Abstract
Magnetic resonance imaging(MRI) based volumetric measurements of medial temporal lobe (MTL) structures can discriminate between normal elderly controls and patients with Alzheimer's disease (AD) of moderate to advanced severity. In terms of clinical utility, however, a more important issue concerns the ability of the technique to differentiate between normal elderly controls and AD patients with the very mildest form of the disease. We performed MRI-based volume measurements of the hippocampus, parahippocampal gyrus, and amygdala in 126 cognitively normal elderly controls and 94 patients with probable AD. The diagnosis of AD was made according to NINDS/ADRDA criteria, and disease severity was categorized by Clinical Dementia Rating (CDR) scores. Patients with CDR = 0.5 were classified as very mild, CDR = 1 as mild, and CDR = 2 moderate disease severity.
Volumes of each structure declined with increasing age in control subjects and did so in parallel for men and women. The volume of each measured MTL structure also declined with age in patients with AD. The volume of each MTL structure was significantly smaller in AD patients than control subjects (P<.001). Of the several MTL measures, the total hippocampal volume measurements were best at discriminating controls from AD cases. The mean hippocampal volumes for AD patients relative to controls by severity of disease were as follows: very mild AD (CDR 0.5) - 1.75 SD below the control mean, mild AD (CDR 1) - 1.99 SD, and moderate AD (CDR 2) - 2.22 SD. Age and gender adjusted normalized MRI-based hippocampal volume measurements provide a sensitive marker of the MTL neuroanatomic degeneration in AD early in the disease process.
Keywords: Alzheimer's Disease, Dementia, MRI, Quantitative MRI, Hippocampus
Alzheimer's disease(AD) is the most common cause of dementia in individuals over 60 years of age(1-3). A well accepted biological concomitant of AD is cerebral atrophy(4). The rationale for quantitative magnetic resonance imaging (MRI) of medial temporal lobe (MTL) atrophy in the diagnosis of AD is: 1) A memory impairment is usually the earliest and most severe clinical manifestation of AD; 2) Medial temporal lobe (MTL) limbic structures are central to the integrity of declarative memory function (5); 3) MTL limbic structures are involved earliest and most extensively in the pathology of AD(6, 7); and 4) several principal MTL limbic structures are amenable to accurate volumetric quantitation by MRI— the hippocampal formation, amygdala, and parahippocampal gyrus (PHG) (8-12). Based on initial studies, MRI-based volume measurements of the MTL have been proposed as a clinically useful test for the diagnosis of AD(13-21). Some limitations of the published data include: 1) Anatomic boundary criteria for the various MTL structures varied significantly among the different studies. 2) Different structures or combinations of MTL structures were evaluated in the various studies. 3) Relatively small numbers of subjects were included in individual studies. 4) Rigorous definitions of the severity of AD often were not employed. Most previous studies have included primarily subjects with AD of moderate severity. Consequently, the differences between the AD patients and control subjects with regard to MTL atrophy have been dramatic. The most important test of the utility of the technique would be in patients with very mild AD in whom the diagnostic decision making process is difficult.
We report a large series of carefully evaluated and longitudinally followed subjects with AD and a large group of prospectively studied normal elderly control subjects. The AD patients were categorized on basis of severity and include a large sample of individuals with very mild AD. As such, we are able to evaluate the utility of volumetric MRI in assisting in the clinical diagnosis of AD at its most mild stages. The goals of this study were 1) to characterize volumetric changes in the hippocampus, amygdala, and PHG in normal aging and in AD, 2) to estimate the ability of these measures to discriminate between normal aging and AD of varying degrees of severity with an emphasis on mild disease.
MATERIALS AND METHODS
Recruitment and Characterization of Subjects
Patients with AD and the cognitively normal control subjects for this study were recruited from the Mayo Clinic Alzheimer's Disease Center (ADC)/Alzheimer's Disease Patient Registry (ADPR) (22-25), which are prospective, longitudinal studies of aging and dementia. Informed consent was obtained for participation in the longitudinal studies which included clinical/cognitive assessment as well as MRI studies, and all studies were approved by the Mayo Institutional Review Board. Patients with a suspected cognitive impairment were identified during general medical examinations by Mayo primary care physicians. A neurologist then performed a detailed neurologic examination and obtained a complete history from the patient and a collateral source. Two sets of neuropsychological tests were administered in two sessions to assess memory, attention, language, visuospatial skills and problem solving (26, 27). One set was used for diagnostic purposes, and the second for research goals. Laboratory studies included a sensitive TSH, vitamin B-12, folic acid, syphilis serology, sedimentation rate, and, if clinically indicated, electroencephalogram, single photon emission computed tomography scan, cerebrospinal fluid analysis, human immunodeficiency virus, Lyme disease titer, antinuclear antigen, extractable nuclear antigen, and a 24-hour urine collection for heavy metals. Patients were not excluded for the presence of ongoing medical problems such as diabetes, hypertension or heart disease. The diagnosis of AD was made according to the NINCDS/ADRDA criteria (1) at a consensus conference attended by behavioral neurologists, nurses, a geriatrician, and neuropsychologists. Disease severity in AD patients was assessed by the Clinical Dementia Rating (CDR) scale; very mild - CDR 0.5; mild - CDR 1; moderate - CDR 2(28). An important distinction is made between establishing a diagnosis of AD and ranking its severity. The former was done according to NINCDS/ADRDA criteria which emphasize a decline in cognitive performance over time as an important benchmark in establishing the diagnosis of AD(1). The CDR score was used as a staging instrument to rank disease severity at a specific point in time. It was therefore possible for patients to meet NINCDS/ADRDA criteria for AD and also be ranked as only very mildly demented (CDR 0.5).
Control subjects were recruited from the same pool of patients coming to Mayo primary care physicians for a general medical examination. Controls were evaluated in the same way as cases with the exception of the additional laboratory studies for cognitive impairment. Their status was reviewed at the consensus conference. The criteria for cognitively normal controls were 1) no active neurological or psychiatric disorders, and 2) like the cases, some had ongoing medical problems, however the illnesses or their treatments did not interfere with cognitive function.
An MRI examination of the brain was performed within 4 months of the clinical assessment, including CDR scoring, in all subjects. For all AD patients in this study, the MRI was therefore performed with close temporal proximity to the initial diagnosis of AD. These MR studies were used in the diagnostic process only to exclude treatable causes of dementia. The volumetric data were not used to aid in the clinical diagnosis of AD.
MR Image Acquisition
All subjects were imaged at 1.5T (Signa, General Electric) using a standardized imaging protocol. The first sequence was a T1-weighted sagittal set of images that was used to measure total intracranial volume and for landmarking subsequent image acquisitions. The other MRI pulse sequence relevant to this report was a T1-weighted 3D volumetric spoiled gradient echo sequence with 124 contiguous partitions, 1.6 mm slice thickness, a 22 × 16.5 cm field of view, 192 views, and 45° flip angle. Volume measurements of the hippocampus, PHG, and amygdala were derived from this pulse sequence.
Image Processing
All image processing steps (including boundary tracing) in every subject were performed by the same trained research assistant who was blinded to all clinical information in order to ensure that the volumetric data were generated in an unbiased fashion. The reformatting and realignment of the MRI images and all anatomic tracing in every subject was reviewed by a three member panel who were likewise blinded to all clinical information, and corrections were made at that time if necessary. This ensured rigorous quality control, as well as uniformity in the subjective aspects of image processing across all the subjects in this study. Validation studies show the intra-rater test-retest coefficient of variation of hippocampal volume measurements to be 1.9% with this method(12).
The 3D MR image data were first interpolated in the slice select dimension to give cubic voxels(29). When necessary the images were reformatted so that the image sections were oriented perpendicularly to the principal axis of the left hippocampal formation. Any rotation of the subject's head with respect to the orthogonal coronal plane was corrected as well during this reformatting and realignment step. The image data were then interpolated in plane to the equivalent of 512 × 512 matrix and magnified × 2. The voxel size of the fully processed image data was 0.316mm3. The borders of the hippocampi, PHG, and amygdala were manually traced with a mouse driven cursor sequentially on each slice from posterior to anterior(29). Having identified the boundaries of these MTL anatomic structures, the number of voxels in each was counted automatically using a summed region of interest function. These were multiplied by voxel volume to give a numeric value in mm3. The processed image files as well as the accompanying region of interest tracing files were saved, for subsequent review.
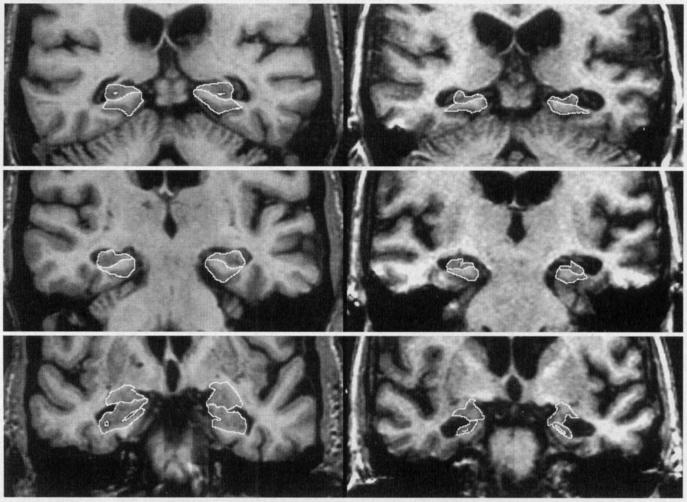
In-plane hippocampal anatomic boundaries were defined to include the CA1-CA4 sectors of the hippocampus proper, the dentate gyrus, and subiculum (10-12, 14, 29, 30) (Fig. 1). The posterior boundary of the hippocampus was determined by the oblique coronal anatomic section on which the crura of the fornices were identified in full profile (31). Thus, essentially the entire hippocampus from tail through head was included in these measurements. These same neuroanatomic hippocampal boundary criteria are employed by many epilepsy research groups (31-37). Subdivision of the hippocampus along its septotemporal axis into three segments labeled head, body, and tail was accomplished as follows: The hippocampal head was defined to encompass those imaging slices extending from the intralimbic gyrus forward to the anterior termination of the hippocampal formation. If the posterior margin of the hippocampal head was labeled as imaging slice x, then the volume of the hippocampal tail was determined by summing the area of the hippocampus on successive slices beginning from the forniceal crura to slice . The volume of the body consisted of the sum of areas on successive slices beginning with slice and extending to slice x-1.
Figure 1. Neuroanatomic Boundaries.
Two columns of images are presented. On the left, cropped oblique coronal MR images through the temporal lobes of a 75 year-old female control, and on the right images from a 73-year-old female AD patient, CDR = 1. In each column, 3 images are present. From the top to bottom, these represent sections at the level of the hippocampal tail, hippocampal body, and hippocampal head. The anatomic outlines of the hippocampus, and PHG are indicated on images of the hippocampal head, and hippocampal body. The outline of the amygdala and hippocampus are indicated in the bottom image of the hippocampal head. Neuroanatomic criteria employed when tracing the boundaries of these 3 MTL structures are indicated in the text.
The posterior boundary of the parahippocampal gyrus (PHG) was defined in a manner identical to that for the hippocampal formation. The superior boundary of the PHG was defined as the gray-white matter interface between the subiculum and the PHG white matter. Medially the PHG was demarcated by CSF in the uncal cistern. Laterally and inferiorly its boundary was the collateral sulcus. The imaging slice immediately preceding that in which the hippocampal intralimbic gyrus first appeared when progressing from posterior to anterior, was defined as the anterior boundary of the PHG. In some cases, a clearly identifiable collateral sulcus was not present along the entire antero-posterior extent of the PHG. For this reason, PHG measurements were not possible in 16 controls and 14 AD patients.
The posterior, superior, medial, and lateral boundaries of the amygdala were defined by gray-white matter borders, or where appropriate CSF in the uncal cistern. The inferior border of the amygdala was either the uncal recess of the temporal horn or the alveus covering the hippocampal head. The anterior boundary of the amygdala is ill-defined in nature, and we defined it operationally to be the most anterior slice on which the head of the hippocampus was present.
Statistical Methods
Individual MTL structure volumes were normalized for intersubject variation in head size by dividing structure volume (in mm3) by the total intracranial volume (TIV in cm3) of that particular subject (10, 14). Associations between normalized MTL volumes, age and gender in normal subjects were evaluated using stepwise regression, including evaluation of nonlinearity and interactions. Stepwise regression was also used to determine if variability was associated with age or gender.
Volumetric percentiles in controls specific for age and gender were obtained using the algorithm described in O'Brien and Dyck(38). Age and gender specific volumetric percentiles among AD patients were determined and converted to W scores (normal deviates) using the inverse of the standard normal distribution (a percentile value of 95 corresponding to a W score of 1.645, for example) (38). The W score value is a covariate adjusted Z-score realtive to the control group. Comparison of W values among MTL anatomic structures for AD patients was performed using ANOVA for repeated measures and paired t tests. In order to identify the MTL limbic structure(s) which best distinguish between AD and controls, we performed a stepwise discriminant analysis including normalized volumes, age, and gender as predictor variables.
RESULTS
Two-hundred-twenty subjects are included in this report—126 controls and 94 AD patients (Table 1). The control and AD patients were well matched on education and gender distribution with approximately a 2:1 female to male ratio in both groups. The age range of controls was 51-89 years and AD patients 50-89 years. Forty-four of the 126 controls were men; 16 of the 36 CDR=0.5 AD patients were men; 10 of the 43 CDR=1 patients were men; 7 of the 15 CDR=2 patients were men. As expected, Dementia Rating Scale(39) and Mini-Mental State Exam (40) scores declined with increasing CDR grade in AD patients.
Table 1.
Characterization of Subjects
| Controls, CDR = 0 (N=126) | AD, CDR = 0.5 (N = 36) | AD, CDR = 1 (N=43) | AD, CDR = 2 (N=15) | |
|---|---|---|---|---|
| Variable | ||||
| mean ± sd | mean ± sd | mean ± sd | mean ± sd | |
| Age | 79.15 ± 6.73 | 72.92 ± 8.43 | 73.47 ± 9.68 | 75.87 ± 8.71 |
| Education | 13.43 ± 2.96 | 13.33 ± 2.91 | 12.98 ± 2.69 | 12.38 ± 2.47 |
| MMSE* | 28.60 ± 1.26 | 21.60 ± 4.36 | 18.16 ± 4.47 | 13.93 ± 5.99 |
| DRS** | 135.14 ± 6.95 | 112.79 ± 13.72 | 101.33 ± 20.75 | 89.62 ± 25.58 |
One case in each CDR group with missing values
One control, three cases in CDR = 0.5, four cases in CDR = 1, two cases in CDR = 2 with missing values
The results from this study are discussed in three parts: 1) descriptive statistics of the control subjects which characterize the relationship between normal aging, gender, and MTL volumes; 2) similar descriptive statistics of the AD patients which are compared to those in controls; 3) analysis of the ability of MTL volume measurements to discriminate between controls and AD subjects with varying disease severity.
Control Subjects
Normalized MTL volumes declined with age in a linear fashion(Table 2, Figure 2). The segment of the hippocampus that demonstrated the greatest decline with age was the head. No significant hemispheric differences in volume loss with age were observed in any MTL structure, except the PHG where the age related volume loss was greater on the left than the right side (P = .024). The mean non-normalized volumetric decline with age was 45.63 mm3/year for the total hippocampus; 27.43 for the hippocampal head; 8.84 for the hippocampal body; 9.68 for the hippocampal tail; 46.65 for the PHG; and 20.75 for the amygdala. Mean total intracranial volume in controls was 1393cm3 (SD 133cm3).
Table 2.
Relationship between normalized volume, age, and gender in controls and AD patients.
| Normalized Structure Volume | Controls |
AD Patients |
||||
|---|---|---|---|---|---|---|
| Intercept | Age | Gender | Intercept | Age | Gender | |
| Total Hippocampus | 6.359 | −0.0357 | 0.263** | 5.135 | −0.029 | --- |
| Hippocampal Head | 3.421 | −0.0185 | --- | 2.991 | −0.020 | --- |
| Hippocampal Body | 1.409 | −0.0074 | 0.097 | 1.186 | −0.006 | --- |
| Hippocampal Tail | 1.442 | −0.0084 | 0.110 | 0.911 | −0.003* | 0.079** |
| Amygdala | 2.414 | −0.0143* | --- | 1.790 | −0.011** | --- |
| PHG | 5.458 | −0.0371 | 0.390 | 4.216 | −0.025 | 0.250* |
+ The entires in the table may be used to predict volumes. For example, the predicted normalized total hippocampal volume for a control is 6.36 - 0.0357 age(in years) + 0.263 gender(1 if female, 0 if male). All associations shown are significant with p<.001 except as indicated.
P<.05
P<.01
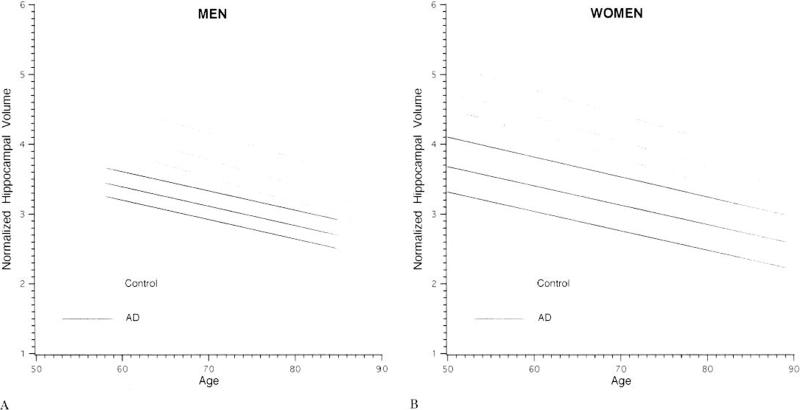
Figure 2. Normalized Hippocampal Volume by Age in Control Subjects and AD Patients.
Regression of the mean normalized hippocampal volume by age in male (A) and female (B) control subjects and AD patients. The upper and lower limits dashed lines, represent the 75th and 25th percentile values for each group. Hippocampal volumes of AD patients are smaller than those of age matched controls. Volumes in both groups decline linearly and in parallel with advancing age. For clinical purposes the position of a memory impaired elderly subject may be plotted and compared to age and gender matched controls and AD patients.
The unnormalized MTL structure volumes of men were generally larger than those of women, while the normalized MTL volumes of women were generally larger than those of men (Table 2, Figure 2). That is, these MTL limbic structures occupied a larger percentage of TIV in women than men. The decline in volume associated with age did not differ significantly between men and women. These associations were used to estimate age and gender specific normal percentiles for TIV-normalized MTL volumes(Table 3).
Table 3.
Age & Gender Specific Normal Percentiles for Hippocampal Volume Normalized by TIV
| |
|
Normal Percentiles |
||||
|---|---|---|---|---|---|---|
| Age | Gender | 1 | 5 | 10 | 20 | 50 |
| 50 | M | 3.736 | 3.953 | 4.059 | 4.184 | 4.491 |
| F | 4.000 | 4.216 | 4.323 | 4.447 | 4.754 | |
| 60 | M | 3.379 | 3.595 | 3.702 | 3.827 | 4.133 |
| F | 3.642 | 3.859 | 3.965 | 4.090 | 4.396 | |
| 70 | M | 3.022 | 3.238 | 3.344 | 3.469 | 3.776 |
| F | 3.285 | 3.501 | 3.608 | 3.733 | 4.039 | |
| 80 | M | 2.664 | 2.880 | 2.987 | 3.112 | 3.418 |
| F | 2.928 | 3.144 | 3.250 | 3.375 | 3.682 | |
| 89 | M | 2.343 | 2.559 | 2.665 | 2.790 | 3.097 |
| F | 2.606 | 2.822 | 2.929 | 3.054 | 3.360 | |
Values in the body of the table represent age and gender specific mean normalized hippocampal volume in controls. The units are mm/cm × 10. The 1st, 5th, 10th, 20th, and 50th percentile values in controls are reported. The presence of hippocampal atrophy in an individual patient can be assessed by comparing the TIV normalized hippocampal volumes of that patient against those of age and gender matched controls reported in this table.
Alzheimer's Disease Patients
A decline in normalized MTL volumes with age was observed among AD patients. The slopes of the age-volume regression lines were not significantly different between patients and controls over the age range studied (Table 2, Figure 2). As with controls, unnormalized volumes were generally larger among men than women, while larger normalized volumes were observed among female AD patients. Associations between MTL volume and age were linear, and did not differ between men and women. The segment of the hippocampus showing the greatest decline with age was the head.
Age and gender specific percentiles for normalized volumes were computed for each of the AD patients, and these were converted to W scores (corresponding to a normal distribution)(Table 4). Thus, values of W below 0 indicate that volume is less than the mean value expected for a normal subject after adjustment for age and gender. A value of −1.96 corresponds to a value which is at the 2.5 percentile among normals.
Table 4.
W Scores* in Alzheimer's Disease Patients
| CDR = 0.5 (N = 36) | CDR = 1 (N = 43) | CDR = 2 (N = 15) | ||||
|---|---|---|---|---|---|---|
| Variable | ||||||
| Mean W Value | SD | Mean W Value | SD | Mean W Value | SD | |
| Total Hippocampus | −1.752 | 0.939 | −1.989 | 1.193 | −2.225 | 1.183 |
| PHG | −0.874 | 1.035 | −0.996 | 1.101 | −0.512 | 1.344 |
| Amygdala | −1.026 | 0.973 | −1.337 | 0.839 | −1.355 | 1.035 |
The W score is the normal deviate relative to controls, adjusted for age and gender. All mean W scores were significantly different from 0 (the expected value for normal subjects), p<.001.
CDR = Clinical Dementia Rating
We assessed the extent to which cases differed from controls, and, for each anatomic structure W scores were significantly less than 0 among AD patients, (P<.001). As would be expected from Table 2, the deficit in volumes relative to controls was not associated with age or gender. We also assessed whether the magnitude of the volumetric deficit in cases relative to controls was greater in some structures than others. The differences among hippocampus, PHG, and amygdala were significant (P = <.001, ANOVA), and all pairwise comparisons (paired t-tests) were also significant (hippocampus vs. amygdala, P<.001; hippocampus vs. PHG, P<.001, amygdala vs. PHG, P=.006)(Table 4). Within the hippocampus, volumes differed significantly among the head, body, and tail (P = <.001, ANOVA), and pairwise differences between the head and body, and body and tail were also significant (P<.001, paired t-tests). The mean TIV of AD patients, 1369 cm3 (SD 138cm3), was not significantly different from that of controls.
When AD patients were categorized by disease severity into those with very mild, mild, or moderate disease, W scores within each group remained significantly less than 0, (P <.001)(Table 4). The MTL structure with the lowest W scores was the hippocampus for all 3 AD groups. Within the hippocampus, W values were most negative for the head(Table 4).W scores for the total hippocampus (P <0.05) and hippocampal head (P<0.001) were significantly different among AD patients of different CDR severity grades (Spearman Rank Correlation). Pairwise comparison of the W scores were also significant for the total hippocampus—CDR 0.5 vs 1.0 (P < 0.01), CDR 1.0 vs 2.0 (P < 0.01) (Rank Sum Test).
Discrimination Between Controls and AD Patients of Varying Severity
Using stepwise linear discriminant analysis (including age, gender, and TIV-normalized volumes as independent variables) to predict AD, the only variables which appeared in the final model were hippocampal volume, hippocampal volume squared, and age. Although all these terms were significant at the .02 level, the prediction equation was dominated by the linear hippocampal volume term, and the accuracy of the prediction was identical to that obtained using hippocampal W scores alone. The sensitivity of hippocampal volumes to distinguish AD patients from controls was assessed by computing the percentage of AD patients with W scores at selected percentiles among controls(Table 5). For example, at a fixed specificity of 80% the sensitivity of hippocampal volume measurements in discriminating controls from cases was 77.8% for CDR=0.5; 83.7% CDR=1; and 86.7% for CDR=2. Discrimination between controls and AD patients was roughly equivalent among the three AD severity groups at the 50th and 20th percentile of normal; discrimination was greater for CDR 1 and 2 than CDR 0.5 patients at the 10th and 5th percentile of norma; and, at the 1st percentile of normal, discrimination improved as patient's disease severity (CDR score) increased.
Table 5.
Diagnostic Discrimination of Normalized Total Hippocampal Volume Adjusted for Age and Gender*
| AD Patients |
Indicated Percentile of Normal |
||||
|---|---|---|---|---|---|
| 50% | 20% | 10% | 5% | 1% | |
| CDR 0.5 (N = 36) | 97.2 | 77.8 | 72.2 | 58.3 | 36.1 |
| CDR 1 (N = 43) | 90.7 | 83.7 | 81.4 | 67.4 | 53.5 |
| CDR 2 (N = 15) | 93.3 | 86.7 | 80.0 | 66.7 | 66.7 |
| Overall (N = 94) | 93.6 | 81.9 | 77.7 | 63.8 | 48.9 |
Percentage of Alzheimer's disease (AD) patients below indicated percentile of normal.
CDR = Clinical Dementia Rating
DISCUSSION
In this study involving a large group of well characterized AD patients and elderly control subjects we have demonstrated that MR volumetric measurements of the hippocampal formation are useful in discriminating between very mild AD and elderly controls. The very mild AD subjects (CDR=0.5) qualify for the diagnosis of probable AD by clinical research criteria yet exhibit only the minimal symptoms necessary for this diagnostic classification. These patients present significant diagnostic difficulties for the clinician and constitute an area where a structural imaging test may be particularly useful.
Control Subjects
All MTL limbic structures measured declined in volume with advancing age. This is consistent with observations of age related brain atrophy in other imaging and autopsy studies (14, 41-46). It is not clear whether this volume loss is an inevitable consequence of aging (14, 41-47). Subjects enrolled as normal controls in this study were community dwelling individuals with no evidence of cognitive impairment. Subjects with other medical conditions-e.g. heart disease, diabetes, hypertension-were included as normal controls. It is possible that medical conditions which increase in prevalence with advancing age and may be associated with brain atrophy such as hypertension, diabetes, or atherosclerosis might account for some of the observed correlation between age and volume loss in these normal control subjects (48-50). Yet, this control population is typical of that which a clinician faces in daily practice. These data do not address the issue of optimal aging or the aging process without any co-morbid illnesses. In analyzing the association between MTL volume and age we recognize that this is a cross-sectional sample and that secular effects of different environmental or socioeconomic conditions experienced by successive age groups may be unrecognized (4).
Alzheimer's Disease Patients
The duration of disease in younger AD patients in this study was not different from that in older AD patients. Our data therefore reflect a cross-sectional sample at the time of the initial diagnosis of AD across an age spectrum from 50-89 years. In AD patients, MTL volumes declined with advancing age in parallel with those of controls. However, the age and gender adjusted normalized MTL volumes of AD patients were significantly smaller than those of controls. This was true for each MTL structure, at all ages, and for both men and women. We hypothesize that the volume loss in AD patients represents the progressive atrophy associated with the degenerative disease, superimposed on that associated with aging.
The analysis of segmental hippocampal volumes in controls demonstrated that age associated volume loss in the head of the hippocampus exceeds that of either the body or the tail. In addition, of the hippocampal segments, the largest volumetric difference between controls and AD patients was found in the hippocampal head. This observation is in agreement with a similar analysis that was performed on autopsy specimens (51). These data would suggest that the head of the hippocampus is more susceptible both to age related atrophy and also more susceptible to the degenerative change associated with AD. The observed differential sensitivity of the hippocampal head to age related and AD related atrophy may be related to differences in the nature of the cortical input between the hippocampal head and the more posterior segments of the hippocampus(52-54).
Discrimination Between Controls and AD Patients of Varying Severity
Although all the MTL limbic structures measured were significantly smaller in AD patients than controls, the structure which best discriminated between AD patients and controls was the total hippocampal volume. When a linear discriminant function model was constructed, essentially all the discriminatory power was found in the hippocampal volume alone. These results are at odds with those published by several other investigators who found that combinations of different volumetric measurements more effectively separated controls from AD patients than measurement of any single structure (15, 16, 19, 55, 56). It is possible that measurement of brain structures other than the ones evaluated here may be useful in a discriminant function analysis. However, the large number of subjects and careful attention to details of technical and neuroanatomic boundary criteria employed in this study should convincingly demonstrate the absence of additional discriminatory power provided by measurements of the PHG or amygdala. Of the three MTL structures measured, the anatomic boundaries presented by MRI are more precise, and less normal anatomic variability exits, for the hippocampus than for the amygdala or PHG (29). Hippocampal measurements therefore have less measurement error and also less “noise” introduced as a function of normal anatomic variation than those of the PHG or amygdala (57).
Hippocampal W values progressively decline (increasing atrophy) with increasing CDR score in Table 4. This suggests that hippocampal volume measurements are a sensitive marker of the degenerative neuroanatomic substrate of the progressively more severe memory impairment seen with advancing CDR scores in AD. Hippocampal volume measurements will not, however, discriminate among different conditions that share MTL atrophy as a common pathologic feature(58). Nevertheless, we believe that this type of MRI-based hippocampal volume measurement maybe helpful to the clinician as an adjunctive piece of diagnostic information in situations when the clinical diagnosis is difficult. A comparison of the normalized hippocampal volume measurements of an individual patient with age and gender specific normal percentiles (as illustrated in Fig. 2 and Table 3) would provide a clinically useful assessment of the presence and severity of hippocampal atrophy. An elderly patient complaining of a memory impairment whose hippocampal volumes fell into the AD range might be more closely scrutinized for a diagnosis of AD, while a patient with a similar complaint whose hippocampal volumes fell into the control range might be reassured that AD was less likely. One caveat however is that the absolute numeric output of any image based volume measurement technique is methodology-dependent(29). Therefore while the associations reported should be generalizable, the specific numeric values may vary among different sites.
In summary, the most encouraging finding in this study was the ability of hippocampal volume measurements to discriminate between controls and AD patients with very mild disease. The mean hippocampal volume in very mild (CDR 0.5) AD patients was 1.75 SD below the control mean and 97.2% of all CDR 0.5 AD patients had hippocampal volumes below the 50th percentile of normal. These data derived from a large number of subjects demonstrate that MRI volume measurements of hippocampal atrophy are a sensitive marker of the pathology of AD in its most mild form. The ability of quantitative MRI volume measurements to predict which currently normal or mildly impaired elderly subjects will develop AD in the future will, however, require longitudinal studies which are in progress.
Acknowledgments
Brenda Maxwell - Typing Ruth Cha - Statistical Analysis
Supported by NIH-NIA-AG11378; AG-08031; AG-06786; NINDS-NS29059; The DANA Foundation; The Alzheimer's Association
Abbreviations
- MRI
Magnetic Resonance Imaging
- TIV
Total Intracranial Volume
- PHG
Parahippocampal Gyrus
- MTL
Medial Temporal Lobe
- AD
Alzheimer's Disease
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Katzman R. Alzheimer's Disease. NEJM. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- 3.Kokmen E, Beard CM, O'Brien PC, Offord KP, et al. Is the incidence of dementing illness changing? A 25-year-time-trend study in Rochester, MN (1960-1984). Neurology. 1993;43:1887–1892. doi: 10.1212/wnl.43.10.1887. [DOI] [PubMed] [Google Scholar]
- 4.DeCarli C, Kaye JA, Horwitz B, Rapoport SI. Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology. 1990;40:872–883. doi: 10.1212/wnl.40.6.872. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR. memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 8.Naidich TP, Daniels DL, Haughton VM, et al. Hippocampal formation and related structures of the limbic lobe: anatomic MR correlation. Part I. Surface features and coronal sections. Radiology. 1987;162:747–754. doi: 10.1148/radiology.162.3.3809489. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr., Gehring D, Sharbrough F, et al. Temporal lobe volume measurement from MR images: accuracy and left-right asymmetry in normal individuals. JCAT. 1988;12(1):21–29. doi: 10.1097/00004728-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR, Jr., Twomey CK, Zinsmeister AR, et al. Anterior temporal lobes and hippocampal formations: normative volumetric measurements for MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Sharbrough FW, Twomey CK, Cascino GD, Hirschorn KA, Marsh WR, Zinsmeister AR, et al. Temporal Lobe Seizures: Lateralization with MR volume measurements of hippocampal formation. Radiology. 1990;175:423–429. doi: 10.1148/radiology.175.2.2183282. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr., Bentley M, Twomey CK, Zinsmeister AR. MR-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990;176:205–209. doi: 10.1148/radiology.176.1.2353093. [DOI] [PubMed] [Google Scholar]
- 13.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 14.Jack CR, Jr., Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 15.Lehericy S, Baulac M, Chiras J, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR. 1994;15:927–937. [PMC free article] [PubMed] [Google Scholar]
- 16.Laakso MP, Soininen H, Partanen K, Helkala E-L, Hartikainen P, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J of Neural Transmission. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- 17.Soininen HS, Partanen K, Pitkanen A, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 18.Pearlson G, Harris GJ, Powers RE, Barta PE, et al. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry. 1992;49:402–408. doi: 10.1001/archpsyc.1992.01820050066012. [DOI] [PubMed] [Google Scholar]
- 19.Killiany RJ, Moss MB, Albert MS, et al. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Arch Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- 20.Convit A, deLeon MJ, Golomb J, George AE, et al. Hippocampal atrophy in early Alzheimer's disease: anatomic specificity and validation. Psychiatric Quarterly. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- 21.Convit A, de Leon MH, Tarshish C, De Santi S, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Smith G, Kokmen E, et al. Memory function in normal aging. Neurology. 1992;42:396–401. doi: 10.1212/wnl.42.2.396. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Ivnik RJ, et al. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 26.Ivnik RJ, Malec JF, Tangalos EG, et al. The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psych Assess. 1990;2:304–312. [Google Scholar]
- 27.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, et al. Mayo's older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 thorugh 97. The Clinical Neuropsychologist. 1992;6:1–103. [Google Scholar]
- 28.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR., Jr MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(Suppl 6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 30.Duvernoy HM. The human hippocampus. An atlas of applied anatomy. JF Bergmann; Munich: 1988. pp. 77–91. [Google Scholar]
- 31.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 32.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gottman M, Watson C, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 33.Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115:1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- 34.Adam C, Baulac M, Saint-Hilaire J, Landau J, et al. Value of magnetic resonance imaging—based measurements of hippocampal formations in patients with partial epilepsy. Arch Neurol. 1994;51:130–138. doi: 10.1001/archneur.1994.00540140036012. [DOI] [PubMed] [Google Scholar]
- 35.Free SL, Bergin PS, Fish DR, et al. Methods for normalization of hippocampal volumes measured with MR. AJNR. 1995;16:637. [PMC free article] [PubMed] [Google Scholar]
- 36.Kuks JBM, Cook MJ, Fish DR, Stevens JM, Shorvon SD. Hippocampal sclerosis in epilepsy and childhood febrile seizures. Lancet. 1993;342:1391–1394. doi: 10.1016/0140-6736(93)92754-h. [DOI] [PubMed] [Google Scholar]
- 37.Murro AM, Park YD, King DW, Gallagher BB, et al. Seizure localization in temporal lobe epilepsy: a comparison of scalp-sphenoidal EEG and volumetric MRI. Neurology. 1993;43:2531–2533. doi: 10.1212/wnl.43.12.2531. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien PC, Dyck PJ. Procedures for setting normal values. Neurology. 1995;45:17–23. doi: 10.1212/wnl.45.1.17. [DOI] [PubMed] [Google Scholar]
- 39.Mattis S. Mental Status Examination for Organic Mental Syndromes in the Elderly Patient. In: Bellak l KT, editor. Geriatric Psychiatry. Grune and Stratton; New York: 1976. [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. Mini Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl. Acad. Sci. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coffey CE, Wilkinson WE, Parashos IA, et al. Quantitative cerebral anatomy of the aging human brain: A cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- 43.Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed x-ray tomography. J Am Geriatr Soc. 1992;40:225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 44.Dekaban AS, Sadowsky BS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 45.Miller AKH, Alston RL, Corsellis JAN. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 46.Zatz LM, Jernigan TL, Ahumada AJ. Changes on computed cranial tomography with aging. Intracranial fluid volume. AJNR. 1982;3:1–11. [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 48.DeCarli C, Murphy DGM, Gillette JA, et al. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR. 1994;15:689. [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy DGM, DeCarli C, Schapiro MB, et al. Age-related differences in volumes of subcortical muclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992;49:839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 50.Salerno JA, Murphy DGM, Horwitz B, et al. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992;20:340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 51.Chang F-LF, Parisi JE, Jack CRJ, Petersen RC. Morphometric analysis of the hippocampus in Alzheimer's Disease: Post-mortem MRI and histological correlates. Annals of Neurology. 1992;32:268. [Google Scholar]
- 52.Rosene DL, Van Hoesen GW. Hippocampal efferent reach widespread areas of cerebral cortex and amygdala in the Rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 53.Van Hoesen GW, Pawdya DN, Butters N. Cortical afferents to the entorhinal cortex of the Rhesus monkey. Science. 1972;175:1471–1473. doi: 10.1126/science.175.4029.1471. [DOI] [PubMed] [Google Scholar]
- 54.Witter MP, Room P, Goenewegen HJ, Lohman AHM. Connections of the parahippocampal cortex in the cat. V. Instrinsic connections; comments on input/output connections with the hippocampus. J Comparative Neurol. 1986;252:78–94. doi: 10.1002/cne.902520105. [DOI] [PubMed] [Google Scholar]
- 55.DeCarli C, Murphy DGM, McIntosh AR. Discriminant analysis of Alzheimer's disease. Arch Neurol. 1994;51:1088–1089. doi: 10.1001/archneur.1994.00540230026006. [DOI] [PubMed] [Google Scholar]
- 56.DeCarli C, Murphy DGM, McIntosh AR, et al. Discriminant analysis of MRI measures as a method to determine the presence of dementia of the Alzheimer type. Psychiatry Research. 1995;57(2):119–130. doi: 10.1016/0165-1781(95)02651-c. [DOI] [PubMed] [Google Scholar]
- 57.Laakso MP, Partanen K, Lehtovirta M, et al. MRI of amygdala fails to diagnose early Alzheimer's disease. NeuroReport. 1995;6:2414–2418. doi: 10.1097/00001756-199511270-00032. [DOI] [PubMed] [Google Scholar]
- 58.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]