Abstract
Purpose
This study aimed to investigate the effects of oral hygiene care by oral professionals on periodontal health in type 2 diabetes mellitus patients.
Materials and Methods
Diabetic participants were recruited at a university hospital and matched at a 1:1 ratio by age and gender, and randomly allocated into intervention (40 people) and control groups (35 people). Tooth brushing instruction, oral health education, and supra-gingival scaling were implemented in all patients at baseline. This program was repeatedly conducted in intervention patients every month for 6 months, and twice at baseline and the sixth month in the control. Oral health was measured by decayed, missing, and filled teeth (DMFT), plaque index, calculus index, bleeding index, patient hygiene performance (PHP) index, tooth mobility, Russel's periodontal index, and community periodontal index (CPI). Diabetes-related factors, oral and general health behaviors, and sociodemographic factors were interviewed as other confounding factors. An analysis of covariance (ANCOVA) was used with SPSS for Windows 14.0.
Results
At baseline, there were no significant differences between the two groups in average of periodontal health (calculus index, bleeding index, Russel's periodontal index, CPI, and tooth mobility), diabetes-related factors (fasting blood glucose, postprandial blood glucose, and HbA1c), and in distribution of sociodemographic factors and health behaviors. In intervention group, plaque index, dental calculus index, bleeding index, and PHP index were reduced fairly and steadily from the baseline. There were significant differences in plaque index, dental calculus index, bleeding index, PHP index, and Russel's periodontal index between the two groups at sixth month after adjusted for baseline status.
Conclusion
Intensive oral hygiene care can persistently improve oral inflammation status and could slow periodontal deterioration.
Keywords: Dental prophylaxis, oral hygiene, periodontal diseases, type 2 diabetes mellitus
INTRODUCTION
The American Society of Diabetes defined periodontal disease as the sixth complication of diabetes in 1997;1,2 Diabetes raises the risks of developing periodontal disease and affects its severity.3,4 Individuals with diabetes tend to have higher values for indices of plaque, dental calculus, and gingival inflammation, and deeper periodontal pockets.5 As compared to healthy people, more individuals with diabetes are likely to have more extensive,6 xerostomia,7 and dental caries.8,9 They are frequently in greater need of periodontal treatment and prophylactic procedures.10 Abrupt periodontal destruction and more severe periodontitis are observed in individuals with uncontrolled diabetes compared to individuals with well controlled blood glucose level.11,12
Regular oral health education performed by dental hygienists significantly improved plaque index, dental calculus index and community periodontal index (CPI) compared to simple education with printed materials for studies of individuals with diabetes and periodontal disease.13,14 Stringent oral hygiene combined with periodontal surgery substantially reduced periodontal pocket depth.15,16 Oral health education significantly reduced gingival sulcus fluid, CPI and plaque index in diabetic individuals with moderate and severe periodontal disease.13 In individuals with well-controlled diabetes, periodontal treatment modalities, ranging from supragingival therapy to non-surgical periodontal treatment, improved periodontal problems without any adverse effects on diabetes when examined after 4 months of the treatment.5 Therefore, it is essential to treat and prevent the periodontal diseases in diabetic patients. To date, few studies have examined in the longer term the effects of regular supragingival care and oral health education among individuals with diabetes.
We, therefore, conducted this study to examine the changes of oral hygiene status and severity of periodontitis by performing intensive oral hygiene care at a 1-month interval for six months in individuals with diabetes.
MATERIALS AND METHODS
Study population
The study design and protocol were reviewed and approved by Yeungnam University Hospital Institutional review board and consent was obtained from the participants. Consenting individuals with Type 2 diabetes were recruited from the Department of Internal Medicine at Yeungnam University Hospital, located in Daegu City, from November 2005 to April 2006. Participants were referred by their endocrinologist to the department of dentistry to receive intensive oral health care after a trained interviewer explained the program to them and obtained informed consent. Participants were randomly assigned to intervention and control groups by order of outpatient visits. In both groups, age and gender were matched at a ratio of 1:1. Finally, 40 patients were included in each group. Five people in the control group were lost to follow up for six months. The final sample size was 75 people.
People in the intervention group were asked to visit once a month to receive regular oral hygiene care for six months. In the control group, there were only two examinations; at baseline and sixth months. Well-controlled diabetic patients referred by an endocrinologist were included in this study. The endocrinologist defined individuals with diabetes as well-controlled, based on review of participants' medical records and clinical criteria regarding HbA1c, and compliance with medication use. They were excluded if they had less than 8 functional teeth in their mouth, had taken antibiotics in the previous six months, or had other general health problems such as cardiovascular, liver, and kidney diseases, or other systemic conditions; including immunologic or psychiatric disorders.
Oral examination
At the first visit, a trained dentist conducted an oral examination, and examined full-mouth intra-oral radiographs, which provided information regarding Russel's periodontal index and the level of alveolar bone resorption.17 The examining dentist was blinded to all clinical measurements prior to the assessment. Assessments of periodontal health status included the number of decayed teeth (DT), number of missing teeth (MT), the number of filled teeth (FT), decayed missing and filled teeth (DMFT) index, tooth mobility, CPI,18 Russel's periodontal index, papillary bleeding index, dental plaque index, dental calculus index, and patient hygiene performance (PHP) index.19 In addition, self-perceived oral health, discomfort in oral cavity, oral health behaviors (smoking, toothbrush frequency, and regular dental visits) were obtained using a self-administered questionnaire. A smoker was defined as an individual who answered 'yes' to the question, "Do you smoke now?".
Intensive oral hygiene care program
At baseline, both intervention and control groups received oral health education that included instructions on tooth brushing and the use of oral health aids (inter-proximal brushes or/and dental floss) from a trained dental hygienist, and received oral health educational brochures. The hygienist then conducted scaling for supra-gingival calculus under the supervision of a trained dentist. All the procedures (oral examination, full-mouth X-ray, education, and supragingival scaling) were repeated monthly for the intervention group and at the end of six months for the control group.
Diabetes-related factors and other information
Information regarding blood glucose level, Oral Glucose Tolerance Test (OGTT), and HbA1c were obtained from the medical records transferred from the Department of Internal Medicine, which had been supervised by endocrinologist. Other diabetes-related factors, including the experience of oral health education as a diabetic patient, diabetes duration, the number of hospital visits for diabetes, the experience of education for managing diabetes, and the type of diabetic treatment, were obtained by questionnaire by the dental hygienist.
A self-administered questionnaire was provided to investigate the sociodemographic factors (age, gender, education, and household income), and general health behavior (smoking, drinking, physical activity).
Statistical analysis
To confirm comparativeness between the intervention and control groups, the distributions of sociodemographic factors, general health behaviors, oral health behaviors at baseline were compared and tested by chi-square test. If the expected frequency was less than 5, Fisher's exact test was done. In addition, the averages of dental caries status, periodontal health, and diabetes-related variables at baseline were also compared and tested using t-test.
To examine the effect of intensive oral hygiene care program, the analysis of covariance (ANCOVA) was implemented to test the differences in periodontal indices after adjusting for baseline values except CPI. SPSS for Windows 14.0 was used for statistical analysis.
RESULTS
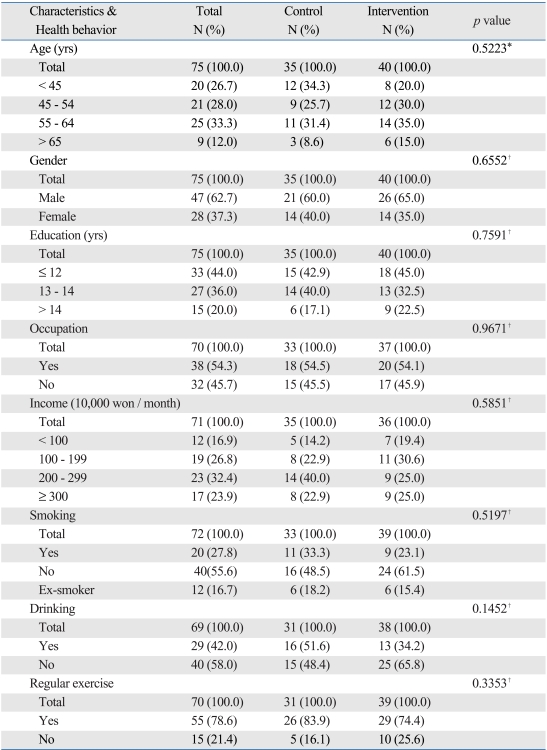
The mean age of patients was 52.2 years and all of them were aged 30 years or older. The proportion of male patients was 62.7%. The proportion of patients whose education level was below that of high school graduates was 44.0%, and this was the highest figure. The proportion of patients who had an occupation was 54.3%. The proportion of patients who responded that their mean monthly household income ranged between KRW 2,000,000 and KRW 2,990,000 was 32.4%. There were no significant differences in baseline characteristics between the two groups (Table 1).
Table 1.
Socio-Demographic Distributions and General Health Behaviors between Control and Intervention Groups at the Baseline
*p value by Fisher's exact test.
†p value by chi-square test.
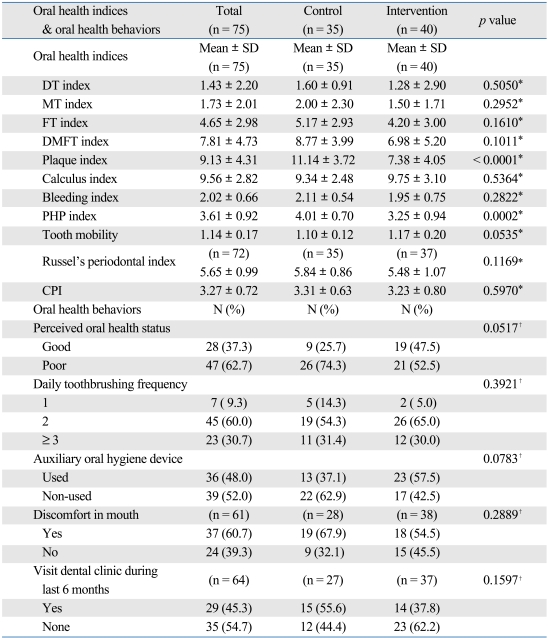
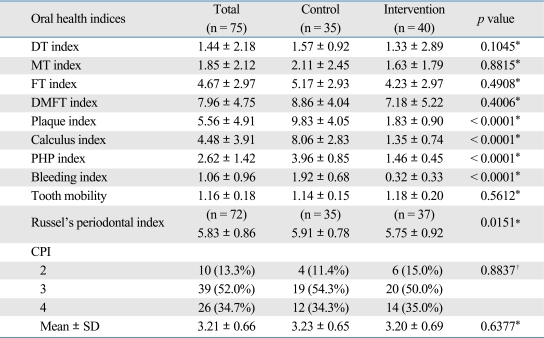
Regarding oral health at baseline, the average of DMFT index was 7.81, and the values of plaque index, dental calculus index and PHP index, indicating oral hygiene status, were 9.13, 9.56, and 3.61, respectively (higher value on the indices indicating worse oral health). The values of bleeding index, tooth mobility, periodontal index (PI) and CPI, indicating the status of periodontal disease, were 2.02, 1.14, 5.65 and 3.27, respectively. These results suggest that most of the patients (87%) who were enrolled in the current study had some periodontal disease. At baseline, plaque index and PHP index were significantly higher in the control group but other factors associated with oral health showed no significant differences between the two groups (Table 2).
Table 2.
Oral Health Status between Control and Intervention Groups at the Baseline
CPI, community periodontal index.
*p value by chi-square test.
†p value by t-test.
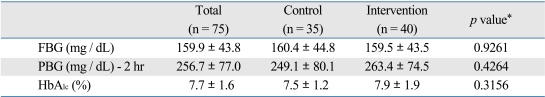
In terms of diabetic information, the mean level of fasting blood glucose, 2 hour post-prandial blood glucose, and HbA1c were 159.9 mg/dL, 256.7 mg/dL, and 7.7%, respectively. There were no significant differences in means for these three values between the two groups (159.5 mg/dL vs. 160.4 mg/dL; 263.4 mg/dL vs. 249.1 mg/dL; and 7.9% vs. 7.5%) (Table 3). Other diabetes-related factors including experience of oral health education as a diabetic patient, diabetes duration, the number of hospital visits for diabetes, and the type of diabetic treatment were not significantly different between the two groups at baseline (not shown in the table).
Table 3.
Diabetes Mellitus Indices between Control and Intervention Groups at the Baseline
FBG, fasting blood gucose; PBG, postprandial blood glucose-2 hr.
*p value by t-test.
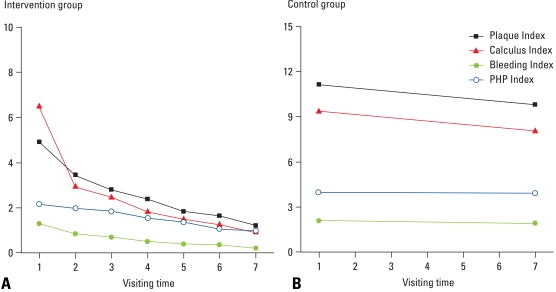
Fig. 1 represents the changes of oral hygiene status depending on the follow-up period in both groups and shows the crude effect of intensive oral hygiene care. In the intervention group, all the parameters such as plaque index, dental calculus index, bleeding index, and PHP index fairly reduced from the baseline and the pattern seemed to persist over time. In contrast, there was slight improvement of the oral hygiene status in control group.
Fig. 1.
Trends of oral hygiene indices for (A) the intervention and (B) control group at follow-up periods.
To examine the effects of the program, the differences of all measured indices between the two groups at 6 months were tested after adjusted for the baseline status using ANCOVA (Table 4). There were significant differences in plaque index, dental calculus index, bleeding index, PHP index, and Russel's periodontal index. However, tooth mobility and CPI did not show any significant differences between the two groups as well as DT, MT, FT, and DMFT index.
Table 4.
Oral Health Status between Control and Intervention Groups after 6 Months
CPI, community periodontal index.
*p value by ANCOVA.
†p value by chi-square test.
DISCUSSION
Oral hygiene care, that included tooth brushing instruction (TBI) through supra-gingival scaling at a month interval for six months implemented by dental hygienist supervised by a dentist and an endocrionologist, dramatically improved gingival health in individuals with well-controlled type 2 diabetes. Furthermore, the periodontal destruction assessed by Russel's periodontal index tended to be slowed down by this program. This study provides empirical evidence that intensive oral hygiene care (oral health education plus supra-gingival scaling) without any periodontal therapy improves gingival health, and may prevent progression of periodontitis in well-controlled diabetes. Therefore, the present results emphasize the importance of oral prophylaxis for individuals with diabetes.
In diabetic people, the management of periodontal disease is more important.20 If advanced glycation end products (AGEs) are accumulated within the body of individuals with diabetes, and these materials promote the chemotaxis and phagocytosis of mononuclear leukocytes and polymorphonuclear leukocytes. This leads to the pathologic changes of subgingiva or the further aggravation of inflammation of the periodontium. The materials that are produced by Gram-negative bacteria further facilitate the infection pathway within the body in the periodontium under this pathologic condition.21,22 It is, therefore, essential to initiate intensive oral health care as earliest as possible.23 Some previous studies24-27 have emphasized that non-surgical periodontal treatment and/or subgingival scaling in individuals with diabetes improved periodontal health and another study proved that periodontal treatment with root planning, subgingival curettage, and extractions as a therapy, if needed, improved glycemic control.28 On the other hand, oral hygiene care without periodontal surgery has positive effects in both patients with type 2 diabetes and type 1 diabetes.13,14,29
The self-recognition of their oral health in diabetes can affect periodontal treatment outcomes.10 A recent study13 showed the effect of oral hygiene instruction, resulting in a significant reduction of CPI among type 2 diabetic patients with mild and moderate periodontitis. In this study, however, CPI was not significant but Russel's periodontal index was. These results suggest that well managed oral hygiene care can improve periodontal health as well as oral hygiene itself and retard periodontal destruction. It is plausible that metabolically well-controlled diabetics as well as healthy control patients respond to non-surgical periodontal therapy.5
One of the important results from the present study was the steadily persistent effect of intensive care on gingival health and periodontal destruction for six months. According to previous studies,5,20,30 a short-term monitoring period of approximately 2-3 months following oral health care was effective in improving periodontal status and in preventing aggravation of periodontal tissue, evidenced by based on the decreased periodontal pocket or periodontal index. The current study indicated that more frequent oral hygiene care results in better periodontal health.
One of the limitations of the current study is that our patients cannot be considered as the representative of total type 2 diabetic people in Korea, because for convenience the patients were recruited from individuals with diabetes who visited a university hospital and then voluntarily expressed their interest in participating in the oral health care program on their endocrinologist's recommendation. However, participants were randomly assigned to the intervention and control groups, which were similar according to the parameters which we evaluated, except plaque index and PHP index. The oral hygiene status in the intervention group might have been a little better at baseline. This situation would underestimate the effects of the intervention on oral hygiene status at follow-up. It can, therefore, be inferred that our study design remains valid for assessing the effect of oral health care on periodontal health. The current study, therefore, demonstrated that the intensive oral health care could improve the oral health status, consequently preventing the aggravation of periodontal disease, although individuals with diabetes had poor oral hygiene status and most of them were affected with periodontal disease. Second, the participants were comparatively well-controlled type 2 diabetic people. The result of this study can not be applied to people who are type 1 diabetic, or have poor glycemic control. Further studies are needed on different type and/or status of diabetic patients. Third, we could not in practice follow the control group people every month only to examine their oral health. Two measurements at baseline and the sixth month may not provide enough information as a control, compared with intervention group. Nevertheless, we were able to find out that there was no significant improvement on gingival health, and that there was a fair amount of periodontal destruction in the control group.
In the current study, intensive oral health care as an intervention, such as oral health education, TBI, and supragingival scaling, was repeatedly implemented at onemonth intervals for six months in well-controlled type 2 diabetic group. The plaque index, dental calculus index, PHP index, bleeding index, and Russel's periodontal index improved compared to controls. These results indicate that the oral hygiene status was persistently enhanced and the perio-dontal deterioration was reduced by intensive oral hygiene care. Therefore, oral hygiene care program is necessary to remove oral inflammation and prevent type 2 diabetes from periodontal complication.
ACKNOWLEDGEMENTS
This research was supported by the Yeungnam University research grants in 2007.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Mealey BL, Oates TW American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 2.Complication of diabetes in the United States. American Diabetes Association. [Accessed in 2007. 9. 1]. Available from: https://www.diabetes.org/diabetes-statistics/complications.jsp.
- 3.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123–131. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 4.Sandler HC, Stahl SS. Prevalence of periodontal disease in a hospitalized population. J Dent Res. 1960;39:439–449. doi: 10.1177/00220345600390030401. [DOI] [PubMed] [Google Scholar]
- 5.Christgau M, Palitzsch KD, Schmalz G, Kreiner U, Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112–124. doi: 10.1111/j.1600-051x.1998.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 6.Seppälä B, Seppälä M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993;20:161–165. doi: 10.1111/j.1600-051x.1993.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 7.Dodds MW, Yeh CK, Johnson DA. Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Community Dent Oral Epidemiol. 2000;28:373–381. doi: 10.1034/j.1600-0528.2000.028005373.x. [DOI] [PubMed] [Google Scholar]
- 8.Hintao J, Teanpaisan R, Chongsuvivatwong V, Ratarasan C, Dahlen G. The microbiological profiles of saliva, supragingival and subgingival plaque and dental caries in adults with and without type 2 diabetes mellitus. Oral Microbiol Immunol. 2007;22:175–181. doi: 10.1111/j.1399-302X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 9.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent. 2004;25:179–184. 186–188, 190. quiz 192. [PubMed] [Google Scholar]
- 10.Sandberg GE, Sundberg HE, Wikblad KF. A controlled study of oral self-care and self-perceived oral health in type 2 diabetic patients. Acta Odontol Scand. 2001;59:28–33. doi: 10.1080/000163501300035742. [DOI] [PubMed] [Google Scholar]
- 11.Ainamo J, Lahtinen A, Uitto VJ. Rapid periodontal destruction in adult humans with poorly controlled diabetes. A report of 2 cases. J Clin Periodontol. 1990;17:22–28. doi: 10.1111/j.1600-051x.1990.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 13.Almas K, Al-Lazzam S, Al-Quadairi A. The effect of oral hygiene instructions on diabetic type 2 male patients with periodontal diseases. J Contemp Dent Pract. 2003;4:24–35. [PubMed] [Google Scholar]
- 14.Karikoski A, Ilanne-Parikka P, Murtomaa H. Oral self-care among adults with diabetes in Finland. Community Dent Oral Epidemiol. 2002;30:216–223. doi: 10.1034/j.1600-0528.2002.300308.x. [DOI] [PubMed] [Google Scholar]
- 15.Westfelt E, Rylander H, Blohmé G, Jonasson P, Lindhe J. The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol. 1996;23:92–100. doi: 10.1111/j.1600-051x.1996.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 16.da Cruz GA, de Toledo S, Sallum EA, Sallum AW, Ambrosano GM, de Cássia Orlandi Sardi J, et al. Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol. 2008;79:1150–1157. doi: 10.1902/jop.2008.070503. [DOI] [PubMed] [Google Scholar]
- 17.Fischer R, Hackl P, Moser F, Schuh E. [Description and results of a training plan for the use of the Russel-Index for an epidemiological study] Osterr Z Stomatol. 1974;71:449–458. [PubMed] [Google Scholar]
- 18.Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN) Int Dent J. 1982;32:281–291. [PubMed] [Google Scholar]
- 19.Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 20.Miller LS, Manwell MA, Newbold D, Reding ME, Rasheed A, Blodgett J, et al. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843–848. doi: 10.1902/jop.1992.63.10.843. [DOI] [PubMed] [Google Scholar]
- 21.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Scannapieco FA. Position paper of The American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841–850. [PubMed] [Google Scholar]
- 23.Jones JA, Miller DR, Wehler CJ, Rich SE, Krall-Kaye EA, McCoy LC, et al. The Department of Veterans Affairs Dental Diabetes Study. Does periodontal care improve glycemic control? J Clin Periodontol. 2007;34:46–52. doi: 10.1111/j.1600-051X.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 24.Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowenguth RA, Greenstein G. Clinical and microbiological response to nonsurgical mechanical periodontal therapy. Periodontol 2000. 1995;9:14–22. doi: 10.1111/j.1600-0757.1995.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mubarak S, Ciancio S, Aljada A, Mohanty P, Ross C, Dandona P. Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol. 2002;29:295–300. doi: 10.1034/j.1600-051x.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- 27.Campus G, Salem A, Sacco G, Maida C, Cagetti MG, Tonolo G. Clinical effects of mechanical periodontal therapy in type 2 diabetic patients. Diabetes Res Clin Pract. 2007;75:368–369. doi: 10.1016/j.diabres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–310. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 29.Britto LR, Katz J, Guelmann M, Heft M. Periradicular radiographic assessment in diabetic and control individuals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:449–452. doi: 10.1016/s1079-2104(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 30.Smith GT, Greenbaum CJ, Johnson BD, Persson GR. Short-term responses to periodontal therapy in insulin-dependent diabetic patients. J Periodontol. 1996;67:794–802. doi: 10.1902/jop.1996.67.8.794. [DOI] [PubMed] [Google Scholar]