Abstract
Diabetic Autonomic Neuropathy (DAN), a major complication of diabetes mellitus, is characterized in part by impaired cardiac parasympathetic responsiveness. Parasympathetic stimulation of the heart involves activation of an acetylcholine-gated K+ current, IKAch, via a (GIRK1)2/(GIRK4)2 K+ channel. Sterol regulatory element binding protein-1 (SREBP-1) is a lipid-sensitive transcription factor. We describe a unique SREBP-1-dependent mechanism for insulin regulation of cardiac parasympathetic response in a mouse model for DAN. Compared to WT mice, Ins2Akita type I diabetic mice demonstrated a decrease in the negative chronotropic response to carbamylcholine characterized by a 2.4 fold decrease in duration of bradycardia; a 52±8% decrease in atrial expression of GIRK1 (P<0.01) and a 31.3±2.1% decrease in SREBP-1 (P<0.05). Myocytes from atria of Akita mice exhibited a markedly decreased carbamylcholine stimulation of IKAch with a peak value of −181±31 pA/pF compared to −451±62 pA/pF (P<0.01) for cells from WT mice. Insulin treatment of Akita mice reversed the impairment in parasympathetic response, increased the expression of GIRK1, SREBP-1 and IKAch activity in atrial myocytes from these mice to levels in WT mice. Insulin treatment of cultured atrial myocytes stimulated GIRK1 expression 2.68±0.12 fold (P<0.01) while overexpression of DN-SREBP-1 reversed this insulin effect. Finally, adenoviral expression of SREBP-1 in Akita atrial myocytes reversed the impaired IKAch to levels in cells from WT. These results support a unique molecular mechanism for insulin regulation of GIRK1 expression and parasympathetic response via SREBP-1 which might play a role in the pathogenesis of DAN in response to insulin deficiency in the diabetic heart.
Keywords: Diabetic autonomic neuropathy, SREBP, insulin deficiency, GIRK channel
INTRODUCTION
Diabetes mellitus is associated with severe debilitating complications that include a Diabetic Autonomic Neuropathy (DAN) characterized by impairment of vascular reflexes, occasional hypotension and decreased sympathetic and parasympathetic responsiveness of the heart.1 Approximately 50% of patients with diabetes for 10 years or more demonstrate an impaired response of the heart to parasympathetic stimulation.2 The presence of DAN is a significant risk factor as demonstrated by a five fold higher 5-year mortality compared with diabetics without DAN.3
Parasympathetic regulation of the heart has both a neuronal component involving vagal stimulation of parasympathetic ganglia in the atria and atrioventricular (AV) node followed by release of acetylcholine and a molecular component involving an intrinsic cardiac signaling pathway which mediates the parasympathetic response to acetylcholine. The latter involves the binding of acetylcholine to M2 muscarinic receptors on the surface of cardiomyocytes and dissociation of the heterotrimeric G-Protein, Gi2, into Gαi2 and Gβγ subunits. Gβγ binds to and activates the G-Protein Coupled Inward Rectifying K+ channel, (GIRK1)2/(GIRK4)2, the channel which is responsible for IKAch (acetylcholine-gated K+ current), resulting in a decrease in the rate of diastolic depolarization and a decrease in heart rate (negative chronotropic effect).4, 5 Changes in levels of expression of M2, and Gαi2 in the heart have been shown to play a role in determining the magnitude of the parasympathetic response.6–8
Sterol regulatory element binding proteins (SREBPs) are a family of three transcription factors that regulate expression of genes involved in lipid homeostasis and glucose metabolism. SREBPs are synthesized in the endoplasmic reticulum as 130-kDa precursor molecules that are transported to the Golgi where they are processed via a two-step sequential proteolytic cleavage to produce a 480 amino acid transcription factor which is transported to the nucleus.9, 10 The expression, transport and processing of these proteins are subject to feedback regulation by sterols. SREBP-1 levels have been shown to be regulated by insulin.10–12
The Ins2Akita diabetic mouse is characterized by a point mutation in the pro-insulin ins2 gene (Ins2Cys96Tyr) which interferes with the transport of ins1 and ins2, resulting in destruction of islet cells and development of the diabetic phenotype.13 Heterozygous males may survive untreated for up to a year making them a good model for the study of secondary effects of diabetes.14
The autonomic dysfunction associated with diabetes mellitus has been attributed to effects of hyperglycemia on neuronal survival and neuronal function.15 Here we show that the Akita type I diabetic mouse demonstrates a decreased response of the heart to parasympathetic stimulation characteristic of DAN associated with a decrease in expression of SREBP-1 and GIRK1 in the atrium. Our data support a new molecular mechanism for the impaired parasympathetic response in the diabetic heart and a unique relationship between insulin, lipid homeostasis and the parasympathetic response which might serve as a new therapeutic target for the treatment of DAN.
METHODS
Detailed experimental protocols are described in the expanded Materials and Methods section in the online data supplement, available at http://circres.ahajournals.org.
Animals
The heterozygous male Akita Ins2Cys96Tyr mice and littermate wild type mice were from Jackson laboratories. All vertebrate animal-related procedures described were approved by the Tufts Medical Center Institutional Animal Care Committee.
Cell culture
Embryonic chick atrial myocytes were cultured as described.16 Atrial myocytes from mouse atria were prepared by a retrograde Langendorf perfusion method as described.17
ECG monitoring in conscious mice
An implantable wireless radiofrequency transmitter was inserted and the ECG signal was recorded with the use of a telemetry receiver and an analog-to-digital acquisition system (Data Sciences International).18
Western blot analysis
To determine GIRK1 and SREBP-1 levels, Western blot analysis was carried out as described previously.17 A GIRK1 specific antibody from Alomone Labs (Jerusalem, Israel) and SREBP-1 antibodies from Santa Cruz were used.
Cellular Electrophysiology
Membrane currents were measured by the patch-clamp technique in whole-cell mode using an LM-EPC7 amplifier as described.17
Echocardiography
Echocardiographic studies were performed as described previously.19
Statistical analysis
All values are expressed as mean ±SEM. Statistical differences between mean values were calculated by ANOVA, followed by Bonferroni's test for unpaired comparisons where appropriate. For comparison of WT and DM mice, Student's t-test was applied. The effect of insulin treatment on diabetic mice was assessed using a paired t-test. A 2-tailed p value ≤0.05 was considered significant.
REUSLTS
Metabolic State and Left Ventricular Structure and Function in Akita Type I Diabetic Mice
Blood glucose in Akita diabetic (DM) mice increased from 294±16 mg/dL (n=13) at 4 weeks of age reaching a plateau varying from 406–732 mg/dL with a mean of 585±18 mg/dL (n=33) at 4 months of age. In WT mice glucose remained stable at 149±8 mg/dL (n=27) at all ages studied. Hemaglobin-A1c was 9.41±0.4 (n=9) in Akita mice compared with 4.37±0.09 (n=7) in WT. Although male Akita heterozygotes demonstrated a marked hyperglycemia, there was no significant difference in blood pH, serum electrolytes and anion Gap between Akita and WT mice four to six month of age (Online Table S1). Echocardiographic analysis demonstrated no significant differences in left ventricular end diastolic dimension, left ventricular end systolic dimension, fractional shortening, ejection fraction and resting heart rate in Akita DM mice compared to WT mice (Online Table S2).
The Ins2Akita mouse demonstrates parasympathetic dysfunction
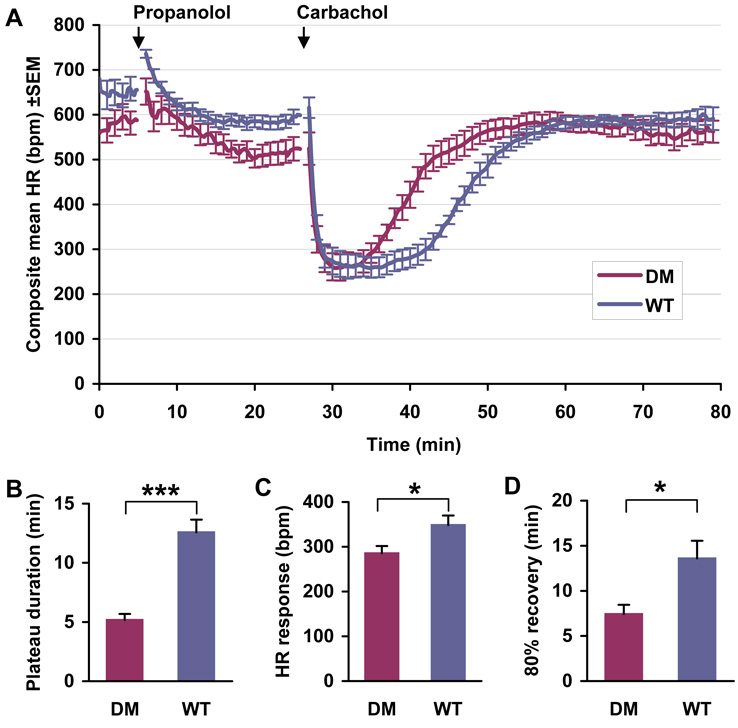
To determine whether type I diabetic Akita mice develop parasympathetic dysfunction, ten conscious male WT mice 6 months of age with an average serum glucose level of 125±20 mg/dL and ten littermate male Akita mice with average serum glucose >500 mg/dL were pretreated with propranolol in order to block β-adrenergic reflex responses to carbamylcholine. Propranolol blockade at this concentration has been shown to persist in mice for up to 2 hours.20 Animals were subsequently challenged with the non-hydrolyzable acetylcholine analogue carbamylcholine, and heart rate determined using implantable ECG transmitters. The effects of carbamylcholine on heart rate were significantly decreased in the Akita mouse. The duration of bradycardia was 5.2±0.51 min in the Akita mouse compared to 12.6±1.07 min in WT mice (P<0.001, Fig. 1A, B). Following carbamylcholine treatment, heart rate recovered to 80% of pretreatment levels significantly faster in Akita mice, 7.4±1.03 min, compared to 13.6±1.96 min in WT mice (P<0.05, Fig. 1A, D), and the slope of recovery of heart rate was much steeper 36.4±4.85 beats/min in the Akita mouse vs. 23.5±2.73 beats/min in the WT mouse (P<0.05). Finally, the absolute decrease in heart rate in response to carbamylcholine was 285.5±16.07 beats/min in Akita mice, compared to 348.5±21.27 beats/min in WT mice (P<0.05, Fig. 1A, C). We also noted that compared to WT there was a tendency to a decreased initial heart rate in the Akita mice, which might be due to a decreased intrinsic heart rate (heart rate in the absence of autonomic stimulation) in the diabetic heart.
Figure 1. Comparison of the heart rate response of WT and Akita mice to parasympathetic stimulation.
A. Continuous ECGs were recorded as described in Methods. Mice were treated with an i.p. injection of propranolol, 1 mg/kg, followed in 20 minutes by an i.p. injection of carbamylcholine, 0.2 mg/kg. Data are the composite mean heart rate (±SEM) of 10 age-matched WT and 10 Akita (DM) mice. B. The plateau time of carbamylcholine induced bradycardia in WT and Akita mice (DM); C. Negative chronotropic response to carbamylcholine; D. Time elapsed for 80% recovery to baseline heart rate determined as described in Methods. *P<0.05, ***P<0.001.
IKAch is decreased in dissociated atrial myocytes from Akita DM mice
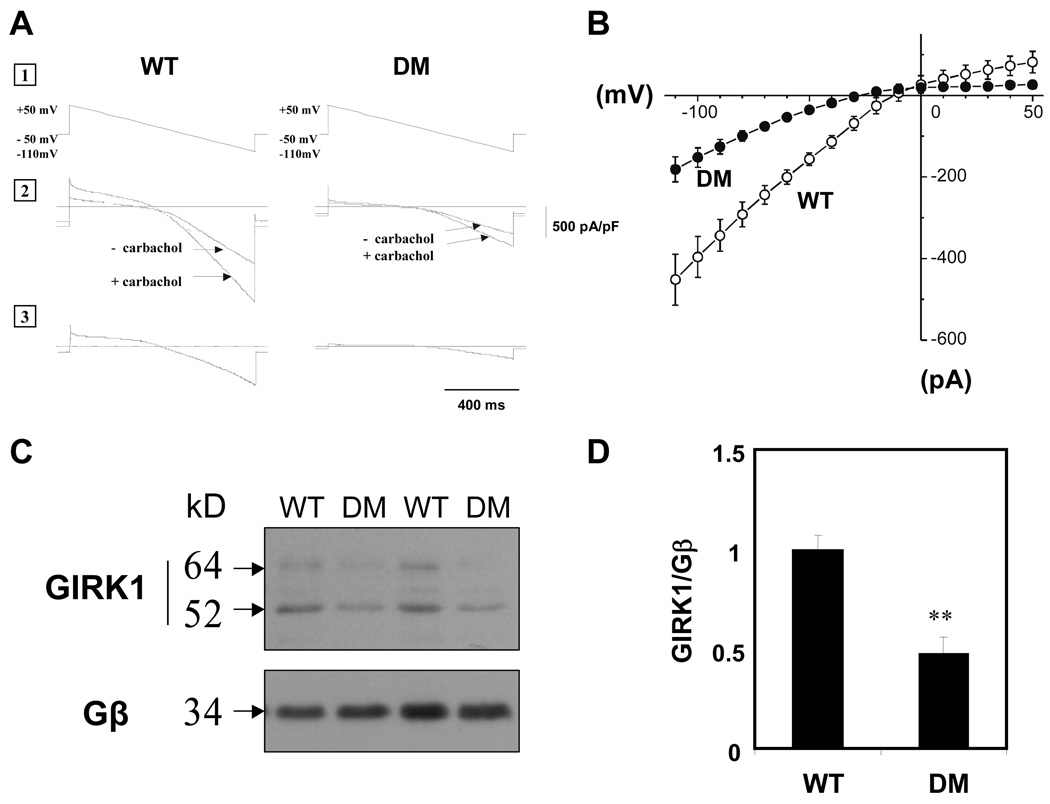
To determine whether the decreased response of heart rate to carbamylcholine in the Akita mouse was associated with a decrease in IKAch, we compared membrane currents in atrial myocytes from Akita and WT mice. Rod shaped cells from atria of Akita mice 4 months of age remained viable for up to 48 hours in culture medium demonstrating clearly defined striations and spontaneous contractions with stable resting membrane potentials. The current voltage (I-V) relationships derived from membrane currents in these cells demonstrated that myocytes from atria of adult Akita mice exhibited a markedly decreased carbamylcholine stimulation of IKAch with a peak value of −181±31 pA/pF (n=5) compared to −451±62 pA/pF (n=5, P<0.01) for cells from WT mice (Fig 2A and B).
Figure 2. IKAch in atrial myocytes and atrial GIRK1 expression are decreased in Akita mice compared to WT.
A. Current-voltage (I-V) relationship of the carbamylcholine-induced whole cell currents elicited from a 1 second voltage ramp with a continuously changing voltage from +50 to −110 mV, from a holding potential of −50 mV (1). Current in a typical atrial myocyte with and without 20 µM carbamylcholine (2). Current generated by subtracting curves (1) from (2) (3). B. I-V plots constructed from a series of data points in Fig. 2A, panel 3. Data are the mean of 5 determinations each from 3 Akita and 3 WT mice. C. Western blot analysis of GIRK1 expression in atria of WT and Akita mice. D. Densitometry analysis of both glycosylated (64 kD) and unglycosylated (52 kD) GIRK1 bands determined by autoradiographs from 6 WT and 6 Akita mice normalized to the expression of Gβ, (**P<0.01).
Atria of Akita diabetic mice demonstrate decreased expression of GIRK1
In order to determine whether the decrease in parasympathetic responsiveness in the Akita mouse heart and the decrease in IKAch in atrial myocytes from these mice were due to a decrease in expression of molecular components of the parasympathetic response pathway, the level of expression of GIRK1 was compared in extracts from atria of WT and Akita mice. Western blot analysis demonstrated two bands which had been previously shown to correspond to the glycosylated and unglycosylated forms of GIRK1.21 Levels of both forms of GIRK1 were decreased by 52±8% (P<0.01) in Akita mice compared to WT (Fig. 2C, D). Although glucose levels in Akita mice ranged from 406–732 mg/dL, there was no correlation between the severity of hyperglycemia and changes in GIRK1 expression.
Insulin treatment reverses the parasympathetic dysfunction in the Akita mouse
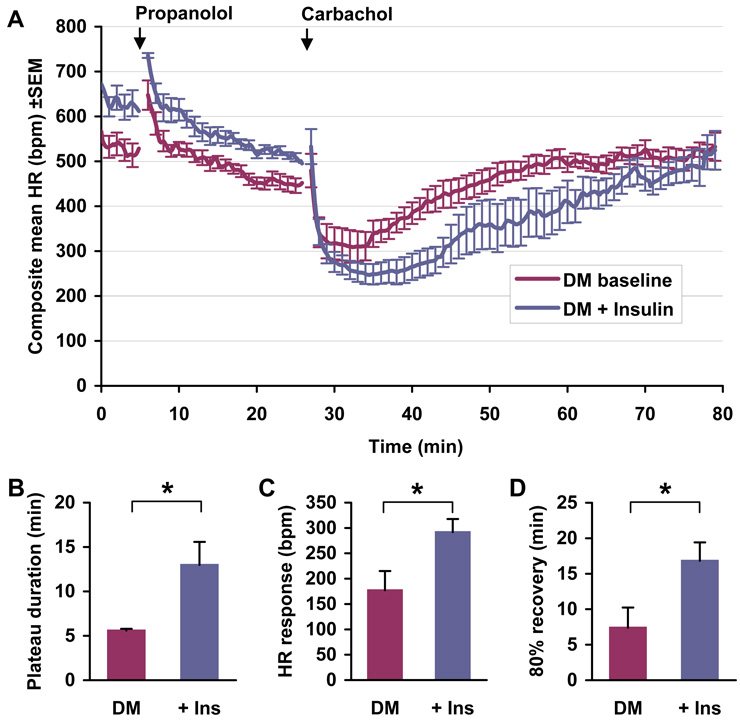
To determine whether the decreased response of the Akita mouse heart to parasympathetic stimulation might be an insulin dependent reversible process, we measured the effect of insulin treatment on parasympathetic responsiveness, IKAch and GIRK1 expression in Akita mice. Implantation of insulin pellets in Akita mice decreased serum glucose from 510±14 to 118±16 mg/dL (n=7) with a 3–4 day time course. Duration of bradycardia was increased by 7.4±2.67 min (n=7, P<0.05) in insulin treated mice, more than 2 fold higher than pretreatment levels (Fig. 3A, B). The recovery time of heart rate to 80% of resting levels following carbamylcholine injection increased from a pretreatment level of 7.4±2.84 min to 16.8±2.59 min in insulin treated mice (n=7, P<0.05, Fig. 3A, D). Finally, the absolute decrease in heart rate in response to carbamylcholine was 114.71±32.51 beats/min greater in insulin treated mice compared to untreated Akita mice (n=7, P<0.05, Fig. 3A, C). Thus insulin treatment significantly reversed the parasympathetic dysfunction in the Akita mouse.
Figure 3. Effect of insulin on the heart rate response of Akita mice to carbamylcholine.
A. Negative chronotropic response of Akita mice (DM) to carbamylcholine prior to and after treatment with slow releasing insulin pellets (+Ins). Glucose levels were monitored on alternative days and maintained constant by the insertion of additional pellets. Continuous ECGs were recorded as described in Methods. Mice were challenged with propranolol and carbamylcholine as described in Fig. 1A. Data are the composite mean heart rate (±SEM) of 7 age-matched Akita mice prior to (DM baseline) and one week following pellet implantation (DM+Insulin). Quantitation of the response to carbamylcholine prior to and following pellet implantation measured as described in Figure 1. B. Plateau time of bradycardia; C. Negative chronotropic response to carbamylcholine; D. Time elapsed for 80% recovery to baseline heart rate. *P<0.05.
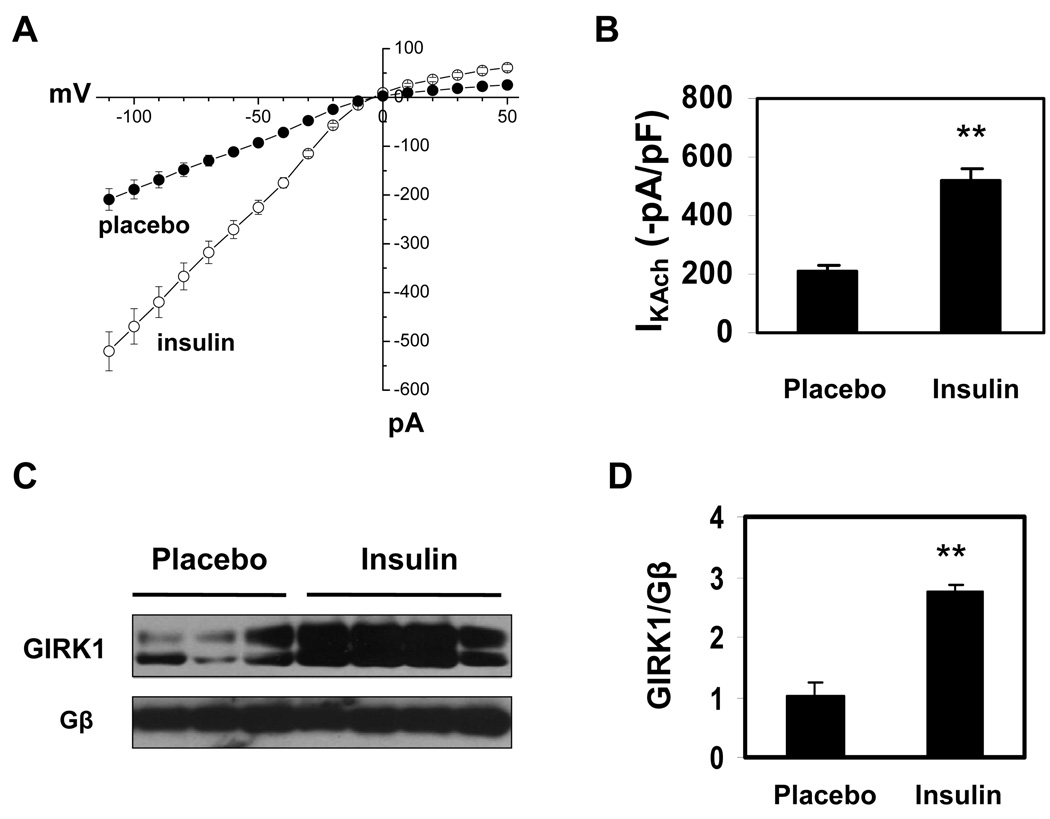
These findings suggested that insulin might increase parasympathetic responsiveness in Akita mice via an effect on IKAch. To test this hypothesis, IKAch was determined in atrial myocytes from placebo and insulin treated Akita mice. Carbamylcholine stimulated peak inward current was −209.1±22.2 pA/pF (n=8) in atrial myocytes from placebo treated mice and −520.2±40.1 pA/pF (n=9, P<0.01; Fig. 4A, B) in atrial myocytes from insulin treated mice which was not significantly different from that seen in WT mice (see Fig. 2B). Given the decreased level of expression of GIRK1 in the atria of Akita mice compared with WT, one explanation for the increase in IKAch in response to insulin might be an increase in the expression of GIRK1. Analysis of extracts of atria from placebo and insulin treated Akita mice demonstrated that insulin treatment increased GIRK1 expression 2.74±0.11 fold (n=4, P<0.01) compared to placebo (Fig. 4C, D).
Figure 4. Effect of insulin on IKAch and GIRK1 expression in Akita mice.
A. IKAch was determined as described in Fig. 2A and I-V plots constructed. Data are the mean of 8 recordings from 3 placebo treated and 9 recordings from 3 insulin treated Akita mice. B. Quantitation of peak inward currents from A. C. Expression of GIRK1 in atria from placebo and insulin treated Akita mice determined by Western blot analysis. D. Densitometry analysis of both glycosylated and unglycosylated GIRK1 bands in C (n=4)*. Data were normalized to the expression of Gβ. **P<0.01.
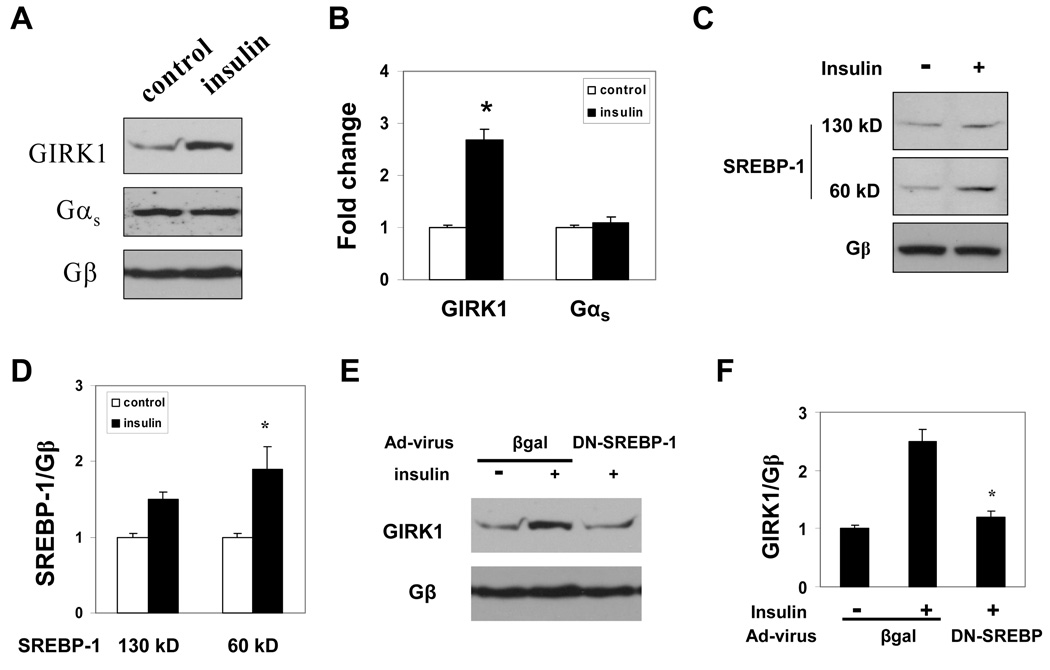
Insulin regulates the expression of GIRK1 in cultured chick atrial myocytes via an SREBP-1 dependent mechanism
Although culture of adult mouse atrial myocytes yielded sufficient cells for measurements of IKACh, the number of cells was not sufficient for western blot analysis to determine the role of insulin in the regulation of either GIRK1 and/or SREBP-1 expression. Furthermore, antibodies have not proven effective for immunohistochemical analysis of either SREBP-1 and GIRK1 expression in mouse atrial tissues. Embryonic chick atrial myocytes constitute an excellent model for the study of the role of SREBP-1 in the regulation of IKACh.17, 22 Hence, embryonic chick atrial myocytes were incubated with either insulin or vehicle and the effect on GIRK1 expression determined. Insulin increased GIRK1 expression 2.68±0.12 fold compared to control (n=4, P<0.01). This effect was specific as demonstrated by the finding that the expression of Gαs was unaffected by insulin (Fig. 5A, B).
Figure 5. Insulin stimulation of GIRK1 expression in atrial myocytes is dependent on SREBP-1.
A. GIRK1 expression in embryonic chick atrial myocytes cultured for 16 hours in the presence and absence of 100 nM insulin determined by Western blot analysis. Only a single GIRK1 band was detected in the cultured atrial myocytes. Blots were sequentially stripped and reprobed with specific antibody to Gαs and Gβ. B. Mean intensity of GIRK1 determined by densitometry scanning of 3 independent experiments similar to that in A (*P<0.05). The relative intensity of GIRK1 normalized to Gβ in the absence of insulin was taken as 1. C. SREBP-1 levels in chick atrial myocytes cultured with and without insulin determined by Western blot analysis. D. Mean intensity of the precursor (130 kD) and processed (60 kD) SREBP-1 determined by densitometry scanning of 3 independent experiments similar to that in C (*P<0.05). E. Effect of DN-SREBP-1 on GIRK1 expression in cultured atrial myocytes. Embryonic chick atrial myocytes were infected with an adenoviral vector expressing GFP plus DN-SREBP-1 or GFP plus β-gal at the time of plating (MOI of 20). On the third culture day cells were incubated with or without insulin for 16 hours, harvested and expression of GIRK1 determined by Western blot analysis. F. Densitometry scanning of 3 experiments similar to that in E. Data are normalized to the expression of Gβ. *P<0.05.
We had previously demonstrated that GIRK1 expression was regulated by the sterol responsive transcription factor SREBP-1.17 Although insulin had been shown to increase SREBP-1 expression in liver and adipocytes,11, 12 the effect of insulin on SREBP-1 expression in the heart had not been determined. Insulin treatment of cultured chick atrial myocytes resulted in a small increase in levels of the 130 kD precursor form of SREBP-1 and a 1.9±0.2 fold increase (n=3, P<0.05) in the 60 kD nuclear form of SREBP-1 (Fig. 5C, D). In order to determine whether this increase in SREBP-1 might play a role in insulin regulation of GIRK1 expression, cultured atrial myocytes were infected with an adenovirus expressing either GFP plus βgal or GFP plus a DN-SREBP-1 followed by incubation for 16 hours with and without insulin. Fluorescence microscopy demonstrated that GFP was expressed in 90% of atrial myocytes infected with Ad-GFP. Expression of Ad-GFP-DN-SREBP-1 decreased insulin stimulation of GIRK1 expression to levels similar to those in control cells in the absence of insulin (Fig. 5E, F) while expression of GFP with βgal had no effect on insulin stimulation of GIRK1 expression. These data support the conclusion that insulin stimulation of GIRK1 expression in atrial myocytes is dependent on SREBP-1.
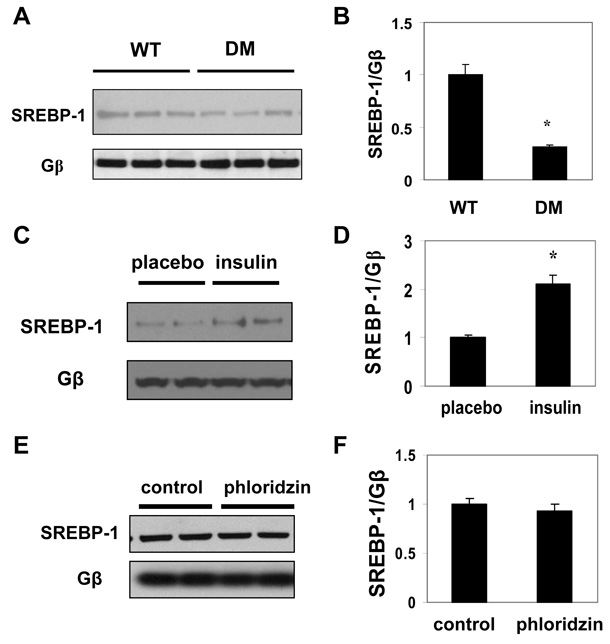
SREBP-1 levels are decreased in the atrium of Akita Mice
The finding that SREBP-1 was regulated by insulin in cultured chick atrial myocytes suggested that insulin deficiency in Akita mice might result in a decrease in SREBP-1 levels in the atrium. Levels of the 60 kD form of SREBP-1 in atria of Akita mice were decreased by 31.3±2.1% (n=3, P<0.05) compared to WT (Fig. 6A, B). To determine whether this decrease in the level of SREBP-1 was associated with the hypoinsulinemia in the Akita mouse, Akita mice were treated with insulin pellets. Insulin treatment increased SREBP-1 by 2.1±0.2 fold (n=3, P<0.05) compared to mice treated with placebo pellets (Fig. 6C, D). Furthermore, in streptozotocin treated mice, with average glucose levels of 457±12 mg/dL (n=10), SREBP-1 expression was also markedly decreased (Fig. S1). To rule out the possibility that the increased level of SREBP-1 in atria of insulin treated Akita mice was due to the resultant decrease in hyperglycemia, Akita mice were treated with phloridzin,23 an agent known to reverse hyperglycemia via the inhibition of glucose reuptake in the kidney, or vehicle 3 times a day for 10 days. Phloridzin treatment decreased glucose levels to an average level of 284±24 mg/dL (n=7) compared to 586±11 mg/dL (n=6) in vehicle treated mice, but had no effect on SREBP-1 levels (Fig. 6E, F), supporting the conclusion that this effect is independent of glucose levels
Figure 6. Insulin regulates SREBP-1 levels in Akita mice.
A. Expression of SREBP-1 in nuclear extracts from atria of age-matched male WT and Akita mice determined by Western blot analysis. B. Densitometry analysis of the autoradiograph in A normalized to the expression of Gβ (*P<0.05). C. Effect of insulin treatment on SREBP-1 in atria of Akita mice. Atria of age-matched male Akita mice treated for 1 week with slow release insulin pellets or placebo pellets were homogenized as in A and the level of SREBP-1 determined by Western blot analysis. D. Mean intensity of SREBP-1 determined as in B from 4 placebo and 4 insulin treated Akita mice (*P<0.05). E. Effect of phloridzin treatment of Akita mice on levels of SREBP-1. Age-matched male Akita mice were treated with phloridzin or vehicle and SREBP-1 determined as in C. F. Mean intensity of SREBP-1 determined as in B from 7 phloridzin treated and 6 vehicle treated Akita mice.
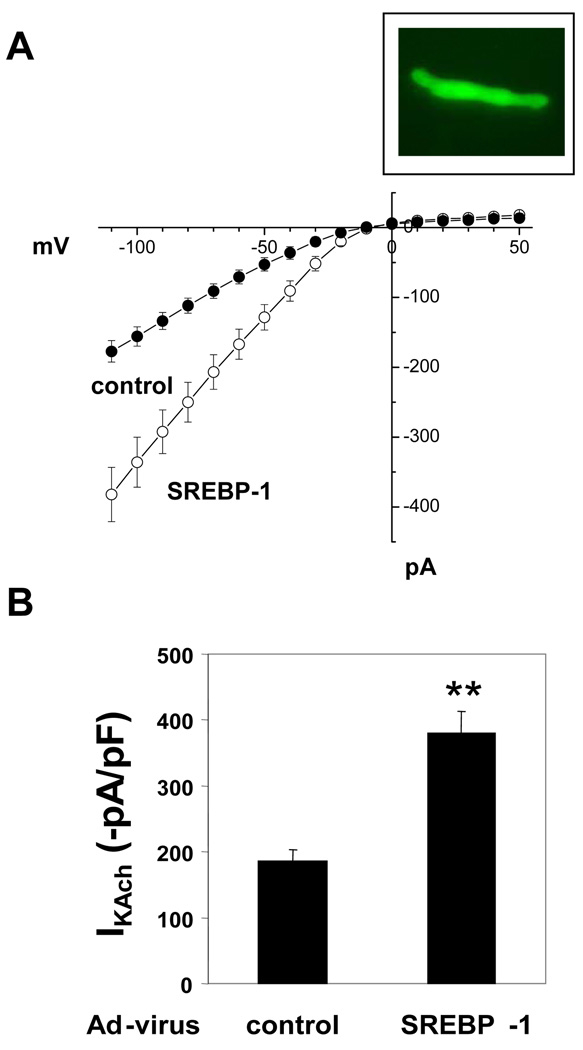
Expression of SREBP-1 in atrial myocytes from Akita mice rescues the impaired IKAch response to carbamylcholine
To determine whether the decreased levels of SREBP-1 in atria of Akita mice might play a role in the impaired response of the Akita mouse to parasympathetic stimulation, the effect of adenoviral expression of SREBP-1 in atrial myocytes from Akita mice on IKAch was determined. Florescence microscopy revealed that 90% of cells infected with adenovirus expressing either GFP plus βgal or GFP plus SREBP-1 were GFP positive (Fig. 7 Insert). Expression of GFP with βgal had no effect on peak inward currents in I-V curves derived from measurements of IKAch (compare DM cells in Figs. 2A, B and control cells in Fig. 7A, B). However, infection of atrial myocytes from Akita mice with adenovirus expressing GFP plus SREBP-1 increased IKAch from −186.2±17 (n=6) to −380.6±32.9 (n=7, P<0.01) which was not significantly different from IKAch in atrial myocytes from WT mice (Fig. 2B). These findings supported the conclusion that SREBP-1 regulates the response of the heart to parasympathetic stimulation and that decreased SREBP-1 in the diabetic heart results in parasympathetic dysfunction.
Figure 7. Adenoviral expression of SREBP-1 restores the impaired IKAch in atrial myocytes from Akita mice to levels similar to those in cells from WT mice.
A. I-V plots of IKAch from dissociated atrial myocytes from Akita mice infected with Ad-GFP-βgal or Ad-GFP plus SREBP-1c (MOI of 20). Forty-eight hours following infection, IKAch was determined in GFP+ cells as described, Fig. 2A; B. Mean of peak inward currents from 7 Ad-GFP-βgal infected cells and 7 Ad-GFP-SREBP-1c infected cells from 4 separate Akita mice. **P<0.01. Insert: Fluorescence in a typical atrial myocyte infected with an adenovirus expressing GFP (90% of cells).
DISCUSSION
The parasympathetic response of the heart involves both a neuronal component carrying input from the central nervous system via the vagus nerve and an intrinsic molecular cardiac response pathway.24 In the current study carbamylcholine was injected into the Akita diabetic mouse in order to bypassing vagal stimulation and directly activate the intrinsic parasympathetic signaling pathway in the heart. Thus the finding of an impaired parasympathetic response to carbamylcholine in the type I diabetic Akita mouse supports the conclusion that the parasympathetic dysfunction in the diabetic heart may not only involve a neuropathy, but may also involve an abnormality of the intrinsic downstream parasympathetic signaling pathway in the heart. This pathway involves acetylcholine binding to the M2 muscarinic receptor, dissociation of the heterotrimeric G-Protein Gi2 into Gαi2 and Gβγ subunits and Gβγ activation of (GIRK1)2/(GIRK4)2, increased IKAch resulting in hyperpolarization of the myocyte membrane and a decrease in heart rate. Changes in the level of expression of Gαi2 have been shown to modulate the parasympathetic response in the heart and in atrial myocytes during embryonic development of the chick heart.7 Viral expression of Gαi2 in the mouse AV-node was shown to increase parasympathetic response.25 The finding that insulin treatment reversed parasympathetic dysfunction and stimulated GIRK1 expression in the atrium of the Akita mouse while increasing IKAch in atrial myocytes from these mice supported a role of decreased GIRK1 expression in the parasympathetic dysfunction in the diabetic heart. Studies comparing GIRK4 expression suggested a similar decrease in GIRK4 expression in atria of Akita mice compared with WT (data not shown). Thus data presented in the current study are the first to suggest that regulation of GIRK expression might constitute a molecular mechanism in the pathogenesis of the parasympathetic dysfunction in the diabetic heart.
Although heart rate is under the control of the SA node in which funny currents (If) and Ca currents as well as IKAch contribute to the negative chronotropic response, in this study we measured IKAch in atrial myocytes since IKAch is a critical mediator of parasympathetic signaling.26 The use of atrial myocytes for measurement of IKAch is supported by the findings of Lomax et al who demonstrated that IKAch has very similar electrophysiologic properties in both SA node and atrium and that the major difference was the distribution of IKAch in a gradient across the superventricular structures of the mouse atrium with the highest density in the SA node.27
A role of lipid metabolism in the regulation of the parasympathetic response of the heart is supported by prior studies in which growth of atrial myocytes in lipoprotein depleted serum (LPDS) resulted in an increased response to muscarinic stimulation in parallel with an increase in the expression of mRNAs coding for M2, Gαi2 and GIRK1.6, 22, 28 It has subsequently been demonstrated that growth of atrial myocytes in LPDS results in an increase in levels of SREBP-1 and that SREBP-1 regulates the expression of Gαi2 in atrial myocytes in response to LPDS.16 Furthermore, both the negative chronotropic response to parasympathetic stimulation and GIRK1 expression were decreased in atria of an SREBP-1 KO mouse.17 Taken together with the findings reported here that atria of type I diabetic Akita mice demonstrate decreased levels of SREBP-1 and GIRK1 and that insulin treatment increased the levels of SREBP-1 and GIRK1 in Akita mice, these data further support the conclusion that insulin might regulate GIRK1 expression via an effect on SREBP-1. The finding that insulin stimulated the expression of GIRK1 in cultured atrial myocytes and that overexpression of a DN-SREBP-1 inhibited insulin stimulated GIRK1 expression in these cells also supported the conclusion that insulin regulation of GIRK1 expression was dependent on SREBP-1.
Decreased IKAch in atrial myocytes from Akita mice would account for the decreased negative chronotropic response of these mice to carbamylcholine. Furthermore, insulin treatment of Akita mice restored the heart rate response to carbamylcholine in the intact mouse and increased IKAch in atrial myocytes from these mice to levels similar to those in atrial myocytes from WT mice. Finally, the finding that viral expression of SREBP-1 in atrial myocytes from Akita mice mimicked the effect of insulin and restored IKAch to levels similar to those in cells from WT mice was consistent with a role of SREBP-1 in insulin regulation of the parasympathetic response. Hence, these data suggest a mechanism for parasympathetic dysfunction in type I diabetes in which decreased insulin levels result in decreased SREBP-1 which in turn results in decreased expression of GIRK1, attenuation of IKAch and parasympathetic dysfunction. The finding that glucose lowering by phloridzin23 had no effect on SREBP-1 levels and that STZ treated mice which demonstrate a less marked hyperglycemia compared with Akita mice supports the conclusion that the decreased SREBP-1, the associated decrease in GIRK1 expression and parasympathetic dysfunction in the type I diabetic Akita mouse are due to hypoinsulinemia and not to hyperglycemia. Insulin regulation of parasympathetic signaling in the heart via SREBP-1 constitutes a unique role for both insulin and SREBP-1 in a pathway not directly related to lipid, glucose or fatty acid metabolism.
The finding that the decreased negative chronotropic response of the heart in the Akita mouse is due only in part to a decrease in the magnitude of the initial carbamylcholine response, but is also due to a decrease in the duration of bradycardia and an increase in recovery of the heart rate from bradycardia, suggested that the impaired parasympathetic response might also involve differences in desensitization of the diabetic heart to carbamylcholine compared to WT. Although this study demonstrates an impaired negative chronotropic response to parasympathetic stimulation, data suggest that the basal heart rate in the Akita mouse is lower relative to WT. One explanation for this finding is that intrinsic heart rate, heart rate in the absence of autonomic stimulation, is decreased in the diabetic heart. Measurement of heart rate in the presence of propranolol and atropine demonstrated a trend to lower intrinsic heart rates in the Akita mice (data not shown). The mechanism for the lower intrinsic heart rate in the Akita mouse remains to be further studied.
These studies suggest that the cardiac autonomic dysfunction in diabetes, previously considered to be secondary to a “neuropathy”, might require a redefinition to include a unique intracardiac molecular abnormality resulting in parasympathetic dysfunction. It is interesting to speculate that parasympathetic dysfunction in the diabetic heart might result in an autonomic imbalance that might predispose the heart to the development of ventricular arrhythmias and sudden death. Hence the regulation of the intrinsic cardiac parasympathetic signaling pathway by insulin and SREBP-1 might offer new therapeutic targets for the treatment and prevention of autonomic dysfunction and sudden death in the diabetic population.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant HL074876 and HL087827 (J.B.G), AHA Scientist Development Grant and NIH grant DK079622 (H.P), NIH grant AI56374 (C.E.M), and JDRF Postdoctoral Fellowship (Y.Z). The cellular electrophysiology studies were carried out in the Tufts University School of Medicine Center for Neuroscience Research supported by NIH grant P30 NS047243.
Footnotes
Subject Code: [16] Myocardial cardiomyopathy disease, [143] Gene regulation, [189] Type 1 diabetes
Conflict of Interest: None
REFERENCES
- 1.Aronson D. Pharmacologic modulation of autonomic tone: implications for the diabetic patient. Diabetologia. 1997;40:476–481. doi: 10.1007/s001250050704. [DOI] [PubMed] [Google Scholar]
- 2.Weston PJ, Gill GV. Is undetected autonomic dysfunction responsible for sudden death in Type 1 diabetes mellitus? The 'dead in bed' syndrome revisited. Diabet Med. 1999;16:626–631. doi: 10.1046/j.1464-5491.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med. 1991;79:495–502. [PubMed] [Google Scholar]
- 4.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 5.Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J Biol Chem. 1997;272:31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- 6.Gadbut AP, Toupin DK, Kilbourne EJ, Galper JB. Low density lipoproteins induce parasympathetic responsiveness in embryonic chick ventricular myocytes in parallel with a coordinate increase in expression of genes coding for the M2 muscarinic receptor, G alpha i2, and the acetylcholine-sensitive K+ channel. J Biol Chem. 1994;269:30707–30712. [PubMed] [Google Scholar]
- 7.Liang BT, Hellmich MR, Neer EJ, Galper JB. Development of muscarinic cholinergic inhibition of adenylate cyclase in embryonic chick heart. Its relationship to changes in the inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1986;261:9011–9021. [PubMed] [Google Scholar]
- 8.Ward SM, Gadbut AP, Tang D, Papageorge AG, Wu L, Li G, Barnett JV, Galper JB. TGFbeta regulates the expression of G alpha(i2) via an effect on the localization of ras. J Mol Cell Cardiol. 2002;34:1217–1226. doi: 10.1006/jmcc.2002.2073. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, Horton JD, Goldstein JL, Brown MS, Shimomura I. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 2001;15:1206–1216. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayo T, Koizumi A. Mapping of murine diabetogenic gene mody on chromosome 7 at D7Mit258 and its involvement in pancreatic islet and beta cell development during the perinatal period. J Clin Invest. 1998;101:2112–2118. doi: 10.1172/JCI1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation. 2002;73:1333–1336. doi: 10.1097/00007890-200204270-00024. [DOI] [PubMed] [Google Scholar]
- 15.Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, Begley U, Kong D, Yu H, Yin L, Hillgartner FB, Osborne TF, Galper JB. Role of sterol regulatory element binding proteins in the regulation of Galpha(i2) expression in cultured atrial cells. Circ Res. 2002;91:32–37. doi: 10.1161/01.res.0000026502.79063.66. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Georgescu SP, Du C, Madias C, Aronovitz MJ, Welzig CM, Wang B, Begley U, Zhang Y, Blaustein RO, Patten RD, Karas RH, Van Tol HH, Osborne TF, Shimano H, Liao R, Link MS, Galper JB. Parasympathetic response in chick myocytes and mouse heart is controlled by SREBP. J Clin Invest. 2008;118:259–271. doi: 10.1172/JCI32011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae EM, Kim WJ, Suk K, Kang YM, Park JE, Kim WY, Choi EM, Choi BK, Kwon BS, Lee WH. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol Immunol. 2008;45:523–533. doi: 10.1016/j.molimm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–H1820. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- 20.Gehrmann J, Meister M, Maguire CT, Martins DC, Hammer PE, Neer EJ, Berul CI, Mende U. Impaired parasympathetic heart rate control in mice with a reduction of functional G protein betagamma-subunits. Am J Physiol Heart Circ Physiol. 2002;282:H445–H456. doi: 10.1152/ajpheart.00565.2001. [DOI] [PubMed] [Google Scholar]
- 21.Corey S, Krapivinsky G, Krapivinsky L, Clapham DE. Number and stoichiometry of subunits in the native atrial G-protein-gated K+ channel, IKACh. J Biol Chem. 1998;273:5271–5278. doi: 10.1074/jbc.273.9.5271. [DOI] [PubMed] [Google Scholar]
- 22.Haigh LS, Leatherman GF, O'Hara DS, Smith TW, Galper JB. Effects of low density lipoproteins and mevinolin on cholesterol content and muscarinic cholinergic responsiveness in cultured chick atrial cells. Regulation of levels of muscarinic receptors and guanine nucleotide regulatory proteins. J Biol Chem. 1988;263:15608–15618. [PubMed] [Google Scholar]
- 23.Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, Groff A, Ferran C, Bonner-Weir S, Sharma A, Weir GC. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta-cell survival during chronic hyperglycemia. Diabetes. 2002;51:413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch E. Innervation of the Vertebrate Heart. Springfield, IL: Charles C. Thomas; 1970. [Google Scholar]
- 25.Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, Eschenhagen T, Marban E. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395–1398. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 26.Wickman K, Krapivinsky G, Corey S, Kennedy M, Nemec J, Medina I, Clapham DE. Structure, G protein activation, and functional relevance of the cardiac G protein-gated K+ channel, IKACh. Ann N Y Acad Sci. 1999;868:386–398. doi: 10.1111/j.1749-6632.1999.tb11300.x. [DOI] [PubMed] [Google Scholar]
- 27.Lomax AE, Rose RA, Giles WR. Electrophysiological evidence for a gradient of G protein-gated K+ current in adult mouse atria. Br J Pharmacol. 2003;140:576–584. doi: 10.1038/sj.bjp.0705474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadbut AP, Wu L, Tang D, Papageorge A, Watson JA, Galper JB. Induction of the cholesterol metabolic pathway regulates the farnesylation of RAS in embryonic chick heart cells: a new role for ras in regulating the expression of muscarinic receptors and G proteins. Embo J. 1997;16:7250–7260. doi: 10.1093/emboj/16.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.