Abstract
Purpose
To evaluate the possible influence of G→T substitution at the Sp1-binding site of the COLIA1 gene on the risk of pelvic organ prolapse (POP).
Materials and Methods
The study group consisted of 15 women with advanced stage POP. Fifteen control subjects with uterine myomas among the postmenopausal women were matched for age and parity. DNA was obtained from peripheral blood leukocytes. The fragments of the first intron of the COLIA1 gene were amplified by real time polymerase chain reaction. The polymorphism was identified using LightCycler Technology with hybridization probes. Sequencing reactions were performed on each template using commercial primer.
Results
Two groups had no significant difference in medical history, surgical, and smoking history. The homozygous peaks in two groups were noted at 57℃ on melting curve analysis. Sequencing reactions confirmed the G/G alleles in the 30 specimens tested. We could not find any polymorphism at the Sp1-binding site in COLIA1 gene with advanced stage POP. Statistical significance was considered to be p < .05.
Conclusion
The polymorphism of the Sp1-binding site in the COLIA1 gene did not contribute to the development of POP in Korea.
Keywords: Polymorphism, prolapse
INTRODUCTION
Pelvic organ prolapse (POP) is a common disease affecting the lives of millions of women.1 In Korea, the prevalence of POP stage greater than stage II in women was reported to be 11.8%.2 Ethnic and racial variations in the incidence of POP have been reported,3-6 however, little is known about its cause.
The causes of POP are multifactorial. The several environmental risk factors that could bring qualitative and quantitative changes in connective tissue which supports the lower pelvic organ could result in POP.7 The connective tissues are composed of extracellular matrix, of which collagen is an important factor.8 Therefore, the changes in collagen has been suggested an important etiology of POP,7 and the use of graft in pelvic reconstructive surgery is growing.9 However, several studies have reported conflicting results showing that collagen in POP patients was either decreased, increased or unaltered.10-12 These results, nevertheless, suggest the possibility of the development of POP by the alteration of the tension of connective tissue caused by the changes in the components of collagen.
Recently, it has been reported that genetic factors contribute to the development of POP. Demographic studies support such possibilities. Several studies found the effect of ethnic and racial variations on the incidence of POP,3,4 and 30% of women with POP had family history.13 Genetic studies on POP patients with family history showed early onset POP with genetic polymorphism of the promoter of LAMC1 gene.1 Nonetheless, to the best of our knowledge, there are only a few studies on the genetic contributions to the development of POP.
Recent studies indicate that a polymorphism of the gene encoding the type I collagen alpha-1 chain (COLIA1 gene) may affect the development of osteoporosis and stress urinary incontinence,14-16 whereas some studies showed that a polymorphism of COLIA1 gene does not increase the risk of development of pelvic floor defect.17,18 This polymorphism is induced by the substitution of a guanidine (G) residue to thymidine (T) residue in the first intron of COLIA1 gene (G→T). Consequently, three different genotypes are generated : the homozygotes G/G, the heterozygotes G/T, and the homozygotes T/T. Such a mutation is important because it affects the recognition area of their transcriptional factor Sp1-binding site, thus influencing the expression of COLIA1 gene. Consequently, the synthesis of COLIA1 protein is increased more than that of COLIA2 protein.15 In the heterozygotes G/T and the homozygotes T/T polymorphisms, the risk of osteoporosis fracture was increased in comparison with the homogyzotes G/G, and such phenomenon was more evident in the homozygotes T/T.14,15 In addition, the risk of stress urinary incontinence was increased in the heterozygotes G/T.16
In the present study, we hypothesized that the polymorphism of transcription factor Sp1-binding site of the COLIA1 gene predisposes Korean women to the development of POP.
MATERIALS AND METHODS
The study protocol was approved by the institutional review board of Yonsei University Health System, Seoul, South Korea. All participants gave written informed consent.
This study was performed in a cohort of 30 Korean women. Patients were recruited and enrolled from the Division of Urogynecology, and the Department of Obstetrics and Gynecology at Yonsei Medical Health System from May 2006 to September 2006. All patients were assessed with a standard history and physical examination before the surgery. A standard urogynecology questionnaire was given to patients. The questionnaire included questions regarding age, parity, body mass index, menopausal status, prior history of hormone replacement therapy, previous history of gynecologic operation or anti-incontinence operation, and medical histories such as hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and herniation of lumber disc. POP was quantified according to the International Continence Society's Pelvic Organ Prolapse Quantification (POP-Q) system.19 The pelvic exam was performed by the same examiner. On all patients participating in this study, urodynamic study including multi-channel cystometry, urethral pressure profilometry, and uroflowmetry using the Dante-5000 (Menuet, Copenhagen, Denmark) was performed. Urethral presssure was measured using a 12 french catheter (Mentor Co, Kedah, Malaysia). The study group consisted of 15 women who underwent hysterectomy for advanced stage POP (stage III to IV). The controls were matched for age and parity among the postmenopausal women with POP-Q stage 0 who underwent hysterectomy for uterine myomas.
Blood samples were stored in tubes containing anticoagulant ethylene diamine tetracetic acid (EDTA) until use. Genomic DNA was extracted from whole blood leukocytes using the High Pure polymerase chain reaction Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). DNA was stored at -80℃ until use. The fragments of the first intron of COLIA1 gene were amplified by real time polymerase chain reaction using 400 ng DNA as a template, by applying the LightCycler FastStart DNA Master Hybridization Probes (Roche Diagnostics GmbH, Mannheim, Germany). For amplification, Taq DNA polymerase (Tibmol, Berlin, Germany) and commercially obtained oligo-nulcleotide primers were used.
The first step of real time polymerase chain reaction was performed with the following primers set: Cola F, 5'-gggggCgTCCCTTCCAA-3' (forward) and Cola R, 5'-AgggCggggggAgAATA-3'(reverse). After denaturation at 95℃ for 10 minutes, 45 cycles of amplification were performed. Denaturation was carried out at 95℃ for 10 seconds, annealing at 58℃ for 10 seconds, and extension at 72℃ for 10 seconds.
To assess the type of polymorphism of the Sp1 binding site of COLIA1 gene, LightCycler FastStart DNA Master Hybridization Probes and LightCycler FastStart Enzyme were mixed, and the melting curve was analyzed using the LightCycler software 4. The amplified DNA was denatured at 95℃ for few seconds, annealed at 40℃ for 30 seconds, and extended at 80℃ for few seconds. It was incubated at 40℃ for 30 seconds, and then cooled. The type of polymorphism of the Sp1 binding site was determined by the analysis of the temperature with maximal absorption of fluorescence on the melting curve.
Sequencing reaction was performed using the ABI PRISM BigDye™ Terminator Cycle Sequencing Kits (Applied Biosystems, Foster city, CA, USA) for confirmation of nucleic acids. The polymerase chain reaction was performed using the PTC-225 Peltier Thermal Cycler (MJ Research, Reno, NV, USA), and the sequence was interpreted using the primers. After the reaction, deoxyribonucleoside triphosphates that did not participate in the reaction were separated from the products using ethanol. The purified polymerase chain reaction product was redissolved in distilled water, and analyzed by the ABI PRISM 3730XL Analyzer (Applied Biosystems, Foster city, CA, USA).
Data analysis was performed with SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). Student t-test, Fisher's exact test, and Pearson chi-square test were used for statistical analysis. Statistical significance was considered at p < .05.
RESULTS
The study group and the control group were similar with respect to demographic and clinical characteristics (Table 1), however, differed significantly in terms of the severity of POP (p = .001). Within the study group, 8 patients had stage III (53.3%) and 7 patients had stage IV (46.7%) POP. All the participants in the control group had stage 0 POP. Eleven women in the study group had stress urinary incontinence (SUI) diagnosed by urodynamic study, whereas 3 women had SUI in the control group (p = .003).
Table 1.
Clinical Characteristics of the Participants
Statistical significance was considered at p < .05.
*Student t-test.
†Fisher's exact test.
‡Matched parameter.
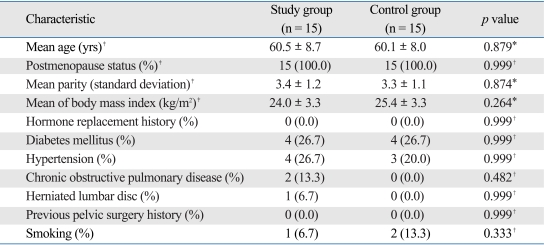
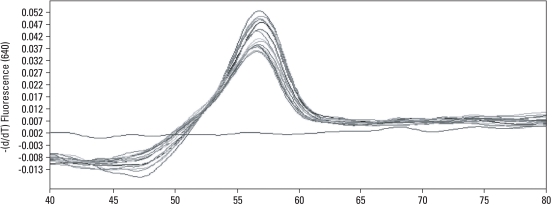
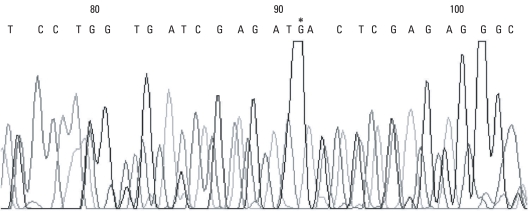
On the LightCycler instrument, the melting curves of the Sp1 binding site of COLIA1 gene in women with POP were similar. The melting curves for women with advanced stage POP showed homozygous peaks at 57℃ (Fig. 1). In the control group, the melting curves also showed homozygous peaks at 57℃ (Fig. 2). Sequencing analysis confirmed that all of the bases mutated in the Sp1 binding site were guanine (Fig. 3).
Fig. 1.
Melting peak analysis of the Sp1 binding site of COLIA1 gene in the study group on the LightCycler instrument.
Fig. 2.
The Sp1 binding site of COLIA1 gene in the control group by melting peak analysis.
Fig. 3.
Sequencing of the Sp1 binding site of the COLIA1 gene. *The guanine residue of Sp1-binding site in COLIA1 gene.
DISCUSSION
Ethnic and racial variations in the incidence of POP have been reported,3-5 and the incidence shown to be higher in Europeans and Latin Americans than Asian or African-American women.3,4,6 Anatomic, physiological, behavioral, and reporting differences have been suggested as possible explanations for the ethnic variation for the development of POP and Geldenhuys reported in their Bantu human study that risk factors mediating the development of POP are not environmental factors, but rather racial factors including the size and shape of pelvis, and the quality of connective tissues and pelvic support.5 Nevertheless, the major cause for the differences in the racial and ethnic variation has not been identified.20-22
In addition, some studies reported that approximately 30% of women with POP are familial, with the patients having multiple affected female family members.13 Recently, genetic etiologies have been considered to significantly contribute to the cause of POP. Nikolova et al.1 reported that a polymorphism in the promoter of LAMC1 may increase the susceptibility to early onset pelvic organ prolapse. However, some studies showed that a polymorphism of COLIA1 gene did not appear to increase the risk of development of pelvic floor defect.17,18
Therefore, we examined whether the incidence of POP was increased in Korean women with polymorphism in the binding site of the transcription factor Sp1 of the COLIA1 gene. First of all, factors such as age, parity, and menopause status were matched between the two groups to reduce statistical bias. The risk factors of POP such as hormone replacement therapy, prior history of pelvic surgery, and medical history including diabetes, hypertension, chronic pulmonary diseases, lumbar vertebral herniation, and smoking did not causes any significant difference between the study group and the control group.
Next, the presence of polymorphism in the binding site of the transcription factor Sp1 in the COLIA1 gene was determined by melting curve analysis. The results showed that none of the cases from both the study and control groups showed polymorphism of the site.
Although varies slightly depending on investigators, the ratio of heterozygotes G/T polymorphism in other countries reaches 22.5% - 42.0%.15-18,23 On the contrary, however, a Japanese study with 404 osteoporosis patients showed the ratio 0%,24 and a study in Korea which included 200 healthy menopausal women showed that the ratio of polymorphism was also 0%,25 therefore, similar to our current study. These findings suggest that the Sp1 binding site polymorphism in the COLIA1 does not significantly contribute to the development of POP.
Our study had a limitation that should be considered when interpreting the result. The participants in the study is relatively small to have statistical significance. A larger sample is needed for further observation. Because of the difficulty of collecting control patients, it was not easy to find a large number of patients who meet all the inclusion criteria such as age, parity, 0 stage in POP-Q scoring system and postmenopausal status.
In our present study, the polymorphism of the transcription factor Sp1 binding site in COLIA1 gene was not founded. Therefore, we conclude that the polymorphism of COLIA1 gene did not contribute to the development of POP in Korea. However, this does not necessarily exclude the possibility that polymorphism plays a role in the development of POP in selected western populations, since the importance of certain genes may be greater in some populations than in others. Furthermore, there is a possibility that the absence of COLIA1 gene Sp1-binding site polymorphism in Korean women may be one possible mechanism that protects Asian women from the development of POP and this merits further in-depth investigation.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 6-2006-0001.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Nikolova G, Lee H, Berkovitz S, Nelson S, Sinsheimer J, Vilain E, et al. Sequence variant in the laminin gamma1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet. 2007;120:847–856. doi: 10.1007/s00439-006-0267-1. [DOI] [PubMed] [Google Scholar]
- 2.Seo JT, Kim JM. Pelvic organ support and prevalence by Pelvic Organ Prolapse-Quantification (POP-Q) in Korean women. J Urol. 2006;175:1769–1772. doi: 10.1016/S0022-5347(05)00993-6. [DOI] [PubMed] [Google Scholar]
- 3.Dietz HP. Do Asian women have less pelvic organ mobility than Caucasians? Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:250–253. doi: 10.1007/s00192-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 4.Swift S, Woodman P, O'Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 5.Geldenhuys FG. [Genital prolapse in the Bantu] S Afr Med J. 1950;24:749–751. [PubMed] [Google Scholar]
- 6.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109:1396–1403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- 7.Weber AM, Buchsbaum GM, Chen B, Clark AL, Damaser MS, Daneshgari F, et al. Basic science and translational research in female pelvic floor disorders: proceedings of an NIH-sponsored meeting. Neurourol Urodyn. 2004;23:288–301. doi: 10.1002/nau.20048. [DOI] [PubMed] [Google Scholar]
- 8.Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 9.Jeon MJ, Bai SW. Use of grafts in pelvic reconstructive surgery. Yonsei Med J. 2007;48:147–156. doi: 10.3349/ymj.2007.48.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goepel C, Hefler L, Methfessel HD, Koelbl H. Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand. 2003;82:659–664. doi: 10.1034/j.1600-0412.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–1661. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 12.Falconer C, Blomgren B, Johansson O, Ulmsten U, Malmström A, Westergren-Thorsson G, et al. Different organization of collagen fibrils in stress-incontinent women of fertile age. Acta Obstet Gynecol Scand. 1998;77:87–94. doi: 10.1034/j.1600-0412.1998.770119.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, Amicarelli F, et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol. 1999;82:63–67. doi: 10.1016/s0301-2115(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 14.Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet. 1996;14:203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- 15.Mann V, Ralston SH. Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone. 2003;32:711–717. doi: 10.1016/s8756-3282(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 16.Skorupski P, Król J, Starega J, Adamiak A, Jankiewicz K, Rechberger T. An alpha-1 chain of type I collagen Sp1-binding site polymorphism in women suffering from stress urinary incontinence. Am J Obstet Gynecol. 2006;194:346–350. doi: 10.1016/j.ajog.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Skorupski P, Miotła P, Jankiewicz K, Rechberger T. Polymorphism of the gene encoding alpha-1 chain of collagen type I and a risk of pelvic organ prolapse--a preliminary study. Ginekol Pol. 2007;78:852–855. [PubMed] [Google Scholar]
- 18.Rodrigues AM, Giräo MJ, da Silva ID, Sartori MG, Martins Kde F, Castro Rde A. COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1471–1475. doi: 10.1007/s00192-008-0662-3. [DOI] [PubMed] [Google Scholar]
- 19.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 20.Thom DH, van den Eeden SK, Ragins AI, Wassel-Fyr C, Vittinghof E, Subak LL, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259–264. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox PS, Webster D. Genital prolapse amongst the Pokot. East Afr Med J. 1975;52:694–699. [PubMed] [Google Scholar]
- 22.Peacock LM, Wiskind AK, Wall LL. Clinical features of urinary incontinence and urogenital prolapse in a black inner-city population. Am J Obstet Gynecol. 1994;171:1464–1469. doi: 10.1016/0002-9378(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 23.Vinkanharja A, Melkko T, Risteli J, Risteli L. New PCR-based method for the Sp1 site polymorphism in the COLIA1 gene. Clin Chem Lab Med. 2001;39:624–626. doi: 10.1515/CCLM.2001.100. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Ota N, Shirai Y, Hata A, Yoshida H, Suzuki T, et al. Ethnic difference in contribution of Sp1 site variation of COLIA1 gene in genetic predisposition to osteoporosis. Calcif Tissue Int. 1999;65:352–353. doi: 10.1007/s002239900711. [DOI] [PubMed] [Google Scholar]
- 25.Han KO, Moon IG, Hwang CS, Choi JT, Yoon HK, Min HK, et al. Lack of an intronic Sp1 binding-site polymorphism at the collagen type I alpha1 gene in healthy Korean women. Bone. 1999;24:135–137. doi: 10.1016/s8756-3282(98)00155-0. [DOI] [PubMed] [Google Scholar]