Abstract
PURPOSE
To test the feasibility and applicability of a handheld probe for Fourier-domain optical coherence tomography (Fd-OCT ) retinal imaging in infants and children.
METHODS
Thirty children ages 7 months to 9.9 years, with (10 of 30) or without (20 of 30) retinal pathology, were imaged with Fd-OCT. Imaging was performed under sedation in 10 of 30 children ages 7 months to 3.7 years. A high-resolution Fd-OCT system (axial resolution: 4.5 m; acquisition speeds: 1000 A-scans/frame, 9 frames/second), constructed at the UC Davis Medical Center, in conjunction with a handheld scanner, was used for retinal imaging.
RESULTS
Useful images were obtained from all selected patients. Image acquisition was possible in a conscious state in children as young as 3 years of age. All children tolerated the tests well. The most challenging situation for young children was the lack of an internal fixation target and the moving scanning line, which usually distracted them from a steady fixation. Despite these problems, image quality was comparable with scans previously obtained from an adult population.
CONCLUSIONS
The flexible handheld scanner in association with high acquisition speed and high-resolution Fd-OCT allows retinal imaging in infants and children. This technology provides high-resolution documentation of retinal structure in a pediatric population for the first time.
The purpose of this study was to test the feasibility and applicability of a handheld probe for Fourier-domain optical coherence tomography (Fd-OCT ) retinal imaging in infants and children. Few reports pertaining to retinal imaging in children using commercially available time-domain OCT systems have been published, with patient ages in these studies as follows: 4 to 17 years, in which 4- and 5-year-old patients were uncooperative1; 9 years (1 case)2; 7 and 9 years (2 cases)3; 9 years (1 case)4; and 7 years (1 case)5; detailed ages not provided.6 To the best of our knowledge, the use of high-speed Fd-OCT systems in infants or children has not been previously reported. We performed such retinal imaging successfully in sedated as well as conscious infants and children. The handheld scanner accommodated the child’s ability to either hold still on a chin rest or be tested in a reclined position.
Methods
Subjects
Thirty children, ages 7 months to 9.9 years, with (10) or without (20) retinal pathology were imaged with retinal Fd-OCT. (One subject had retinal disease in one eye and a normal retina in the other.) Clinical diagnoses were maculopathy (6), retinal dystrophy (4), and posttraumatic choroidal neovascularization (1). Examination under chloral hydrate sedation (10 patients aged 7 months to 3.7 years) was used only for standard-of-care diagnostic procedures and not primarily for this research study. One patient with a retinal dystrophy (Leber’s congenital amaurosis) had horizontal pendular nystagmus. Pupils were dilated in 27 of 30 subjects: 3 children did not want eye drops but were cooperative enough for successful retinal imaging.
Written informed consent and/or assent were obtained from all participants and/or guardians. The project was approved by the Research Ethics Board at The Hospital for Sick Children in Toronto and the UC Davis Medical Center Institutional Review Board, and conducted in accordance with the Tenets of Helsinki.
Fd-OCT Image Acquisition
We used a custom-built, high-speed, high-resolution Fd-OCT system (axial resolution, 4.5 m; acquisition speed, 9 frames/second, 1000 A- scans/frame),7 constructed at UC Davis with a sample arm handheld scanning head (Bioptigen Inc., Durham, NC) for image acquisition in children younger than 10 years of age. The handheld probe was used either like a handheld camera in supine children under sedation or children too young to be positioned on a chin rest or mounted on a slit-lamp post for seated patients (Figure 1). Children under sedation were under the constant care of a registered nurse. An eyelid speculum was used when possible.
FIG 1.
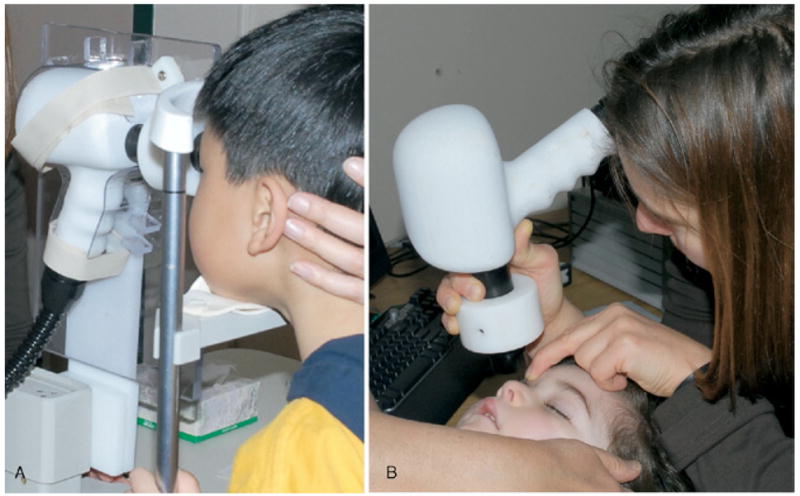
Retinal image acquisition. (A) Conscious 5-year-old child, fitted with a chin rest, with scanner mounted on a slit-lamp post. (B) Sedated 2-year-old infant and the handheld scanner.
Horizontal line scans of 6 – 8 mm or volumetric scans of 6 6 mm centered in the fovea were obtained through the macular area. Images were postprocessed as described in detail elsewhere,8 and retinal layers were identified based on previously published data.9
Results
We were able to obtain retinal images from all 30 children enrolled in the study. Children who were not sedated deeply did not tolerate a speculum placement. In those cases, the nurse and/or the examiner held open the child’s eye (Figure 1). The first aim was to locate the macular area without fundus viewing. The retina was “screened” with a “volumetric” scan mode, which permits image acquisition over an area of 6 6 mm and reconstruction of an OCT fundus image. Once the macular mound was located, a macular line or a volumetric scan followed.
Children who could sit quietly were imaged with a chin rest with a mount for the scanner. Smaller or more restless children were asked to lie down on an examination stretcher and were imaged with the handheld probe. We imaged children as young as 2.9 years with this approach. Participants were instructed to look straight ahead or at an external fixation target for the nontested eye. The most challenging situation for young children, especially those younger than 6 years of age, was the lack of an internal fixation target and the moving scanning line, which usually distracted them from a steady fixation. External fixation targets were successful in only a few cases. The localization of the macular area was easier in conscious than in sedated children because of lack of simultaneous fundus viewing.
Retinal layer identification was possible in all subjects. Images obtained using the handheld mode contained movement artifacts caused by the examiner and/or the child. It still allowed retinal layer identification but reduced the image quality for further possibility of 3D reconstruction.
Macular Scans Obtained from Controls
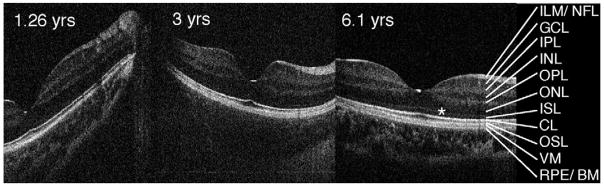
Scans from control subjects of different ages were obtained under sedation or conscious conditions. The resolution of the images was similar in all tested subjects (Figure 2). Macular scans shown in Figure 2 demonstrate well-formed macular layering. Retinal layers are labeled as previously published.8 The small sample size in this pilot study did not allow us to address potential age-related changes of different retinal layers. Macular scans of patients with retinal diseases that illustrate the usefulness of high-resolution imaging in children are available in e-Supplement 1 (available at jaapos.org).
FIG 2.
Fd-OCT horizontal line scans through the macula of control subjects. Subjects were tested sedated (ages 1.26 and 3 years; imaging in supine position) or conscious (age 6.1 years, imaging with chin rest). CL, connecting cilia; GCL, ganglion cell layer; ILM/NFL, internal limiting membrane/nerve fiber layer; INL, inner nuclear layer; IPL, inner plexiform layer; ISL, inner segment layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OSL, outer segment layer; RPE/BM, retinal pigment epithelium/Bruch’s membrane; VM, Verhoeff’s membrane.*Outer limiting membrane.
Discussion
The application of high-resolution retinal imaging in infants and children without and with sedation using a flexible setup is described here for the first time. The high-speed acquisition allowed imaging in young children with a low attention span or intermittent fixation as well as in one patient with nystagmus and reduced vision. In addition, very cooperative children younger than 6 years of age could be imaged without pupil dilation.
These cases highlight the benefit of having high-speed, high-resolution retinal imaging modalities for pediatric assessments. Microstructural retinal abnormalities can now be detected and correlated with visual function in this population. The ability to recognize small intraretinal abnormalities is an important objective measure in cases where nonorganic vision loss is suspected. Identification and quantification of retinal layer changes and localization of intraretinal deposits is an important investigative tool in children with early stages of retinal dystrophy (e-Supplement 1).
The handheld retinal imaging system with the flexible probe has overcome the current technical limitations of using OCT systems in a supine position. The probe can be mounted to a slit lamp or articulating arm or held, depending on the child’s cooperativeness and ability. Further improvements of this technology, such as simultaneous fundus viewing and a fixation target, will advance the applicability in a routine clinical setting. The use of such a device will contribute to our further understanding of normal retinal morphology, development, and aging, and prove useful in early detection of retinal diseases in children.
Supplementary Material
Acknowledgments
The authors wish to thank Drs. Asim Ali, J. Raymond Buncic, Peter Kertes, Stephen Kraft, Wai-Ching Lam, Alex V. Levin, and Nasrin Najm-Tehrani for patient recruitment; Yesmino Elia for study coordination; Lindsay Hampton, Carmelina Trimboli, and Cynthia VandenHoven for fundus photography; Yasmin Shariff and Beverly Griffiths for support of studies under sedation; Jonathan Klingenberg and Andrew Baziw for technical setup; Tom Wright for help with data analysis; Carole Panton for editorial comments; and the patients and their families who made this study possible.
Supported by the Mira Godard Fund (EH), the Sick Kids Research Institute’s Restracomp Fund (CG), NIH (NEI 014743) (JSW), the Albrecht Fund (JSW), in collaboration with Bioptigen, Inc, and a Research to Prevent Blindness Senior Scientist Award (J.S.W).
Footnotes
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, May 2007.
Literature Search
PubMed and MEDLINE were searched for the period 1990 to the present for the following terms: OCT, optical coherence tomography, infant, pediatric, and children.
References
- 1.Salchow DJ, Hutcheson KA. Optical coherence tomography applications in pediatric ophthalmology. J Pediatr Ophthalmol Strabismus. 2007;44:335–49. doi: 10.3928/01913913-20071101-01. [DOI] [PubMed] [Google Scholar]
- 2.Skarmoutsos F, Sandhu SS, Voros GM, Shafiq A. The use of optical coherence tomography in the management of cystoid macular edema in pediatric uveitis. J AAPOS. 2006;10:173–74. doi: 10.1016/j.jaapos.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Harvey PS, King RA, Summers CG. Spectrum of foveal development in albinism detected with optical coherence tomography. J AAPOS. 2006;10:237–42. doi: 10.1016/j.jaapos.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Timoney P, Darcy F, McCreery K, Reardon W, Brosnahan D. Characterization of optical coherence topography findings in Kenny-Caffey syndrome. J AAPOS. 2007;11:291–3. doi: 10.1016/j.jaapos.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Gao H, Salam GA, Chern S. Spontaneous separation of idiopathic epiretinal membrane in a 7-year-old child. J AAPOS. 2007;11:393–4. doi: 10.1016/j.jaapos.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 6.El-Dairi MA, Holgado S, O’Donnell T, Buckley EG, Asrani S, Freedman SF. Optical coherence tomography as a tool for monitoring pediatric pseudotumor cerebri. J AAPOS. 2007;11:564–70. doi: 10.1016/j.jaapos.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Zawadzki RJ, Bower BA, Zhao M, et al. Exposure time dependence of image quality in high-speed retinal in vivo Fourier-domain OCT. In: Manns F, Soederberg PG, Ho A, et al., editors. Proceedings of the SPIE, Volume 5688. XV. Bellingham, WA: Ophthalmic Technologies; 2005. pp. 45–52. [Google Scholar]
- 8.Zawadzki RJ, Jones SM, Olivier SS, Zhao M, Bower BA, Izatt JA, et al. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in-vivo imaging. Optics Express. 2005;13:8532–46. doi: 10.1364/opex.13.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–63. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.