Abstract
Background
Pretreatment with retrorsine crosslinks host hepatocyte DNA and prevents proliferation after partial hepatectomy (PH), allowing selective expansion of transplanted progenitors. Shortcomings are length of protocol and carcinogenicity of retrorsine.
Methods
This report describes a rapid liver repopulation protocol using mitomycin C (MMC) to block proliferation of rat hepatocytes in response to PH. One week post-MMC treatment, dipeptidyl peptidase IV negative (DPPIV−) host rats were given a PH followed by injection of late gestation, newborn or adult total liver isolates from DPPIV+ rats. For allogeneic transplantation, host rats received injections of anti-CD3 antibody before and after PH.
Results
Host liver staining 2–9 weeks post-transplantation revealed well-defined donor hepatocyte colonies with strong canalicular DPPIV activity. At the same cell dose, fetal and newborn isolates produced more colonies than adult liver isolates. Hepatocyte colonies also co-expressed marker proteins characteristic of adult hepatocytes and showed polarized localization of plasma membrane proteins. Host livers contained large clusters of sinusoids lined by DPPIV+ endothelial cells co-expressing the endothelial cell marker, RECA-1 but lacked the canalicular marker leucine aminopeptidase. Colonies containing donor hepatocytes, endothelial cells and bile ducts, were also observed. Similar levels of engraftment and expansion were achieved with allogeneic liver cell isolates by using anti-CD3 antibody treatment.
Conclusions
The MMC transplantation model provides a rapid method for engraftment and expansion of hepatocytes, endothelial cells and cholangiocytes and should be applicable to investigations centering on the role of endothelial cells in liver regeneration and the identification and characterization of putative endothelial, hepatocyte and cholangiocyte progenitors.
Keywords: mitomycin C, partial hepatectomy, rat, dipeptidyl peptidase IV, histochemical stain
INTRODUCTION
Demonstration of differentiation along a hepatocyte or cholangiocyte lineage following transplantation into the liver of a suitable host has been accepted as the most definitive method for assessing the differentiation potential of putative hepatic progenitors. Application of this approach requires a unique marker that allows transplanted hepatic progenitors to be distinguished from host liver cells, a requirement satisfied by Thompson et al. with the discovery of a mutant strain of F344 rats lacking an enzymatically active form of the cell surface exoprotease, dipeptidyl peptidase IV (DPPIV) (1). Thompson et al. and later Sigal et al., Coleman et al. and others subsequently showed that donor hepatocytes from wild type F344 rats transplanted into DPPIV− hosts could be differentiated from host hepatocytes by indirect immunofluorescence (IIF) with monoclonal antibodies (MAbs) specific for enzymatically active DPPIV or by a histochemical stain for DPPIV activity (1–3). This DPPIV model system has since been used in large numbers of investigations in the rat aimed at demonstrating the ability of progenitor cells from the liver, pancreas, and bone marrow to undergo hepatocytic or ductal differentiation (4).
In a number of recent investigations, amplification of DPPIV+ donor cells isolated from wild type F344 rats was achieved by treating DPPIV− hosts with retrorsine and partial hepatectomy (PH) prior to transplantation (5, 6). Laconi et al. demonstrated that treatment with retrorsine, a DNA-crosslinking pyrrolizidine alkaloid, effectively inhibited liver regeneration following PH, thereby providing donor cells with a selective growth advantage that allowed them to proliferate and eventually replace retrorsine compromised host hepatocytes (5). Although the retrorsine/PH transplantation model has proven to be a valuable method for expanding transplanted progenitors, the limited availability, high cost, potent hepatotoxicity and carcinogenicity of retrorsine (7) promoted a search for reliable alternatives. We were also interested in reducing the time needed to generate donor colonies large enough for analysis as the protocol described by Laconi et al. takes a minimum of 9 weeks (5), a turn-around time that necessitates the expensive expedient of maintaining a constant supply of treated rats to avoid long delays in repeating or modifying experiments.
In the present report we describe a rapid transplantation model using mitomycin C (MMC) that avoids some of the limitations encountered with retrorsine. MMC was chosen because it is a readily available and well-characterized agent with well-established basic research and clinical applications. Our results show that donor derived DPPIV+ bile ducts and colonies of both hepatocytes and endothelial cells can be generated within 4 weeks following injection of MMC. We further show that with the addition of a pre-transplant treatment with anti-CD3 antibody, the MMC/PH protocol can be used to potentiate the engraftment and expansion of allogeneic liver cell transplants.
MATERIALS AND METHODS
Animals
Donor cells were isolated from embryonic day (ED) 16, late gestation (ED 18/19), newborn and adult DPPIV+ F344 and ACI rats (Harlan Sprague Dawley). Five to six week old host F344 rats expressing an enzymatically inactive form of DPPIV were obtained from a breeding colony maintained at Rhode Island Hospital or were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were fed rat chow ad libitum and kept in an alternating twelve-hour light and dark cycle environment. All animal work was conducted under protocols approved by the Rhode Island Hospital Animal Care and Usage Committee.
Cell isolation and transplantation of hepatocytes into host rats treated with retrorsine or MMC/PH
Adult DPPIV+ hepatocytes were isolated by collagenase perfusion as previously described (8). Total newborn and fetal DPPIV+ liver cell suspensions were isolated as previously described (6). Viability of cell isolates was greater than 80% as determined by trypan blue dye exclusion. Fetal (ED 16, 18/19), newborn or adult total liver isolates, from DPPIV+ rats, were suspended in Hank’s balanced salt solution (5×105 cells in 500 µl or 1×107 cells in 500 µl for allogeneic transplants) and transplanted into the livers of DPPIV- rats by intrasplenic injection. Prior to transplantation, DPPIV- host rats were treated with MMC (A.G. Scientific, San Diego, CA; 1 mg/ml in saline) by a single intrasplenic injection, a single intraperitoneal injection, or two sequential intraperitoneal injections one week apart. Doses of MMC tested ranged from 0.5 to 4 mg/kg. One week after injection of MMC, antibiotic therapy with gentamicin at 2–5 mg/kg was initiated. Gentamicin was administered four days a week for 2–4 weeks by intraperitoneal injection. One week after treatment with MMC, host rats were given a 2/3 PH (9) followed by intrasplenic injection of normal adult, newborn or fetal hepatocytes. For allogeneic transplants of ACI newborn or fetal hepatocytes, host rats were injected i.p. with 5 µg of anti-CD3 antibody/25 g body weight (e-Bioscience, Inc., San Diego, CA) for 4 consecutive days prior to transplantation and again at 3 weeks post-transplantation. For comparative purposes, donor hepatocyte colonies were also generated by transplantation of late gestation (ED18–19) fetal liver isolates into DPPIV− rats treated with two i.p. doses of retrorsine (Sigma-Aldrich, St. Louis, MO; 30 mg/kg) two weeks apart followed five weeks later by PH (6). To quantify the effect of antibiotic therapy on the survival of MMC treated rats transplanted with adult hepatocytes, survival curves generated by the method of Kaplan and Meier were analyzed by the Mentel-Haenszel test using GraftPad Prism 3.0 software (San Diego, CA).
Phenotypic analysis
Frozen sections were prepared from liver tissue excised from animals sacrificed at 2–13 weeks post-PH. Following fixation in acetone, sections were stained histochemically for DPPIV according to Piazza et al. (10) or were double labeled as previously described by Erickson et al. (11) using an IIF protocol with antibodies specific for DPPIV (1) and the following markers: Pan-Cadherin, a cell-cell adhesion molecule (AbCam, Cambridge, MA); Desmoplakin I, a desmosomal plaque protein (12); H.4, a hepatocyte specific marker (13); OC.10, a cholangiocyte specific marker (13); γ-glutamyl transpeptidase (GGT), a phase II enzyme expressed by preneoplastic hepatocytes and cholangiocytes but gernerally not by normal adult hepatocytes or endothelial cells (Rabbit antibodies recognizing rat GGT were a gift from Marie Hanigan, University of Oklahoma Health Sciences Center, Oklahoma City, OK); Leucine amino peptidase (LAP), a canalicular marker (14); CYP2E1 (AbCam, Cambridge, MA), an enzyme constitutively expressed by hepatocytes (15); LYRIC, a tight junction protein (16); transferrin receptor, a cell surface glycoprotein (17); RECA-1 (AbCam, Cambridge, MA), a pan-endothelial monoclonal antibody (18). Detection of canalicular ATPase was performed as previously described using ATP, lead nitrate and magnesium sulfate (6). Glucose-6-phosphatase activity was detected using a previously described histochemical protocol (6). Glycogen stores in hepatocytes were detected with a Periodic Acid-Schiff (PAS) staining system from Sigma-Aldrich (St. Louis, MO).
Mitotic index after partial hepatectomy
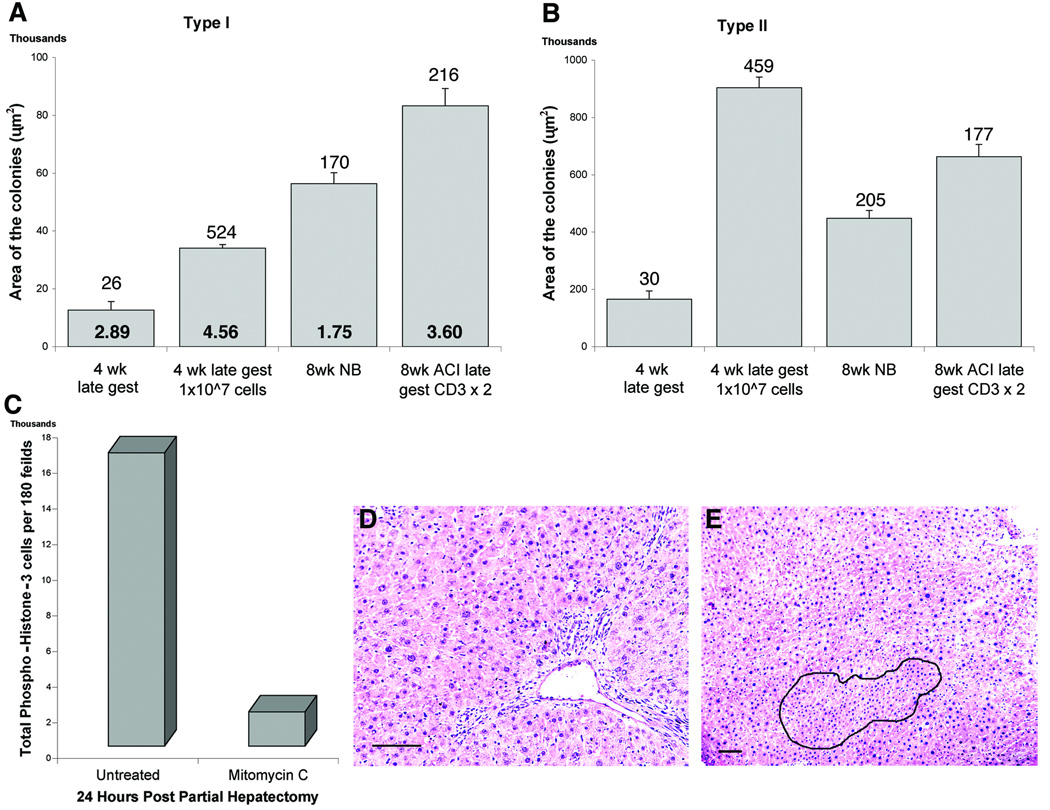
The effect of MMC on the regenerative response post-PH was determined by the number of cells labeled by IIF with a monoclonal antibody specific for phospho-histone-3, a marker for mitotic cells (19). Mitotic index was defined as the average number of phospho-histone-3 positive cells within the area defined by the photographic field viewed with a 20X objective. Mitotic index for animals injected with either saline or MMC at 7 days prior to PH were based on counts from 180 fields of frozen sections prepared at 24 hrs post-PH.
Changes in the size of type I and type II colonies
Acetone fixed frozen sections (5 µm) were cut three sections per slide with a 100 µm interval between each slide. The area of donor derived DPPIV+ colonies visualized by a histochemical stain for DPPIV was determined using Image-Pro Plus 5.0 software (Media Cybernetics, Inc., Bethesda, MD) to analyze digital images acquired with a 4X objective using a Nikon Microphot FX microscope (Nikon Instruments, Inc., Melville, NY). Statistical analysis was performed using Kruskal Wallis ANOVA followed by the Mann-Whitney-Bonferroni test for multiple comparisons (6).
RESULTS
DPPIV- host rats transplanted with DPPIV+ hepatocytes after treatment with MMC/PH contained donor derived hepatocyte colonies but showed a high level of mortality by 4–6 weeks post-transplant
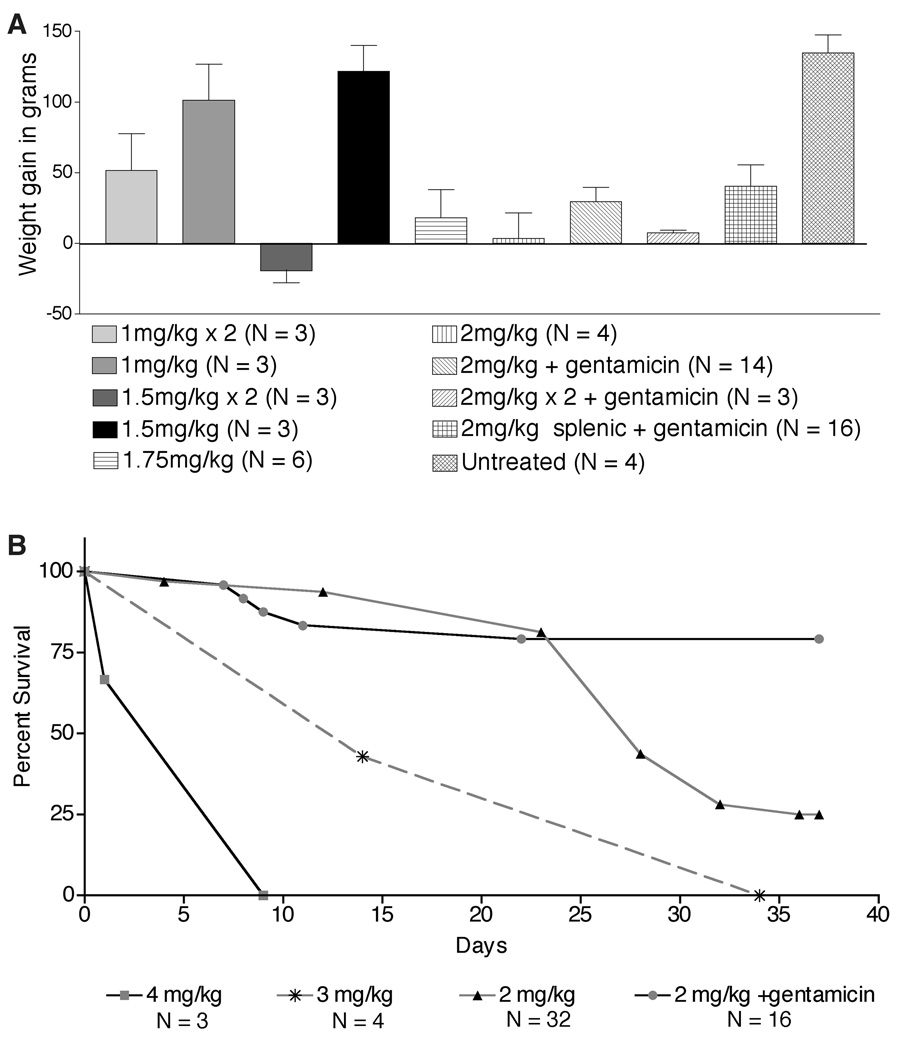
When frozen sections from livers excised from rats surviving for 4–9 weeks post-transplant were stained histochemically for DPPIV, the largest DPPIV+ colonies (up to 32 cells) and the largest number of DPPIV+ colonies were found in animals receiving a single 2 mg/kg dose of MMC by intrasplenic injection (on average, approximately 1 colony/section). In general, increasing the dose of MMC resulted in an increase in morbidity and a decreased or negative weight gain (Figure 1A). At doses above 2 mg/kg, 100% of the animals were moribund within 4–6 weeks post-PH (Figure 1B), with typical symptoms being labored breathing, hunched posture, rough fur, closed eyes, emaciated appearance, protruding penis and low activity levels. Even without PH, morbidity was >75% by 4–6 weeks after injection of MMC indicating that the adverse symptoms were not related to surgical trauma.
Figure 1.
Dose effects of MMC on weight gain and survival. A: Weight gain post-MMC treatment. Rats were weighed at MMC treatment (5 weeks old) and at four weeks post-PH (10 weeks old for untreated rats). B: Survival curves. Survival curves generated with Prism software show the percentage of surviving rats as a function of time post-transplantation of adult hepatocytes. p values indicate that survival was significantly improved by treatment with gentamicin (each group when compared to 2mg/kg +gentamicin had p < 0.005).
Antibiotic therapy after PH greatly reduced morbidity without adversely affecting the formation of DPPIV+ donor colonies
Like many anti-neoplastic drugs, MMC induces reversible bone marrow cytotoxicity in both humans and laboratory animals (20). In mice and rats treated with single or multiple i.p. injections of MMC at 1.0–2.5 mg/kg, significant multi-lineage bone marrow suppression has been reported by 1–2 days post-injection (20–22). Since it is well known that immunocompromised rats are susceptible to lung infections, (23, 24), we initiated treatment with gentamicin (25, 26) to see if antibiotic therapy would decrease morbidity. As shown in Figure 1B, approximately 80% of rats treated with gentamicin four days/week for 4–6 weeks appeared healthy at 5 weeks post-PH, exhibited no signs of respiratory distress, displayed normal behavior and showed positive weight gain (Figure 1A). Notably, in recent experiments, we achieved the same decrease in morbidity with only two weeks of gentamicin treatment at half the dose (2.5 mg/kg).
Transplantation of liver isolates from fetal, newborn and adult DPPIV+ rats into DPPIV− hosts treated with the combined MMC/PH/gentamicin protocol generated two distinct types of DPPIV+ colonies that differed in organization, morphology and distribution of DPPIV
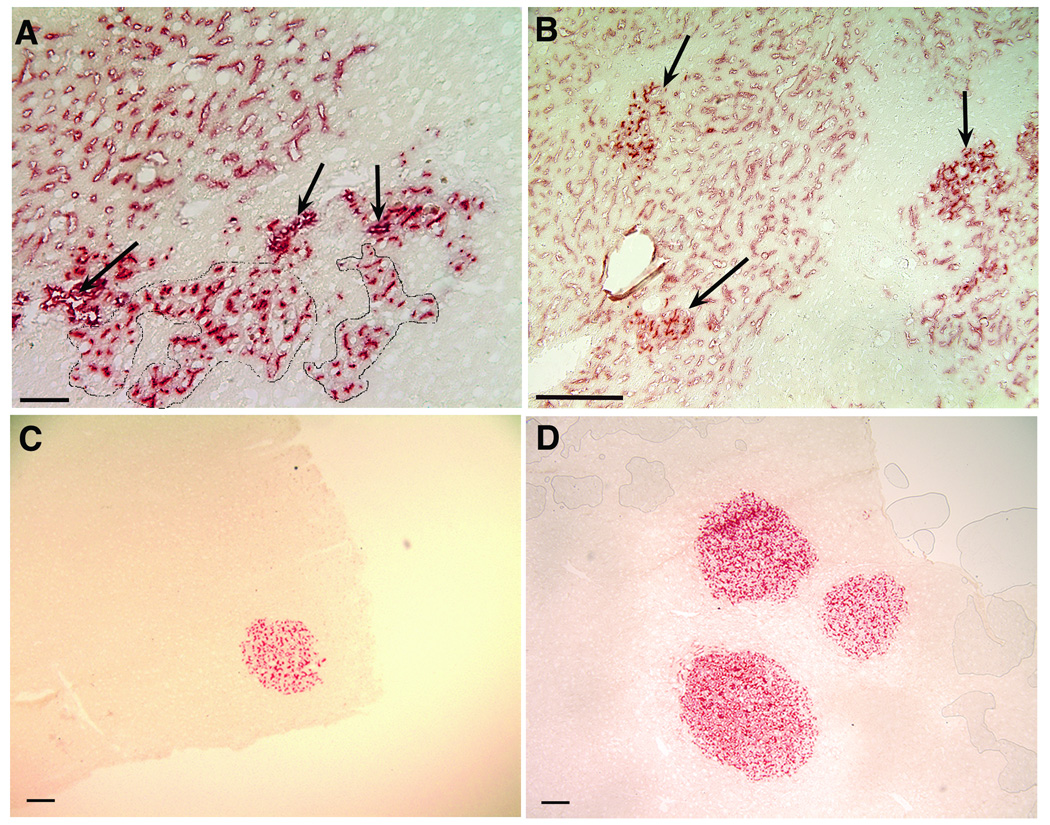
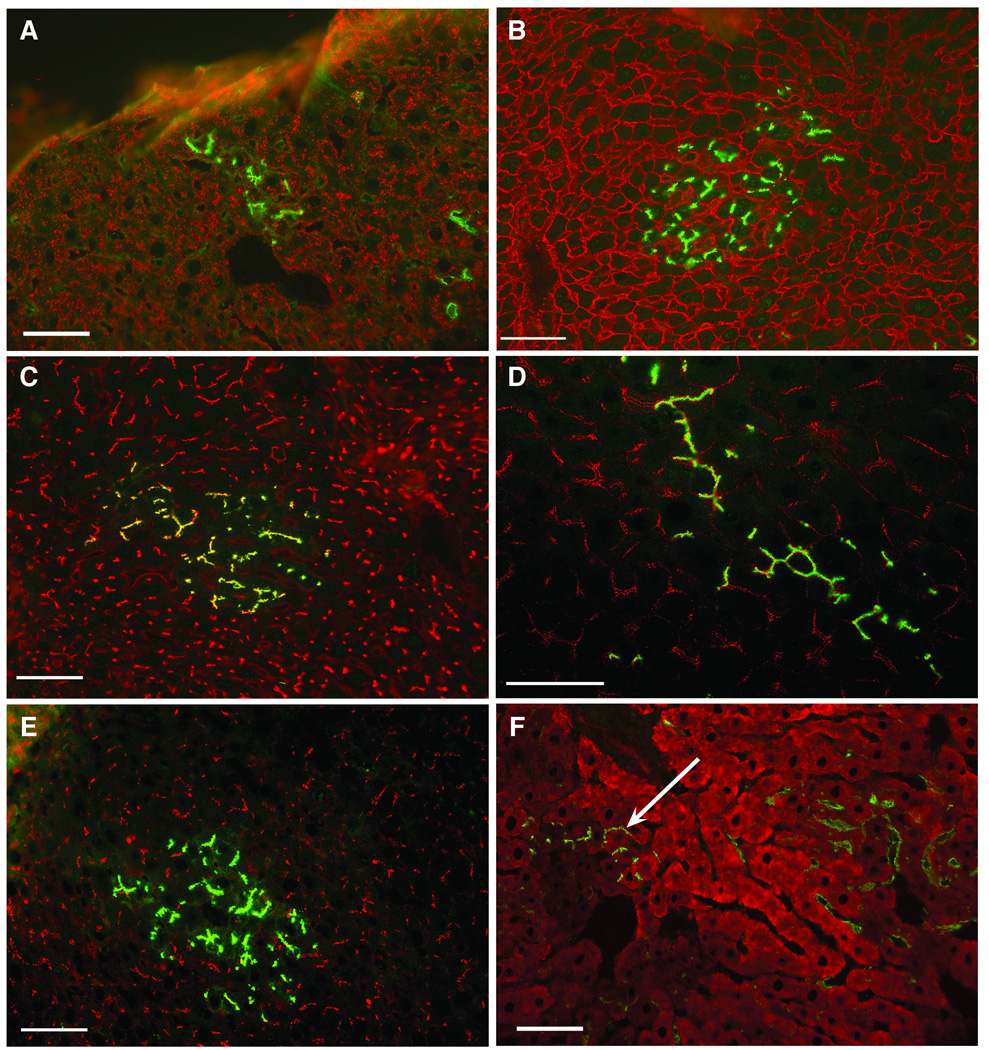
Examination of frozen sections histochemically stained for DPPIV revealed two types of DPPIV+ colonies: small, compact colonies histologically identical to the surrounding liver, containing well defined bile canaliculi strongly positive for DPPIV (type I colonies, Figure 2A, circled areas) and loosely organized clusters of DPPIV+ cells (type II colonies, Figure 2A). Subsequent analysis showed that type I donor colonies were composed of large cells with phenotypes characteristic of adult hepatocytes. Cells in type I colonies were negative for OC.10, a cholangiocyte marker expressed in both fetal and adult rat liver (13) but were positive for the hepatocyte marker, H.4 (Figure 3A, Table 1). Type I colonies also showed plasma membrane reactivity with pan-cadherin antibodies, canalicular localization of LAP and a distribution of desmoplakin I (desmosomal plaques) and LYRIC (tight junctions) identical to that in the surrounding host liver (Figure 3B–E and Table 1). CYP2E1 was expressed at similar levels in type I colonies and the surrounding host hepatocytes (Figure 3F arrow and Table 1). Although most of the donor derived hepatocytes were negative for GGT, many of the type I colonies in animals receiving DPPIV+ hepatocytes contained a small percentage of cells displaying strong canalicular GGT reactivity (Figure 4A, arrow head), a phase two enzyme usually found only on bile ducts in normal adult rat liver (27). Scattered clusters of DPPIV−/GGT+ host hepatocytes were also frequently observed (Figure 4A, short arrow).
Figure 2.
Histochemical detection of DPPIV+ donor cell colonies in acetone-fixed frozen sections from livers of DPPIV- host rats. A: DPPIV+ donor cell colonies in MMC/PH treated host rats at 9 weeks post-transplantation of late gestation fetal liver cells. The arrows designate donor derived bile ducts. In circled areas below the line of ducts are type I hepatocyte colonies with well-defined DPPIV+ bile canaliculi. A large cluster of sinusoids lined by DPPIV+ endothelial cells (type II colony) is located in the upper left corner (bar=50µm). B: MMC/PH treated host rats 4 weeks post-transplantation of ACI newborn liver cells. Type I hepatocyte colonies with strong canalicular DPPIV activity (arrows) can be seen within large clusters of sinusoids lined by DPPIV+ donor endothelial cells. Sinusoids within hepatocyte colonies were also positive for DPPIV (bar=200µm). C: DPPIV+ type I hepatocyte colony in a MMC/PH treated host rat at 3 weeks post-transplantation of ED16 fetal liver cells (bar=200µm). D: Three DPPIV+ donor hepatocyte colonies at three weeks post-transplantation of ED16 fetal liver cells into a retrorsine/PH treated host rat (bar=200µm).
Figure 3.
Phenotypic analysis of type I donor colonies present at 5 weeks post-transplantation of adult (A, D and F) and newborn (B, C and E) liver isolates. Acetone fixed frozen sections were double labeled by IIF with an antibody specific for enzymatically active DPPIV (green). Donor hepatocytes were positive for the following epitopes/proteins (red): A: H.4, a cytoplasmic hepatocyte epitope, B: Pan-Cadherin (plasma membrane); C: LAP (bile canaliculi), D: DP.I (desmosomal plaques); E: LYRIC, a tight junction protein; F: CYP2E1. The arrow indicates a small hepatocyte colony positive for both DPPIV (green) and CYP2E1 (red). Yellow fluorescence in B and C result from the overlap in red and green fluorescence. There is no overlap in red and green fluorescence in D and E because DP.1 and LYRIC are on the cytoplasmic face (red) and DPPIV on the extracellular face (green) of the plasma membrane (bars=50 µm).
Table 1.
Phenotype of Donor Colonies
| Syngeneic | Allogeneic | |||
|---|---|---|---|---|
| Type I | Type II | Type I | Type II | |
| DPPIV | + | + | + | + |
| H.4 | + | NDa | + | ND |
| E−Cadherin | + | ND | + | ND |
| Pan−Cadherin | + | + | + | + |
| LAPb | + | − | + | − |
| Desmoplakin I | + | − | + | − |
| LYRIC | + | − | + | − |
| CYP2E1 | + | ND | + | ND |
| GGT | + | − | + | − |
| RECA−1 | − | + | − | + |
| Transferrin receptor |
− | − | − | − |
| Albumin | + | ND | + | ND |
| ATPase | + | ND | + | ND |
| G−6−Pase | + | ND | + | ND |
| PAS | + | ND | + | ND |
ND = not determined
Abbreviations: LAP, leucine amino peptidase; GGT, γ−glutamyl transpeptidase; RECA−1, rat endothelial cell antigen 1; G−6−Pase, glucose−6−phosphatase; PAS, Periodic Acid−Schiff stain
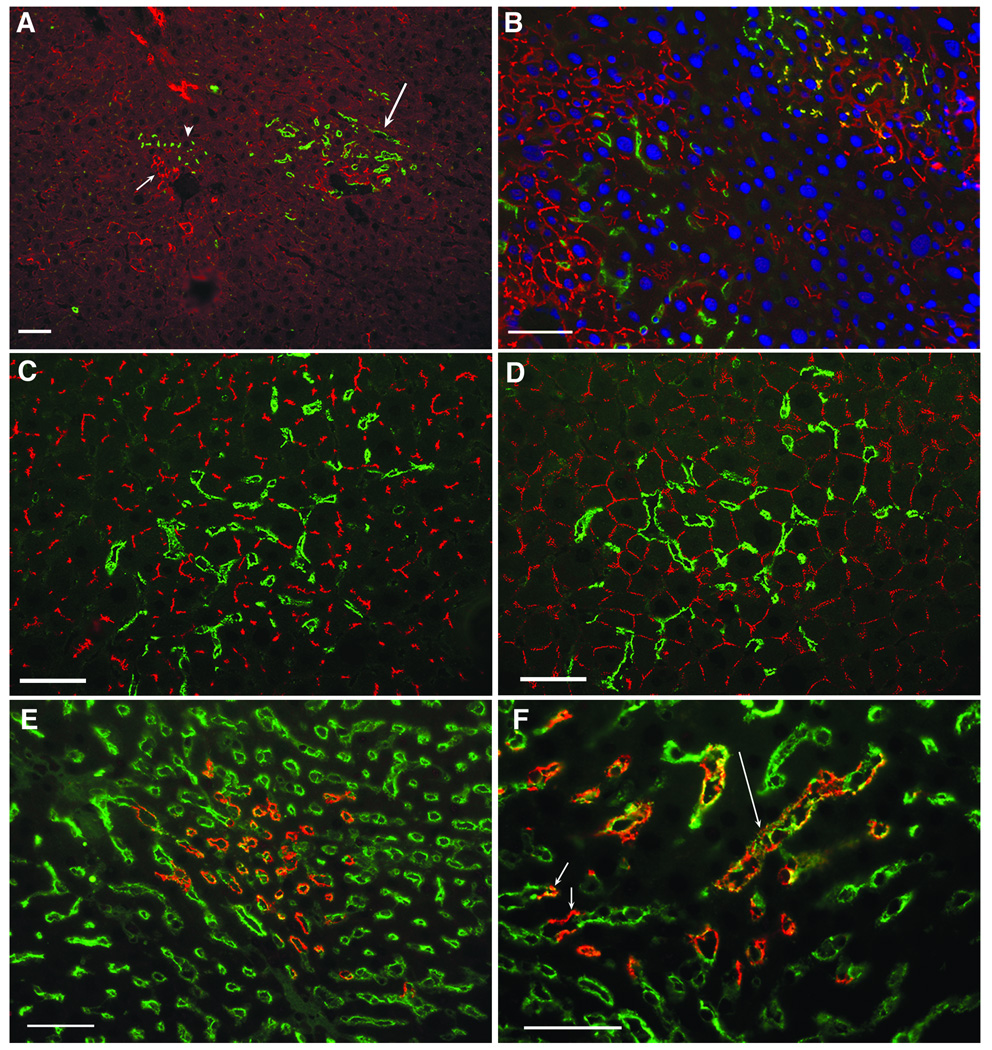
Figure 4.
Phenotypic analysis of type II donor colonies present at 5 weeks post-transplantation of adult (A and D) and newborn (B, C, E and F) liver isolates. Acetone fixed frozen sections were double labeled by IIF with a MAb specific for enzymatically active DPPIV (green). Donor hepatocytes were positive for the following epitopes/proteins (red); A, GGT: A DPPIV+/GGT− type I colony (arrow head) is located adjacent to a cluster of DPPIV−/GGT+ host hepatocytes (short arrow). DPPIV+/GGT− type II colonies (long arrow) are also shown. B, GGT: Type I colonies in the upper right contained a subpopulation of cells displaying a strong canalicular GGT reactivity. These cells appeared yellow due to overlap in green DPPIV and red GGT fluorescence. Type II colony on the left was negative for GTT as indicated by the lack of overlap in the red and green fluorescence. Nuclei were counterstained with DAPI (blue). C and D, LAP and DP.1: These membrane proteins were strongly expressed by hepatocytes (red) but were not detected on DPPIV+ endothelial cells (green). E: RECA-1 (green) was strongly expressed on the surface of DPPIV+ endothelial cells (yellow to orange) in type II colonies. Donor endothelial cells displayed both orange and yellow fluorescence, the color depending upon the intensity of the green RECA-1 labeling and the degree of overlap of the green and red fluorescence. F. Higher magnification view of panel E showing the presence of sinusoids partially (green and orange/yellow, short arrow) or completely (orange/yellow long arrow) lined by donor and host or completely by host (green) endothelial cells (bars=50µm).
Double IIF labeling showed that DPPIV+ cells in type II colonies (Figure 4C, green) were negative for the canalicular marker, LAP (Figure 4C, red) (14) and for GGT (Figure 4A, long arrow). When type II colonies were double IIF labeled for DPPIV and desmoplakin I (Figure 4D) the punctate canalicular pattern of desmoplakin membrane fluorescence seen in type I colonies was not apparent. Taken together, these findings suggested that DPPIV reactivity in type II colonies was present in GGT−/LAP-negative endothelial cells or was localized in the sinusoidal membranes of donor DPPIV+ hepatocytes, a membrane domain that is normally positive for DPPIV but negative for both GGT and LAP (14). To distinguish between these two possibilities, double IIF labeling was performed with a rabbit anti-DPPIV antibody and a pan-endothelial monoclonal antibody specific for RECA-1 (18). As shown in Figure 4E and F, DPPIV+ cells in type II colonies were strongly positive for RECA-1, indicating that the type II colonies were composed of DPPIV+ donor endothelial cells that completely (long arrow) or partially (short arrow) lined sinusoids in the host liver. The endothelial origin of type II colonies was also consistent with a previous report documenting the absence of GGT on liver endothelial cells (28).
Transplantation of late gestation fetal and newborn liver isolates produced mixed colonies with both donor hepatocytes and endothelial cells
In general, type I colonies formed in animals receiving fetal or newborn liver isolates were larger and more frequent than in animals transplanted with adult hepatocytes. Mixed colonies with both donor derived hepatocytes and endothelial cells were observed more frequently in host rats receiving fetal versus adult liver isolates or at the higher dose (1×107 versus 5×105) of donor cells. When the areas of type I and type II colonies were determined by image analysis, the area encompassed by type II colonies was consistently larger (8–26-fold) that the area occupied by type I hepatocyte colonies (Figure 5A and B).
Figure 5.
A and B: Relative size of type I and type II colonies, respectively. The area of donor colonies was determined by morphometric analysis using Image-Pro Plus software. Numbers above each bar indicate the number of colonies that were measured. Note the ten-fold difference in the scale of the Y-axis in Panels A and B. In Panel A, numbers within each bar show the number of type I colonies in microscopic fields with at least one type I colony. Error bars indicate the standard error of the mean. C: Image analysis of frozen sections of MMC versus saline treated rats stained for mitotic cells by IIF for phospho-histone-3. D: Hematoxylin and eosin stained frozen section from an MMC/PH treated rat at 3 weeks post-PH. E: Hematoxylin and eosin stained frozen section from an MMC/PH treated rat at 6 weeks post-PH. The encircled area designates a colony of small hepatocyte progenitors (bars=100µm).
Treatment with MMC is required for colony formation
To determine if MMC was promoting the expansion of engrafted donor cells, ED16 fetal liver cells were transplanted into host rats receiving a PH without MMC. At four weeks post-transplantation, examination of frozen sections histochemically stained for DPPIV did not reveal any type I or type II colonies. Colonies were also not observed in sham injected host rats previously treated with MMC and PH. This suggested that MMC treatment was required for colony formation. Expansion of donor cells in retrorsine/PH treated host rats results from the mitoinhibition that occurs secondary to DNA alkylation and crosslinking by retrorsine. To see if the same mechanism was operative in MMC/PH treated rats, frozen sections from rats given a PH after injection of saline or MMC were stained with an antibody specific for phospho-histone-3, a marker for mitotic cells. Image analysis of frozen sections stained by IIF for phospho-histone-3 revealed a nine-fold difference in the number of positive cells in MMC versus saline treated rats (Figure 5C), suggesting MMC/PH, like retrorsine/PH, causes the selective proliferation of transplanted donor cells in response to PH.
Type I hepatocyte colonies predominate in retrorsine/PH treated host rats transplanted with fetal hepatocytes
Type I donor colonies present at four weeks post-transplantation of fetal liver cells from DPPIV+ rats into MMC/PH treated (Figure 2C) rats contained well defined DPPIV+ bile canaliculi and were histologically identical (Figure 2D) to donor hepatocyte colonies in retrorsine/PH treated DPPIV− hosts. Type II colonies were rarely seen in retrorsine/PH treated host rats receiving fetal or adult hepatocytes.
Endogenous colonies of small DPPIV− hepatocytes were present in MMC/PH treated host rats
Frozen sections stained with hematoxylin and eosin (Figure 5D and E), were examined for the presence of oval cells and small hepatocytes, a progenitor population that in the absence of exogenous donor hepatocytes regenerates the liver of retrorsine/PH treated rats (6, 29, 30). At the time points examined, colonies of small hepatocyte progenitors were present within the parenchyma (Figure 5E, outlined area) but periportal areas were devoid of oval cells or immature oval cell ducts (Figure 5D).
Treatment with anti-CD3 antibody promoted the engraftment and expansion of allogeneic liver cells
Anti-CD3 antibodies have been used successfully to treat acute allograft rejection in humans (31), to suppress autoimmunity in diabetic mice (32) and to induce anergy in CD8+T cells (33). To determine if treatment with anti-CD3 would allow allogeneic transplantation, colony formation was assessed following transplantation of ACI liver isolates (RT1av1 haplotype) into F344 DPPIV− host rats (RT1lv1 haplotype) (34) treated with MMC, anti-CD3 and PH. These two rat strains that differ at major histocompatibility loci have been used extensively in previous studies of the immune mechanisms responsible for allograft rejection (35–37). Since we were uncertain about the efficiency of engraftment, host rats were injected with 1×107 cells, 20 times the standard dose. As show in Figure 2B, at four weeks post-PH, type II colonies of DPPIV+ endothelial cells occupied large areas of the host parenchyma. Numerous type I hepatocyte colonies with well-defined canaliculi were also present. However, by 8 weeks there was a marked decrease or complete loss of DPPIV+ donor colonies. This suggested a recovery of T cell function during the interval between 4 and 8 weeks post-transplant. By giving host rats a second treatment with anti-CD3 antibody three weeks post-PH, we were able to extend the period of immunosuppression to 8 weeks. At this time point, the repopulation of the DPPIV− host liver with DPPIV+ hepatocyte colonies was more extensive than in rats receiving syngeneic transplants. Significantly, extensive type II colonies and groups of donor-derived bile ducts were present in most sections examined (data not shown).
To determine if the high levels of liver replacement with allogeneic isolates could be attributed to the 20-fold increase in the number of transplanted cells, we examined the size of donor colonies present at 4 weeks post-transplantation of 1×107 F344 DPPIV+ liver cells. Results in Figure 5A show a significant increase in type I colony size at the higher cell dose. This can be seen by comparison of columns 1 and 2 in Figure 5A. Type I colonies also appeared to increase in size with increasing time (Figure 5A, columns 1 and 3). It seems likely that the increase in colony size with cell dose and time was caused at least in part by the merging of neighboring colonies, an occurrence that would be facilitated by an increase in the number of colonies. Interpreting the results for type II colonies was more difficult since cell dose was based on the number of hepatocytes in each isolate not the number of endothelial cells. As a consequence, relationships between colony size and time post-PH or cell dose could not be determined with any certainty. Taking these limitations into consideration, there still appeared to be an increase in colony size at the higher cell dose (Figure 5B, columns 1 and 2). To determine if there was a positive relationship between cell dose and frequency of type I colonies, we calculated the number of type I colonies in microscopic fields with at least one type I colony. As shown in Figure 5, at 4 weeks there was a 1.6–2.1-fold increase in frequency (columns 2 and 4 versus columns 1 and 3, respectively) at a dose of 1×107 versus 5×105 cells.
DISCUSSION
Transplantation of DPPIV+ donor liver cells into DPPIV− rats treated with MMC/PH/gentamicin offers a rapid alternative to the retrorsine/PH transplantation protocol for assessing the differentiation potential of putative hepatic progenitors and the engraftment efficiency of non-parenchymal cells. The key to the successful implementation of this protocol was the initiation of antibiotic therapy following PH, a treatment that significantly improved long term survival. The positive response to antibiotics observed in the present study suggests that systemic or pulmonary infections acquired as a consequence of transient MMC-induced neutropenia could be a major factor in the pronounced morbidity observed in untreated animals at 4–6 weeks. Since rat chow supplemented with antibiotics can be obtained commercially (Land O’Lakes Purina Feed, Richmond, IN), this has potential as a more convenient alternative to i.p. injection.
By four weeks post-transplantation of fetal liver cells into DPPIV− MMC/PH treated host rats, large areas of the host liver contained sinusoids lined by DPPIV+ or a mixture of DPPIV+ and DPPIV− endothelial cells (Figure 4E and F). Clusters of DPPIV+ sinusoids lined by RECA-1 positive endothelial cells were also reported by Sandhu et al. six months post-PH transplantation of ED16 fetal liver epithelial cells (38) but at a much lower frequency and extent than in the present investigation. Endothelial cell engraftment is also infrequent in retrorsine/PH treated host rats (6, 30, 38), an indication that endothelial megalocytosis and G2/M arrest induced by retrorsine (39, 40) are either not sufficient or are unnecessary for establishing endothelial chimerism.
Previous studies by Matsumoto et al. showing the essentiality of endothelial cells for liver development (41) raises the possibility that in mixed colonies, the donor hepatocytes may benefit from de novo secretion of growth factors or cytokines initiated during the endothelial cell engraftment. These investigators showed that liver development in mice with VEGFR2 deficient endothelial precursors incapable of forming blood vessels was arrested by the failure of the liver epithelial cells to migrate into the septum transversum. A similar inhibition of liver development was observed in vitro when the growth of ED9.5 liver buds from mutant mice was compared to liver buds from wild type controls (41). In a recent report, LeCouter et al. found that treatment with VEGF induced hepatocyte proliferation in vivo but did not stimulate growth in vitro unless endothelial cells were present (42). Further analysis showed that hepatocyte proliferation in co-cultures treated with VEGF was due at least in part to hepatocyte growth factor secretion by endothelial cells.
In agreement with Sandhu et al. (38), we found higher numbers of donor derived bile ducts in MMC/PH treated host rats transplanted with fetal/newborn versus adult liver isolates. The number of donor derived ducts was also dose dependent, with high doses of fetal liver isolates yielding higher frequencies of both donor derived DPPIV+/OC.10 positive ducts and hepatocytes and a substantial increase in the numbers of sinusoids lined by donor endothelial cells. We have recently demonstrated that fetal cholangiocytes retain a high capacity for differentiation along a hepatocyte lineage (6). This raises the possibility that a portion of the donor derived ducts and hepatocytes in MMC/PH treated rats receiving fetal liver isolates could arise from immature cholangiocytes present at high levels in total fetal liver isolates (6) but at much lower levels in adult liver isolates. This differential results at least in part from the greater resistance of the biliary tree in adult versus fetal liver to dissociation by collagenase.
Recently, monocrotaline (MCT), a pyrrolizidine alkaloid structurally related to retrorsine, has been proposed as a substitute. Witek et al. found that two 30 mg/kg doses of MCT combined with a PH increased liver repopulation by donor hepatocytes 30-fold relative to host rats receiving only a PH (15% vs 0.5%) (43). However, none of the rats treated with doses of MCT above 37 mg/kg survived longer than 7 weeks, a mortality attributed to severe interstitial lung disease at higher doses. Pulmonary toxicity is a well recognized characteristic of MCT that has led to its use for almost 50 years as a model for pulmonary hypertension (44, 45). In a related study, Joseph et al. reported a dramatic increase in repopulation of host rats treated with a 200 mg/kg dose of MCT in combination with three injections of carbon tetrachloride but did not indicate whether pulmonary damage was caused by this treatment (46). Although MMC induced pulmonary hypertension is a well known but infrequent complication for cancer patients receiving high dose MMC for breast, liver or lung cancer (47–50), hypertension is uncommon in animals even at significantly higher dose levels (51).
To extend the usefulness of the MMC/PH protocol, we examined two different strategies for potentiating engraftment of allogeneic (ACI) liver cells in F344 DPPIV− host rats. The first involved the administration of donor ACI leukocytes immediately post-transplantation of an ACI liver isolate, an approach used by Yan et al. (52) to induce long-term acceptance of kidney or liver allografts. After three attempts with little or no engraftment, we abandoned this approach in favor of anti-CD3 antibody injections, a treatment known to be an effective means for suppressing T cell mediated allograft rejection (53). Daily administration of low doses of anti-CD3 antibody for four days (32) proved to be highly effective in promoting engraftment, expansion and survival of allogeneic cells for up to four weeks. We further showed that survival of allografts could be extended for up to eight weeks by a second administration of anti-CD3 injections at three weeks post-transplant.
The extensive engraftment of endothelial cells in MMC/PH and MMC/anti-CD3/PH treated host rat livers suggests the application of this protocol for investigations into the biological and immunological consequences of endothelial cell chimerism in liver allografts. In liver transplantation, endothelial cells in the allograft play a central role in both acute and chronic rejection responses, serving either as targets of the host immune reaction or as active participants that aid in the recruitment of host immune cells (54). Paradoxically, endothelial cells can also protect against vascular rejection by reducing alloreactivity of the transplanted organ, an effect that appears to result in part from the expression of inhibitory molecules such as ILT3 and ILT4 that induce a tolerogenic response (55). Endothelial chimerism resulting in the replacement of damaged graft endothelial cells with host progenitors (56, 57) has been suggested as a third mechanism for decreasing alloreactivity (58, 59) with the most extensive chimerism (>30% replacement) occurring in conjunction with vascular rejection (60). A possible strategy for exploring some of these issues would be to generate a chimeric liver to use the MMC/anti-CD3/PH protocol to populate the liver of donor rats (allograft donors) with allogeneic endothelial cells from host rats (allograft recipients). In effect, this would recreate the post-transplantation chimerism that is thought to suppress allograft rejection, a supposition that could be directly tested by showing a reduction in the alloreactivity of chimeric versus non-chimeric liver allografts.
In summary, MMC in combination with PH and antibiotic therapy appears to be an effective and rapid alternative to retrorsine/PH. With this protocol, donor derived hepatocyte colonies suitable for gene expression analysis at the RNA or protein level can be generated within four weeks of transplantation. When combined with anti-CD3 treatment, this protocol also supports the engraftment and expansion of liver cell allografts. The extensive engraftment of endothelial cells is an additional novel feature that could be exploited to study the role of endothelial cell chimerism in allograft survival.
ABBREVIATIONS
- PH
partial hepatectomy
- MMC
mitomycin C
- DPPIV−
dipeptidyl peptidase IV negative
- DPPIV+
dipeptidyl peptidase IV positive
- DPPIV
dipeptidyl peptidase IV
- MAbs
monoclonal antibodies
- ED
embryonic day
- GGT
γ-glutamyl transpeptidase
- LAP
Leucine amino peptidase
- IIF
indirect immunofluorescence
Footnotes
Sponsored by grants CA42715, CA93840 and RR017695
REFERENCES
- 1.Thompson NL, Hixson DC, Callanan H, et al. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem J. 1991;273(Pt 3):497. doi: 10.1042/bj2730497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigal SH, Rajvanshi P, Reid LM, Gupta S. Demonstration of differentiation in hepatocyte progenitor cells using dipeptidyl peptidase IV deficient mutant rats. Cell Mol Biol Res. 1995;41(1):39. [PubMed] [Google Scholar]
- 3.Coleman WB, McCullough KD, Esch GL, et al. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151(2):353. [PMC free article] [PubMed] [Google Scholar]
- 4.Hixson DC. Animal models for assessing the contribution of stem cells to liver development. In: Sell S, editor. Stem Cell Handbook. Towanda, NJ: Humana Press; 2003. p. 353. [Google Scholar]
- 5.Laconi S, Curreli F, Diana S, et al. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31(6):1069. doi: 10.1016/s0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]
- 6.Simper-Ronan R, Brilliant K, Flanagan D, et al. Cholangiocyte marker-positive and -negative fetal liver cells differ significantly in their ability to regenerate the livers of adult rats exposed to retrorsine. Development. 2006;133(21):4269. doi: 10.1242/dev.02589. [DOI] [PubMed] [Google Scholar]
- 7.Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine alkaloids--genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 2004;36(1):1. doi: 10.1081/dmr-120028426. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Faris RA, Hixson DC. Long-term culture and characteristics of normal rat liver bile duct epithelial cells. Gastroenterology. 1993;104(3):840. doi: 10.1016/0016-5085(93)91021-9. [DOI] [PubMed] [Google Scholar]
- 9.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following surgical removal. Arch Pathol. 1931;12:186. [Google Scholar]
- 10.Piazza GA, Callanan HM, Mowery J, Hixson DC. Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J. 1989;262(1):327. doi: 10.1042/bj2620327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson BM, Thompson NL, Hixson DC. Tightly regulated induction of the adhesion molecule necl-5/CD155 during rat liver regeneration and acute liver injury. Hepatology. 2006;43(2):325. doi: 10.1002/hep.21021. [DOI] [PubMed] [Google Scholar]
- 12.Comegys MM, Carreiro MP, Brown JF, et al. C-CAM1 expression: differential effects on morphology, differentiation state and suppression of human PC-3 prostate carcinoma cells. Oncogene. 1999;18(21):3261. doi: 10.1038/sj.onc.1202666. [DOI] [PubMed] [Google Scholar]
- 13.Hixson DC, Brown J, McBride AC, Affigne S. Differentiation status of rat ductal cells and ethionine-induced hepatic carcinomas defined with surface-reactive monoclonal antibodies. Exp Mol Pathol. 2000;68(3):152. doi: 10.1006/exmp.2000.2302. [DOI] [PubMed] [Google Scholar]
- 14.Mowery J, Hixson DC. Detection of cell-CAM 105 in the pericanalicular domain of the rat hepatocyte plasma membrane. Hepatology. 1991;13(1):47. [PubMed] [Google Scholar]
- 15.Gordon GJ, Coleman WB, Grisham JW. Induction of cytochrome P450 enzymes in the livers of rats treated with the pyrrolizidine alkaloid retrorsine. Exp Mol Pathol. 2000;69(1):17. doi: 10.1006/exmp.2000.2308. [DOI] [PubMed] [Google Scholar]
- 16.Britt DE, Yang DF, Yang DQ, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp Cell Res. 2004;300(1):134. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Faris RA, McEntire KD, Thompson NL, Hixson DC. Identification and characterization of a rat hepatic oncofetal membrane glycoprotein. Cancer Res. 1990;50(15):4755. [PubMed] [Google Scholar]
- 18.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66(4):459. [PubMed] [Google Scholar]
- 19.Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106(6):348. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 20.Molyneux G, Gibson FM, Gordon-Smith EC, et al. The haemotoxicity of mitomycin in a repeat dose study in the female CD-1 mouse. Int J Exp Pathol. 2005;86(6):415. doi: 10.1111/j.0959-9673.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto K, Furuya T, Tobe M. Effect of mitomycin C on bone marrow cells in mice. J Toxicol Sci. 1984;9(1):51. doi: 10.2131/jts.9.51. [DOI] [PubMed] [Google Scholar]
- 22.Futamura Y, Matsumoto K. Characteristics of peripheral blood monocytes and bone marrow macrophages from rats treated with mitomycin C, 5-fluorouracil or phenylhydrazine. J Toxicol Sci. 1995;20(1):1. doi: 10.2131/jts.20.1. [DOI] [PubMed] [Google Scholar]
- 23.Cassell GH, Davis JK, Lindsey JR. Control of Mycoplasma pulmonis infection in rats and mice: detection and elimination vs. vaccination. Isr J Med Sci. 1981;17(7):674. [PubMed] [Google Scholar]
- 24.Baker DG. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev. 1998;11(2):231. doi: 10.1128/cmr.11.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83(2):417. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 26.Karen Grant RC. A Layman's Guide to the Health and Nursing Care of Rats. vol 2006:2000–2006. [Google Scholar]
- 27.Gupta S, Rajvanshi P, Malhi H, et al. Cell transplantation causes loss of gap junctions and activates GGT expression permanently in host liver. Am J Physiol Gastrointest Liver Physiol. 2000;279(4):G815. doi: 10.1152/ajpgi.2000.279.4.G815. [DOI] [PubMed] [Google Scholar]
- 28.Hanigan MH, Frierson HF., Jr Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem. 1996;44(10):1101. doi: 10.1177/44.10.8813074. [DOI] [PubMed] [Google Scholar]
- 29.Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156(2):607. doi: 10.1016/S0002-9440(10)64765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon GJ, Butz GM, Grisham JW, Coleman WB. Isolation, short-term culture, and transplantation of small hepatocyte-like progenitor cells from retrorsine-exposed rats. Transplantation. 2002;73(8):1236. doi: 10.1097/00007890-200204270-00008. [DOI] [PubMed] [Google Scholar]
- 31.Cole MS, Stellrecht KE, Shi JD, et al. HuM291, a humanized anti-CD3 antibody, is immunosuppressive to T cells while exhibiting reduced mitogenicity in vitro. Transplantation. 1999;68(4):563. doi: 10.1097/00007890-199908270-00020. [DOI] [PubMed] [Google Scholar]
- 32.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91(1):123. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodle ES, Hussein S, Bluestone JA. In vivo administration of anti-murine CD3 monoclonal antibody induces selective, long-term anergy in CD8+ T cells. Transplantation. 1996;61(5):798. doi: 10.1097/00007890-199603150-00021. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Li XH, Miyamoto M, Sano T, Hattori Y, Yamashita A. Induction of transplantation tolerance in adult rats by vascularized spleen transplantation. Transplantation. 1997;64(4):650. doi: 10.1097/00007890-199708270-00018. [DOI] [PubMed] [Google Scholar]
- 35.Part 1: Mouse and Rat. Bethesda, Maryland: Federation of American Societies for Experimental Biology; 1979. Inbred and Genetically Defined Strains of Laboratory Animals; p. 418. [Google Scholar]
- 36.Wang JJ, Hendrich KS, Jackson EK, Ildstad ST, Williams DS, Ho C. Perfusion quantitation in transplanted rat kidney by MRI with arterial spin labeling. Kidney Int. 1998;53(6):1783. doi: 10.1046/j.1523-1755.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 37.Foster RD, Pham S, Li S, Aitouche A. Long-term acceptance of composite tissue allografts through mixed chimerism and CD28 blockade. Transplantation. 2003;76(6):988. doi: 10.1097/01.TP.0000079827.91675.A3. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol. 2001;159(4):1323. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean EK. The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev. 1970;22(4):429. [PubMed] [Google Scholar]
- 40.Shah M, Patel K, Sehgal PB. Monocrotaline pyrrole-induced endothelial cell megalocytosis involves a Golgi blockade mechanism. Am J Physiol Cell Physiol. 2005;288(4):C850. doi: 10.1152/ajpcell.00327.2004. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294(5542):559. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 42.LeCouter J, Moritz DR, Li B, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299(5608):890. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 43.Witek RP, Fisher SH, Petersen BE. Monocrotaline, an alternative to retrorsine-based hepatocyte transplantation in rodents. Cell Transplant. 2005;14(1):41. doi: 10.3727/000000005783983278. [DOI] [PubMed] [Google Scholar]
- 44.Schultze AE, Roth RA. Chronic pulmonary hypertension--the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1998;1(4):271. doi: 10.1080/10937409809524557. [DOI] [PubMed] [Google Scholar]
- 45.Lalich JJ, Merkow L. Pulmonary arteritis produced in rat by feeding Crotalaria spectabilis. Lab Invest. 1961;10:744. [PubMed] [Google Scholar]
- 46.Joseph B, Kumaran V, Berishvili E, Bhargava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44(6):1411. doi: 10.1002/hep.21416. [DOI] [PubMed] [Google Scholar]
- 47.Lenci G, Muller-Quernheim J, Lorenz J, Schweden F, Ferlinz R. [Toxic lung damage caused by mitomycin C] Pneumologie. 1994;48(3):197. [PubMed] [Google Scholar]
- 48.Cheirsilpa A, Leelasethakul S, Auethaveekiat V, et al. High-dose mitomycin C: activity in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1989;24(1):50. doi: 10.1007/BF00254105. [DOI] [PubMed] [Google Scholar]
- 49.Okuno SH, Frytak S. Mitomycin lung toxicity. Acute and chronic phases. Am J Clin Oncol. 1997;20(3):282. doi: 10.1097/00000421-199706000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Buzdar AU, Legha SS, Luna MA, Tashima CK, Hortobagyi GN, Blumenschein GR. Pulmonary toxicity of mitomycin. Cancer. 1980;45(2):236. doi: 10.1002/1097-0142(19800115)45:2<236::aid-cncr2820450207>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Verweij J, Kerpel-Fronius S, Stuurman M, et al. Mitomycin C-induced organ toxicity in Wistar rats: a study with special focus on the kidney. J Cancer Res Clin Oncol. 1988;114(2):137. doi: 10.1007/BF00417827. [DOI] [PubMed] [Google Scholar]
- 52.Yan Y, Shastry S, Richards C, et al. Posttransplant administration of donor leukocytes induces long-term acceptance of kidney or liver transplants by an activation-associated immune mechanism. J Immunol. 2001;166(8):5258. doi: 10.4049/jimmunol.166.8.5258. [DOI] [PubMed] [Google Scholar]
- 53.Isaacs JD. T cell immunomodulation--the Holy Grail of therapeutic tolerance. Curr Opin Pharmacol. 2007;7(4):418. doi: 10.1016/j.coph.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Rifle G, Mousson C, Herve P. Endothelial cells in organ transplantation: Friends or foes? Transplantation. 2006;82(1 Suppl):S4. doi: 10.1097/01.tp.0000231368.36476.4a. [DOI] [PubMed] [Google Scholar]
- 55.Cortesini R, Suciu-Foca N. ILT3+ ILT4+ tolerogenic endothelial cells in transplantation. Transplantation. 2006;82(1 Suppl):S30. doi: 10.1097/01.tp.0000231437.12890.64. [DOI] [PubMed] [Google Scholar]
- 56.Koopmans M, Kremer Hovinga IC, Baelde HJ, de Heer E, Bruijn JA, Bajema IM. Endothelial chimerism in transplantation: Looking for needles in a haystack. Transplantation. 2006;82(1 Suppl):S25. doi: 10.1097/01.tp.0000231446.41051.98. [DOI] [PubMed] [Google Scholar]
- 57.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952. [PubMed] [Google Scholar]
- 58.Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339(23):1657. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 59.Aird WC. Endothelium and allotransplantation. Transplantation. 2006;82(1 Suppl):S6. doi: 10.1097/01.tp.0000231345.23918.bd. [DOI] [PubMed] [Google Scholar]
- 60.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH. Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet. 2001;357(9249):33. doi: 10.1016/S0140-6736(00)03569-8. [DOI] [PubMed] [Google Scholar]