Summary
Leptin plays a pivotal role in regulation of energy balance. Via unknown central pathways leptin also affects peripheral glucose homeostasis and locomotor activity. We hypothesized that specifically Pro-opiomelanocortin (POMC) neurons mediate those actions. To examine this possibility we applied Cre-Lox technology to express leptin receptors (ObRb) exclusively in POMC neurons of the morbidly obese, profoundly diabetic, and severely hypoactive leptin receptor deficient Leprdb/db mice. We here show that expression of ObRb only in POMC neurons leads to a marked decrease in energy intake and a modest reduction in body weight in Leprdb/db mice. Remarkably, blood glucose levels are entirely normalized. This normalization occurs independently of changes in food intake and body weight. In addition, physical activity is greatly increased despite profound obesity. Our results suggest that leptin signaling exclusively in POMC neurons is sufficient to stimulate locomotion and prevent diabetes in the severely hypoactive and hyperglycemic obese Leprdb/db mice.
Keywords: Leptin Receptor, POMC, Melanocortin, Hypothalamus, Glucose Balance, Locomotor Activity
Introduction
Leptin, an adipocyte-derived hormone, acts on the central nervous system to regulate energy balance by activating the long form of the leptin receptor (ObRb or LEPR-B)(Friedman and Halaas, 1998). The absence of leptin or leptin receptors in Lepob/ob mice or Leprdb/db mice respectively, results in morbid obesity, hyperphagia, severe hyperglycemia and insulin resistance, and marked hypoactivity (Chen et al., 1996; Coleman, 1978; Lee et al., 1996; Pelleymounter et al., 1995; Tartaglia et al., 1995; Zhang et al., 1994). Among several leptin-responsive brain regions, the arcuate nucleus of the hypothalamus (ARC) is a key area for mediating leptin actions on energy homeostasis. Consistent with this, leptin receptor mRNA is densely expressed in the ARC of mice and rats (Elmquist et al., 1998; Mercer et al., 1996), and injection of leptin directly into the ARC is sufficient to acutely reduce food intake (Satoh et al., 1997). Moreover, unilateral restoration of leptin receptor expression in the ARC of leptin receptor deficient Leprdb/db mice leads to long term reduction of body weight and food intake (Coppari et al., 2005), and arcuate nucleus-specific Lepr gene therapy is sufficient to attenuate the obesity phenotype of leptin receptor deficient Koletsky fak/fak rats (Morton et al., 2003).
The ARC contains at least two subsets of leptin responsive neurons, namely the anorexigenic POMC neurons and the orexigenic Agouti-related peptide (AgRP) neurons. POMC neurons are depolarized by leptin, leading to release of α-melanocyte stimulating hormone (α-MSH), a POMC-derived neuropeptide that mediates its anorexigenic effects through activation of melanocortin receptors (Cone, 2005; Cowley et al., 2001; Schwartz et al., 2000). AgRP is an endogenous melanocortin receptor antagonist that potently stimulates feeding (Ollmann et al., 1997). Consistent with this, AgRP neurons are inhibited by leptin (van den Top et al., 2004). Mice lacking leptin receptors only in POMC or AgRP neurons are mildly obese, demonstrating that both groups of cells are partly required for maintenance of body weight homeostasis by leptin (Balthasar et al., 2004; van de Wall et al., 2007).
In addition to its role in energy homeostasis, leptin can regulate peripheral glucose and insulin homeostasis via the central nervous system. For example, leptin-deficient Lepob/ob mice exhibit profound diabetes that can be fully prevented by low doses of leptin that do not affect body weight and food intake (Pelleymounter et al., 1995). In addition, intracerebroventricular leptin can acutely stimulate glucose uptake in skeletal muscle (Cusin et al., 1998; Haque et al., 1999; Kamohara et al., 1997; Minokoshi et al., 1999) and inhibit hepatic glucose production (Pocai et al., 2005; van den Hoek et al., 2008). Moreover, leptin dramatically improves insulin sensitivity in human lipodystrophy and in lipodystrophic mouse models which are characterized by low serum leptin levels and by severe insulin resistance (Oral et al., 2002; Petersen et al., 2002; Shimomura et al., 1999). Genetic studies in mice suggest that the arcuate hypothalamic nucleus plays a major role in mediating effects of leptin on glucose balance and on voluntary locomotor activity (Coppari et al., 2005), however the specific arcuate neurons responsible remain unspecified.
Long-term impairment of central melanocortin receptor action in mice results in marked obesity, hyperinsulinemia, and late-onset hyperglycemia (Huszar et al., 1997). Insulin resistance is present before the onset of obesity in melanocortin-4-receptor deficient mice (Fan et al., 2000). In addition, ventricular infusion of α-MSH into rats enhances acute insulin-stimulated peripheral glucose uptake and reduces hepatic glucose production, while a melanocortin receptor antagonist exerts opposite effects (Obici et al., 2001). Furthermore, loss of glucose-sensing by POMC neurons and of glucose-dependent α-MSH release, leads to impaired whole body glucose tolerance (Parton et al., 2007). Combined, these studies suggest a role of POMC neurons and the downstream melanocortin pathway in influencing peripheral insulin sensitivity and glucose balance. However the extent to which POMC neuronal action can prevent severe hyperglycemia is unknown.
Given that arcuate POMC neurons express leptin receptors and that both leptin and the melanocortin system can influence glucose and insulin homeostasis, we hypothesized that POMC neurons mediate those leptin actions. In addition, yet unspecified arcuate neurons can control locomotor activity by leptin. To that end, we generated mice expressing leptin receptors exclusively in POMC neurons of the severely diabetic and hypoactive Leprdb/db mice.
Results
Generation of mice expressing leptin receptors in POMC neurons
Mice expressing HA-tagged ObRb in POMC neurons were generated by mating HA-ObRb transgenic mice with transgenic Pomc-Cre mice (Figure 1A). The HA-tag at the N-terminal of ObRb was introduced to facilitate detection of the leptin receptor protein. To first assess whether HA-ObRb was expressed in areas known to contain POMC neurons, we performed HA immunohistochemistry (IHC). As expected, HA immunoreactive (IR) cells were found in the ARC of HA-ObRb/Pomc-Cre mice (Figure 1B), but not in brains from HA-ObRb control mice (Figure 1B). Double HA and β-Endorphin IHC analyses on brains from three HA-ObRb/Pomc-Cre mice showed that 44% (+/− 12%) of POMC neurons (3,760+/−440) in each animal expressed HA-ObRb. This percentage may be underestimated due to reduced sensitivity of the HA antibody combined with low HA-ObRb expression. Importantly, the vast majority (87% +/−9%) of detectable HA IR neurons were POMC neurons (Figure 1C).
Figure 1. Generation of Mice Expressing HA-tagged Leptin Receptors in POMC Neurons.
A) Strategy for generation of HA-ObRb/Pomc-Cre mice. B) HA-ObRb expressing neurons of HA-ObRb/Pomc-Cre mice are located in the arcuate nucleus of the hypothalamus, as shown by HA immunohistochemistry (IHC). Right Images: High magnification of areas marked in the left two photomicrographs. C) HA-ObRb is expressed in POMC neurons of HA-ObRb/Pomc-Cre mice, as demonstrated by double-fluorescent IHC for HA (red) and β-Endorphin (green). High magnification images are shown on the right. Arc, arcuate nucleus; 3v, third ventricle; ME, median eminence. Scale bars, 50 µm.
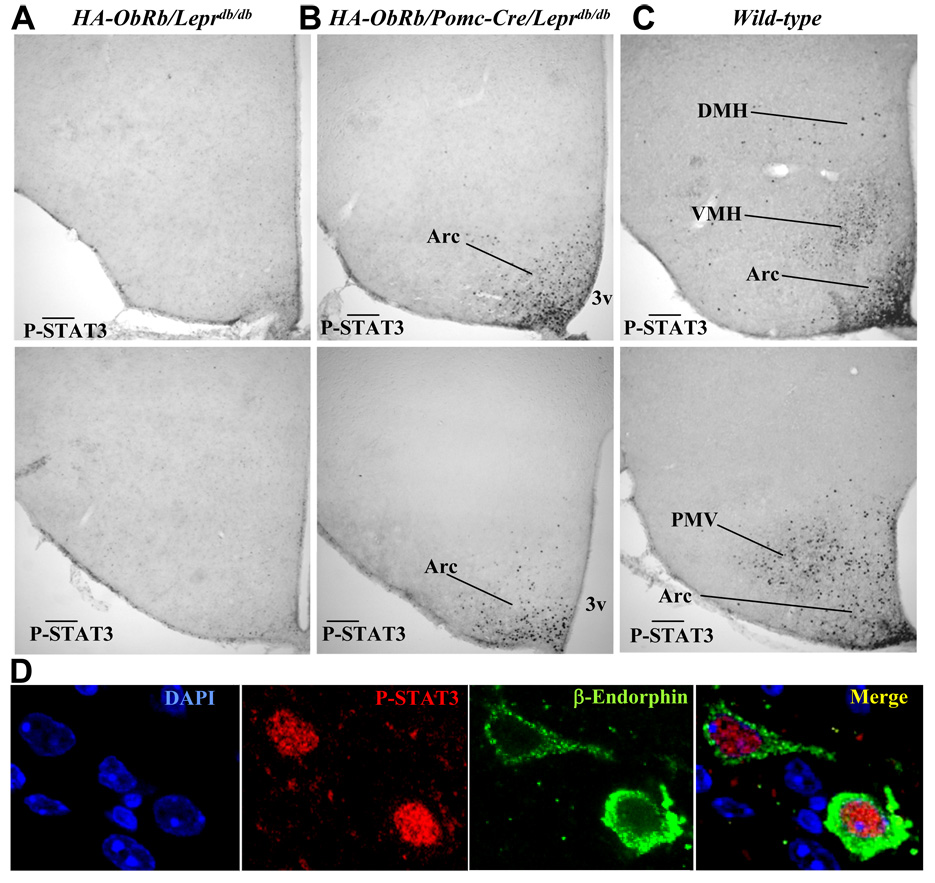
The HA-tag did not interfere with STAT3 activation by ObRb in transfected cells (not shown). To determine whether HA tagged leptin receptors are also functional in vivo, we generated mice that express HA-ObRb in POMC neurons but lack endogenous long form receptors (HA-ObRb/Pomc-Cre/Leprdb/db) and measured leptin-dependent STAT3 phosphorylation in the brain. As predicted, leptin did not induce P-STAT3 in the hypothalamus of HA-ObRb/Leprdb/db control mice (Figure 2A) or Pomc-Cre/Leprdb/db control mice (not shown). In leptin-treated Leprdb/db mice we counted a baseline of 106+/−39 P-STAT3 IR neurons in the entire arcuate nucleus. In contrast, 1877+/−612 P-STAT3 positive cells were found in the arcuate of leptin-treated HA-ObRb/Pomc-Cre/ Leprdb/db mice (Figure 2B) and 9540+/−885 in wild type mice (Figure 2C). Importantly, P-STAT3 was not detected in the ventromedial (VMH), the dorsomedial (DMH), and the premammillary (PMV) nuclei of HA-ObRb/Pomc-Cre/Leprdb/db mice, regions known to express P-STAT3 in leptin-treated wild type mice (Figure 2C)(Munzberg et al., 2004). A small group of POMC neurons exists in the nucleus of the solitary tract (NTS) of the caudal hindbrain (Palkovits and Eskay, 1987). Despite lack of detectable HA positive neurons in this region (not shown), P-STAT3 IR cells were detected in the NTS of HA-ObRb/Pomc-Cre/Leprdb/db mice (not shown). The expression of HA-ObRb in the NTS is thus lower compared to the ARC, but still sufficient to activate STAT3.
Figure 2. HA-tagged Leptin Receptors in POMC Neurons are Functional in vivo.
A–C) Representative photomicrographs of P-STAT3 IHC in brains from leptin-treated (i.p. 4mg/kg, 45 min) HA-ObRb/Leprdb/db (A), HA-ObRb/Pomc-Cre/Leprdb/db (B), and wild type (C) mice. Matched medial (top) and caudal (bottom) arcuate sections are shown. Arc, arcuate nucleus; DMH, dorsomedial nucleus; VMH, ventromedial nucleus; PMV, ventral premammillary nucleus. Scale bars, 200 µm. D) Leptin activates nuclear P-STAT3 in two POMC neurons of HA- ObRb/Pomc-Cre/Leprdb/db mice as shown by confocal microscopy.
Counts of β-Endorphin and P-STAT3 positive neurons (Figure 2D) in leptin-treated HA- ObRb/Pomc-Cre/Leprdb/db mice showed that 56% (+/−24%) of POMC neurons expressed P-STAT3, and that 88% (+/−8%) of the P-STAT3 IR cells were POMC neurons. Further, 60% (+/−5%) of POMC neurons in leptin-treated wild type mice and less than 2% of POMC neurons in Leprdb/db mice expressed P-STAT3.To assess the expression level of functional HA-ObRb in POMC neurons of transgenic mice relative to endogenous leptin receptors in POMC neurons of wild type mice, leptin-dependent STAT3 phosphorylation in individual POMC neurons was quantified. We found a 50% decreased level of P-STAT3 in HA-ObRb/Pomc-Cre/Leprdb/db mice relative to control mice (Supplemental Figure 1). These data combined demonstrate that HA-ObRb receptors in POMC neurons are functional in vivo, and suggest that HA-ObRb levels in POMC neurons of transgenic HA-ObRb/Pomc-Cre/Leprdb/db mice are modestly lower compared to wild type mice.
Leptin signaling in POMC neurons of Leprdb/db mice reduces body weight and food intake
Body weights of HA-ObRb/Pomc-Cre/Leprdb/db mice were slightly decreased (10–15%) as compared to Leprdb/db mice, but remained greatly increased (65–90%) relative to wild type mice (Figure 3A and 3B). Fat mass and circulating leptin levels tended towards a modest decrease in HA-ObRb/Pomc-Cre/Leprdb/db mice versus Leprdb/db mice (Figure 3E and 3F, Table 1). Body length (Table 1) and lean mass was not different among these groups (not shown). Food intake was markedly reduced (30–40%) in HA-ObRb/Pomc-Cre/Leprdb/db mice compared to Leprdb/db control mice, but remained significantly higher than wild type mice (Figure 3C and 3D). Whole body energy expenditure as assessed by indirect calorimetry was not different between HA-bRb/Pomc-Cre/Leprdb/db and Leprdb/db mice (Table 1). The respiratory exchange ratio (RER) was modestly increased in female, but not male, HA-ObRb/Pomc-Cre/Leprdb/db mice relative to Leprdb/db animals. Altogether, the data suggest that the decreased body weight of HA-ObRb/Pomc-Cre/Leprdb/db mice is primarily due to reduced energy intake.
Figure 3. Expression of HA-ObRb in POMC Neurons of Leprdb/db Mice Reduces Body Weight and Food Intake.
A)-B) Body weight curves. C)-D) Cumulative food intake (7–9 wks). E)-F) Adipose mass (12 wks of age). A)-D) Data were collected from 11 female and 14 male Leprdb/db mice, 11 female and 17 male HA-ObRb/Pomc-Cre/Leprdb/db mice, and 5 female and 9 male wild type mice. Fat mass was measured by Echo-MRI in 5 female and 4 male Leprdb/db mice, 5 female and 7 male HA-ObRb/Pomc-Cre/Leprdb/db mice, and 8 female and 6 male wild type mice. All animals are littermates. *, p < 0.05; **, p < 0.01, ***, p< 0.001.
Table 1.
Body Length, O2 Consumption, CO2 Production, RER, Leptin, and Triglycerides. Serum analyses and body length were measurements in 18 weeks old mice. O2 Consumption, CO2 production, and RER were measured at 13 weeks of age. The number of mice per group is shown in parentheses. *, p < 0.05.
| Leprdb/db | HA-ObRb/Pomc-Cre/Leprdb/db | |
|---|---|---|
| Nose/anus length (cm) | ||
| Males | 10.6 ± 0.2 (5) | 10.5 ± 0.2 (7) |
| Females | 10.7 ± 0.1 (7) | 10.7 ± 0.1 (8) |
| Leptin (ng/ml) | ||
| Males | 100.4 ± 8.5 (17) | 91.7 ± 5.4 (12) |
| Females | 106.8 ± 6.7 (11) | 96.1 ± 6.9 (11) |
| Triglycerides (mg/dl) | ||
| Males | 223.1 ±21.9 (7) | 236.9 ± 33.3 (11) |
| Females | 220.3 ± 19.2 (10) | 180.1 ± 21.3 (8) |
| Respiratory Exchange Ratio | ||
| Males | 0.86 ± 0.03 (6) | 0.91 ± 0.03 (4) |
| Females | 0.80 ± 0.04 (7) | 0.89 ± 0.03 (5) |
| VO2 (ml/day) | ||
| Males | 7639 ± 730 (6) | 5844 ± 885 (4) |
| Females | 5566 ± 552 (7) | 5408 ± 691 (5) |
| VCO2 (ml/day) | ||
| Males | 6613 ± 758 (6) | 5335 ± 779 (4) |
| Females | 4406 ± 728 (7) | 4984 ± 521 (5) |
Normalization of glucose by ObRb in POMC neurons in Leprdb/db mice
Expression of HA-ObRb in POMC neurons of Leprdb/db mice dramatically improves blood glucose levels. Remarkably, in five weeks old HA-ObRb/Pomc-Cre/Leprdb/db mice blood glucose levels were fully normalized (Figure 4A–4D). This normalization was also observed in overnight fasted female and male HA-ObRb/Pomc-Cre/Leprdb/db mice (not shown). To examine whether this reduction in circulating glucose in HA-ObRb/Pomc-Cre/Leprdb/db mice was dependent of energy intake, eight weeks old female Leprdb/db mice were pair-fed (~30% intake reduction) to ad libitum fed HA-ObRb/Pomc-Cre/Leprdb/db littermates for three weeks (Figure 4F). As shown in Figure 4E, this food restriction did not reduce hyperglycemia in Leprdb/db mice. During the 3 weeks, the average body weight of Leprdb/db mice fell 3.5 grams (42.1 (+/−1.9) to 38.6 (+/−1.2)). In comparison, ad libitum fed Leprdb/db mice with the same age gained 8.6 grams (40.4 (+/−1.4) to 49.0 (+/−1.2)) during 3 weeks (Figure 3A), altogether demonstrating that the pair-feeding paradigm greatly impacted the metabolic status of Leprdb/db mice and that this alone was insufficient to attenuate hyperglycemia. Therefore, expression of leptin receptors in POMC neurons of Leprdb/db mice is sufficient to correct hyperglycemia independently of changes in energy intake and body weight.
Figure 4. Expression of HA-ObRb in POMC Neurons of Leprdb/db Mice Normalizes Circulating Glucose Levels and Increases Insulin Sensitivity.
A) Fed glucose levels and C) body weight of 5 wks old female Leprdb/db mice (n=7), HA-ObRb/Pomc-Cre/Leprdb/db mice (n=8), and wild type mice (n=8). B) Fed glucose levels and D) body weight of 5 wks old male fed Leprdb/db mice (n=11), HA-ObRb/Pomc-Cre/Leprdb/db mice (n=12), and wild type mice (n=14). E) Glucose levels in food-restricted Leprdb/db mice. Over a period of 3 weeks, 8 wks old female Leprdb/db mice (n=4) were pair-fed to ad libitum fed HA- ObRb/Pomc-Cre/Leprdb/db littermates (n=4). Wild type littermates (n=6) were fed ad libitum. F) Cumulative intake over the 3 wks.G) Fasted serum insulin levels of 12 wks old female Leprdb/db mice (n=7), HA-ObRb/Pomc-Cre/Leprdb/db mice (n=8), and wild type mice (N=8). H) Insulin tolerance tests in 13 weeks old female Leprdb/db mice (n=7), HA-ObRb/Pomc-Cre/Leprdb/db mice (n=8), and 4 wild type mice. (*, Leprdb/db vs HA-ObRb/Pomc-Cre/Leprdb/db). Glucose was administered to wild type mice after 30 min to prevent severe hypoglycemia caused by the high insulin dose (1.5 U/kg). Shown are changes from baseline. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, not significant.
Serum insulin levels were reduced by 60% in HA-ObRb/Pomc-Cre/Leprdb/db mice compared to Leprdb/db control mice, but elevated relative to control animals (Figure 4G). Insulin tolerance was significantly improved in HA-ObRb/pomc-Cre/Leprdb/db mice relative to Leprdb/db mice over time (p=0.021), and trended towards a decrease (0–30 min) compared to wild type mice (p=0.097)(Figure 4H). In 12 weeks old fasted HA-ObRb/pomc-Cre/Leprdb/db mice, blood glucose levels were markedly reduced as compared to Leprdb/db mice (Supplemental Figure 2A), despite only a slight decrease in weight (Supplemental Figure 2B). Fed glucose was similarly attenuated (not shown). To further assess whether the reduced glucose levels occurred independently of reduced body weight, we selected mice from the Leprdb/db and HA-ObRb/Pomc-Cre/Leprdb/db groups such that the average body weight of both subgroups were not different (Supplemental Figure 2D). Despite equal body weights, glucose remained reduced in HA-ObRb/Pomc-Cre/Leprdb/db mice (Supplemental Figure 2C), thus further supporting the notion that leptin signaling in POMC neurons of Leprdb/db mice is sufficient to reduce circulating glucose levels independent of body weight. Expression of HA-ObRb in POMC neurons of lean non-Leprdb/db mice did not affect body weight or glucose levels on the mixed (FVB/C57BLKSJ) genetic background (Supplemental Figure 3A and 3B). Similarly, HA-ObRb/Pomc-Cre mice on a pure FVB background were indistinguishable from wild type littermates with regard to body weight, food intake, circulating glucose and insulin, and glucose- and insulin-tolerance (Supplemental Figures 4 and 5).
Leptin receptors in POMC neurons of Leprdb/db mice increases islet mass
Pancreatic islets in Leprdb/db mice have altered morphology and impaired function (Baetens et al., 1978; Leiter et al., 1983). To indirectly assess islet function in HA-ObRb/Pomc-Cre/Leprdb/db mice, we investigated islet morphology, islet size, and total endocrine mass (Supplemental Figure 6). Total endocrine mass was increased by 2.5 fold in HA-ObRb/Pomc-Cre/Leprdb/db mice as compared to both Leprdb/db and wild type mice, and was caused by increased islet size. The total number of islets was similar among the three groups (not shown). Islets from HA- ObRb/Pomc-Cre/Leprdb/db mice appeared structurally intact. In contrast, Leprdb/db islets exhibited variable size and irregular morphology. The total islet mass did not correlate with circulating insulin levels between Leprdb/db and HA-ObRb/Pomc-Cre/Leprdb/db groups, indicating differential β-cell regulation and/or functionality.
Leptin receptors in POMC neurons increases activity
Restoration of leptin-receptors in arcuate neurons greatly increases voluntary locomotor activity in Leprdb/db mice (Coppari et al., 2005), but the specific neurons mediating this action remain unknown. We therefore measured ambulatory movements of HA-ObRb/Pomc-Cre/Leprdb/db mice and found a remarkable increase (~100%) compared to Leprdb/db mice (Figure 5). The activity of HA-ObRb/Pomc-Cre/Leprdb/db mice was not statistically different from lean wild type mice, although there was a trend towards a reduction.
Figure 5. Increased Activity of Leprdb/db Mice Expressing HA-ObRb in POMC Neurons.
A) Examples of locomotor activity from one Leprdb/db, one HA-ObRb/Pomc-Cre/Leprdb/db and one wild-type mouse. Black bar depicts lights-off period. B) Average nocturnal, C) diurnal, and D) 24 hours locomotor activity in 13 weeks old male Leprdb/db mice (n=4), HA-ObRb/Pomc- Cre/Leprdb/db mice (n=6), and wild type mice (n=5).*, p < 0.05; NS, not significant.
Effects of leptin signaling in POMC neurons on neuropeptides
HA-ObRb/Pomc-Cre/Leprdb/db mice had modestly increased hypothalamic POMC mRNA (Figure 6A) consistent with a role of leptin signaling to stimulate Pomc gene expression (Munzberg et al., 2003; Thornton et al., 1997). Moreover, total hypothalamic α-MSH peptide levels were 3.5 fold increased in HA-ObRb/Pomc-Cre/Leprdb/db mice relative to Leprdb/db mice, and were similar to wild type levels (Figure 6D). The increase in α-MSH was observed both in the arcuate and in POMC fibers innervating the DMH (Figure 6G). NPY and AgRP neuropeptide levels were unchanged despite slight changes in AgRP and NPY mRNA (Figure 6B–C, 6E–F).
Figure 6. Hypothalamic POMC mRNA and α-MSH Peptide Levels are Increased in Leprdb/db Mice Expressing Leptin Receptors Exclusively in POMC Neurons.
A) - C) Arcuate POMC, NPY, and AgRP mRNA from 18 weeks old control Leprdb/db mice (n=7) and HA-ObRb/Pomc-Cre/Leprdb/db mice (n=5). D) - F) Hypothalamic α-MSH, NPY, and AgRP neuropeptide levels as measured by EIA’s in 18 weeks Leprdb/db mice (n=3), HA-ObRb/Pomc- Cre/Leprdb/db mice (n=3), and wild type mice (n=3). Numbers are depicted as percent of Leprdb/db levels. *, p< 0.05; **, p<0.01; NS, not significant. G) Representative images of hypothalamic α-MSH immuno-staining in 18 wks old HA-ObRb/Pomc-Cre/Leprdb/db and Leprdb/db mice. Scale bar, 200 µm.
Discussion
Through actions in the CNS, leptin affects a wide number of biological systems and processes, including feeding behavior, body weight balance, neuroendocrine function, insulin sensitivity, glucose homeostasis, and physical activity, demonstrating pleiotrophic properties of the hormone. Little is however known about which specific neuronal groups mediate each of leptin’s actions. We show here that expression of ObRb only in POMC neurons is sufficient to correct diabetes and drastically stimulate locomotor activity of the leptin receptor-deficient Leprdb/db mice.
Restoration of leptin receptors in arcuate neurons normalizes blood glucose levels and voluntary locomotor activity in mice lacking all leptin receptors (Coppari et al., 2005). However the specific arcuate neurons responsible remained unspecified. We here extend those findings by identifying POMC neurons as the likely mediators of those effects of leptin. Interestingly, deletion of ObRb from only POMC neurons of lean mice does not lead to hyperglycemia (Balthasar et al., 2004) or hypoactivity (Shi et al., 2008), suggesting that leptin receptors in POMC neurons are not required for normal regulation of glucose balance and locomotion. In contrast, our studies demonstrate that leptin receptor signaling in POMC neurons is sufficient to exert remarkable control of serum glucose concentrations and locomotion. These data combined could be explained by the existence of several groups of leptin responsive neurons that are each capable of independently regulating glucose homeostasis and physical activity.
The specific mechanism by which leptin signaling in POMC neurons leads to normalization of blood glucose is unknown, but likely involves neuroendocrine processes and/or autonomic actions. Our data suggest that the normalization is a direct consequence of leptin action in POMC neurons and is not secondary to differences in energy consumption or body weight. Consistent with this, central leptin administration can acutely increase muscle glucose uptake (Kamohara et al., 1997) and decrease hepatic glucose production (van den Hoek et al., 2008) through altered autonomic output (Haque et al., 1999). Furthermore, the central melanocortin pathway can increase sympathetic drive to peripheral tissues and enhance insulin-dependent glucose disposal (Brito et al., 2007; Heijboer et al., 2005; Rahmouni et al., 2003). Therefore, since HA- ObRb/Pomc-Cre/Leprdb/db mice have greatly elevated hypothalamic levels of α-MSH peptides, increased activity of the melanocortin system may contribute to the normo-glycemic phenotype, which likely results in part from increased insulin sensitivity and enhanced islet function/capacity. Whether the increased islet mass is a direct result of leptin action via POMC neurons and CNS regulation (Imai et al., 2008), or is an indirect consequence of altered metabolic state or incretin action (Karaca et al., 2009), remains to be elucidated. Moreover, detailed experiments are required to determine the specific role of the melanocortin system and the relative contribution of the pancreas and insulin-sensitive tissues, such as muscle and liver, in the normalization of serum glucose levels.
Exercise training increases insulin-sensitivity and improves glucose homeostasis (Hespel et al., 1996; Ryder et al., 2001). It is therefore possible that the increased insulin sensitivity and whole body glucose balance of HA-ObRb/Pomc-Cre/Leprdb/db mice is enhanced by increased physical activity. A role of skeletal muscle AMP-activated protein kinase (AMPK) in mediating exercise-dependent stimulation of insulin sensitivity and glucose uptake has been reported (Musi and Goodyear, 2003). In addition, central administration of melanocortin receptor agonists is sufficient to activate muscle AMPK phosphorylation in mice (Tanaka et al., 2007). The extent to which increased locomotion may contribute to the lowered circulating glucose concentration remains to be determined.
The marked increase in locomotion of mice expressing ObRb exclusively in POMC neurons could be due to their improved metabolic status or to a direct effect of leptin action via POMC neurons. In favor of the latter possibility, genetically obese rodents such as melanocortin-4-receptor knockout mice have reduced physical activity before they become obese (Ste Marie et al., 2000). In addition, AgRP-deficient mice are hyperactive (Wortley et al., 2005) and recombinant AgRP decreases locomotor activity in normal mice (Tang-Christensen et al., 2004), further suggesting a role of the melanocortin pathway. Moreover, deletion of leptin-receptors from AgRP neurons results in a modest reduction in activity (van de Wall et al., 2007) and constitutive STAT3 signaling specifically in AgRP neurons increases locomotion (Mesaros et al., 2008). We did not find altered levels of AgRP in HA-ObRb/Pomc-Cre/Leprdb/db mice, but the increase in hypothalamic α-MSH peptides supports the possibility of a role of the melanocortin pathway in stimulating locomotor activity in these mice.
In contrast to the striking effects of leptin-signaling in POMC neurons on blood glucose and locomotor activity in Leprdb/db mice, regulation of body weight appears only partially mediated by POMC neurons. At 12 weeks, HA-ObRb/Pomc-Cre/Leprdb/db mice are ~10% lighter relative to mice lacking all leptin receptors. It is however possible that the difference in weight between HA-ObRb/Pomc-Cre/Leprdb/db and Leprdb/db mice would be greater if Leprdb/db mice had not been diabetic and therefore loose calories through the urine. The contribution of POMC neurons in leptin’s regulation of body weight might thus be underestimated. However, our result showing a modest contribution of POMC neurons on body weight is consistent with data from non-diabetic 12 weeks old mice where deletion of leptin receptors from POMC neurons results in a 10% weight increase (Balthasar et al., 2004). Therefore, both deletion- and restoration-data show that leptin signaling elsewhere in the CNS is required for leptin’s full effect on long-term body-weight regulation. In further support of this, deletion of ObRb in the ventromedial hypothalamus (VMH) in 12 wks old mice increases body weight by ~10% and a combined loss in POMC and VMH neurons increases weight by 25%, suggesting additive effects (Dhillon et al., 2006). Further, deletion of leptin receptors selectively from AgRP neurons results in a 16% increase in body weight in 8–12 weeks old mice and combined ObRb deletion from both AgRP and POMC neurons increases body weigh by ~28% (van de Wall et al., 2007). Body weight regulation by leptin is therefore likely distributed between several brain nuclei and cell types, each having partial, but additive roles.
We found a marked 30–40% reduction in energy intake in mice expressing leptin receptors solely in POMC neurons relative to Leprdb/db mice, but did not detect changes in whole body O2 consumption. The greatly increased locomotor activity therefore does not appear to significantly impact whole body energy expenditure, and the reduction in food intake likely accounts for the decrease in body weight of HA-ObRb/Pomc-Cre/Leprdb/db mice. This suggests that POMC neurons play a partial role in attenuating food intake by leptin, but have little if any role in leptin’s action to increase energy expenditure (Hwa et al., 1997).
In addition to the hypothalamus, a small group of POMC neurons exists in the nucleus of the solitary tract (NTS) of the caudal brainstem (Palkovits and Eskay, 1987) . The Cre-Loxp strategy was expected to induce expression of HA-ObRb both in arcuate and NTS POMC neurons. Indeed, STAT3 phosphorylation was detected in both sites of leptin-treated HA-ObRb/Pomc-Cre/Leprdb/db animals. However in normal mice, NTS POMC neurons do not appear respond to leptin as measured by lack of leptin-inducible STAT3 phosphorylation (Huo et al., 2006), although another group reported the opposite results using a similar strategy (Ellacott et al., 2006). The reason for the discrepancy between the two studies is yet unclear. Regardless, since leptin receptors are expressed in NTS neurons of HA-ObRb/Pomc-Cre/Leprdb/db mice, a possible role of these cells in mediating some of the phenotypic changes in HA-ObRb/Pomc-Cre/Leprdb/db animals cannot be excluded. However, restoration of endogenous leptin receptors solely in the arcuate nucleus, accomplished by stereotaxic delivery of adeno viruses expressing FLPe-recombinase in otherwise leptin-receptor deficient mice, also results in a partial reduction in body weight and food intake, and a normalization of serum glucose levels and of locomotor activity (Coppari et al., 2005). Since these findings are directly comparable to the phenotype of our HA-ObRb/Pomc-Cre/Leprdb/db mice we conclude that arcuate POMC neurons, but not NTS POMC neurons, are responsible for mediating those effects of leptin.
Approximately 50% of hypothalamic POMC neurons express functional HA-leptin receptors in the transgenic mice, even though the Cre-Loxp strategy might have predicted a higher fraction. The reason for this is unclear. However, we found that in wild type mice only about 60% of POMC neurons respond to leptin as measured by STAT3 phosphorylation. Furthermore, unilateral, not bilateral, restoration of endogenous leptin receptors in arcuate neurons of leptin-receptor deficient mice (Coppari et al., 2005) results in a phenotype strikingly similar to that of our HA-ObRb/Pomc-Cre/Leprdb/db mice. These data combined therefore suggest that leptin signaling in ~50% of hypothalamic POMC neurons are sufficient to normalize blood glucose levels and stimulate physical activity.
In conclusion, our data show that leptin signaling specifically in hypothalamic POMC neurons is sufficient to correct diabetes and greatly increase locomotion in Leprdb/db mice, revealing a remarkable novel regulatory capacity of POMC neurons.
Experimental Procedures
Animal Care
Animal care and procedures were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. Mice were housed at 22°C–24°C using a 14 hr light/10 hr dark cycle with chow food (Teklad F6 Rodent Diet 8664, Harlan Teklad, Madison, WI).
Generation of HA-OBRb Transgenic Mice
A 6.8 kb HA-ObRb STOP transgene was generated consisting of the following elements (5’ to 3’): 1) a 1.6 kb CAG promoter that combines the human cytomegalovirus (CMV) enhancer and a chicken β-actin promoter and the first intron; 2) a Loxp site; 3) a STOP sequence containing a false translational stop sequence and an SV40 splice donor/poly(A) site; 4) a Loxp site; 5) a hemagglutinin (HA) tagged murine leptin receptor (HA-ObRb) cDNA sequence (the HA epitope (TyrProTyrAspValProAspTyrAla) was inserted after residue 42 of the leptin receptor); and 6) a bovine growth hormone polyadenylation (PA) sequence. Briefly, the 6.8 kb construct was produced by first cloning the HA-ObRb cDNA on a NotI/HindIII fragment from a plasmid generously given by Dr. J. Flier (Harvard Medical School, Boston, MA) into pGEM-11Zf(+) (Promega, Madison, WI). The PA sequence with flanking HindIII and SfiI restriction sites was amplified from pcDNA3.1Zeo(-) (Invitrogen, Carlsbad, CA) using the following primer set: 5’-CCCAAGCTTAAGTTTAAACCGCTGATCAGC-3’ and 5’- CCCAAGCTTGGCCATGCAGGCCGCCATAGAGCCCACCGCATCC-3’, and cloned into a HindIII site downstream of the HA-OBRb cDNA. A SalI/NotI fragment encompassing the Loxp- flanked STOP sequence from plasmid cAct-XstopXnz (a generous gift from Dr. Anderson, CIT, CA)(Tsien et al., 1996) was then inserted upstream of the HA-ObRb cDNA. Finally, the CAG promoter isolated from pDRIVE-CAG (InvivoGen, San Diego, CA) was cloned into a SalI site located upstream of the Loxp-flanked STOP sequence. Transgenic mice were generated by microinjecting the 6.8kb SfiI HA-ObRb STOP DNA fragment into fertilized one-cell embryos (FVB strain) by the transgenic facility at BIDMC.
Generation of HA-OBRb/Pomc-Cre Mice
HA-ObRb transgenic mice (FVB background) were mated with transgenic Pomc-Cre mice (FVB) which have been described earlier (Balthasar et al., 2004; van de Wall et al., 2007) and kindly supplied by Dr. Lowell (BIDMC, Boston, MA).
Generation of HA-ObRb/Pomc-Cre/Leprdb/db Mice
Leprdb/+ (C57BLKSJ, stock # 00642) mice were purchased from Jackson Laboratories (Bar Harbor, ME). HA-ObRb/Pomc-Cre mice (FVB) were then mated with Leprdb/+ mice. HA- ObRb/Pomc-Cre/Leprdb/db mice were obtained by inter-mating of HA-ObRb/Pomc-Cre/Leprdb/+ mice. Only littermates with the same mixed genetic background (FVB/C57BLKSJ) were compared, except for Supplemental Figures 4 and 5 where pure FVB non-Leprdb/db mice were studied.
Materials
Murine leptin was purchased from Dr. E. Parlow (NIDDK, Torrance, CA) and human insulin was from Eli Lilly (Indianapolis, IN). Supplies for immunohistochemistry (IHC) were from Sigma-Aldrich (St. Louis, MO) and the ABC Vectastain Elite kit from Vector Laboratories (Burlingame, CA). The phospho-specific-(Y705)-STAT3 rabbit antibody was from New England Biolabs (Beverly, MA), the anti-HA mouse antibody from Covance Inc. (Berkeley, CA), and the rabbit anti-β-endorphin antibody was a kind gift from Dr. Ronnekleiv (Oregon Health and Science University, Portland, OR)(Qiu et al., 2003). Sheep anti-α-MSH antiserum was from Chemicon International, Inc. (Temecula, CA). The biotinylated donkey-anti-mouse antibody and the biotinylated donkey-anti-rabbit antibody were from Jackson Immunology Research Laboratories (West Grove, PA). Fluorescent donkey anti-rabbit, fluorescent donkey anti-sheep, and fluorescent donkey anti-mouse immunoglobulin conjugates were from Molecular Probes (Eugene, OR), and donkey serum was from Invitrogen Life Technologies, Inc (Carlsbad, CA).
Immunohistochemistry (IHC)
Twenty-five µm coronal brain sections were generated as described earlier (Huo et al., 2006). For non-fluorescent HA IHC, brain sections were pretreated with citrate buffer for 30 minutes at 80C. Sections were incubated with anti-HA antibody (1:250) and biotinylated anti-mouse antibodies (1:1,000), followed by avidin-biotin-complex labeling, and developed with Nickel-diaminobenzidine (DAB). For double fluorescence HA and β-Endorphin IHC, sections were incubated with anti-HA (1:250) and anti-β-Endorphin (1:5,000), and then with fluorescent-labeled (red or green) secondary antibodies. P-STAT3 IHC was performed as described earlier (Huo et al., 2006). For α-MSH fluorescence IHC, sections were incubated with anti α-MSH (1:20,000) and fluorescent-labeled secondary antibodies generating green fluorescence. Results were visualized using fluorescent or bright-field light, and captured with a digital camera (AxioCam, Carl Zeiss, Thornwood, NY) mounted on a Zeiss microscope (Axioscope2). To visualize double-labeled cells, Adobe Photoshop software (Adobe, San Jose, CA) was used to merge fluorescence via RGB channels. Single- and double-labeled cells were counted bi-laterally in every 5th section of one brain series and multiplied with five to obtain a total for the entire hypothalamus as we have done earlier (Huo et al., 2006). Confocal laser scanning microscopy was performed using a Zeiss LSM510 system.
Quantification of Leptin-Induced STAT3 Phosphorylation
HA-ObRb/Pomc-Cre/Leprdb/db and wild type littermates were injected i.p. with leptin (45 min). Brain sections were subjected in parallel to double IHC for P-STAT3 (DAB) and β-Endorphin (fluorescent)(Huo et al., 2006). Digital images were obtained with a Zeiss Axioscope. Nuclear DAB staining in POMC neurons from three anatomically comparable brain sections from each mouse was quantified using ImageQuant software (GE Healthcare, Piscataway, NJ). In total, P-STAT3 was quantified in 204 random POMC neurons from wild type mice (N=3) and in 234 POMC neurons from transgenic mice (N=3).
Blood Composition, Insulin- and Glucose-Tolerance Tests
Tail vein blood was collected at 12:00 PM ± 2 hr from either ad libitum fed or fasted mice. Blood glucose was assayed with OneTouch Ultra Blood Glucose Monitoring System (Fisher Scientific, Morrison Plains, NJ). ELISA’s were used to measure serum insulin and leptin (Crystal Chem. Inc., Downers Grove, IL) and triglycerides (Thermo Fisher Scientific Inc., Waltham, MA). For ITT’s, food was removed for 5 hours and blood glucose concentrations were measured at 15, 30, 60, 90, and 120 minutes after i.p. injection of insulin (1.0 or 1.5 U/kg). For GTT’s, food was removed for 15 hours and blood glucose concentrations were measured at 15, 30, 60, 90, and 120 minutes after i.p. injection of D-glucose (2 mg/g).
Pancreas Histology
Mice (11–20 wks) were sacrificed by cervical dislocation. Pancreata were fixed in 10% phosphate-buffered formalin and paraffin embedded. Each pancreas was cut in serial 5 µm sections and every 20th section was HE stained and analyzed. Following digital image acquisition using a CCD camera on a Zeiss microscope, islet and exocrine areas (after exclusion of vessels and connective tissue) were measured in each section with Adobe Photoshop and Axiovision software. At 5x magnification, 1 pixel equals 2.126 square µm’s. For each pancreas, the total number of islets, total islet (endocrine) area, and total exocrine area’s were determined. The relative islet mass ((total islet area)/(total islet area + exocrine area) ×100) and the mean islet size (total islet area/number of islets) were then calculated.
Oxygen Consumption and Locomotor Activity
Oxygen consumption, CO2 production, and physical activity were measured in 13-week mice using a comprehensive lab animal monitoring system (CLAMS, Columbia Instruments, Columbus, Ohio). Mice were acclimated in the monitoring chambers for 2 days followed by data collection for 3 days.
Hypothalamic Neuropeptide mRNA
Mice were euthanized by cervical dislocation. Brains were removed, arcuate-enriched tissue isolated, and cDNA were generated as described earlier (Huo et al., 2006). Real-time PCR was performed in a 96-well plate according to the manufacturer’s instructions (Stratagene). The primers (Invitrogen) and probes (Biosearch Technologies, Novato, CA) were designed with the assistance of PrimerExpress software (Foster City, CA) as follows: mPOMCF (5'-ACCTCACCACGGAGAGCA-3'), mPOMCR (5'-GCGAGAGGTCGAGTTTGC-3'), and mPOMCP [5'-6-carboxy- fluorescein (Fam)- TGCTGGCTTGCATCCGGG-BHQ-1–3']; mNPYF (5'-CTCCGCTCTGCGACACTAC-3'), mNPYR (5'-AATCAGTGTCTCAGGGCT-3'), and mNPYP [5'-6-carboxy- fluorescein (Fam)- CAATCTCATCACCAGACAG-BHQ-1–3']; mAgRPF (5'-GCGGAGGTGCTAGATCCA-3’), mAgRPR (5'- AGGACTCGTGCAGCCTTA −3'), and mAgRPP [5'-6-carboxy- fluorescein (Fam)- CGAGTCTCGTTCTCCGCG -BHQ-1–3']. PCR reactions were run in a volume of 25.0 µl using 1.0 µl cDNA. A standard curve was generated from duplicate measurements of serial dilutions of arcuate cDNA. The housekeeping gene cyclophilin was used for normalization.
Hypothalamic Neuropeptides
The extraction and purification of the peptides from whole hypothalamic tissues were performed as previously described (Gamber et al., 2005; Guo et al., 2004). The samples were then assayed by enzyme immunoassays (EIA) for α-MSH, NPY (both in house assays), and AgRP (Phoenix Pharmaceuticals, Burlingame, CA). The sensitivities of the assays were 2, 8, and 9 pg per sample, respectively.
Statistical Analyses
Data are presented as means ± SEM and significance level was set at p ≤ 0.05. Analyses were done by Students T-tests. For insulin tolerance tests, treatment groups were analyzed using general linear models and individual differences between the treatment groups were identified by one-way ANOVA followed by the protected least significant-differences technique (SAS version 8.2; SAS Institute, Cary, NC).
Supplementary Material
Acknowledgements
This work was supported by grants from the American Diabetes Association and The Richard and Susan Smith Family Foundation Pinnacle Program Project (7-05-PPG-02), the National Institutes of Health (DK60673 and DK65743), the Endocrine Society (all to C.B.), and by the Boston Obesity Nutrition Research Center (DK46200). We are particularly grateful to Dr. Brad Lowell (BIDMC, Boston, MA) for providing us with the Pomc-Cre mice. We also thank Dr. Oline Ronnekleiv (Oregon Health and Science University, OR) for the β-Endorphin antibody. The HA-tagged leptin receptor cDNA was kindly given by Dr. Jeffrey Flier (Harvard Medical School, Boston, MA). Finally, we thank Lesli Larsen, Daniel Woo, Gage Hackford, and Won Yoon Chung for assistance with animals.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baetens D, Stefan Y, Ravazzola M, Malaisse-Lagae F, Coleman DL, Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978;27:1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC., Jr The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cusin I, Zakrzewska KE, Boss O, Muzzin P, Giacobino JP, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Diabetes. 1998;47:1014–1019. doi: 10.2337/diabetes.47.7.1014. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gamber KM, Macarthur H, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Guo L, Munzberg H, Stuart RC, Nillni EA, Bjorbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci U S A. 2004;101:11797–11802. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48:1621–1626. doi: 10.1007/s00125-005-1838-8. [DOI] [PubMed] [Google Scholar]
- Hespel P, Vergauwen L, Vandenberghe K, Richter EA. Significance of insulin for glucose metabolism in skeletal muscle during contractions. Diabetes. 1996 45 Suppl 1:S99–S104. doi: 10.2337/diab.45.1.s99. [DOI] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Fawzi AB, Graziano MP, Ghibaudi L, Williams P, Van Heek M, Davis H, Rudinski M, Sybertz E, Strader CD. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol. 1997;272:R1204–R1209. doi: 10.1152/ajpregu.1997.272.4.R1204. [DOI] [PubMed] [Google Scholar]
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- Karaca M, Magnan C, Kargar C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 2009 doi: 10.1016/j.diabet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Leiter EH, Coleman DL, Ingram DK, Reynolds MA. Influence of dietary carbohydrate on the induction of diabetes in C57BL/KsJ-db/db diabetes mice. J Nutr. 1983;113:184–195. doi: 10.1093/jn/113.1.184. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 Signaling in AgRP Neurons Promotes Locomotor Activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Musi N, Goodyear LJ. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol Scand. 2003;178:337–345. doi: 10.1046/j.1365-201X.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Eskay RL. Distribution and possible origin of beta-endorphin and ACTH in discrete brainstem nuclei of rats. Neuropeptides. 1987;9:123–137. doi: 10.1016/0143-4179(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Gilbert M, Zierath JR. Skeletal muscle and insulin sensitivity: pathophysiological alterations. Front Biosci. 2001;6:D154–D163. doi: 10.2741/ryder. [DOI] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett. 1997;224:149–152. doi: 10.1016/S0304-3940(97)00163-8. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 2008;294:E630–E639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Masuzaki H, Yasue S, Ebihara K, Shiuchi T, Ishii T, Arai N, Hirata M, Yamamoto H, Hayashi T. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab. 2007;5:395–402. doi: 10.1016/j.cmet.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschop M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- van de Wall, E., Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, Mackenzie RG, Allison DB, Dun NJ. Collective and Individual Functions of Leptin Receptor Modulated Neurons Controlling Metabolism and Ingestion. Endocrinology. 2007 doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoek AM, Teusink B, Voshol PJ, Havekes LM, Romijn JA, Pijl H. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J Neuroendocrinol. 2008;20:120–127. doi: 10.1111/j.1365-2826.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.