Abstract
Background
Levator ani syndrome is characterized by anorectal discomfort/pain, whose treatment is unsatisfactory. We hypothesized that Botulinum toxin relieves spasm and improves symptoms.
Aim
Randomized, placebo-controlled, crossover study to examine efficacy and safety of botulinum toxin.
Methods
Twelve patients with levator ani syndrome (≥ 1year) received anal intra sphincteric injections of 100 units botulinum toxin A and placebo at 90 day intervals using EMG guidance. Daily frequency, severity, duration, and intensity of pain (VAS) were recorded. Anorectal manometry, balloon expulsion, and pudendal nerve latency tests were performed to examine the physiological changes and adverse effects.
Results
Seven patients (male/female = 4/3) completed and three had incomplete data, but all ten were analyzed in an ITT analysis; two others dropped out. After botulinum toxin, the mean frequency, intensity, and duration of pain was unchanged (p=0.31) compared to baseline. The 90 day mean VAS pain score was 6.79 ± 0.27 versus baseline score of 7.08 ± 0.29 (p=0.25). Anal sphincter pressures, rectal sensory thresholds pudendal nerve latency and balloon expulsion times were unchanged after drug or placebo.
Conclusions
Injection of botulinum toxin into anal sphincter is safe but does not improve anorectal pain in levator ani syndrome.
Keywords: Botulinum toxin, Levator Ani Syndrome, Anorectal Pain, Anorectal Manometry
Introduction
Levator ani syndrome is characterized by episodes of prolonged anorectal discomfort/pain of unclear origin.1 Its prevalence is not known but estimates in the range of 2-5% have been quoted.1,2,3 These estimates could be higher either because other local anorectal conditions were not systematically excluded or because a population based assessment has not been performed.
The pathophysiology of levator ani syndrome is poorly understood. In one study, during an episode of pain, undulations of anal sphincter muscle were seen with anal endosonography suggesting a hypertonic overactive sphincter.4 Another study of anal prolonged manometry reported that painful episodes were associated with paroxysmal anal spasm5. These observations suggested that spasm or overactivity of the anal sphincter muscle may be a mechanism for pain.4,5,6 Consequently, relieving anal spasm may be therapeutically beneficial5,6 and has been the goal of many studies for this condition. 7
The treatment of levator ani syndrome has been unsatisfactory.7 Several uncontrolled therapies have been tried including rectal massage, electrogalvanic stimulation, caudal block and biofeedback therapy.2,7 However, none of these therapeutic treatments have been tested through a randomized controlled study.
Botulinum toxin A, is one of seven neurotoxins produced by the anaerobic bacterium, clostridium botulinum.8,9 When injected into the muscle, the toxin enters the nerve terminal by endocytosis. Once inside the neuron, the toxin inhibits the release of acetylcholine by acting as a zinc-dependent protease that selectively cleaves a synaptic protein named SNAP-25.9 The injected muscles become weak over 2-20 days and may recover over 2-4 months as new terminal axons sprout and restore neurotransmission.10 Thus, the toxin produces chemo-denervation that can last for 14 to 16 weeks.11,12
Botulinum toxin has been used for the treatment of various gastrointestinal disorders including achalasia,12 diffuse esophageal spasm,13 chronic anal fissure,14,15 obstructive defecation,16 cricopharyngeal dysfunction,17 and sphincter of Oddi dysfunction.18 In patients with chronic anal fissure the results are promising,14,15 but for obstructive or dyssynergic defecation, botulinum toxin treatment has been disappointing.16,19
In a pilot study of 5 subjects with levator ani syndrome, we found that at six week botulinum toxin relieved symptoms in 3/5 patients. Hence, we tested the hypothesis that botulinum toxin relieves anorectal pain in patients with levator ani syndrome. The aim of this study was to perform a randomized, placebo controlled, cross over study to investigate the effects of intra anal sphincteric injection of botulinum toxin in patients with levator ani syndrome.
Methods
Subjects
Twelve (male/female = 7/5) patients with symptoms of chronic anorectal pain for at least one year and who fulfilled ROME II criteria for levator ani syndrome1 were enrolled in this study. All patients underwent comprehensive biochemical and metabolic work up which excluded common gastrointestinal, neurologic and metabolic disorders, including constipation, fecal incontinence, inflammatory bowel disease, diabetes, hemorrhoids, anal fissure, and other anorectal mucosal diseases. A flexible sigmoidoscopy or colonoscopy was performed to exclude colorectal problems. Anal endosonography and abdominal and pelvic CAT scan were performed to exclude any structural problems that cause anorectal pain.
Study protocol
Patients underwent a baseline physiological assessment that consisted of anorectal manometry, balloon expulsion test, saline continence test and pudendal nerve latency using standard protocols.20 Additionally, a flexible sigmoidoscopy or colonoscopy was performed to test for mucosal disease and an anal endosonography, in order to exclude anorectal structural disorders.
Patients were asked to score their overall intensity of anorectal pain on a visual analog scale (VAS) of 0-10, where 0 represented no pain and 10 severe pain. In addition, they were asked to maintain a daily pain diary in which they recorded the frequency, severity, and duration of each pain episode. The frequency of pain was scored on a scale of 0-4 using the following description; if the pain was episodic and occurred only during the day it was scored as 1, if it was continuous and occurred only during the day it was rated as 2, if it occurred intermittently during the day and night it was scored as 3 and if it was continuous and occurred during the day and night it was scored as 4. The severity of the pain was scored on a 4 point Likert-like scale with 0 = none, 1 = mild, 2 = moderate and 3 = severe. The duration of pain was also scored on a 3 point Likert-like scale. The duration was scored as 1 if the pain lasted for 1-15 minutes, 2 if it lasted for 15-30 minutes and 3 if it was longer than 30 minutes. Patients were asked to maintain and complete the pain diaries before injection and throughout the entire study period.
Next, they attended the motility lab and were randomized to receive an injection of either botulinum toxin A (Botox®, Allergan pharmaceuticals, CA) or placebo into the anal sphincter. One week later, the patients underwent a repeat assessment of anorectal manometry, balloon expulsion test, saline continence test, pudendal nerve latency tests, and anal endosonography. On this visit they were also asked to score their overall pain intensity on a VAS.
Three months later, the patients returned to the laboratory for a second injection of either botulinum toxin or placebo, and also completed the pain intensity VAS. One week later, physiological tests of anorectal function and anal endosonography were performed, and a VAS for pain intensity was completed. Patients returned for a final follow up visit, 3 months after their last injection (180 days). At this visit, they also returned their daily pain diaries.
Protocol for Intra anal sphincteric injection
Twenty five units of botulinum toxin A (Botox®, Allergan Pharmaceuticals, Los Angeles, CA) were injected into each of 4 quadrants (total dose 100 Units). After application of topical 2% lidocaine gel to the anal canal, a slit proctoscope was advanced into the rectum. The anal mucosa which bulged into the slit proctoscope was then pierced by a special 27 gauge electromyography (EMG) needle (Allergan Pharmaceuticals, Los Angeles, CA) that allows simultaneous recording of EMG signals as well as injection of materials (Fig. 1). The needle was connected to an amplifier and recorder. We attached a special extension adapter to the needle with a three way stop cock and a luer lock (Fig. 1). A 5cc solution was prepared containing 100 units of botulinum toxin A. The anal mucosa was pierced to a depth of 5-10 mm between 1-2 cm from the anal verge. After insertion of the needle, we first recorded the EMG signals from the anal sphincter. Next, the patient was asked to squeeze and relax on two separate occasions. Undulations in the anal EMG signals provided confirmation that the needle was in the muscle plane. Next, 25 units of botulinum toxin was administered. The needle and the proctoscope were removed. The proctoscope was reinserted such that the slit was now positioned at 90° to the previous injection site. Thus, rotating the proctoscope in a clockwise manner, botulinum toxin was injected in all four quadrants. Mild pain and occasionally minimal bleeding was encountered. These resolved quickly in all subjects with no long term sequelae.
Figure 1.
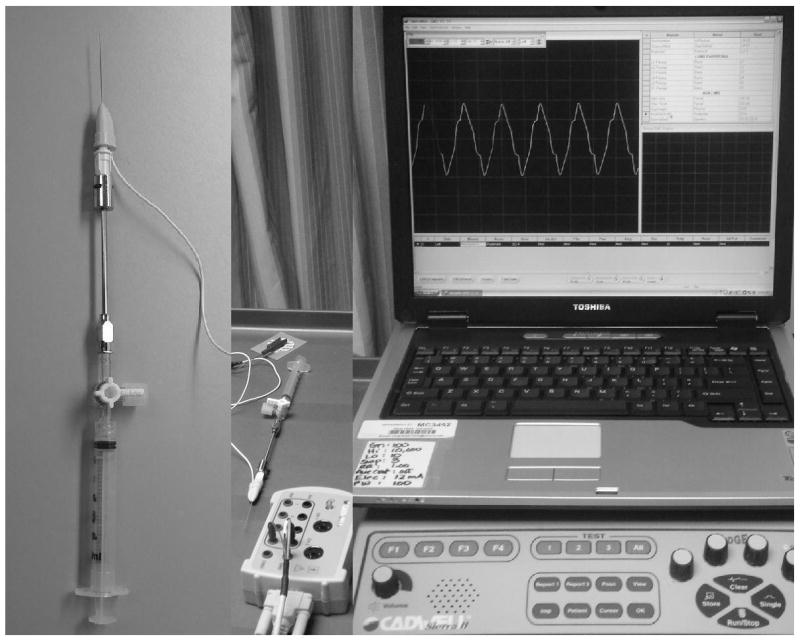
This figure describes the set up and methods used for botulinum toxin injection into the anal sphincter. A special EMG needle was attached to a metal needle extender and connected to a syringe containing botulinum toxin via a 3 way stop cock. The needle was also connected via an electrode to a neurophysiology recorder (Cadwell Sierra II Wedge) and the EMG signals were displayed on a monitor.
Data Analysis
We measured and compared the resting and squeeze anal sphincter pressures, rectal sensory thresholds for first perception, desire to defecate and urgency to defecate, pudendal nerve terminal motor latency time, balloon expulsion time and percentage of saline retention between baseline and after each of the two injections.
The overall pain intensity score (VAS) were compared between baseline and at day 7 and at day 90 after each injection. The total sum of the cores for the frequency, severity and duration of pain episodes per day was used to calculate the daily total pain score.
From this data, a weekly total pain score (0-10) was calculated for each patient by averaging their daily scores. The mean weekly total pain score was compared between baseline period and following each injection.
Statistical analysis
With a proposed sample size of 24 patients, the paired t-test at the 0.05 significance level can detect a mean change of at least 0.71SD in overall pain measurements (VAS scales) with a power of 0.80 assuming a correlation of 0.3 between baseline and post-botox pain. If r=0.7 then a mean change of at least 0.47 SD can be detected. For comparing the effect of botulinum toxin versus placebo, a difference at the 0.05 level can detect a mean change of 0.7 SD for pain with a power of 0.80, and for anal resting sphincter pressure a s.d of 9.4 assuming r=0.7. If there is a carry-over effect the latter two may be higher with a S.D of 24 and 1.3.
A paired student's t-test was used to compare the manometric data between baseline and after each injection. The pain scores were analyzed and compared using Wilcoxon's rank sum or Mann Whitney tests as appropriate. The data are expressed as mean ± standard error. We performed an intention-to-treat analysis and wherever data were unavailable, the last observation was carried forward. An interim analysis was planned after enrollment of 50 % of subjects.
Results
Subjects
Seven patients (male/female = 4/3, mean age 63 yrs (range 45-73)) completed the study. Three subjects (male/female= 2/1) did not complete the study and their last observation was carried forward. Two patients did not receive any injection because they failed to show up for their first injection, although, they signed consent and underwent baseline physiological tests. The other subject dropped out of the study after the first injection, because he developed a recurrent anal fissure (that was not present at the time of evaluation or first injection) that required surgery. Thus, in total data from ten subjects were analyzed for the interim analysis. Because this analysis showed no improvement in symptoms, further recruitment was terminated.
Weekly total pain scores
Weekly total pain scores were calculated from the patients' daily pain diaries for the baseline period and for the last 4 weeks (week 4) and for next four weeks (week 8) following each injection. The patients' mean frequency, mean severity, and mean duration of anorectal pain were unchanged after botulinum toxin when compared to baseline (p=0.31) or placebo (p=0.28). The mean weekly total pain score for week 4 of the botulinum toxin phase was 6.79 ± 0.28; whereas, at baseline it was 6.83 ± 0.37 [Figure 2].
Figure 2.
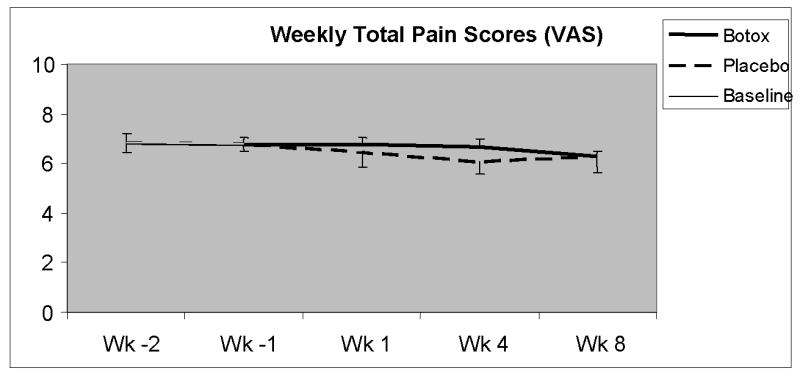
Weekly total pain scores at baseline and during each 8 week period following injection. (Mean ± SE).
Overall pain intensity score (VAS)
The pain intensity scores (0-10) were analyzed at baseline and again on days 8 and 90. The baseline score was 7.08 ± 0.29 after botulinum toxin the VAS pain intensity score on day 8 was 6.79 ± 0.27, and on day 90 it was 6.79 ± 0.27. Thus, no significant change was observed (p=0.25) [Figure 3].
Figure 3.
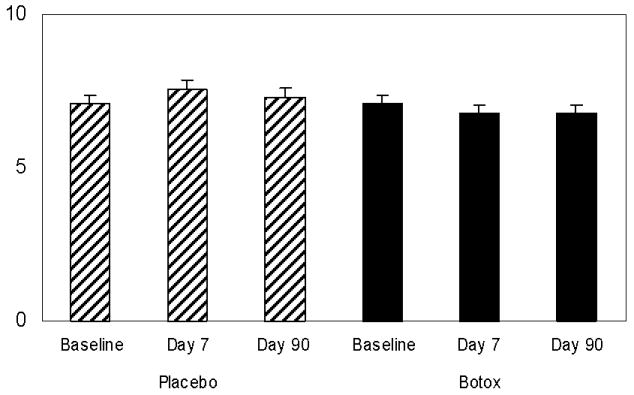
The effects of botulinum toxin or placebo on anorectal pain intensity score (VAS), at baseline, and at 7 and 90 days following each injection. Mean ± SEM.
Anal sphincter pressures
The resting anal sphincter pressure did not show any appreciable change after botulinum toxin injection. The maximum squeeze sphincter pressure did not change after botulinum toxin when compared to either baseline (p=0.91) or placebo (p=0.43). See table 1.
Table 1. The effects of Botox injections on anal sphincter function, pudendal nerve latency (PNTML), balloon expulsion, rectal sensory thresholds and % saline retention. (Mean ± SEM.).
| Test | Baseline | Botox | Placebo |
|---|---|---|---|
| Resting Pres. (mm Hg) | 61 ± 6 | 62 ± 6 | 67 ± 8 |
| Max. Squeeze Pres. (mm Hg) | 161 ± 21 | 142 ± 22 | 144 ± 22 |
| PNTML (ms) | 2.08 ± 0.32 (Right) 2.26 ± 0.27 (Left) |
2.13 ± 0.26 (Right) 2.28 ± 0.28 (Left) |
2.11 ± 0.28 (Right) 2.19 ± 0.24 (Left) |
| Balloon Expulsion Time (s) | 31 ± 5 | 60 ± 20 | 45 ± 6 |
| Desire to Defecate (cc) | 123 ± 34 | 104 ± 29 | 130 ± 38 |
| Urgency to Defecate (cc) | 158 ± 32 | 139 ± 30 | 174 ± 34 |
| % Saline Retention (cc) | 77 ± 38 | 60 ± 33 | 54 ± 43 |
Pudendal Nerve Terminal Motor Latency (PNTML)
The pudendal nerve latency time did not change significantly after botulinum toxin either on the right side (2.13 ± 0.26, p=0.70) or on the left side (2.28 ± 0.28, p=0.51) when compared to baseline (2.08 ± 0.95 (R), 2.26 ± 0.82 (L)). See table 1.
Rectal sensory thresholds
The threshold for desire to defecate, and urge to defecate were unchanged after botulinum toxin injections when compared to baseline (p=0.1) or placebo (p=0.25). See table 1.
Balloon expulsion test
The amount of time required to expel a balloon increased after botulinum toxin but was not significantly different when compared to baseline (p=0.27) or placebo (p=0.50). See table 1.
Saline continence test
The percentage of saline retention did not change significantly after botulinum toxin injection (59.58 ± 32.28, p=0.84) when compared to baseline (77.50 ± 37.91) (Table 1).
Anal endosonography
Anal endosonography showed no change in internal or external anal sphincter morphology when compared to baseline, after each of the two injections.
Discussion
Chronic anorectal pain is a frustrating clinical problem that comprises of at least two functional anorectal disorders that include levator ani syndrome and proctalgia fugax.1,7 Despite advances in several functional anorectal disorders, these two problems continue to pose a therapeutic challenge and their pathophysiology is poorly understood. 7 Although a number of therapeutic approaches have been tried, there has been limited success, and there is a dearth of randomized controlled clinical trials.
Encouraged by our pilot studies, we performed a randomized controlled trial of intra sphincteric injection of botulinum toxin and assessed both subjective and objective parameters of anorectal function. Our trial was designed to enroll 25 subjects with an interim analysis, after enrollment of 12 subjects. This analysis revealed that unlike our pilot studies, intra sphincteric botulinum toxin injection did not improve anorectal pain. The total pain score as assessed by the subjects on a visual analog scale was similar after injection of botulinum toxin or placebo. Likewise, the rating of pain intensity was similar between botulinum toxin and placebo. Thus, botulinum toxin injection does not appear to relieve anorectal pain in levator ani syndrome.
In this study, we designed a novel approach to ensure that the botulinum toxin was delivered into the anal sphincter muscle and not to scar tissue, fat or a non-muscular plane. To facilitate this we used a technique of simultaneously assessing EMG of the anal sphincter along with botulinum toxin injection. However, in spite of such technical advances and accurate localization of injection, we found that botulinum toxin was ineffective in relieving anal pain. Whether this is because of a lack of efficacy of botulinum toxin or whether the pathophysiology of pain in these patients involves mechanisms other than anal spasm or whether the injection should be delivered to another site, for example, the puborectalis muscle as opposed to the anal sphincter muscle, or whether the dose of botulinum toxin used when compared to the muscle mass was inadequate merits further appraisal. Furthermore, because anal sphincter not only responds to cholinergic but also nitrergic stimulation and VIP,21 chemo-denervation of a single pathway with botulinum toxin may have produced minimal or no effect on anal sphincter spasm.
Interestingly, 100-units of botulinum toxin injection appear to be safe, with regards to anal sphincter function. Although, there are some reports of changes in anal sphincter physiology following botulinum toxin injection,22,23 this is the first and most comprehensive assessment of all four aspects, notably anal sphincter morphology, manometric function, and neurophysiologic function as well as rectal sensorimotor function. The resting sphincter pressure which predominantly reflects external anal sphincter function decreased by about 10%, but this was not significant. The sensory thresholds for a desire to defecate decreased slightly both with botulinum toxin and placebo, but there was no significant difference. The ability of the subject to retain an infusion of saline was also not different between botulinum toxin and placebo phases. The balloon expulsion time was more prolonged after botulinum toxin injection, but this value was not significantly different. Finally, we did not observe any changes in the pudendal nerve terminal motor latency time suggesting that botulinum toxin did not induce pudendal neuropathy, at least in the short term. The lack of significance for some of these physiological parameters could be due to type II error, given the small number of subjects and the large standard deviations. However, generally the anal sphincter function, rectal sensory function and neuromuscular function were well preserved after botulinum toxin injection, suggesting that from a physiological standpoint this compound is safe.
Anal endosonography also showed no morphological changes in the sphincter apparatus after each injection. This is the first study that has assessed these changes following botulinum toxin. Whether some of the transient neuromuscular changes and possible edema and mild inflammation that followed botulinum toxin injections led to some delay in the balloon expulsion time or altered neuromuscular condition requires further study.
The limitations of this study include the small number of subjects who were examined and the cross over study design. As discussed, the study was terminated earlier following an interim analysis. We chose a 3 month period before crossing over our subjects, because the effects of botulinum toxin can last up to 12 weeks,8,12 and also to allow sufficient time for the effects of the drug to wear off. As evident from our results, there was less likelihood of any carry over effect, because both subjectively and objectively there was no change after botulinum toxin. Thus, the crossover design was unlikely to play a significant role in the outcome of our results. However, three subjects dropped out of the study, and even, when excluded, in a per protocol analysis (data not reported), there was no difference between the two groups.
In conclusion, we found that botulinum toxin injection into the anal sphincter was safe, but it was not effective in improving anorectal pain caused by levator ani syndrome.
Acknowledgments
This study was supported in part by an ACG clinical research grant and by Allergan Pharmaceuticals, CA who provided Botulinum toxin solutions and needles. Dr. Rao was also supported by NIH 2RO1-DK0570100-06A.
The authors acknowledge the superb secretarial assistance of Mrs. Kimberly Klein and technical assistance of Ms. Mary Stessman, RN and Ms. Joan Kempf, LPN. Portions of this work were presented at the annual ACG meeting and published as an abstract in Am J Gastroenterol 2004;99:S114.
Contributor Information
Satish SC Rao, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City IA, USA.
Jessica Paulson, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City IA, USA.
Mariana Mata, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City IA, USA.
Bridget Zimmerman, Clinical Research Center, University of Iowa Carver College of Medicine, Iowa City IA, USA.
References
- 1.Whitehead WE, Wald A, Diamant NE, et al. Functional disorders of the anus and rectum. Gut. 1999;45 2:1155–9. doi: 10.1136/gut.45.2008.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant SR, Salvatti EP, Rubin RJ, et al. Hereditary internal anal sphincter myopathy causing proctalgia fugax and constipation. Gastroenterology. 1991;100:805–10. doi: 10.1016/0016-5085(91)80030-d. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 4.Kamm MA, Hoyle CHV, Burleigh DE, et al. Hereditary internal anal sphincter myopathy causing proctalgia fugax and constipation. Gastroenterology. 1991;100:805–10. doi: 10.1016/0016-5085(91)80030-d. [DOI] [PubMed] [Google Scholar]
- 5.Rao SSC, Hatfield RA. Paroxysmal anal hyperkinesis: A characteristic feature of proctalgia fugax. Gut. 1996;39:609–612. doi: 10.1136/gut.39.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckardt VF, Dodt O, Kanzler G, et al. Anorectal function and morphology in patients with sporadic proctalgia fugax. Dis Colon Rectum. 2004;39:755–62. doi: 10.1007/BF02054440. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Trabuco E. Functional and Chronic anorectal and pelvic disorders. Gastroenterol Clin North Am. 2008;37:685–96. doi: 10.1016/j.gtc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J, Brin MF. Therapeutic uses of botulinum toxin. N Engl J Med. 1991;324:1186–94. doi: 10.1056/NEJM199104253241707. [DOI] [PubMed] [Google Scholar]
- 9.Blasi J, Chapman ER, Linke, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–3. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 10.Alderson K, Holds JB, Anderson RI. Botulinum induced alteration of nerve-muscle interactions in the human orbicularis oculi following treatment for blepharospasm. Neurology. 1991;41:1800–05. doi: 10.1212/wnl.41.11.1800. [DOI] [PubMed] [Google Scholar]
- 11.Scott AB, Suzuki D. Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov Disorders. 1988;3:333–5. doi: 10.1002/mds.870030409. [DOI] [PubMed] [Google Scholar]
- 12.Pasricha PJ, Ravich WJ, Hendrix TR, et al. Intrasphincteric botulinum toxin for the treatment of achalsia. N Engl J Med. 1995;332:774–8. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 13.Fishman VM, Parkman HP, Schiano TD, et al. Symptomatic improvement in achalsia after botulinum toxin injection of the lower esophageal sphincter. Am J Gastroenterol. 1996;91:1724–30. [PubMed] [Google Scholar]
- 14.Gui D, Cassetta E, Anastasio G, et al. Botulinum toxin for chronic anal fissure. Lancet. 1994;344:1127–8. doi: 10.1016/s0140-6736(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 15.Jost WH, Schimrigk K. Use of botulinum toxin in anal fissure. Dis Colon Rectum. 1993;344:1127–8. doi: 10.1007/BF02050639. [DOI] [PubMed] [Google Scholar]
- 16.Hallan RI, Williams NS, Melling J, et al. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet. 1988;2:714–7. doi: 10.1016/s0140-6736(88)90188-2. [DOI] [PubMed] [Google Scholar]
- 17.Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Annals of Otology, Rhinology & Larngology. 1994;103:31–5. doi: 10.1177/000348949410300105. 1994. [DOI] [PubMed] [Google Scholar]
- 18.Pasricha PJ, Miskovsky EP, Kalloo AN. Intrasphincteric injection of botulinum toxin for suspected sphincter of Oddi dysfunction. Gut. 1994;35:1319–21. doi: 10.1136/gut.35.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SSC. Dyssynergic Defecation and Biofeedback Therapy. Gastroenterol Clin N Am. 2008;37:569–586. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao SSC, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 21.Rattan S, Regan RF, Patel CA, et al. Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology. 2005;129:1954–66. doi: 10.1053/j.gastro.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Arroyo A, Perez F, Serrano P, et al. Long-term results of botulinum toxin for the treatment of chronic anal fissure: prospective clinical and manometric study. doi: 10.1007/s00384-004-0644-y. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis SK, Abbott JA, Lenart MB, et al. Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust N Z J Obstet Gynaecol. 2004;44:46–50. doi: 10.1111/j.1479-828X.2004.00163.x. [DOI] [PubMed] [Google Scholar]


