Abstract
Diffuse peritubular capillary C4d deposition in renal allograft biopsies is associated with donor specific antibodies (DSA) and graft failure. The significance of focal C4d+ is unclear. We reviewed 368 biopsies from 301 patients performed for renal dysfunction or proteinuria over 5-years. Diffuse C4d+, focal C4d+, and C4d- detected by immunofluorescence occurred in 9.5%, 20.9% and 69.4% of biopsies, respectively. Patients were similar in gender, age, cause of renal disease, donor source, HLA mismatch, serum creatinine at baseline and interval from transplantation to biopsy. Diffuse and focal C4d+ were associated with acute cellular rejection (p<0.001). Transplant glomerulopathy was associated with diffuse C4d+. DSA at the time of biopsy, were positive in 79.3% of diffusely C4d+ patients, 68.8 % of those with focal C4d+ (P=0.27) and 9.9% of patients with C4d- (P< 0.001, compared to either the focal or diffuse groups, respectively). Allograft survival at 40 months was lower in diffuse C4d+ compared to the C4d- group (P=0.014), but not when compared to the focal C4d+ group. There was a clear trend towards worse graft survival in patients with focal C4d+ in this time interval, but focal C4d+ compared to both diffuse C4d+ and C4d-groups was not statistically significant (p=0.08).
Keywords: Kidney, allograft, C4d, focal, diffuse, acute rejection, donor-specific antibodies
Introduction
Antibody mediated rejection (AMR) may occur early or late, may be acute or chronic and is associated with poor renal allograft function and survival. Three AMR conditions are now recognized: hyperacute, subacute and chronic AMR [1]. Diagnosis of acute AMR requires a) histopathological evidence of either acute tubular injury (with no other identifiable cause for it), glomerulitis, endotheliitis, or capillaritis with neutrophil or mononuclear cell infiltrate, capillary thrombosis b) serologic evidence of circulating antidonor antibodies and c) diffuse C4d positivity in peritubular capillaries (PTC) [1–4]. Implementation of C4d immune detection in renal allograft biopsies in the last decade has greatly facilitated the diagnosis of AMR. For example, diffuse PTC C4d+ staining by IF on frozen sections, defined as >50% of peritubular capillaries staining positive, is highly associated with serum donor specific anti-HLA antibodies (DSA) and is reported to have 95% sensitivity and 96% specificity for circulating DSA [5]. Diffuse PTC C4d+ correlates strongly with graft dysfunction and with chronic antibody mediated rejection, reportedly detected in about 50% of cases with transplant glomerulopathy [5–8]. However, the significance of focal C4d+ defined as <50% of PTC staining positive, remains poorly understood. Most published studies either exclude biopsies with focal C4d+ or group these with diffusely positive C4d+ biopsies. Some authors suggest that graft loss occurs more frequently in patients with diffuse C4d+ compared to focal C4d+ and C4d− groups [9, 10]. Other studies show that patients have inferior one year graft survival in the setting of either diffuse or focal C4d+ staining [11, 12]. Notably, such results are derived from patients on a variety of immunosuppressive protocols and using different methodologies of C4d detection in kidney allografts (immunofluorescence versus immunohistochemistry). The clinical significance of focal C4d+ in allograft recipients remains controversial and better understanding of focal C4d+ is needed, emphasized in the most recent Banff (2007) report [2].
In this study, we evaluated all renal allograft biopsies obtained for cause performed at our center over a period of five years. Our aim was to evaluate biopsies with focal versus diffuse C4d+, compare to C4d− biopsies, correlate with histological findings, presence or absence of donor specific antibodies (DSA) and graft survival and function.
MATERIALS AND METHODS
Study population and biopsies
We retrospectively reviewed all kidney allograft biopsies performed at Washington University/Barnes-Jewish Hospitals between August 2002 and June 2007. The initial date was chosen based on the implementation of routine C4d staining in allograft biopsies at our institution. The closing date was selected to allow a follow-up period at least six months after biopsy. The study was approved by the Institutional Review Board at Washington University in St Louis (IRB 05–0951). All samples were indication biopsies for graft dysfunction and / or significant proteinuria (>1 g/24h). There were no ABOincompatible transplant biopsies included in the study cohort. A total of 428 renal allograft biopsies were performed during this period. Of these, 368 biopsies (from 301 patients) were included in the study based on the following criteria: (1) availability of adequate tissue for histological diagnosis and C4d staining (2) at least 6 month of clinical follow-up available after biopsy, including all previous and subsequent allograft biopsies. Those with assessment of DSA were further analyzed.
Follow-up and demographic data were obtained from our computerized renal transplant data base (OTTR, 5.2.5 Omaha, NE). Specific information not available in this data base was additionally obtained from chart review.
The following clinical data were analyzed: patient demographics, cause of renal disease, history of previous transplants, donor source, HLA mismatching, time from transplantation to biopsy, renal function baseline at the time of biopsy and change from baseline to the end of follow-up period, and graft failure, defined as return to permanent dialysis or re-transplantation. Follow-up of all patients was sought either to final graft failure or to January 2008 in the surviving grafts.
More than 90% received induction with rabbit-antithymocyte globulin (Thymoglobulin, Genzyme, Cambridge, MA). Patients with a 2-haplotype living related renal allograft did not receive induction therapy. In the majority of patients maintenance immunosuppression included a calcineurin inhibitor, antimetabolite and prednisone. The majority received tacrolimus. All patients were included regardless of induction therapy; the questions of interest were the outcomes of patients with no versus focal versus diffuse C4d staining on indication biopsies.
C4d staining
C4d staining was performed by immunofluorescence (IF) on frozen sections using a standard protocol. Briefly, monoclonal antibody to C4d (Serotec, Brentwood, New Hampshire, U.S.A) was used in a 1:40 dilution and detection was with Fluorescein isothiocyanate (FITC) (rabbit anti-mouse from DAKO, Carpinteria, CA). Slides were counterstained with 3% Evan’s Blue (from Health Scientific, Saint Louis). Evan’s Blue decreases FITC background which appears red under the fluorescent light.
Biopsies were categorized in 3 groups based on the extent of C4d staining as follows: Diffuse: PTC C4d+ involving >50% of surface area. Focal: PTC C4d+ involving 10%–50% surface area and Negative: <10% of surface area stained. Definitions were based on the revised Banff criteria in which 10% of PTC staining is considered negative when performed by immunofluorescence [2]. Patients were considered C4d+: if at least one biopsy demonstrated focal or diffuse positivity. The index biopsy was the one which showed the most severe C4d+ staining. There were 2 patients who initially were focally C4d+ and later became diffusely C4d+ and 3 patients who initially had diffuse C4d+, but later became focally C4d+. These cases were categorized as diffuse. Those patients considered C4d negative had <10% C4d staining on all biopsies performed.
Histopathology
All renal biopsies were evaluated by light microscopy and immunofluorescence. Electron microscopy (EM) was available in biopsies suspected of glomerular disease, n=56 (15.2%). Adequacy criteria included presence of at least one artery and 7 or more glomeruli. All biopsies were reevaluated without knowledge of the clinical outcome, or immunofluorescence results and histopathological data and classified according to revised Banff 1997 criteria for acute cellular rejection (ACR), AMR, transplant glomerulopathy (TG), capillaritis, glomerulitis and interstitial fibrosis/tubular atrophy (IF/TA) [2, 3].
Transplant glomerulopathy (TG) was defined as glomerular capillary wall thickening on light microscopy showing widening of the subendothelial space and or formation of new lamina densa layers by electron microscopy in the absence of electron dense deposits [13]. Chronic calcineurin inhibitor toxicity was diagnosed when hyaline arteriolar thickening, “striped” pattern of fibrosis and or isometric vacuolization of tubular epithelial cells was present [14]. Plasma cell rich infiltrate was defined when at least 300 plasma cells per 20 high power fields were identified [15].
The presence of capillaritis as a histopathological surrogate marker of humoral rejection was examined in all biopsies. Scoring was performed as previously described [16]. Recurrent glomerular disease was considered when patients had pre-transplant histological diagnosis of the same pathology; if glomerular disease in the allograft was different including TG, it was considered de novo disease.
Detection of HLA Specific Antibodies
Results of donor-specific antibodies were limited to the analysis of 132 patients, who had sera obtained at the time of biopsy or within 7 days of biopsy. The presence of HLA class I and class II antibodies was initially determined using enzyme-linked immunosorbent assay (ELISA, One-Lambda) and specificity was confirmed by flow cytometry (Luminex, Austin, TX, USA). Assays were performed according to manufacturer’s instructions.
Statistical analysis
Descriptive statistical values were presented as means with standard deviation (SD). Categorical variables were summarized as counts and percentages. Categorical data were compared with the use of Chi-square, continuous variables using Fisher’s exact test and ANOVA analysis, Tukey HSD was used for post hoc analysis, correlations were calculated using Pearson’s correlation. The cumulative survival curve was obtained by the Kaplan-Meier method, and the difference between the survival curves was compared by the log-rank test. Cox regression was also used for multivariate analysis. The SPSS software package (Version 14.0, SPSS Inc, Chicago, IL) was used for statistical analysis and calculation of cross tables. A p value of less than 0.05 for two-sided univariate tests was considered significant.
RESULTS
Patient demographics, C4d staining, and clinical findings
There were no significant differences among patient demographic variables and the three C4d staining categories. This included cause of end stage renal disease, history of previous transplant, donor source, HLA mismatching, time from transplant to biopsy, mean serum creatinine level at baseline, time of biopsy, and change from baseline to the end of follow-up among those who had not returned to dialysis (Table 1).
Table 1.
Patient demographics compared to C4d status.
| Variable | DIFFUSE, N=32 | FOCAL, N=57 | NEGATIVE, N=212 | P VALUE* |
|---|---|---|---|---|
| Age at biopsy (years) | 43.9± 14.8 | 45.2±12.8 | 48.8±14.4 | 0.07 |
| Gender (male:female) | 18:14 | 36:21 | 130:82 | 0.82 |
| Primary kidney disease | ||||
| Glomerular disease | 14(43.8%) | 14(24.6%) | 70(33%) | 0.17 |
| Diabetes mellitus | 4(12.5%) | 12(21.1%) | 39(18.4%) | 0.60 |
| Hypertension | 5(15.6%) | 9(15.8%) | 38(17.9%) | 0.90 |
| Polycystic Kidney Dis | 1(3.1%) | 9(15.8%) | 19(9%) | 0.13 |
| Congenital/other | 8(25%) | 13(22.8%) | 46(21.7%) | 0.91 |
| Re-transplantation | 1(3.0%) | 10(17.5%) | 19(9.0%) | 0.06 |
| Type of graft (living: deceased) | 14:18 | 23:34 | 109:103 | 0.31 |
| Mean HLA mismatch | 3.6±1.5 | 3.5±.1.6 | 3.1 ±1.8 | 0.18 |
| HLA DR mismatch | 1.2±0.6 | 1.0±0.7 | 1.0±−0.7 | 0.29 |
| Time after transplant to biopsy (months) | 60.9±63.8 | 53.6±54.7 | 49.1±53.8 | 0.49 |
| Mean baseline serum creatinine (mg/dL) | 1.61±.56 | 1.63±.57 | 1.66±.75 | 0.84 |
| Mean serum creatinine at biopsy (mg/dL) | 3.0±1.5 | 3.16±1.3 | 2.99−±1.7 | 0.48 |
| Mean serum creatinine change in mg/dL (among patients who did not return to dialysis) | 0.82±0.8 (18) | 0.59±1.0(32) | 0.47±0.9(137) | 0.30 |
not significant in all groups
C4d staining and histopathological findings
Of the 301 patients included in this analysis who underwent biopsy, 247 had one biopsy, 42 patients had two biopsies, 11 had three, and one patient had four biopsies. We identified 112/368 (30.4%) biopsies from 89/301 patients (29.6%) with C4d+ staining. Of these, 9.5% had diffuse C4d+ (35/368) and 20.9% had focal C4d+ (77/368). These correspond to 32 and 57 patients respectively. C4d− biopsies were present in 256/368 (69.4%) biopsies, representing 212 patients. Representative sections from the three C4d patterns are shown in Figure 1. The main histopathological diagnoses in all three groups are shown in Table 2. Acute cellular rejection was present in 111/368 (30.2%) biopsies. The frequency of acute cellular rejection was significantly less in C4d- biopsies compared to either diffuse or focal C4d+ biopsies (23.8% versus 40% and 49.4% respectively, p<0.001).
Figure 1. Immunofluorescence staining for C4d in renal allograft biopsies.
A: Diffuse C4d+ involving >50% of peritubular capillaries. In this case almost 100% of surface area is involved (x200). B: Focal C4d+ is seen in <50% of surface area (x400). C: Negative C4d staining (x200).
Table 2.
Histopathology and C4d staining of biopsies
| Biopsy findings | C4d staining | Total (n=368) |
P value | ||
|---|---|---|---|---|---|
| Diffuse(n=35) | Focal(n=77) | Negative(n=256) | |||
| Normal | 2 (5.5%) | 4(5.1%) | 22(8.6%) | 28(7.6%) | 0.56 |
| All ACR | 12(40%) | 38(49.4%) | 61(23.8%)* | 111(30.2%) | <0.001 |
| IA/IB | 12(34.2%) | 24(31.2%) | 47(18.4%) | 83(22.5%) | 0.01 |
| IIA/IIB | 2 (5.7%) | 13(16.8%) | 13(5%) | 28(7.6%) | 0.01 |
| III | 0(0%) | 1 (1.3%) | 1(0.4%) | 2(0.4%) | 0.57 |
| Borderline | 2(5.7%) | 4(5.1%) | 10(3.3%) | 16(4.3%) | 0.81 |
| Plasma cell rich infiltrate | 3(8.5%) | 3(5.7%) | 9(4.9%) | 15(4.1%) | 0.49 |
| Glomerular diseases | 5(14.3%) | 9(11.7%) | 43(16.8%) | 57(15.5%) | 0.54 |
| Transplant Glomerulopathy | 7(20%) | 4(5.1%)* | 14(5.4%)* | 25(6.7%) | 0.01 |
| IF/TA | 2(5.7%) | 17(22%) | 42(16.4%) | 61(16.5%) | 0.11 |
| CNI toxicity | 5(14.3%) | 10(13.1%) | 56(21.8%) | 71(19.3%) | 0.16 |
| ATI | 0(0%) | 4(5.1%) | 26(10.0%) | 30(8.3%) | 0.08 |
| AIN | 3(8.5%) | 4(5.1%) | 25(9.8%) | 32(8.7%) | 0.45 |
| Interstitial hemorrhage | 3(8.5%) | 2(2.6%) | 3(1.2%)* | 8(2.1%) | 0.02 |
| Capillaritis | 22(62.9%) | 45(58.4%) | 68(26.6%)* | 135(36.7%) | <0.001 |
| Glomerulitis | 20(57.1%) | 26(33.8%)* | 46(18%)* | 92(25%) | <0.001 |
ACR: acute cellular rejection, IF/TA: interstitial fibrosis/ tubular atrophy, CNI: calcineurin inhibitor, ATI: acute tubular injury, AIN: acute interstitial nephritis
Significantly less in these groups
Transplant glomerulopathy was more common in patients with diffuse C4d+ than in either C4d- or focal C4d+ (20% versus 5.4% versus 5.1%, p=0.01). Cortical necrosis and interstitial hemorrhage were noted in 3 of the 35 (8.5%) biopsies with diffuse C4d+, in 2/77 (2.6%) with focal C4d+, and 3/256 (1.2%) in C4d- biopsies. The difference was only significant between diffuse C4d+ vs C4d negative groups (p=0.02).
Capillaritis was significantly more common in both diffuse and focal C4d + biopsies than in C4d− biopsies (p<0.001), but there was no statistically significant difference when focal C4d+ was compared to diffuse C4d+ (p=0.88) (Table 2).
The frequency of glomerulitis was significantly less in C4d- biopsies compared to either diffuse or focal C4d+ biopsies (18% versus 33.8% and 57.1% respectively, p<0.001). There was also significant difference between focal C4d+ and diffuse C4d+ (p=0.02) demonstrating intermediate incidence and severity of glomerulitis in focal C4d+.
Glomerulitis and capillaritis significantly correlated with C4d+ (r = 0.28, p <0.001 and r = 0.30, p < 0.001, respectively). There was significant correlation between capillaritis and glomerulitis (r=0.74, p<0.001), as well.
Glomerular disease was diagnosed in 57/368 (15.5%) of all biopsies from 48/301 (16%) patients (one without EM). Of these patients 28/48 (58%) had recurrent glomerular disease, there were 5 patients with diffuse C4d+, 6 with focal C4d+ and 17 C4d− patients There was no statistical difference based on C4d staining in recurrent (p=0.36) or de novo glomerular disease (p=0.11).
HLA data
Of the 132 patients (132/301, 43.8%) who were tested for DSA at the time of biopsy there were 29 patients with diffuse C4d+, 32 with focal C4d+ and 71 with C4d− staining. Donor-specific antibodies were detected more frequently in diffuse or focal C4d+ groups than in C4d− patients: 79.3% (23/29) versus 68.8% (22/32) versus 7/71 (9.9%) respectively, p<0.001. There was no difference between focal and diffuse C4d+ groups in regards to presence of serum DSA (p=0.27). The findings are summarized in Table 3. All three criteria for antibody mediated rejection (C4d, histology and DSA) were present in 58.6% (17/29) of diffuse C4d + biopsies and 50% (16/32) of focal C4d+ (p=0.33).
Table 3.
C4d staining and donor-specific antibodies
| Variable | Diffuse C4d+ (N=29) | Focal C4d+ (N=32) | C4d− (N=71) |
|---|---|---|---|
| DSA+ | 23/29 (79.3%) | 22/32 (68.8%) | 7/71 (9.9%) |
| Class I | 6 (20.7%) | 5(15.6%) | 0 |
| Class II | 11 (37.9%) | 10 (31.3%) | 3(4.2%) |
| Class I and II | 6 (20.7%) | 7 (21.9%) | 4 (5.7%) |
Given that only a portion of the patients in the cohort had available DSA, we analyzed outcomes in patients with both C4d and DSA data available separately, in addition to all the patients in the study. There were significant differences between the three groups when those with positive DSA during the follow up period who lost the allograft and returned to dialysis were analyzed: 12/29 (41.4%) with diffuse C4d +, 12/32 (37.5%) with focal C4d+ and 14/71 (19.7%) of C4d− patients returned to dialysis (p=0.043). Post hoc analysis showed that the difference was statistically significant only between diffuse C4d+ and C4d− groups. Cumulative renal allograft survival was significantly lower in the DSA+ group than in that in DSA− group (p=0.042).
The presence of donor specific antibodies correlated significantly with TG (r=0.36, p<0.01). Of 11 patients with TG who had DSA data available, 10/11 (91%) had positive DSA results. Of these 11 patients, 5 (45%) had diffuse C4d+, four (36%) had focal C4d+ and 2 (18%) were C4d−. Thus, of those with DSA screening available only one patient with TG was both DSA and C4d negative.
C4d and clinical outcomes
During the follow-up period 14/32 (43.8%) of patients with diffuse C4d+, 25/57 (43.9%) with focal C4d+, and 63/212 (29.7%) with C4d− biopsies lost their grafts.
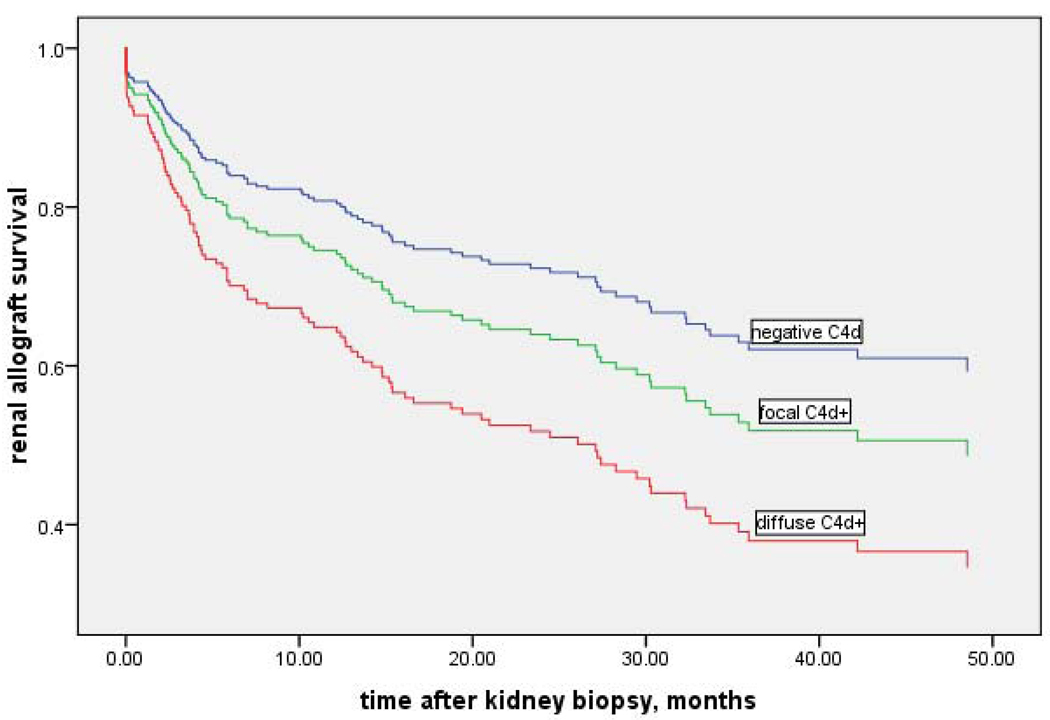
To analyze the impact of C4d deposition on graft function we compared the renal graft survival rate without dialysis or repeat transplantation between the three groups (Figure 3). In the Kaplan-Meier analysis the Log Rank test of group differences was significant P=0.038, indicating that C4d− patients have better graft survival from the time of biopsy than those with focal or diffuse C4d+ staining (mean 43.4 months vs. 35.0 months vs. 22.5 months, respectively). The survival difference was only significant in diffuse C4d + versus the C4d− group (p=0.014). Focal C4d+ survival was not significantly different compared to diffuse C4d+ group (P=0.25) or to the C4d− group (P=0.09).
Figure 3. Kaplan -Meier curve.
Cumulative renal allograft survival at 40 months for diffuse C4d+ vs focal C4d+ vs C4d−was 12 % vs 51% vs 64%. Statistical difference was found between diffuse C4d+ and both focal C4d+ and C4d− groups (p<0.01), but not when focal C4d+ was compared with C4d− biopsies (p=0.08).
The survival at 20 month interval for diffuse vs focal C4d+ was 62% vs 63% vs 72%. There was no statistical significance in the 3 groups at 20 months, (p=0.32). Specifically, the p value for focal C4d+ vs C4d− was p=0.69. The survival at 30 months was as follows: diffuse C4d+ vs focal C4d+ vs C4d− was 37% vs 58% vs 67%; statistical significance was found only between diffuse C4d+ and C4d− groups (p<0.01); focal C4d+ vs C4d – was not statistically significant (p= 0.21).
At 40 months, graft survival for diffuse C4d+ vs focal C4d+ vs C4d− were 12% vs 51% vs 67% respectively; diffuse C4d+ and both focal C4d+ and C4d− were statistically significant (p<0.01). Focal C4d+ vs C4d− was not statistically significant (p=0.08). However, these results demonstrate a clear trend towards worse graft survival in patients with focal C4d+ in this time interval.
To evaluate the most important independent risk factors for the survival of renal allografts multivariate analysis was done using the Cox regression model which included all index biopsies and independent variables (age, gender, acute cellular rejection, HLA mismatch, capillary deposition of C4d, allograft type, transplant number). Only peritubular capillary C4d deposition had a significant impact on graft outcome. Using Cox regression, the hazard ratio of graft loss was 2.03 (1.13–3.64) for diffuse C4d+ compared with focal C4d+ and C4d negative combined (p=0.017).
Graft survival following diagnosis of acute cellular rejection in diffuse C4d+, focal C4d+ and C4d− groups was also compared. Only biopsies with an acute rejection episode were used in the evaluation. There were 12/32 (37.5%) patients in the diffuse C4d+ group, 26/57 (45.6%) in the focal C4d+ group and 51/212 (24.1%) patients in C4d− group with acute rejection. The mean time from an acute cellular rejection episode to graft loss was 27.6 months in the diffuse C4d+ group, 39.3 month in the focal C4d+ group and 38.9 month in the C4d− group, which was not statistically significant.
Discussion
Diffuse C4d+ staining in renal biopsies is associated with antibody mediated rejection [17] and circulating DSA and correlates with graft dysfunction [5, 7, 8]. But there are limited data on focal C4d+ staining in renal biopsies and correlation with serum DSA and patient outcomes. Most reports either exclude focal C4d+ biopsies or group them with diffuse C4d+ biopsies. In several previous studies [9, 11] where focal C4d+ results were analyzed, DSA data were not available. Historically the correlation between concurrent circulating DSA and C4d staining is variable.
In this study, we compared the clinical and pathological findings in patients with diffuse C4d+, focal C4d+ and C4d− biopsies in all renal allograft biopsies performed at our center over 5 years and included DSA when available (nearly 44% of biopsies). We found correlation of both focal and diffuse C4d+ with presence of DSA. DSA was detected more often in focal C4d+ group compared to C4d− group, but was not significantly different when compared with diffuse C4d+. Donor specific antibody was only present in 7/71 (9.9%) patients in the C4d− group. Our results are in part consistent with previous reports. [5, 10, 18]. We defined focal C4d+ as 10–50% of peritubular capillaries staining consistent with the recent recommendations by the most current Banff 2007 classification [2]. The same criteria were used by Worthington et al [10], with C4d staining using paraffin blocks. But in other studies C4d+ was defined as staining in 25 %–50% of peritubular capillaries, making the results and outcomes in focal C4d+ groups difficult to compare [19, 20].
The prevalence of C4d+ patients reported in the literature varies. This is in part because, different criteria to define C4d positivity as well as different methods of C4d detection (IF versus IHC, frozen versus paraffin sections) for C4d+ were utilized. Most studies report C4d+ prevalence 17% to 37% [12, 20–23]. Others find C4d+ in over 60 % of all evaluated biopsies [24, 25]. In our study C4d+ in both focal and diffuse groups is 30.4%, which is consistent with the majority of studies.
The presence of C4d+ in peritubular capillaries has been strongly associated with chronic antibody mediated rejection and is present in about 50% of reported transplant glomerulopathy cases [6].
Transplant glomerulopathy was found infrequently in our study, but was more prevalent in the patients with diffuse C4d+. That 11/25 (44%) of renal allograft biopsies with evidence of TG had some degree of C4d+, suggests that TG is immune mediated as others have advocated [26, 27]. Transplant glomerulopathy is also strongly associated with circulating antibodies to donor HLA and has a poor prognosis [24]. A recent study reported that 64% of patients with TG have circulating DSA [28]. We found that 10/11 (91%) patients with TG on biopsy had DSA+ and only 2 of these patients were from the C4d− group.
Although numerous studies report that C4d+ patients have inferior graft survival compared to C4d – patients [22, 23, 29], the distinction between focal and diffuse C4+ patients is unclear and controversial. In the present study, renal allograft function in both diffuse and focal C4d+ groups deteriorated more rapidly compared to the C4d− group; focal C4d+ was intermediate between those with diffuse and C4d− biopsies, even though not statistically significant. The light microscopic findings were similar among focal and diffuse C4d+ biopsies including morphologic evidence for AMR such as ATN, glomerulitis and capillaritis. Our results support recent studies which showed that biopsy findings and clinical course in patients with focal PTC C4d+ staining are similar to those with diffuse C4d+ [11, 19, 20]. However others have shown that graft loss occurs more frequently in patients with diffuse C4d+ as compared to focal C4d+ and C4d− groups 1 year after diagnosis [9, 10]. Such differences may reflect slightly different histopathological selection criteria. For example, in the study by Worthington etal, 7 patients with ATN only were included; of these 2 had focal C4d+, 1 had diffuse C4d+ and 4 were C4d− (10).
Our study is also unique in that we evaluated the incidence of recurrent or de novo glomerulonephritis as it relates to C4d staining. The strength of the present study is that it evaluates a relatively large group of patients using the revised Banff criteria for focal C4d + evaluation and IF staining. We were able to correlate separately focal and diffuse C4d+ with DSA and patient outcomes including recurrent or de novo glomerulonephritis.
The limitations of our study are: that it is retrospective and therapies were not identical for treatment of rejection, DSA data were available in 44% of patients and C4d− patients were less commonly assessed for DSA. Only 34 % C4d- patients were tested for DSA, while 90% diffuse C4d+ and 56% focal C4d+ were tested. This relates to the fact that in earlier years, serum samples were obtained only when preliminary pathologic diagnosis on biopsy was suggestive of AMR. More recently, DSA screening is ordered routinely in virtually every patient undergoing an allograft biopsy.
Although survival data showed significant difference only between diffuse C4d + and the C4d− group, there was no difference between survival and focal C4d+ and diffuse C4d+. There was a clear trend towards worse outcome in focal C4d+ versus the C4d− group. Lack of statistical significance most likely represents a Type II error due to small sample size. In addition, longer follow-up period is needed to determine whether this is true. The mean time interval of graft survival from the time of biopsy was related to the degree of C4d+: it was longest in the C4d− group, shorter in the focal C4d+ group and shortest in the diffuse C4d+ group. These findings are similar to those reported by Magil et al [11] who calculated the odds for worsening of renal function occurring at 48 months after transplantation and found that it was dependent on the intensity of C4d staining(diffuse>focal>negative).
Given our results of poor graft survival in the focal C4d+ group, high correlation of focal C4d+ with DSA, and an overall similar histology and graft survival as compared to diffuse C4d+, we propose that not only diffuse, but also focal C4d+ is a poor prognostic factor for AMR and graft survival. The impact of focal C4d+ biopsies should be considered in further treatment strategies.
Figure 2. Cox proportional hazard curve.
Probability of graft survival of the three C4d groups shows worse survival in diffuse C4d+ and intermediate survival in focal C4d+ compared to the C4d− group, controlled for acute cellular rejection, gender and transplant type and number of transplants.
Acknowledgement
This work was supported in part by NIH P30 DK079333
References
- 1.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 2.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J.Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria-an addition to the Banff 97 classification of renal allograft rejection. Am J. Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Mauiyyedi S, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology and pathologic classification. J Am Soc Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 6.Rotman S, Collins AB, Colvin RB. C4d detection in allografts: current concepts and interpretation. Transplant Rev. 2005;19:65–77. [Google Scholar]
- 7.Crespo M, Pascual M, Tolkoff-Rubin N, et al. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;15(71):652–658. doi: 10.1097/00007890-200103150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Rahman MH, Racusen LC, et al. C4d and C3d staining in biopsies of ABO-and HLA-incompatible renal allografts: correlation with histologic findings. Am J. Transplant. 2006;6:1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 9.Poduval RD, Kadambi PV, Josephson MA, et al. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation. 2005;79:228–235. doi: 10.1097/01.tp.0000148987.13199.10. [DOI] [PubMed] [Google Scholar]
- 10.Worthington JE, McEwen A, McWilliam LJ, et al. Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation. 2007;83:398–404. doi: 10.1097/01.tp.0000251430.11723.b6. [DOI] [PubMed] [Google Scholar]
- 11.Magil AB, Tinckam KJ. Focal peritubular capillary C4d deposition in acute rejection. Nephrol Dial Transplant. 2006;21:1382–1388. doi: 10.1093/ndt/gfk028. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz M, Regele H, Schillinger M, et al. Risk factors for capillary C4d deposition in kidney allografts: evaluation of a large study cohort. Transplantation. 2004;15(78):447–452. doi: 10.1097/01.tp.0000128344.94808.03. [DOI] [PubMed] [Google Scholar]
- 13.Ivanyi B. Transplant capillaropathy and transplant glomerulopathy: ultrastructural markers of chronic renal allograft rejection. Nephrol Dial Transplant. 2003;18:655–660. doi: 10.1093/ndt/gfg139. [DOI] [PubMed] [Google Scholar]
- 14.Mihatsch MJ, Ryffel B, Gudat F. The differential diagnosis between rejection and cyclosporine toxicity. Kidney Int Suppl. 1995;52:S63–S69. [PubMed] [Google Scholar]
- 15.Charney DA, Nadasdy T, Lo AW, Racusen LC. Plasma cell-rich acute renal allograft rejection. Transplantation. 1999;68:791–797. doi: 10.1097/00007890-199909270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gibson IW, Gwinner W, Bröcker V, et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clinicopathological correlates. Am J. Transplant. 2008;8:819–825. doi: 10.1111/j.1600-6143.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- 17.Watschinger B, Pascual M. Capillary C4d deposition as a marker of humoral immunity in renal allograft rejection. J Am Soc Nephrol. 2002;13:2420–2423. doi: 10.1097/01.asn.0000029941.34837.22. [DOI] [PubMed] [Google Scholar]
- 18.Böhmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 19.Mengel M, Bogers J, Bosmans JL, et al. ESPRIT group. Incidence of C4d stain in protocol biopsies from renal allografts: results from a multicenter trial. Am J. Transplant. 2005;5:1050–1056. doi: 10.1111/j.1600-6143.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 20.Nickeleit V, Zeiler M, Gudat F, et al. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242–251. doi: 10.1681/ASN.V131242. [DOI] [PubMed] [Google Scholar]
- 21.Regele H, Böhmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–2380. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 22.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura N, Tomita M, Hasegawa M, et al. Complement C4d deposition in transplanted kidneys: preliminary report on long-term graft survival. Clin Transplant. 2005;19(Suppl 14):27–31. doi: 10.1111/j.1399-0012.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 24.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 25.David-Neto E, Prado E, Beutel A, et al. C4d-positive chronic rejection: a frequent entity with a poor outcome. Transplantation. 2007;84:1391–1398. doi: 10.1097/01.tp.0000288807.52520.5e. [DOI] [PubMed] [Google Scholar]
- 26.Vongwiwatana A, Gourishankar S, Campbell PM, et al. Peritubular capillary changes and C4d deposits are associated with transplant glomerulopathy but not IgA nephropathy. Am J.Transplant. 2004;4:124–129. doi: 10.1046/j.1600-6143.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 27.Sijpkens YW, Joosten SA, Wong MC, et al. Immunologic risk factors and glomerular C4d deposits in chronic transplant glomerulopathy. Kidney Int. 2004;65:2409–2418. doi: 10.1111/j.1523-1755.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 28.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7:1743–1752. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 29.Regele H, Exner M, Watschinger B, et al. Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16:2058–2066. doi: 10.1093/ndt/16.10.2058. [DOI] [PubMed] [Google Scholar]