Abstract
Taste loss or alterations can seriously impact health and quality of life due to the resulting negative influence on eating habits and nutrition. Infection and inflammation are thought to be some of the most common causes of taste perception disorders. Supporting this view, neuro-immune interactions in the peripheral gustatory system have been identified, underlying the importance of this tissue in mucosal immunity, but we have little understanding of how these interactions influence taste perception directly or indirectly. This limited understanding is evident by the lack of even a basic knowledge of the resident immune cell populations in or near taste tissues. The present study characterized the distribution and population of the major immune cells and their subsets in healthy human anterior, lingual, fungiform papillae (FP) using immunohistochemistry. Dendritic cells (DCs) were the predominant innate immune cells in this tissue, including four subtypes: CD11c+ DCs, DC-SIGN+ immature DCs, CD83+ mature DCs, and CD1a+ DCs (Langerhans cells). While most DCs were localized beneath the lamina propria and only moderately in the epithelium, CD1a + Langerhans cells were exclusively present within the epithelium and not in sub-strata. A small number of macrophages were observed. T lymphocytes were present throughout the FP with CD4+ T cells more prevalent than CD8+ T cells. Very few CD19+ B lymphocytes were detected. The results show that DCs, macrophages, and T lymphocytes are the constitutive guardians of human FP taste tissue, with DCs and CD4 T cells being dominant, while B lymphocytes are rare under normal, healthy conditions These observations provide a basic anatomical foundation for the immune response in the healthy human tongue as a basis for subsequent disease-related studies, but none of the present data indicate that the immune cell populations identified are, in fact, altered in individuals with abnormal taste perception.
Keywords: Taste tissue, taste bud, dendritic cell, Langerhans cell, lymphocyte, fungiform papillae, cell population
1. Introduction
Taste disorders have many causes and can seriously detract from general health, feeding, nutrition, and quality of life (Deems et al., 1991; Graham et al., 1995; Schiffman and Graham, 2000). Infection and inflammation are thought to contribute to or exacerbate taste disorders (Cullen and Leopold, 1999; Heald et al., 1998; Mistretta, 1984; Pribitkin et al., 2003). Among the more prominent examples, patients with chronic inflammatory and autoimmune diseases report taste dysfunctions (Mann, 2002). Also, inflammation induced by radiation therapy to the oral cavity for treatment of head and neck cancer is strongly correlated with either hypogeusia or ageusia and the consequent loss of interest in feeding (Conger and Wells, 1969; Esses et al., 1988; Nelson, 1998; Yamashita et al., 2006). Perhaps more telling, the exogenous use of cytokines, such as IFN, is associated with taste disorders and causes apoptosis of taste cells (Abdollahi and Radfar, 2003; Wang et al., 2007). Inflammatory processes are necessary for injured tissue to heal, but they can also alter normal cellular function. The basic knowledge of inflammatory processes in taste disorders is not well understood. In addition, no study has identified the presence, distribution, and populations of immune cells in the taste system, despite mounting evidence that immune responses play a role in the regeneration of injured taste receptor cells and in the maintenance of normal gustatory perception (Cavallin and McCluskey, 2005, 2007a, b; Phillips and Hill, 1996).
The peripheral taste system is composed of three main types of papillae containing taste buds that are distributed in the lingual mucosa, as well as taste buds that are not in papillae within the soft palate and pharynx. Located in the oral cavity, the peripheral gustatory system is continuously exposed to numerous dietary antigens and various pathogenic or commensal bacteria. To cope with these challenges, the oral mucosa is populated by a significant representation of the overall cellular population of immune system. The immune cells contained in oral mucosa include different proportions of macrophages, dendritic cells (DCs) and lymphocytes. Studies on the composition and distribution of immune cell populations in oral mucosa have been reported in human buccal and gingival tissues (Colasante et al., 1992; Haque et al., 1997; Ishii, 1987; Walton et al., 1998), but studies of gustatory tissues are lacking. In the present study, immunohistochemical methods were used to characterize and enumerate specific immune cell populations in healthy human anterior, lingual, fungiform papillae (FP). We found that DCs/Langerhans cells, macrophages, and T lymphocytes are normal constituents within healthy human FP, while B lymphocytes are rare. This work will provide fundamental data for the subsequent study of the role of immune and inflammatory processes in patients with gustatory perceptual and behavioral dysfunctions, and also help us better understand the role of immune activity in maintaining healthy gustatory function, as well as in treatment of taste disorders related to immune responses and inflammation. We emphasize that while these data are an important description of the normative immune state of taste tissue, the cellular changes that occur in response to various pathologic conditions and their role in taste abnormalities remain to be established.
2. Methods
2.1. Human Subjects
Twelve healthy adult subjects (6 males and 6 females; age 35.8 ± 9.6 years) were recruited through the Department of Otolaryngology at Thomas Jefferson University and were evaluated by the physicians. The evaluations were performed to ensure that all the subjects had normal taste perception and were free of systemic inflammation, immune-related diseases, and gustatory lingual disease, and were not on any medication for two weeks. None of the subjects presented with any clinical complaints and reported abnormal taste perceptions of bitter, sweet, sour, and salty. The taste perception was evaluated by standard clinical tool developed at the Monell Smell and Taste Clinical Research Center in Thomas Jefferson University. The collection of human tissue was conducted after subjects provided informed consent, in accordance with the Declaration of Helsinki according to protocols approved by the Institutional Review Board of Thomas Jefferson University and the Office of Regulatory Affairs at the University of Pennsylvania.
2.2. Tissue Sampling
Four human lingual FP were collected from anterior tongue with micro-scissors from each of the volunteers by a licensed surgeon as described elsewhere (Rossier et al., 2004), in total 48 biopsies were collected for this study. Biopsies were immediately placed in 4% paraformaldehyde for 1–2 hr at 4°C, processed through a series of 10–30% sucrose solutions for cryoprotection, and then embedded in M1 matrix medium (Shandon) and frozen using an acetone dry ice bath. Frozen sections were cut at a thickness of 10 µm through the entire biopsy, mounted on StarFrost slides (Mercedes Medical, Sarasota FL) and stored at −20°C until analyzed.
2.3. Immunohistochemistry
We used previously described immunohistochemistry techniques (Yee and Rawson, 2005) to identify immune cell populations in FP. Briefly, primary and secondary antibodies were selected based on their specificity and sensitivity and our previous experience in their use with human tissue. Frozen sections were dried in an oven at 37°C for 30 min and rehydrated 10 min with 0.1 M phosphate buffered saline (PBS) at pH 7.0. Endogenous peroxidase was blocked by 3% hydrogen peroxide for 10 min. Tissue sections were then washed in PBS for 3 × 5 min, incubated in Superblock® (Pierce) for 1 hr at room temperature (RT) to block nonspecific binding sites. Antibodies made in mouse specifying the following cell surface markers were employed as primary antibodies and incubated with the tissue sections for 2 hr at RT: HLA-DR II, CD1a, CD11c, CD83, DC-SIGN, CD64, CD4, CD8, and CD19 (all from Pharmingen, San Diego, CA). After a 3 × 5 min PBS wash, tissue sections were incubated for 1 hr with secondary biotinylated antibody then in pre-diluted Streptavidin-HRP complex (Anti-Ig HRP Detection Kits, BD Pharmingen) for 45 min. Immunoreactivity was detected using diaminobenzidine (DAB) as the chromogen. Sections were counterstained with hematoxylin and 0.3% (v/v) diluted ammonia prior to dehydration and coverslipping. Controls for non specific binding were performed by substituting primary antibody with an isotypic control IgG or excluding primary antibodies. Each antibody was used on 48 sections from the 12 subjects.
2.4. Computer-assisted image analysis and cellular quantification
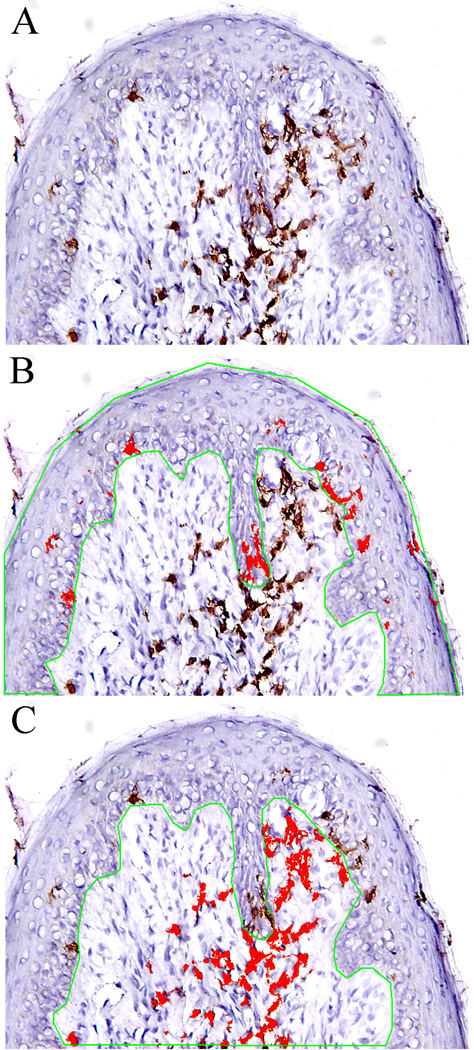
The applied method for cellular quantification used was similar to those previously reported (Phipps et al., 2004; Robert et al., 1995). Brightfield images were captured at a magnification of 10x using the Nikon DMX 1200C attached to a Nikon Eclipse 80i microscope. The density of specific immunoreactive cells in both the epithelium and lamina propria of fungiform papillae were quantitatively measured using Image-Pro Plus image analysis software 6.0. This software allows analysis of brown or orange stained areas of tissue sections (obtained after immunohistochemistry with DAB staining) with light-blue counterstaining. On the computer screen, two masks were used. One mask was used to define a threshold to determine the total areas of the epithelium and laminar propria in the measured section. In the color-cube based feature under count/size menu, a second mask was applied over the captured image based on the color range of DAB reactive immune cells. The mask areas within the epithelium and lamina propria were measured and summed and the size for each region was calculated, as shown in Figure 1. The cell population was expressed as the ratio of the masked color area to the total area of tissue region measured. The accuracy of the computer evaluation of color was assessed by human eye appraisal of each section. For each subject, four different fungiform papillae biopsies were analyzed and the values were averaged. The data in the results are the combination of the twelve pooled data sets. In a preliminary experiment, the protocol was normalized by performing multiple morphometric assessments of sections with different densities of positive staining by an observer blind to the antibody used.
Figure 1.
Quantitative analysis of specific cells in fungiform papillae. A. Specifically stained cells (brown) in fungiform papillae; B, Total area of epithelium (the area within the green line) and area of stained cells (red) in epithelium selected by using computer-assisted image analysis. C. Total area of lamina propria and area of stained cells in lamina propria.
2.5. Statistical analysis
The two-sample t-test was employed using the SAS (Inc., 1999) software package to compare the differences of cell density (CD11c, CD209, CD4, CD64, CD83, MHC, and CD8) in the epithelial and lamina propria compartments. Diagnostic tests were performed to check for significant violations in the distribution assumptions. As a means of validating the results from the t-tests, the non-parametric Wilcoxon rank sum test was also applied. The Wilcoxon test requires no distribution assumptions (Rosner, 1990). An evaluation of the residual values from the two-sample t-test on all the main outcome variables revealed no outliers, influential points, or significant violations in the constant variance assumption. Consistent results were obtained from using the Wilcoxon non-parametric test and two-sample t-test. For each subject, four different fungiform papillae biopsies were assessed and analyzed, and the values were then pooled and averaged as one cohort data, and were used for the statistical analysis. N = 12. A p level < 0.05 was defined as a significant difference.
3. Results
3.1. Histological Features of Fungiform Papillae
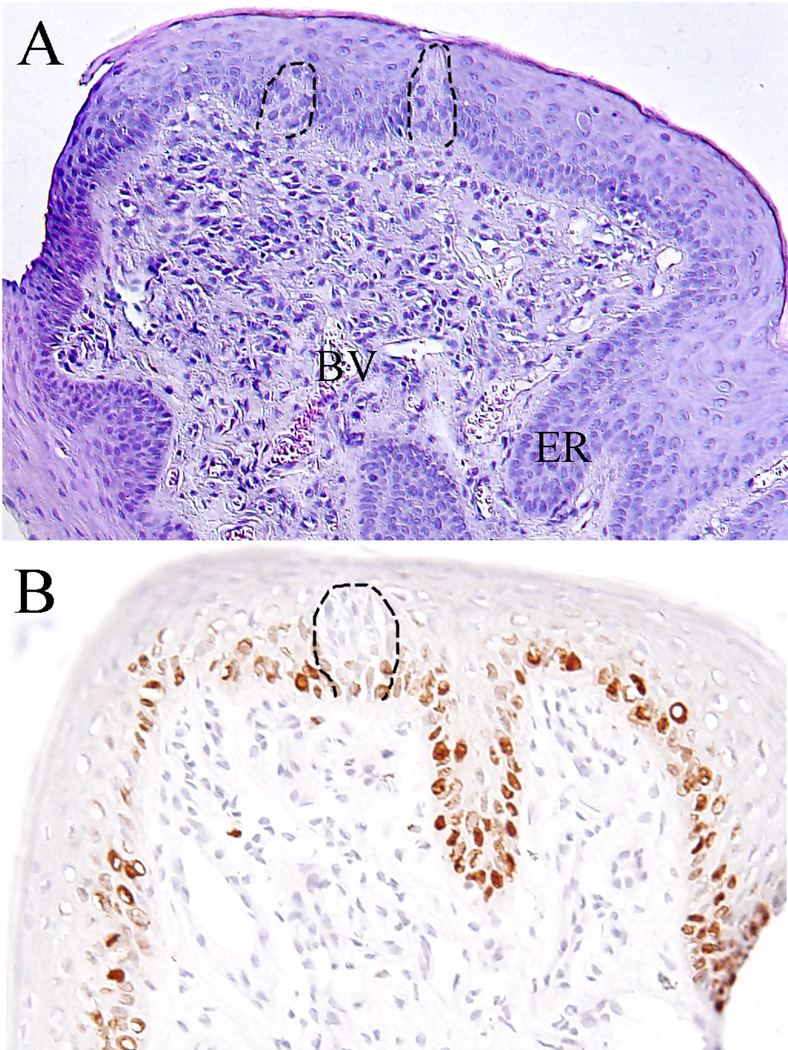
Examination of hematoxylin stained sections of healthy human FPs revealed an outer layer of parakeratinized, stratified squamous epithelium and underlying lamina propria (Fig 2A). Within the epithelium, up to two taste buds typically were observed at the basement membrane of any fungiform slice extending to the apical layers, with the taste bud pore opening toward what would be the oral cavity. In some cases, the epithelium appeared to extend into the underlying lamina propria forming epithelial ingrowths. Along the basement membrane is a layer of basal cells with a high level of cellular proliferation as indicated by Ki67 immunoreactivity (Fig 2B). Located below the epithelium, the lamina propria is composed of loose connective tissue, scattered with small blood vessels and various types of immune cells including DCs, monocytes, macrophages, and lymphocytes.
Figure 2.
Tissue structure of fungiform papillae. A. Showing the keratinized, stratified epithelium and underlying lamina propria. Note the taste buds embedded in epithelium (dotted line), epithelium ridges (ER) extending to connective tissue, small blood vessels (BV) and various types of cells. HE, 10 ×10 magnification; B. Antibody ik67 labeled proliferative cells of basal layer of epithelium. 10 × 20 magnification.
3.2. MHC class II
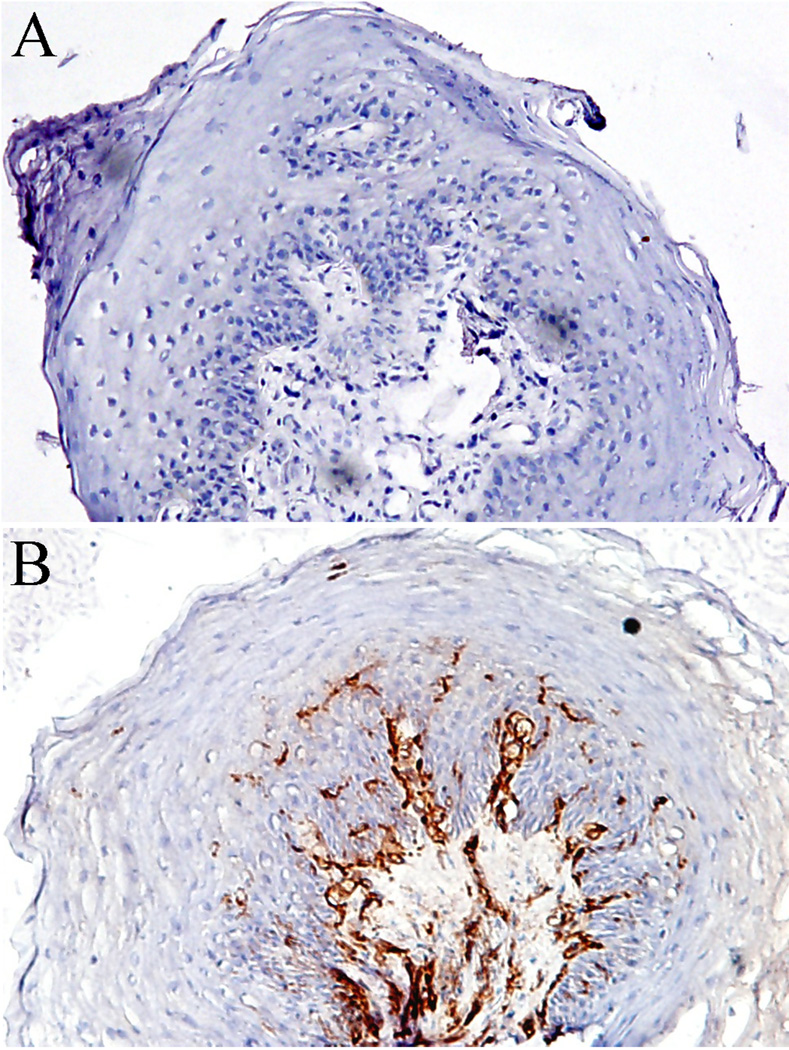
To explore the general presence and distribution of immune cells in tissues, especially antigen presenting cells (APCs), MHC class II expressing cells in FPs were identified using a monoclonal antibody for HLA DR-II. Our results show that MHC class II molecules were consistently present in intraepithelial and subepithelial immune cells with the characteristic morphology of DCs and mononuclear cells. The MHC class II expressing cells were more prevalent in the lamina propria than in the epithelium, which corresponded with the distribution pattern of most immune cells detected in the tissues. The MHC Class II molecule was not detected in any constitutive epithelial cells, as shown in Figure 3.
Figure 3.
MHC class II expression in fungiform papillae. A. Negative control performed by skipping primary antibody; B. MHC class II expression and distribution. Frozen sections of human fungiform papillae were labeled with mouse against human HLA DR antibody. Note that the molecules present in intraepithelial and subepithelial cells but not in epithelial cells. 10 × 20 magnification.
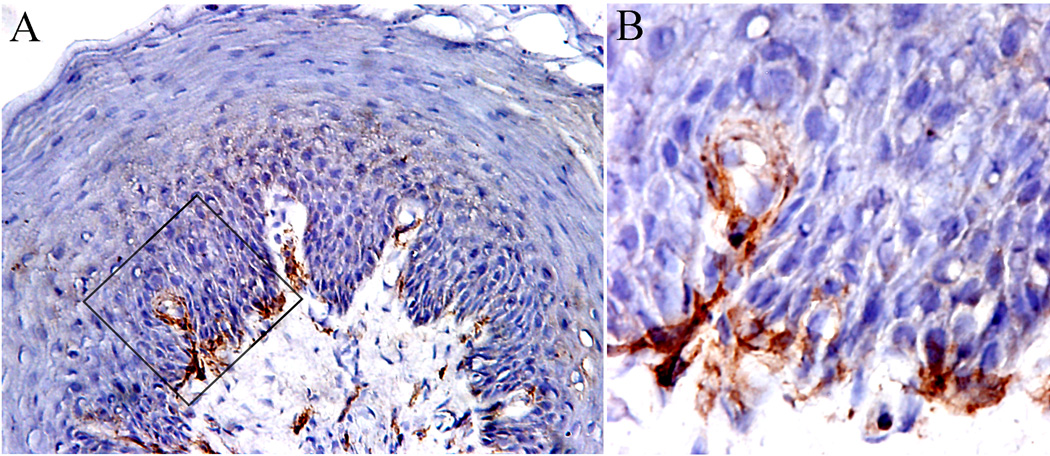
3.3. CD11c+ DCs
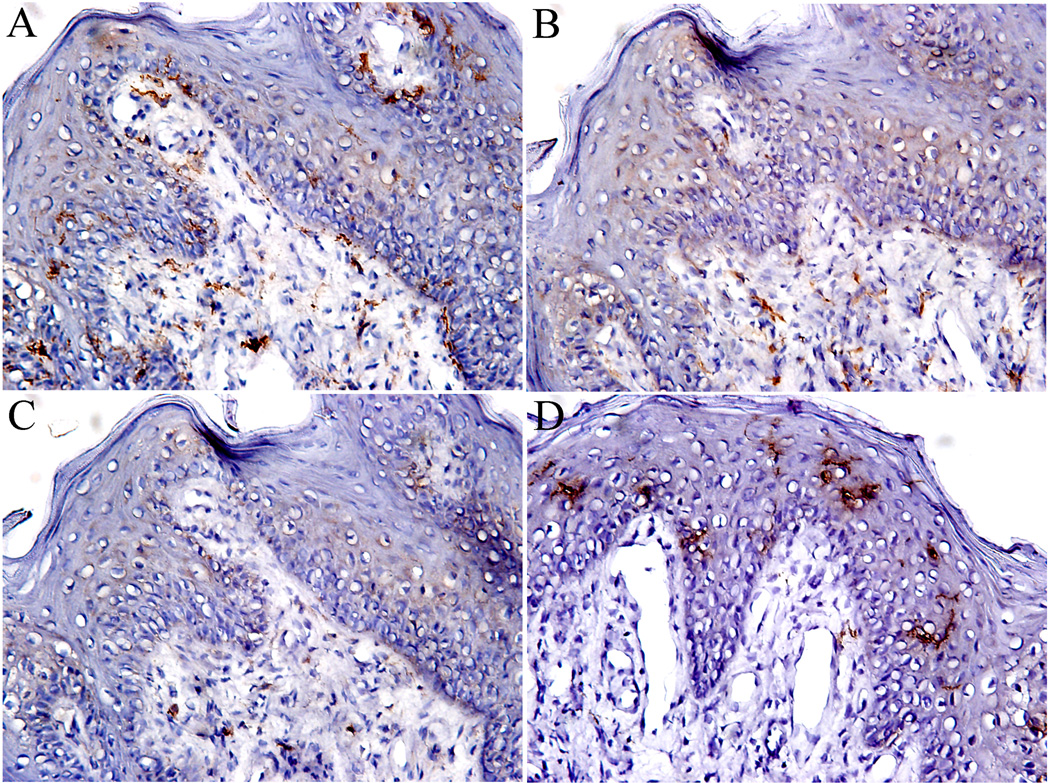
CD11c+ DCs were observed consistently both in the epithelium and connective tissues of FP, and the expression levels were significantly higher in lamina propria than in epithelium, as shown in Figure 4A. These cells tended to be scattered throughout the tissue, but they also clustered together in some areas, especially under the basement membrane of FP.
Figure 4.
Dendritic cells in fungiform papillae. A. CD11c staining dendritic cells; B. DC-SIGN staining immature dendritic cells; C. CD83 staining mature dendritic cells, and D. CD1a staining Langerhans cells. Note the difference in distributions of CD1a Langerhans cells, which are mainly present in epithelium, and CD11c dendritic cells, which are distributed both in epithelial and connective compartments. 10 × 20 magnification.
3.4. DC-SIGN+ and CD83+ DCs
DCs in different developmental stages were identified with DC-SIGN and CD83, which are expressed on immature DCs ( imDC) and mature DCs (mDC), respectively (Figure 4B and 4C). While DC-SIGN+ imDCs and CD83+ mDCs were present in both the epithelium and the lamina propria, DC-SIGN+ DCs were mainly localized within the lamina propria.
3.5. Langerhans cells
Langerhans cells, another immature counterpart of DCs, are highly specialized APCs located in the skin, mucosa, and lymphoid tissues. CD1a+ Langerhans cells were distributed exclusively in the epithelium of FPs and displayed a typical dendritic morphology with abundant processes (Figure 4D). Langerhans cells were rarely observed in the lamina propria, indicating an epithelial specialization.
3.6. Macrophages
CD64+ macrophages were observed in both the epithelium and lamina propria. The cell population was significantly higher in lamina propria than that in the epithelical compartment. In lamina propria, the macrophages are usually distributed just beneath the epithelium (Figure 5A), and, occasionally, we observed CD64+ macrophages that apparently had migrated through the basement membrane to reside within the epithelium (Figure 5B).
Figure 5.
CD64+ macrophages in fungiform papillae. A. Macrophages were mainly distributed in the lamina propria, 10 × 20 magnification; B. High magnification of image showing macrophages occasionally migrate through the basement membrane into the epithelium. 10 × 60 magnification.
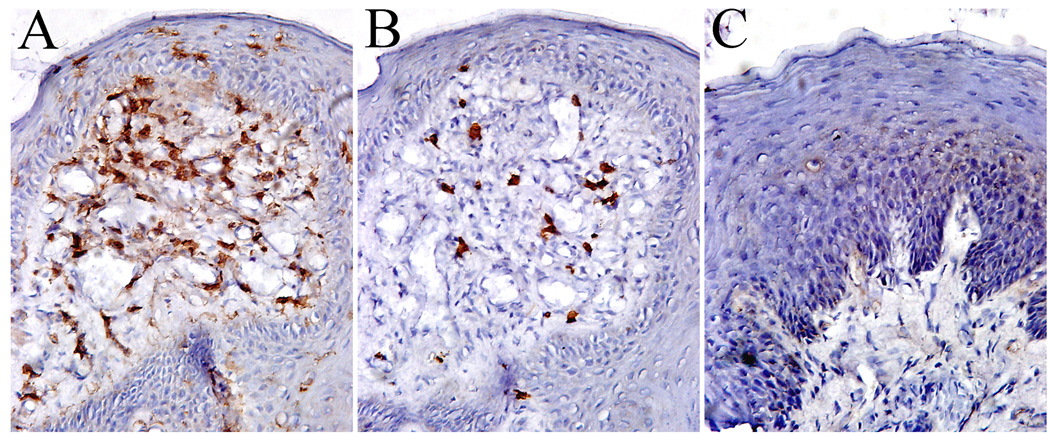
3.7. Lymphocytes
The distribution and population of CD4+ helper T cells, CD8+ suppressor/cytotxic T cells, and CD19+ B cells were identified in epithelium and lamina propria of human FPs. CD4+ T cells were observed scattered throughout the FPs with the majority of cells localized in the lamina propria (Figure 6A). The CD4+ T cells were significantly higher in number than CD8+ T cells (Figure 6B) both in epithelium and lamina propria in most FP sections. Very few CD19+ B cells were observed (Figure 6C).
Figure 6.
Lymphocytes in fungiform papillae. A. CD4 helper T cells; B. CD8 cytotoxic T cells; C. Showing no CD19 staining B lymphocytes. 10 × 20 magnification.
3.8. Quantification of the cell populations
An analysis of the relative density of each identified cell type in the epithelium vs. the lamina propria is summarized in Table 1. In the epithelium, the most numerous cells were MHC class II+ cells, followed by CD1a+ Langerhans cells >CD11c+ DCs >CD4+ T cells > CD64+ macrophages > DC-SIGN+ imDCs >CD8+ T cells > CD83+ mDCs. In the lamina propria, the population of immune cells were MHC-II+ cells > CD4+ T cells >CD11c+ DCs > CD64+ macrophages > DC-SIGN+ imDCs > CD8+ T cells > CD83+ mDC cells. All the immune cells we examined were significantly more prevalent in lamina propria than in the epithelium, with exception of CD1a+ Langerhans cells, which were only found in the epithelium. The distribution and density of FP immune cells in males and females are not significantly different in this sample.
Table 1.
Cell Density in Human Fungiform Papillae
| Cell types | Cell density 1in | |||
|---|---|---|---|---|
| Epithelium | Lamina propria | |||
| x ± SD | Range | x ± SD | Range | |
| MHC-II | 5.49 ± 2.80 | 2.30 – 11.23 | 27.30 ± 6.37** | 18.65 – 40.65 |
| CD1a | 4.42 ± 2.80 | 1.13 – 7.90 | ND | |
| CD11c | 1.35 ± 0.68 | 0.58 – 2.83 | 5.35 ± 2.2** | 2.2 – 9.3 |
| CD83 | 0.18 ± 0.14 | 0 – 0.33 | 0.52 ± 0.42* | 0 – 1.5 |
| DC-SING | 0.40 ± 0.39 | 0.05 – 1.39 | 2.57 ± 0.98 ** | 1.20 –4.15 |
| CD64 | 1.03 ± 0.92 | 0 – 2.4 | 3.02 ± 1.864** | 0 – 5.8 |
| CD4 | 1.16 ± 0.78 | 0.36– 1.8 | 6.20± 3.31** | 1.11– 13.92 |
| CD8 | 0.35 ± 0.12 | 0.27 – 0.68 | 1.36 ± 0.83** | 0.48 – 2.17 |
| CD19 | ND | ND | ||
The percentage of stained area to the total area of section observed.
ND = not detectable.
p < 0.05,
p < 0.001 when compared to the same cell number in epithelium.
4. Discussion
This study identifies the populations and distributions of the principal immune cells and their subsets in the FP of healthy subjects to help us better understand the potential interactions among the immune system and the gustatory perceptual system. This picture of immune function in or near the gustatory system will benefit future studies of inflammatory processes on taste, which can negatively affect patient nutrition. All the biopsies of FP were collected from volunteers who had neither signs of systemic inflammation nor clinical signs of significant oral diseases and no reported taste losses; therefore, we believe the results are representative of immune cell populations in human gustatory tissue under normal, healthy conditions. These data lay the foundation for future studies to examine the impact of age, infection or injury on these parameters.
We first examined the expression of MHC class II molecules to verify the general population and distribution of APCs in the taste tissue. MHC class II immunoreactivity was intense in the intraepithelial and subepithelial cells. There was no MHC Class II expression by epithelial cells themselves. The MHC class II expression in the lamina propria was predominant, which fits the distribution pattern of most immune cells detected in tissues. In addition to the immune cells, the MHC class II expressing cells in lamina propria also include vascular cells and other cells. This is in agreement with previous studies in non-gustatory oral epithelium (Harley et al., 2003).
Accumulating evidence suggests that mucosal DCs play a key role in orchestrating the immune balance between immunity against pathogens and immune tolerance for self-tissues and harmless antigenic components. The heterogeneity of mucosal DCs has been well described and it is currently believed that the subsets of DCs differ with respect to their biological functions (Iwasaki, 2007). Therefore, we focused on morphometric assessment of the subtypes and localization of DCs in mucosa of human anterior lingual gustatory tissue. We demonstrate there are four different subpopulations of DCs that populate the mucosa of FP: (1) prototypical DCs that express CD11c markers, (2) imDCs that express DC-SIGN, (3) mDCs that express CD83, and (4) Langerhans cells that express CD1a. The distributions of these CDs in mucosa of human FP differ. CD11c+ DCs are distributed throughout the epithelium and lamina propria with the population in lamina propria is 4 fold higher than in epithelium. They are the most numerous DCs in the lamina propria. CD11c is a unique marker for DCs of myeloid origin which are potent APCs and can activate T cells (Shortman and Liu, 2002). Similar distribution patterns were observed for CD83+ mDCs and DC-SIGN+ imDCs, with both cell types scattered mainly in the lamina propria of FP and less prevalently in the epithelium; however, the total population of DC-SIGN+ DCs was significantly higher than CD83+ DCs in general.
It is well known that imDCs and mDCs are different stages of DCs and they play distinct roles in immune response and regulation. After Ag exposure, imDCs migrate to draining lymph nodes where they differentiate into mDCs to present Ag to T cells (Banchereau and Steinman, 1998). The imDCs are characterized by a high capacity of phagocytic activity of pathogens, an important function in immune defense, and mDCs are featured by their strong capacity for antigen presentation. The fact that the majority of DCs are immature in FP suggests that this group of cells may have an important role in the defense against infection by pathogens. The distribution of CD1a+ expressing Langerhans cells in FP is unique, as they are only present in the epithelium and not in the lamina propria. It is worth noting that oral Langerhans cells differ from those within the nasal mucosa in their distribution and constitutive expression of receptors (Allam et al., 2006; Allam et al., 2003).
T cells are predominant in FP. This result is consistent with the previous studies in the oral mucosa (van Loon et al., 1989). As previously reported, the predominant populations detected in buccal mucosa were CD3, CD4 and HLA-DR positive cells, with few scattered B cells (CD20) and macrophages (CD68) (Haque et al., 1997) and in gingival mucosa CD3 and CD4 were also the dominant populations both in epithelial and stromal compartments with no B cells apparent in the epithelium and a few in the underlying connective tissue (Colasante et al., 1992). In the present study the distribution patterns of CD4+ and CD8+ cells were similar; we observed significantly more CD4+ cells than CD8+ cells. The predominant T cells and high ratio of CD4/CD8 in FP suggests that helper T cells are selected into this site and their preponderance here may be important in immune surveillance in this tissue under the normal, healthy conditions and immune response in some diseases.
The roles of immune response and inflammation in the physiology of taste activity or in taste disorders have been previously noted. The activation of an immune response with LPS could promote recovery from gustatory neuron injury and of taste function (Phillips and Hill, 1996). This LPS-induced recovery of normal taste function was mediated by increased activation of macrophages or T cells (Cavallin and McCluskey, 2005). Some immune regulatory molecules responded differently in response to gustatory neural injury (Cavallin and McCluskey, 2007a, b). There are, however, no reports on the mechanisms of how the immune system regulates the health of the gustatory system. Because taste buds are embedded within the epithelium and there is, so far, no evidence of immune cells in taste buds under normal conditions, either in the present study (data not shown) and in other reports (Cavallin and McCluskey, 2005), the capacity for direct action of immune cells on taste cells is low.
In summary, the results presented here show that some of the key cells of the immune system (i.e., T lymphocytes, macrophages, DCs and Langerhans cells) normally reside near human gustatory tissue in FP. These immune cells form a tight network throughout the epithelium and the underlying lamina propria, being ideally situated to protect the local tissue. These results will help us to understand the impact of inflammation on gustatory perception and the interaction between the immune and gustatory systems. These normative data do not, however, indicate what, if any, alterations in the immune cell populations occur with various disease states. We are presently comparing these data to observations from patients receiving radiotherapy, a consistent and dramatic cause of taste loss
Acknowledgments
This work was supported by NIH DC 02995 to PASB and P50DC 06760 to PASB and NER. We thank Biostatistician Jesse Chittams for data analysis, Linda Wysocki for her technical help in histology, and Luba Dankulich, Suzanne Alarcon, and Anne Ledyard for their invaluable assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdollahi M, Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;4:10–31. [PubMed] [Google Scholar]
- Allam JP, Niederhagen B, Bucheler M, Appel T, Betten H, Bieber T, Berge S, Novak N. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61:166–172. doi: 10.1111/j.1398-9995.2005.00965.x. [DOI] [PubMed] [Google Scholar]
- Allam JP, Novak N, Fuchs C, Asen S, Berge S, Appel T, Geiger E, Kochan JP, Bieber T. Characterization of dendritic cells from human oral mucosa: a new Langerhans' cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:141–148. doi: 10.1067/mai.2003.1607. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Lipopolysaccharide-induced up-regulation of activated macrophages in the degenerating taste system. J Neurosci Res. 2005;80:75–84. doi: 10.1002/jnr.20438. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Upregulation of intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 after unilateral nerve injury in the peripheral taste system. J Neurosci Res. 2007a;85:364–372. doi: 10.1002/jnr.21128. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Upregulation of the chemokine monocyte chemoattractant protein-1 following unilateral nerve injury in the peripheral taste system. Neurosci Lett. 2007b;413:187–190. doi: 10.1016/j.neulet.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Colasante A, Rosini S, Piattelli A, Artese L, Aiello FB, Musiani P. Distribution and phenotype of immune cells in normal human gingiva: active immune response versus unresponsiveness. J Oral Pathol Med. 1992;21:12–16. doi: 10.1111/j.1600-0714.1992.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Conger AD, Wells MA. Radiation and aging effect on taste structure and function. Radiat Res. 1969;37:31–49. [PubMed] [Google Scholar]
- Cullen MM, Leopold DA. Disorders of smell and taste. Med Clin North Am. 1999;83:57–74. doi: 10.1016/s0025-7125(05)70087-0. [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB., Jr Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- Esses BA, Jafek BW, Hommel DJ, Eller PM. Histological and ultrastructural changes of the murine taste bud following ionizing irradiation. Ear Nose Throat J. 1988;67:478–484. 487-478, 493. [PubMed] [Google Scholar]
- Graham CS, Graham BG, Bartlett JA, Heald AE, Schiffman SS. Taste and smell losses in HIV infected patients. Physiol Behav. 1995;58:287–293. doi: 10.1016/0031-9384(95)00049-o. [DOI] [PubMed] [Google Scholar]
- Haque MF, Harris M, Meghji S, Speight PM. An immunohistochemical study of oral submucous fibrosis. J Oral Pathol Med. 1997;26:75–82. doi: 10.1111/j.1600-0714.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Harley R, Gruffydd-Jones TJ, Day MJ. 2003. Characterization of immune cell populations in oral mucosal tissue of healthy adult cats. J Comp Pathol. 2003;128:146–155. doi: 10.1053/jcpa.2002.0619. [DOI] [PubMed] [Google Scholar]
- Heald AE, Pieper CF, Schiffman SS. Taste and smell complaints in HIV-infected patients. AIDS. 1998;12:1667–1674. doi: 10.1097/00002030-199813000-00015. [DOI] [PubMed] [Google Scholar]
- Inc. S.I. SAS/STAT user's Guide, Version 8. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Ishii T. Immunohistochemical demonstration of T cell subsets and accessory cells in oral lichen planus. J Oral Pathol. 1987;16:356–361. doi: 10.1111/j.1600-0714.1987.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Mann NM. Management of smell and taste problems. Cleve Clin J Med. 2002;69:329–336. doi: 10.3949/ccjm.69.4.329. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Aging effects on anatomy and neurophysiology of taste and smell. Gerodontology. 1984;3:131–136. doi: 10.1111/j.1741-2358.1984.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Nelson GM. Biology of taste buds and the clinical problem of taste loss. Anat Rec. 1998;253:70–78. doi: 10.1002/(SICI)1097-0185(199806)253:3<70::AID-AR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Phillips LM, Hill DL. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol. 1996;271:R857–862. doi: 10.1152/ajpregu.1996.271.4.R857. [DOI] [PubMed] [Google Scholar]
- Phipps S, Habib FK, McNeill A. Quantitative morphometric analysis of individual resected prostatic tissue specimens, using immunohistochemical staining and colour-image analysis. BJU Int. 2004;94:919–921. doi: 10.1111/j.1464-410X.2004.05060.x. [DOI] [PubMed] [Google Scholar]
- Pribitkin E, Rosenthal MD, Cowart BJ. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003;112:971–978. doi: 10.1177/000348940311201110. [DOI] [PubMed] [Google Scholar]
- Robert M, Costa P, Bressolle F, Mottet N, Navratil H. Percentage area density of epithelial and mesenchymal components in benign prostatic hyperplasia: comparison of results between single biopsy, multiple biopsies and multiple tissue specimens. Br J Urol. 1995;75:317–324. doi: 10.1111/j.1464-410x.1995.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. Boston: PWS-KENT Publishing Company; 1990. [Google Scholar]
- Rossier O, Cao J, Huque T, Spielman AI, Feldman RS, Medrano JF, Brand JG, le Coutre J. Analysis of a human fungiform papillae cDNA library and identification of taste-related genes. Chem Senses. 2004;29:13–23. doi: 10.1093/chemse/bjh002. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54 Suppl 3:S54–S63. doi: 10.1038/sj.ejcn.1601026. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- van Loon LA, Krieg SR, Davidson CL, Bos JD. Quantification and distribution of lymphocyte subsets and Langerhans cells in normal human oral mucosa and skin. J Oral Pathol Med. 1989;18:197–201. doi: 10.1111/j.1600-0714.1989.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Walton LJ, Macey MG, Thornhill MH, Farthing PM. Intra-epithelial subpopulations of T lymphocytes and Langerhans cells in oral lichen planus. J Oral Pathol Med. 1998;27:116–123. doi: 10.1111/j.1600-0714.1998.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 2007;27:10703–10713. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Nakagawa K, Tago M, Nakamura N, Shiraishi K, Eda M, Nakata H, Nagamatsu N, Yokoyama R, Onimura M, Ohtomo K. Taste dysfunction in patients receiving radiotherapy. Head Neck. 2006;28:508–516. [Google Scholar]
- Yee KK, Rawson NE. Immunolocalization of retinoic acid receptors in the mammalian olfactory system and the effects of olfactory denervation on receptor distribution. Neuroscience. 2005;131:733–743. doi: 10.1016/j.neuroscience.2004.11.011. [DOI] [PubMed] [Google Scholar]