar is 200 µm.
GscGfp/+ cells were cultured in 100 ng/ml activin for five days and (A) transfected with β-catenin siRNA as indicated or (B) treated with Dkk1 as indicated. At the end of day 5 of the culture period RNA was prepared and qRT-PCR was performed to measure the relative abundance of Lhx1 and Chrd message.