Abstract
First line immunosuppressive treatment in steroid-resistant nephrotic syndrome in children is still open to discussion. We conducted a controlled multicentre randomized open label trial to test the efficacy and safety of cyclosporin A (CSA) versus cyclophosphamide pulses (CPH) in the initial therapy of children with newly diagnosed primary steroid-resistant nephrotic syndrome and histologically proven minimal change disease, focal segmental glomerulosclerosis or mesangial hypercellularity. Patients in the CSA group (n=15) were initially treated with 150 mg/m2 CSA orally to achieve trough levels of 120–180 ng/ml, while patients in the CPH group (n=17) received CPH pulses (500 mg/m2 per month intravenous). All patients were on alternate prednisone therapy. Patients with proteinuria >40 mg/m2 per hour at 12 weeks of therapy were allocated to a non-responder protocol with high-dose CSA therapy or methylprednisolone pulses. At week 12, nine of the 15 (60%) CSA patients showed at least partial remission, evidences by a reduction of proteinuria <40 mg/h per m2. In contrast, three of the 17 (17%) CPH patients responded (p<0.05, intention-to-treat). Given these results, the study was stopped, in accordance with the protocol. After 24 weeks, complete remission was reached by two of the 15 (13%) CSA and one of the 17 (5%) CPH patients (p=n.s.). Partial remission was achieved by seven of the 15 (46%) CSA and two of the 15 (11%) CPH patients (p<0.05). Five patients in the CSA group and 14 patients in the CPH group were withdrawn from the study, most of them during the non-responder protocol. The number of adverse events was comparable between both groups. We conclude that CSA is more effective than CPH in inducing at least partial remission in steroid-resistant nephrotic syndrome in children.
Keywords: Child, Cyclophosphamide, Cyclosporin A, Focal segmental glomerulosclerosis, Minimal change nephropathy, Nephrotic syndrome
Introduction
Idiopathic nephrotic syndrome in children is characterized by minimal change nephropathy (MCD) in more than 70% of all cases [1]. While approximately 90% of these patients respond to steroid treatment within 4 weeks, in patients with focal segmental glomerulosclerosis (FSGS) response rates to steroids are as low as 30%. Steroid resistance itself has a poor prognosis for renal survival [2, 3]. Furthermore, recent studies discuss a rise in the incidence of steroid resistance [4] and an increasing rate of FSGS in children [5]. Studies testing therapeutic options in children with steroid-resistant nephrotic syndrome (SRNS) are still limited. Uncontrolled trials [6–11] as well as a meta-analysis of three small randomized controlled trials [12] showed a positive effect of cyclosporin A (CSA) therapy in children with SRNS. Nevertheless, CSA is therapeutically effective in only a proportion of patients with SRNS [8, 13].
Earlier randomized controlled trials with cyclophosphamide (CPH) given orally (p.o.) showed no effects compared to unspecific therapy [2, 14] in SRNS. A subsequent small randomized trial showed some benefits with intravenous (i.v.) CPH therapy (similar to the lupus erythematodes nephritis therapy) [15, 16]. One trial, only published as an abstract, compared p.o. and i.v. CPH therapy and found no significant difference [17]. Steroid resistance in SRNS may be relative and, in some children, can be overcome with high-dose methylprednisolone (MPR) usually in combination with CPH or CSA [18–21]. Azathioprin and chlorambucil seem to be without therapeutic effect [12]. Although often used in the treatment of SRNS, data on the use of i.v. CPH and MPR pulses are still controversial, and until now randomized controlled trials were lacking.
The German Arbeitsgemeinschaft für Pädiatrische Nephrologie (APN) set up a trial for the standardized initial treatment of SRNS aimed at testing p.o. CSA versus i.v. CPH pulses. The study tested the hypothesis that CSA and CPH are differently effective in the therapy of SRNS because CSA and CPH interfere with separate mechanisms in the immune defense. The study therapy with CSA was mainly based on data available in the literature, while CPH pulse therapy was planned parallel to the treatment of lupus nephritis [22]. Nevertheless, the protocol committee tried to adjust the therapeutic protocol to the clinical practice of the members of the APN.
Methods
The study was designed as a multicentre controlled randomized open label trial to test the effectiveness of CSA and CPH pulses in combination with prednisone (PRD) as initial therapy in children and adolescents with primary SRNS. All centres for pediatric nephrology in Germany and Austria were invited to participate. In total, 23 centres in Germany and one centre in Austria participated.
Patients
Patients considered for enrollment presented with the first episode of nephrotic syndrome (gross proteinuria >40 mg/m2 body surface area per hour, equivalent to 1 g/m2/24 h) and hypoalbuminemia (<25 g/l). All patients had to be treated according APN treatment protocol [23] or the International Study of Kidney Disease in Children (ISKDC) therapy scheme [24] and had to receive at least 4 weeks of daily PRD without reaching complete remission. Minimal change nephropathy, FSGS or diffuse mesangial proliferation (MP) was verified by kidney biopsy. As proof of steroid resistance, patients received in addition to an oral application of PRD, three i.v. PRD pulses (500 mg/m2 i.v.) on alternate days. Steroid resistance was defined as the absence of complete remission within 14 days after the last pulse. To exclude patients with vasculitis or renal function impairment, we required that the participants had normal levels of the serum C3 complement and an endogenous creatinine clearance of over 70 mg/ml per minute per 1.73 m2 body surface area (BSA) at the time of determining steroid resistance. Prior to enrolment parental informed consent and, where applicable, patient’s assent was obtained. Patients with hereditary, syndromic or secondary SRNS were excluded as were patients who had received pre-treatment with immunosuppressive drugs other than PRD and prednisolone and not according APN [23] or ISKDC treatment protocol [24].
To exclude mutations in NPHS2 or WT1 genes (exon 8 and 9) as a cause for sporadic nephrotic syndrome, each patient’s DNA was analysed by direct exon sequencing (Friedhelm Hildebrandt and coworkers). The methods are described elsewhere [25–28].
Study treatment
Patients were randomly assigned at a ratio of 1:1 to arm A (p.o. CSA) or arm B (i.v. CPH pulses). To exclude centre-specific effects, randomization was stratified by centre. Restricted randomization was done centrally and concealed according to centre-specific computer-generated random lists. The investigator sent the patient enrolment form to the study coordinator by fax and was in turn informed by phone, e-mail and fax about the randomization result.
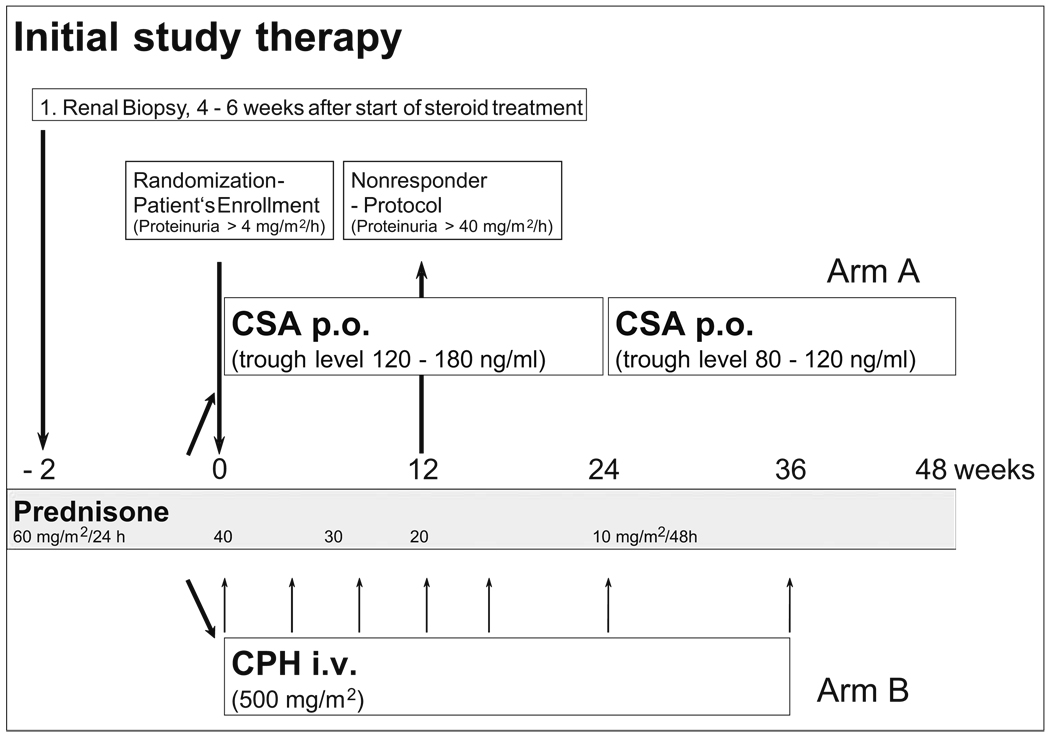
Patients in arm A initially received CSA (Sandimmun optoral, Novartis Pharma GmbH, Nuremberg, Germany) in a dose of 150 mg/m2 BSA per day in two single doses p.o aimed at obtaining constant trough levels of 150 ng/ml [range of 120–180 ng/ml according fluorescence polarization immunoassay (TDx) measurements] (Fig. 1).
Fig. 1.
Flowchart of the initial study therapy in patients with primary steroid-resistant nephrotic syndrome (SRNS). CSA Cyclosporin A, CPH cyclophosphamide, p.o. oral application, i.v. intravenous application
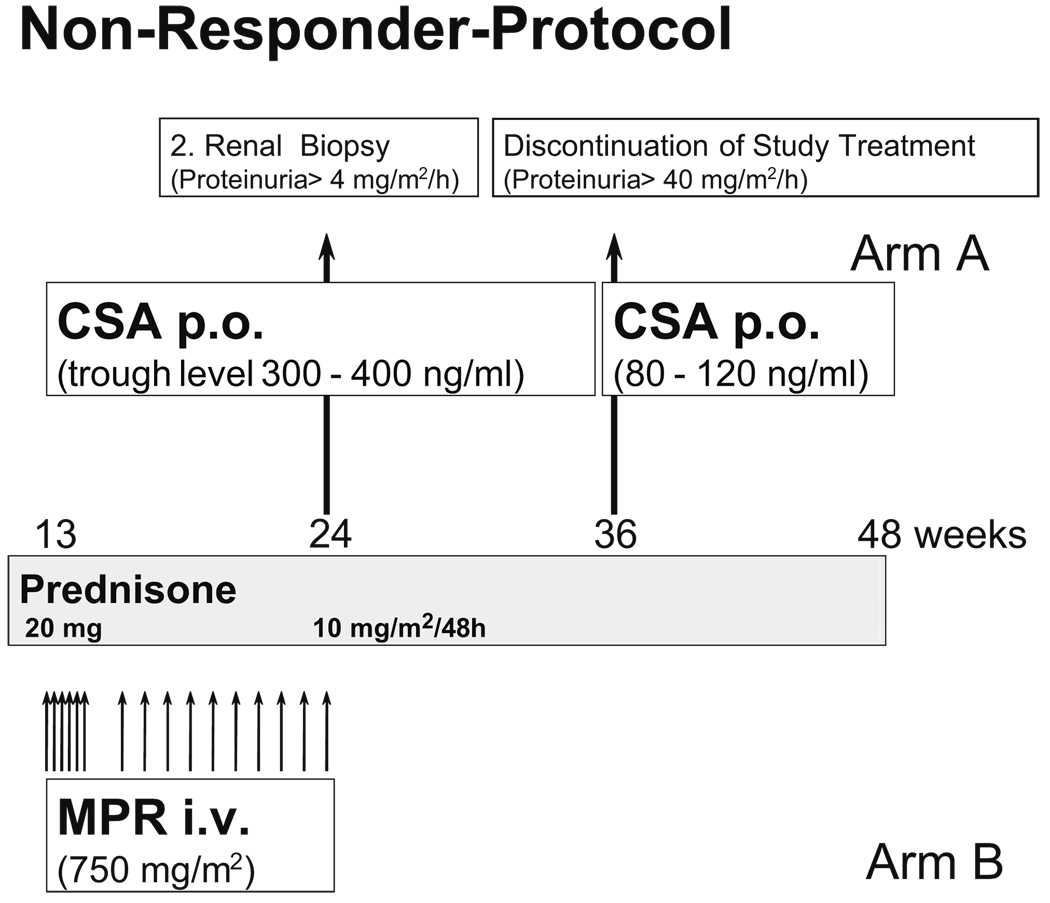
If there were no observed decrease in proteinuria to <40 mg/m2 per hour during the CSA therapy within the first 12 weeks, patients were enrolled in the non-responder protocol (Fig. 2) in which increased CSA doses with a constant trough level of 350 ng/ml (range 300–400 ng/ml) were administered (Fig. 2).
Fig. 2.
Flowchart showing the study therapy for patients not responding to the initial study therapy after 12 weeks (non-responder protocol). Patients not responding to cyclosporin A (CSA) in group A were treated with high-dose CSA. Patients in group B not responding to cyclophosphamide pulses were treated with methyprednisolone pulses (MPR). Inclusion criterion was proteinuria above 40 mg/m2 body surface area (BSA) per hour
When there was no indication of complete remission after 12 weeks of high-dose CSA therapy, a second kidney biopsy was planned to analyse the morphological progression of the disease and to detect changes caused by the medication. The patients left the study as per protocol withdrawal if there was no therapy effect after a 36-week therapy.
Arm B patients were given CPH (Endoxan, Baxter-Oncology, Frankfurt/Main, Germany) pulse therapy in a dose of 500 mg/m2 BSA in a 4-h infusion. The infusion was repeated after 4, 8, 12, 16, 24 and 36 weeks (Fig. 1). Following CPH infusion, the leukocyte count was measured twice a week. In the absence of a decrease in white blood cells to <4000/µl, the next dose of CPH was increased by 250 mg/m2 BSA. The maximum dose was 1 g/m2 BSA. In the case of a decrease in leukocytes to <2000/µl, the next dose was reduced by 250 mg/m2 BSA. If there were no decrease of proteinuria to < 40 mg/m2 per day after 12 weeks of therapy, patients in arm B entered a non-responder protocol with high-dose MPR-therapy that started with 750 mg/m2 BSA i.v. three times a week for 2 weeks; this was followed by one pulse a week for another 12 weeks (Fig. 2).
Similar to patients in arm A, patients without complete remission after 12 weeks of MPR treatment underwent renal biopsy. In patients without signs of remission, study therapy was stopped in week 36.
Patients in both arms also received PRD p.o. every second day in tapering doses until week 48 (Fig. 1, Fig. 2).
Definitions, endpoints and duration
Nephrotic syndrome was defined as proteinuria [urinary protein excretion >40 mg/m2 per hour (=1 g/m2 per 24 h)], hypoalbuminemia (serum albumin level <25 g/l) and edema.
Case definition for study enrollment was a pediatric patient with the first episode of idiopathic nephrotic syndrome, receiving at least 4 weeks of daily prednisone according the APN or ISKDC therapy scheme, followed by three prednison pulses without reaching complete remission within 14 days after the last pulse. Minimal change nephropathy, FSGS or diffuse mesangial proliferation (MP) were bioptically proven.
Complete remission demanded a protein excretion of ≤4 mg/m2 per hour (100 mg/m2 per day) at three successive analyses. First morning spot urine was used for protein quantification. A negative semi-quantified dip-stick colour test for albumin in spot urine was interpreted as proteinuria <4 mg/m2 per hour. For the analysis of remission rate, dip stick results were confirmed by proteinuria quantification in a urine sample timed over at least 12 h (≤4 mg/m2 per hour).
Partial remission was defined as the resolution of edema, an increase of serum albumin concentration to >35 g/l and persisting proteinuria between 4 and 40 mg/m2 per hour.
No response was defined as persisting proteinuria above 40 mg/m2 per hour.
The APN treatment protocol for initial therapy of nephrotic syndrome suggests treatment with 60 mg/m2 BSA PRD divided in three single doses, with the largest dose in the morning (maximum dose 80 mg/day) for 6 weeks. This is to be followed by 40 mg/m2 BSA over a 48-h period as a single dose in the morning (maximum 60 mg/day) for another 6 weeks [23].
The ISKDC treatment protocol for initial therapy of nephrotic syndrome suggests 60 mg/m2 BSA PRD per day for 4 weeks, followed by 40 mg/m2 BSA PRD intermittently on 4 of 7 days per week for 4 weeks [24].
The primary endpoint of the study was complete remission of the nephrotic syndrome (NS)—i.e. continuous reduction of the proteinuria to <4 mg/m2 per hour within 24 weeks under the initial therapy regimen with CSA or CPH pulses. Secondary endpoints were partial remission of the NS by the initial therapy with CSA or CPH pulses in week 24, efficacy of the non-responder protocol, incidence and significance of adverse drug reactions (ADR) and differences in the renal histology after 24 weeks of therapy in patients with an absence of partial or complete remission. The planned recruitment time was 36 months and was extended because the calculated number of 60 patients was not reached.
Sample size and statistical analysis
The underlying scenario for the sample size estimation assumed a large difference of 38% in the outcome between the two treatment arms. This followed the results from randomized trials on CSA showing complete remission in up to 40% of the cases [13, 29] and the heterogeneous results on CPH in the literature [16, 30]. Therefore, a sample size of 28 patients per group in the final analysis was required to verify such a difference using a (two-sided) significance level of 5% with a statistical power of 80%. Assuming a drop-out rate of 10% during the trial, at least 60 patients had to be recruited. Constraints of patient recruitment were given by the low incidence of SRNS in Germany. It was estimated that 20 patients per year meeting our case definition could be recruited for the study, leading to period of 3 years for patient enrolment.
Descriptive information on continuous variables is given as mean, standard deviation and range, if not mentioned otherwise. Analyses comparing the two treatment arms were performed paying attention to the intention-to-treat principle. Differences in the efficacy of both therapy arms were tested by Fisher’s exact test. A p value <0.05 was considered to indicate statistically significance. Differences in continuous variables between both groups were tested by unpaired t tests incorporating Welch’s correction in the case of different group-specific variances. Further differences in categorical variables between both groups were tested by Fisher’s exact test in an explorative manner. The statistical procedures were carried out using GRAPH PAD prism software ver. 3 (GraphPad Software, San Diego, CA) and SAS software ver. 9.1 (SAS Institute, Cary, NC).
Safety
The incidence and severity of adverse events were documented and analysed. Individual reasons for withdrawal from the protocol were the following: parental request, severe ADR, application of non-approved drugs according to the protocol and a constant decrease of the glomerular filtration rate to <40 ml/min per 1.73 m2 BSA.
As a safety measure, the protocol stated that the trial would be discontinued if the number of children who achieved complete or partial remission with the initial trial therapy by 12 weeks (the time point for entry to the nonresponder protocol) was significantly greater in one therapy arm compared to the other.
Legal aspects
The study protocol was approved by the ethics committee of the Medical Faculty of the University of Erlangen–Nuremberg. Local ethics committees in charge of the individual study centres also approved the protocol. Written informed consent of the parents and, if possible, assent of the patients were obtained. The study was conducted according the Declaration of Helsinki and national German laws.
Results
Patients
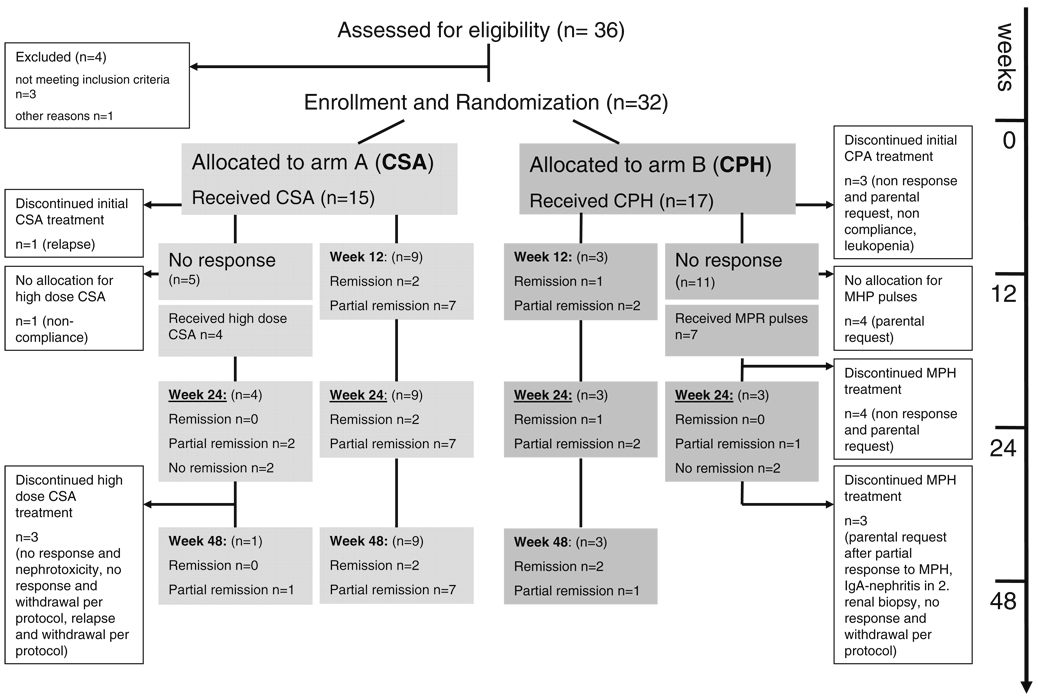
Between January 2001 and November 2004 a total of 37 patients were enrolled to the study. Of these, 15 patients (11 male, four female) were randomized to arm A to be treated with CSA, and 17 children (eight male, nine female) were randomized to arm B to be treated with CPH. Between the two groups there was no difference in age at manifestation of nephrotic syndrome, time between diagnosis and enrollment, calculated glomerular filtration rate, extent of proteinuria, serum albumin, or number of patients with arterial hypertension or on angiotensin converting enzyme (ACE) inhibitors (Table 1). In arm A, eight of the 15 patients presented with FSGS in contrast to arm B, where 13 of the 17 patients had FSGS. All patients were treated according to the APN treatment protocol for at least 4 weeks (Table 1).
Table 1.
Patient characteristics at enrollment
| Patient characteristics at enrollment | Arm A (CSA) | Arm B (CPH) |
|---|---|---|
| Number of patients | 15 | 17 |
| Sex (male:female) | 11:4 | 8:9 |
| Age at first manifestation (years) | 6.22 ± 5.11 (1.58–14.83) | 6.69 ± 3.93 (2.5–15.83) |
| Age at enrollment (years) | 6.99 ± 5.48 (1.67–15.50) | 6.84 ± 3.90 (2.67–16.00) |
| Time between manifestation and enrollment (months) | 2.34 ± 0.7 (1.25–4.0) | 2.38 ± 0.88 (1.5–4.45) |
| Calculated glomerular filtration rate (ml/min per 1.73 m2) | 190.9 ± 82.0 (94.22–369.3) | 184.5 ± 74.7 (57.96–343.8) |
| Protein excretion (mg/m2 per hour) | 217.3 ± 213.3 (28.0–900.0) | 253.6 ± 257.6 (12.10–1022) |
| Serum albumin (g/l) | 21.9 ± 10.4 (6.4–40.5) | 27.1 ± 10.4 (13.6–44.0) |
| Number of patients with arterial hypertension at study enrollment | 9 | 9 |
| Number of patients on ACE inhibitors | 6 | 6 |
| Number of patients on addition anti-hypertensive drugs | 7 | 10 |
| Histological diagnosis (number of patients) | ||
| MCD | 6 | 4 |
| FSGS | 8 | 13 |
| MP | 1 | 0 |
Data are given, where appropriate, as mean ± standard deviation, with the range given in parenthesis
CSA Cyclosporin A; CPH cyclophosphamide; ACE angiotensin converting enzyme; MCD minimal change nephropathy; FSGS focal segmental glomerulosclerosis; MP mesangial proliferation
Mutation analysis in the NPHS2 gene and WT1 gene
In arm A, mutation analysis for NPHS2 or WT1 mutations was performed on 12 of the 15 patients. There were no patients with heterozygous, compound heterozygous or homozygous mutations. In arm B, mutation analysis was performed on 14 of the 17 patients. Four patients showed the heterozygous sequence variant of unknown significance, R229Q, in exon 5; one patient showed a heterozygous mutation, G17A(h) = R6Q(h) and one patient showed a heterozygous mutation G413A(h) =R138Q in exon 3. Both heterozygous mutations alone are not sufficient to explain the nephrosis phenotype.
Withdrawals from the study
In total, five of the 15 patients enrolled in arm A were withdrawn from the study; in arm B, 14 of the 17 enrolled patients left the study. The time points and reasons for withdrawal are given in Fig. 3. Interestingly, in arm B, five patients were withdrawn during CPH treatment and another six patients during non-responder treatment by the parents or per protocol because the therapy was not successful. Only one patient with calcineurin inhibitor toxicity in the follow-up biopsy in arm A and one patient with severe leukopenia in arm B left the protocol due to treatment side effects.
Fig. 3.
Flowchart summarizing the allocation, treatment, response to therapy and withdrawals from the protocol. CSA Cyclosporin A, CPH cyclophosphamide, MPR methylprednisolone
Efficacy of CSA and CPH
In arm A (CSA), two of the 15 enrolled patients achieved complete remission by 12 weeks and maintained this at 24 weeks. In arm B (CPH), only one of the 17 enrolled patients achieved complete remission by 12 weeks and maintained this at 24 week (p=0.58). In addition. partial remission was seen in seven children treated with CSA at 12 and 24 weeks compared with two children treated with CPH at 12 and 24 weeks (p=0.04). The trial was stopped according to protocol after the enrollment of 32 children because nine of the 15 children treated with CSA compared with three of the 17 treated with CPH had responded with complete or partial remission (p=0.027) by 12 weeks.
Efficacy of the non-responder protocol
In arm A, five patients of the 15 enrolled reached week 12 without any response to CSA therapy (Fig. 3); four of these patients entered the non-responder protocol and were treated with high-dose CSA. One patient did not enter the non-responder protocol because non-compliance was suspected as the reason for non-response. Two patients reached partial remission in week 24, but only one patient showed a lasting partial remission until week 48; the other patient relapsed and left the study according the protocol at week 36. In arm B, 11 of the 17 enrolled patients did not respond to CPH treatment until week 12. By this time, three patients had already left the study. Seven of these 11 patients entered the non-responder protocol and were treated with MPR pulses: one patient reached partial remission by MPR in week 24, two patients did not show any response until week 24 and four patients were withdrawn from the study before week 24 by the parents because of no response.
Parameters influencing efficacy
Data on this aspect of the study are given in Table 2. All six patients with heterozygous sequence variations or heterozygous mutations of unknown significance were randomized in arm B. No patient with the polymorphic sequence variant R229Q responded to CPH and MPR. During follow-up, two of these patients were successfully treated with CSA, one did not respond to CSA and one experienced renal failure. The patient with heterozygous NPHS2 mutations R6Q(h) achieved partial remission with CPH therapy. One patient with heterozygous NPHS2 mutation R138Q did not respond to CPH and MPR therapy; in the follow-up period partial remission was achieved by CSA.
Table 2.
Therapy success in relation to mutations and polymorphisms in NPHS2, proteinuria at enrollment, histology and concomitant medication (n.a. not applicable)
| Arm A (CSA) | Arm B (CPH) | |||
|---|---|---|---|---|
| No response or relapse | Complete or partial remission | No response | Complete or partial remission | |
| Number of patients | 6 | 9 | 14 | 3 |
| Polymorphic sequence variant in NPHS2 R229Q | 0 | 0 | 4 | 0 |
| Heterozygous NPSH2 mutation | 0 | 0 | 1 | 1 |
| Protein excretion at enrollment (mg/m2 per hour) | 227 (166–900) | 94 (28–319) | 243 (12–1022) | 15 (31–63) |
| Number of patients with protein excretion of <40 mg/m2 per hour at enrollment | 0 | 1 | 1 | 2 |
| Serum albumin (g/l) | 15.1 (6.4–26.7) | 26.7 (6.7–40.5) | 24.2 (13.6–44.0) | 39.0 (37.0–42.8) |
| Number of patients with serum albumin of >25 g/1 at enrollment | 2 | 5 | 7 | 3 |
| Cumulative CPH dose (pulse 1–3) | n.a. | n.a. | 2041 (1903–2243) | 1814 (926–2300) |
| Leukocyte nadir (/µl) | n.a. | n.a. | 4700 (880–13800) | 4000 (3900–4500) |
| Patients on ACE inhibitors | 5 | 7 | 7 | 1 |
| Histological diagnosis | ||||
| MCD | 2 | 4 | 2 | 2 |
| FSGS | 4 | 4 | 12 | 1 |
| MP | 0 | 1 | 0 | 0 |
Data are given as median and range (in parenthesis) where pplicable, otherwise as the number of patients
In terms of impact of the histological diagnosis, cumulative dose of CPH, leukocyte nadir after CPH pulses and concomitant therapy with ACE inhibitors, such as like enalapril, ramipril or captopril were not different between responding and non-responding patients.
Due to the case definition of the study, patients not reaching complete remission by steroid treatment were eligible for enrollment. We therefore analysed whether there was a difference in proteinuria and serum albumin at enrollment between patients responding and not responding to study therapy. In arm A, patients responding to CSA therapy had a lower proteinuria at enrollment than non-responding patients, although the difference was not statistically significant. In arm A, one patient showed proteinuria between 4 and 40 mg/m2 per hour, no edema and a serum albumin of 28 mg/dl at the time of enrollment. This patient reached complete remission.
Interestingly, the three responding patients in arm B also had an initially lower proteinuria than the rest of the patients enrolled in this arm who did not respond to CPH. At enrollment, two patients later responding to CPH and one patient showing no response to CPH, but later to MPH, had proteinuria below 40 mg/m2 per hour, no edema and serum albumin above 25 mg/dl.
Safety
The number of adverse events in arm A and in arm B was similar for the whole study period as well as for the initial treatment with CSA or CPH (Table 3). In the non-responder protocol, the number of adverse events was also not different (data not shown). Eight and six severe adverse events were reported in arm A and arm B, respectively. One patient in arm A experienced hyperkalemia in week 20, probably caused by the intake of CSA. In a second patient, recurring abscess formation was reported in week 12, with a probable causal relation to CSA intake. Further reported serious adverse events during CSA therapy were enteritis (two cases), severe hypertension and edema, noncompliance, appendicitis and planned adenotonsilectomy (all one each). Two patients reaching partial remission with high-dose CSA showed a 45 and 200% increase in serum creatinine, respectively. Two patients not reaching partial remission on high-dose CSA showed a doubling of serum creatinine. Histological progress to FSGS and histological signs of calcineurin inhibitor toxicity were diagnosed, respectively. In arm B, one patient developed severe leucopenia after two CPH pulses and was withdrawn from the study. One patient in arm B was withdrawn in week 12, according to the protocol, because the glomerular filtration rate fell below 40 ml/ ml per minute per 1.73 m2. Renal failure was related to the progression of disease. Peritoneal dialysis had to be started later on. No other severe adverse event (bronchitis, urinary tract infection, nephrotic crisis) was related to the study medication. In both arms, the most frequent adverse events were infections, arterial hypertension and Cushing syndrome. As expected, patients in arm A developed hypertrichosis and gingiva hyperplasia. Nausea and emesis were seen in both arms. One patient in arm B developed peritonitis; no patient fell sick with thromboembolic events.
Table 3.
Number of adverse events and serious adverse events
| Arm A (CSA) | Arm B (CPH/MPH) | |
|---|---|---|
| Whole study period: | ||
| Total number | 113 | 105 |
| Number per month and patient | 1.25 ± 0.94 (0.29–4.0) | 1.34 ± 0.91 (0.33–3.0) |
| Serious adverse events | 8 | 6 |
| Initial treatment with CSA or CPH: | ||
| Total number | 76 | 66 |
| Number per month and patient | 1.22 ± 1.08 (0.29–4.0) | 1.61 ± 1.04 (0.33–3.0) |
| Selected adverse events | ||
| Infection | 17 | 15 |
| Arterial hypertension | 12 | 12 |
| Hypertrichosis | 11 | 3 |
| Cushing Syndrom | 10 | 16 |
| Gingiva hyperplasia | 7 | 0 |
| Diarrhea | 5 | 4 |
| Emesis | 4 | 7 |
| Tremor | 2 | 0 |
| Cephalgia | 4 | 2 |
| Nausea | 4 | 6 |
| Aszites | 2 | 7 |
| Hair loss | 1 | 1 |
| Peritonitis | 0 | 1 |
Numbers are given as the total number or as mean ± standard deviation and range (in parenthesis)
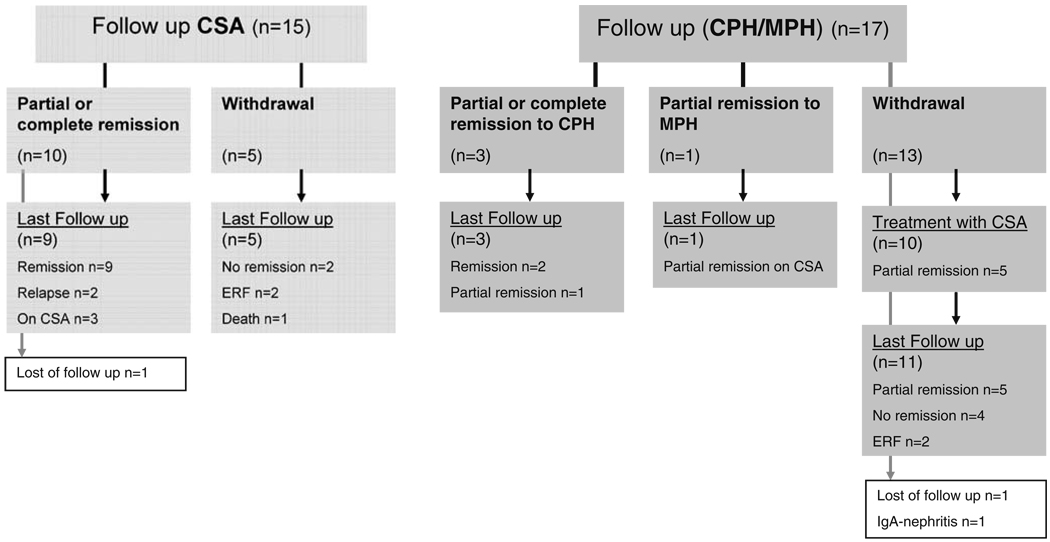
Follow-up
Follow-up data are available for 29 of the 32 enrolled patients. Data are shown as a flowchart in Fig. 4. Children responding to CSA therapy showed a favourable outcome at the last follow-up with relapses in only two children, no impairment of renal function or ongoing proteinuria. Two of the children responding to CPH showed ongoing remission at the last follow-up.
Fig. 4.
Flowchart summarizing the follow-up of patients from the protocol. CSA Cyclosporin A, CPH cyclophosphamide, MPR methylprednisolone, ERF end stage renal failure
Children withdrawn from arm A had a poorer outcome. One of these children died in the follow-up period from septicaemia, two patients developed end stage renal failure and two patients were still proteinuric at the last follow-up.
In arm B, ten of the 13 patients withdrawn from the study were switched to CSA therapy. Five of these patients had reached partial remission at the last follow-up (mean 9.5, range 4–27 months) after study withdrawal.
Discussion
The trial was conducted to compare the efficacy and safety of CSA and CPH in the initial treatment of steroid-resistant nephrotic syndrome in children. To the best of our knowledge, this is the first randomized trial comparing CSA and CPH.
The primary endpoint of this study-complete remission after 24 weeks of therapy-was only reached by two patients in arm A and one patient in arm B. Nevertheless, the number of patients reaching complete or partial remission was significantly higher under the CSA regimen than the CPH regimen. This is the reason why the trial had to be prematurely stopped, in accordance with the protocol. Response to CPH or CSA could be seen as early as week 12 and was similar to the results achieved in week 24 or 48. Therefore, early response to immunosuppressant treatment falls in line with the remission rate at later time points. This result is important because the primary endpoint in this study-normalization of proteinuria back in the physiological range (<4 mg/m2 per hour)-is often very hard to achieve. In daily clinical care, even partial remission seems to improve long-term renal survival [31] and is perhaps the more realistic therapeutic aim to achieve.The finding that only 13% of our patients treated with CSA reached complete remission is in accordance with data from adult patients that showed complete remission in 12% of steroid-resistant FSGS patients [32]. In terms of the pediatric population, two randomized controlled trials in which CSA therapy was compared with placebo or no treatment reported complete remission in up to 40% of the patients [13, 29]. This may be explained by higher CSA trough levels of up to 500 ng/ml in both studies. In addition, both of these studies were not analysed according to the intention-to-treat principle, which may lead to an overestimation of the effect [33]. A meta-analysis of these studies and of another smaller study by Garin et al. [34] confirmed the positive effect of CSA, with complete remission in 36% of the patients [12]. In a large case study by Niaudet et al. [7], 48% of MCD patients and 30% of FSGS patients achieved complete remission between 14 and 60 months after the initiation of treatment with 150–200 mg/m2 CSA. This result appears to be in agreement with our follow-up findings showing complete remission over the time period in all patients even though at 24 weeks the vast majority presented with partial remission. Therefore, the number of complete remissions may have been higher in the case of a later primary end point. Interestingly, FSGS and MCD patients showed similar response rates in our study, although the number of patients is too small to carry out a reliable subgroup analysis on the histology.
The low success of our CPH treatment, with a response rate of 17% in an intention-to-treat-analysis, is in part in contrast to the success reported in uncontrolled studies. Intravenous CPH pulse therapy, most often after steroid pulse treatment, has been used in uncontrolled trials with success rates of up to 80% [30]. However, smaller case series form Turkey [35] and Saudi Arabia [36] found limited or no effect of CPH pulses in primary SRNS. In a small randomized open label trial, monthly CPH pulses over a 6-month period showed a beneficial effect in the induction of remission in patients with steroid-resistant MCD compared to oral CPH [16]. Nevertheless, in a randomized trial (published as an abstract) comparing i.v. CPH pulses and PRD over a 6-month period with i.v. dexamethason, p.o. CPH and PRD, there was no significant effect [17]. It may be that the restriction to 12 weeks of initial therapy with CPH was in part the reason for the very low response rate in our study. If we look at the follow-up of patients not responding to CPH, it is interesting that up to 50% of these patients achieved partial remission with CSA therapy.
In our study only patients with primary steroid resistance naïve to other immunosuppressive or cytotoxic therapies were enrolled. Steroid resistance was diagnosed after treatment with prednisone over 4 weeks and three subsequent steroid pulses. This may be a limitation of our study. Four patients entered the study with a proteinuria of less than 40 mg/m2 per hour, without edema and a serum albumin above 25 mg/dl. One responded to CSA, two to CPH and one to high-dose MPH. It is possible that these patients would have remitted under longer steroid treatment alone. In the literature, there are proposals to go on with steroid treatment over 6 months before defining steroid resistance [37]. In total, patients responding to CPH presented with lower proteinuria at the time of enrollment. There was also a trend for CSA responders to show lower levels of proteinuria at enrollment. Nevertheless, the number of patients and the differences between the groups are too small and heterogeneous to use the amount of proteinuria as a prognostic marker for the responsiveness of second line treatments.
The use of high-dose CSA in an experimental non-responder protocol was based on the positive experience reported in cases of post-transplantation recurrence of FSGS [38] and reported remission rates of >80% in children with steroid- and CPH-resistant FSGS by CSA doses of up to 20 mg/kg per day [39]. Because case series have demonstrated the effectiveness of high-dose MPR treatment, in part in combination with alkylating agents [40–42], we offered MPH pulses in an experimental non-responder protocol for patients who failed to remit on CPH. In Germany, MPR pulse therapy for the treatment of SRNS is not well established, which may explain in part the low acceptance of this part of the protocol by the parents. Because of the low number of patients treated in this protocols and the lack of randomization to the non-responder protocol, conclusions from these episodically results should only be drawn with utmost care.
Genetic aspects were not the main topic of this study, nevertheless screening for NPHS2 mutations (Podocin) and mutations in WT1, exon 8 and 9 were included to the protocol to exclude genetical forms of SRNS. Based on the data of Ruf [27] and Weber [43], who analysed cohorts with sporadic SRNS from Europe, North Africa and India, up to six patients in our cohort could have been carriers of homozygous or compound heterozygous mutations. In 26 patients with well-defined SRNS, we found no patient with homozygous and compound heterozygous mutations. All patients with heterozygous mutations or sequence variations were allocated to the CPH group. Based on the response of one patient to CPH and another four patients to subsequent CSA treatment, we do not see an effect of these genetic variants on the therapeutic efficacy.
In addition to the efficacy, we analysed the safety of the treatment. Comparing both arms we could not detect a difference in the safety aspects. Nevertheless, SRNS patients are prone to complications, and it may be difficult to determine whether an adverse event is study related or disease related. Data on the long-term safety, especially in the CPH arm, can not be given at the present time.
In conclusion, we found a significantly higher response rate to CSA therapy in comparison to CPH pulse therapy. Although the rate of complete remission at 6 months was not different, the rate of complete and partial remission in CSA patients was significantly higher than in CPH patients. Higher doses of CSA may be helpful in patients not responding to the usual doses of CSA therapy. The shortterm safety profile of both therapies was comparable. Cyclosporin A therapy is superior to CPH therapy in inducing at least partial remission in children with primary SRNS secondary to MCD or FSGS. As such, CSA is indicated as first line therapy in children with SRNS.
Acknowledgements
We thank all the members of the Arbeitsgemeinschaft für Pädiatrische Nephrologie for their help in the design and realization of this project. For their help in coordinating the study, we thank K. Benz, K.-D. Nüsken, B. Geiβler and K. Escherich. We very much appreciate the willingness of our patients and their families to participate in this trial. This project was supported in part by a grant of Novartis Pharma, Nuremberg, Germany.
Footnotes
Conflict of interest statement None to declare.
Contributor Information
Christian Plank, Department of Pediatrics, University Erlangen-Nuremberg, Loschgestraβe 15, 91054 Erlangen, Germany, e-mail: christian.plank@uk-erlangen.de.
Veronica Kalb, Department of Pediatrics, University Erlangen-Nuremberg, Loschgestraβe 15, 91054 Erlangen, Germany.
Bernward Hinkes, Department of Pediatrics, University Erlangen-Nuremberg, Loschgestraβe 15, 91054 Erlangen, Germany; Department of Pediatrics, University of Michigan, Ann Harbor, MI, USA.
Friedhelm Hildebrandt, Department of Pediatrics, University of Michigan, Ann Harbor, MI, USA.
Olaf Gefeller, Institute for Medical Informatics, Biometry and Epidemiology, University Erlangen-Nuremberg, Erlangen, Germany.
Wolfgang Rascher, Department of Pediatrics, University Erlangen-Nuremberg, Loschgestraβe 15, 91054 Erlangen, Germany.
References
- 1.International Study of Kidney Disease in Children. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98:561–564. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 2.Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatr Nephrol. 1996;10:590–593. doi: 10.1007/s004670050167. [DOI] [PubMed] [Google Scholar]
- 3.Abrantes MM, Cardoso LS, Lima EM, Penido Silva JM, Diniz JS, Bambirra EA, Oliveira EA. Predictive factors of chronic kidney disease in primary focal segmental glomerulosclerosis. Pediatr Nephrol. 2006;21:1003–1012. doi: 10.1007/s00467-006-0138-y. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int. 2005;68:1275–1281. doi: 10.1111/j.1523-1755.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 5.Chesney R. The changing face of childhood nephrotic syndrome. Kidney Int. 2004;66:1294–1302. doi: 10.1111/j.1523-1755.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- 6.Tejani A, Suthanthiran M, Pomrantz A. A randomized controlled trial of low-dose prednisone and ciclosporin versus high-dose prednisone in nephrotic syndrome of children. Nephron. 1991;59:96–99. doi: 10.1159/000186526. [DOI] [PubMed] [Google Scholar]
- 7.Niaudet P, Fuchshuber A, Gagnadoux MF, Habib R, Broyer M. Cyclosporine in the therapy of steroid-resistant idiopathic nephrotic syndrome. Kidney Int Suppl. 1997;58:S85–S90. [PubMed] [Google Scholar]
- 8.Gregory MJ, Smoyer WE, Sedman A, Kershaw DB, Valentini RP, Johnson K, Bunchman TE. Long-term cyclosporine therapy for pediatric nephrotic syndrome: a clinical and histologic analysis. J Am Soc Nephrol. 1996;7:543–549. doi: 10.1681/ASN.V74543. [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Tejani C, Tejani A. One-center experience with cyclosporine in refractory nephrotic syndrome in children. Pediatr Nephrol. 1999;13:26–32. doi: 10.1007/s004670050557. [DOI] [PubMed] [Google Scholar]
- 10.El-Husseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, Taha N, Hassan N, Sayed-Ahmad N, Sobh M. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant. 2005;20:2433–2438. doi: 10.1093/ndt/gfi059. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoud I, Basuni F, Sabry A, El-Husseini A, Hassan N, Ahmad NS, Elbaz M, Moustafa F, Sobh M. Single-centre experience with cyclosporin in 106 children with idiopathic focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2005;20:735–742. doi: 10.1093/ndt/gfh766. [DOI] [PubMed] [Google Scholar]
- 12.Hodson EM, Habashy D, Craig JC. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst. 2006 doi: 10.1002/14651858.CD003594.pub3. Rev:CD003594. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7:56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 14.International study of Kidney Disease in Children. Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet. 1974;2:423–427. [PubMed] [Google Scholar]
- 15.Balow JE, Boumpas DT, Fessler BJ, Austin HA., 3rd Management of lupus nephritis. Kidney Int Suppl. 1996;53:S88–S92. [PubMed] [Google Scholar]
- 16.Elhence R, Gulati S, Kher V, Gupta A, Sharma RK. Intravenous pulse cyclophosphamide—a new regime for steroid-resistant minimal change nephrotic syndrome. Pediatr Nephrol. 1994;8:1–3. doi: 10.1007/BF00868243. [DOI] [PubMed] [Google Scholar]
- 17.Mantan M, Bagga A, Siriam C, Hari P. Efficacy of IV pulse cyclophosphamide (CP) vs iv steroids & oral CP for steroid resistant nephrotic syndrome. Pediatr Nephrol. 2004;19:C73. doi: 10.1007/s00467-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 18.Tune BM, Kirpekar R, Sibley RK, Reznik VM, Griswold WR, Mendoza SA. Intravenous methylprednisolone and oral alkylating agent therapy of prednisone-resistant pediatric focal segmental glomerulosclerosis: a long-term follow-up. Clin Nephrol. 1995;43:84–88. [PubMed] [Google Scholar]
- 19.Tune BM, Lieberman E, Mendoza SA. Steroid-resistant nephrotic focal segmental glomerulosclerosis: a treatable disease. Pediatr Nephrol. 1996;10:772–778. doi: 10.1007/s004670050216. [DOI] [PubMed] [Google Scholar]
- 20.Waldo FB, Benfield MR, Kohaut EC. Methylprednisolone treatment of patients with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 1992;6:503–505. doi: 10.1007/BF00866483. [DOI] [PubMed] [Google Scholar]
- 21.Waldo FB, Benfield MR, Kohaut EC. Therapy of focal and segmental glomerulosclerosis with methylprednisolone, cyclosporine A, and prednisone. Pediatr Nephrol. 1998;12:397–400. doi: 10.1007/s004670050473. [DOI] [PubMed] [Google Scholar]
- 22.Niaudet P. Treatment of lupus nephritis in children. Pediatr Nephrol. 2000;14:158–166. doi: 10.1007/s004670050034. [DOI] [PubMed] [Google Scholar]
- 23.Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr. 1993;152:357–361. doi: 10.1007/BF01956754. [DOI] [PubMed] [Google Scholar]
- 24.International Study of Kidney Disease in Children. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr. 1981;98:561–564. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 25.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A. Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2002;13:388–393. doi: 10.1681/ASN.V132388. [DOI] [PubMed] [Google Scholar]
- 26.Ruf RG, Schultheiss M, Lichtenberger A, Karle SM, Zalewski I, Mucha B, Everding AS, Neuhaus T, Patzer L, Plank C, Haas JP, Ozaltin F, Imm A, Fuchshuber A, Bakkaloglu A, Hildebrandt F APN Study Group. Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int. 2004;66:564–570. doi: 10.1111/j.1523-1755.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 27.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F Arbeitsgemeinschaft Für Pädiatrische Nephrologie Study Group. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–732. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 28.Mucha B, Ozaltin F, Hinkes BG, Hasselbacher K, Ruf RG, Schultheiss M, Hangan D, Hoskins BE, Everding AS, Bogdanovic R, Seeman T, Hoppe B, Hildebrandt F Members of the APN Study Group. Mutations in the Wilms’ tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatr Res. 2006;59:325–331. doi: 10.1203/01.pdr.0000196717.94518.f0. [DOI] [PubMed] [Google Scholar]
- 29.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, Pasquali S, Castellani A, Della Casa-Alberighi O. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. doi: 10.1038/ki.1993.194. [DOI] [PubMed] [Google Scholar]
- 30.Bajpai A, Bagga A, Hari P, Dinda A, Srivastava RN. Intravenous cyclophosphamide in steroid-resistant nephrotic syn-drome. Pediatr Nephrol. 2003;18:351–356. doi: 10.1007/s00467-003-1095-3. [DOI] [PubMed] [Google Scholar]
- 31.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 32.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 33.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. Br Med J. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garin EH, Orak JK, Hiott KL, Sutherland SE. Cyclosporine therapy for steroid-resistant nephrotic syndrome. A controlled study. Am J Dis Child. 1988;142:985–988. doi: 10.1001/archpedi.1988.02150090083029. [DOI] [PubMed] [Google Scholar]
- 35.Buyukcelik M, Cengiz N, Dursun H, Soran M, Bayazit AK, Noyan A, Anarat A. Intravenous pulse cyclophosphamide therapy in focal segmental glomerulosclerosis. Clin Nephrol. 2006;65:7–12. doi: 10.5414/cnp65007. [DOI] [PubMed] [Google Scholar]
- 36.Al Salloum AA. Pulse cyclophosphamide therapy for steroid-resistant focal segmental glomerulosclerosis in children. Ann Saudi Med. 2004;24:27–30. doi: 10.5144/0256-4947.2004.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 38.Raafat RH, Kalia A, Travis LB, Diven SC. High-dose oral cyclosporin therapy for recurrent focal segmental glomeruloscle-rosis in children. Am J Kidney Dis. 2004;44:50–56. doi: 10.1053/j.ajkd.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Ingulli E, Singh A, Baqi N, Ahmad H, Moazami S, Tejani A. Aggressive, long-term cyclosporine therapy for steroid-resistant focal segmental glomerulosclerosis. J Am Soc Nephrol. 1995;5:1820–1825. doi: 10.1681/ASN.V5101820. [DOI] [PubMed] [Google Scholar]
- 40.Mendoza SA, Tune BM. Treatment of childhood nephrotic syndrome. J Am Soc Nephrol. 1992;3:889–894. doi: 10.1681/ASN.V34889. [DOI] [PubMed] [Google Scholar]
- 41.Kirpekar R, Yorgin PD, Tune BM, Kim MK, Sibley RK. Clinicopathologic correlates predict the outcome in children with steroid-resistant idiopathic nephrotic syndrome treated with pulse methylprednisolone therapy. Am J Kidney Dis. 2002;39:1143–1152. doi: 10.1053/ajkd.2002.33382. [DOI] [PubMed] [Google Scholar]
- 42.Yorgin PD, Krasher J, Al-Uzri AY. Pulse methylprednisolone treatment of idiopathic steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2001;16:245–250. doi: 10.1007/s004670000494. [DOI] [PubMed] [Google Scholar]
- 43.Weber S, Gribouval O, Esquivel EL, Morinière V, Tête MJ, Legendre C, Niaudet P, Antignac C. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]