Abstract
To determine the contributions of galactose and fructose to glucose formation, six subjects (26±2 y; BMI 22.4±0.2 kg/m2) (Mean±SE) were studied during fasting conditions. Three subjects received a primed constant intravenous infusion of [6,6-2H2]glucose for 3 h followed by oral bolus ingestion of galactose labeled to 2% with [U-13C]galactose (0.72 gm/kg); the other three subjects received a primed constant intravenous infusion of [6,6-2H2]glucose followed by either a bolus ingestion of fructose alone (0.72 gm/kg) (labeled to 2% with [U-13C]fructose) or co-ingestion of fructose (labeled with [U-13C]fructose) (0.72 gm/kg) and unlabeled glucose (0.72 gm/kg). Four hours after ingestion, subjects received 1 mg of glucagon intravenously to stimulate glycogenolysis. When galactose was ingested alone, the area under the curve (AUC) of [13C6] glucose and [13C3] glucose were 7.28 ± 0.39 and 3.52 ± 0.05 mMole/l per 4 h respectively. When [U-13C] fructose was ingested with unlabeled fructose or unlabeled fructose plus glucose, no [13C6] glucose was detected in plasma. The AUC of [13C3] glucose after fructose and fructose plus glucose ingestion were 20.21 ± 2.41 and 6.25 ± 0.34 mMol/l per 4h respectively. Comparing the AUC for the 13C3 vs. 13C6 enrichments, 67% of oral galactose enters the systemic circulation via a direct route and 33% via an indirect route. In contrast, fructose only enters the systemic circulation via the indirect route. Finally, when ingested alone, fructose and galactose contribute little to glycogen synthesis. Following the co-ingestion of fructose and glucose with the resultant insulin response from the glucose, fructose is a significant contributor to glycogen synthesis.
Keywords: galactose, fructose, stable isotopes, gluconeogenesis, metabolism
Background
Fructose and galactose are the predominant non-glucose carbohydrates consumed on a daily basis by children and adolescents in the form of lactose and sucrose. Galactose is a unique dietary sugar in that its exclusive source is milk lactose. In infants, who are exclusively breast fed, galactose alone provides u 20% of their total caloric intake.
It has long been assumed that galactose consumption is associated with first pass hepatic clearance and intravenously administered labeled galactose has been used as a test of hepatic function (1, 2). Following lactose consumption, systemic plasma galactose concentrations in the fed newborn and in adults increase from 0 to 25 µM to 200–500 µM (3), as compared to plasma glucose concentration which increases from 5 to 8 mM following glucose consumption. Ingestion of 0.5 g/kg of galactose alone increases the plasma glucose by u 1 mM (i.e. u 20%), while plasma concentrations of galactose increases to u 2 mM (4). Similar results were obtained by Sunehag AL et al. (5) that reported in healthy subjects peak plasma galactose concentrations of u 2.3 mM after ingesting galactose at a rate of 33 µmol·kg−1·min−1. Under these conditions, galactose becomes the nearly exclusive carbon source for glucose production although the absolute rate of glucose production remains essentially unchanged (5).
Fructose constitutes an important source of carbohydrate intake in Western societies with adults consuming u 100 g/day in the form of sucrose plus fructose (6, 7). In rats, 55% of ingested fructose is taken up by the liver on a first pass basis. Similarly, in humans 50% of intravenously administered fructose is taken up by the liver (8). In the liver, fructose is phosphorylated to fructose-1-phosphate by fructokinase; aldolase B of the liver reversibly splits fructose-1-phosphate into glyceraldehyde and dihydroxyacetone-phosphate, a member of the glycolysis sequence, then glyceraldehyde is phosphorylated to glyceraldehyde-3-phosphate by the action of the triokinase, another intermediate of the glycolytic pathway (9). When fructose is co-ingested with glucose, the absorption of fructose is thought to be enhanced, suggesting an important role of the ingested sugars in the regulation of this process (10). The conversion of fructose to glucose has been estimated to be from 20% to 100% in humans after oral, intravenous or intragastric administration of labeled fructose (11–16). Recently, Gopher et al. (13)challenged the traditionally held pathway by which fructose is thought to be metabolized. Using high resolution NMR spectroscopy, they reported a direct conversion of u 50% of [U-13C]fructose into [U-13C]glucose in healthy children after an oral dose of [U-13C]fructose suggesting a direct conversion pathway not previously recognized in humans.
The present studies were undertaken to determine: 1) the contribution of galactose or fructose to glucose formation by the direct pathway (hexose → glucose) and the indirect pathway (hexose → 3 carbon substrates → glucose); and 2) if the co-ingestion of glucose with fructose modifies the contribution of fructose to glucose formation.
Materials and Methods
Tracers
[U-13C]galactose (99 atom %13C), [U-13C]fructose (99 atom % 13C), [6,6-2H2]glucose (99 atom %2H), and [1-2H]glucose (99 atom % 2H) were obtained from Cambridge Isotope Laboratories (Andover, MA). The labeled compounds were tested for sterility and pyrogenicity by the company and the investigation pharmacy at Texas Children’s Hospital, Houston, TX, and dissolved in 0.45% saline. The solution was subsequently filtered through a 0.2-µm Millipore filter (Millipore Corporation, Bedford, MA) into sterile syringes. Sterile isotope solutions were prepared less than 48 h before study and maintained at 4°C until used.
Study Design
The protocol was approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine in Houston, Texas. Informed consent was obtained from each subject.
Subjects
Six healthy adult volunteers were recruited; see Table 1. All were free of any identified diseases as assessed by a medical history, physical examination, and normal screening laboratory studies including hemoglobin, plasma glucose, aspartate transaminase, alanine transaminase, bilirubin, lactate dehydrogenase, and a negative pregnancy test in all women with child bearing potential. Three subjects participated in Protocol A whereas three additional subjects were studied on two occasions in Protocols B and C.
Table 1.
Subjects
| Protocol A | |||||
| Subject | Age (years) | Sex | Weight (kg) | Height (cm) | BMI (kg/m2) |
| 1 | 24 | M | 71 | 177 | 22.7 |
| 2 | 34 | F | 68 | 171 | 23.1 |
| 3 | 29 | M | 68 | 174 | 22.5 |
| 29 ± 3 | 69 ± 1 | 174 ± 2 | 22.7 ± 0.2 | ||
| Protocols B and C | |||||
| Subject* | Age (years) | Sex | Weight (kg) | Height (cm) | BMI (kg/m2) |
| 1 | 26 | F | 55 | 157 | 22.3 |
| 2 | 18 | M | 66 | 175 | 21.6 |
| 3 | 30 | F | 61 | 160 | 23.8 |
| 25 ± 4 | 61 ± 3 | 164 ± 6 | 22.6 ± 0.7 | ||
Mean ± SE.
Protocols: A, Galactose bolus; B, Fructose bolus; C Fructose + Glucose bolus.
The same subjects participated in both protocols.
Protocol design
The subjects were admitted to the Metabolic Research Unit (MRU) at the Children’s Nutrition Research Center the evening prior to study. During the week prior to each study, the subjects consumed a diet consisting of approximately 50% carbohydrate, 15% protein, and 35% fat, as instructed by the dietician. At 17:00 h on the evening of admission, two IV catheters were introduced in the subject’s antecubital fossa or forearm vein under Emla® (AstraZeneca Pharmaceuticals LP, Wayne, PA) cream analgesia, one for isotope infusion and the other for blood sampling. Subjects were fed a supper meal of 10 kcal/kg (same composition as mentioned above) at 18:00 h and were subsequently fasting, except for water ad libitum overnight.
At 06:00 h on the day of the study (−180 min), a baseline blood sample (3 ml) was obtained and the subjects received primed constant infusions of [6,6-2H2]glucose (10 µmol·kg−1, 0.16 µmol·kg−1·min−1) for a total of 8 h to measure the rate of appearance glucose into the plasma space (Protocols A, B and C). Blood samples were obtained at specific time points (−120, −60, −45, −30, −15, and 0 min). Beginning at zero time (09:00 h), the subjects consumed a drink bolus (0.72 gm/kg) of galactose labeled to 2% with [U-13C]galactose (Protocol A), a drink bolus (0.72 gm/kg) of fructose labeled to 2% with [U-13C]fructose (Protocol B) or a single bolus of fructose labeled to 2% with [U13C]fructose (0.72 gm/kg) and unlabeled glucose (0.72 gm/kg) (a total of 1.44 gm/kg) (Protocol C). Blood samples were obtained at 15, 30, 45, 60, 80, 90, 100, 110, 120, 150, 180, 210, 220, 230, and 240 minutes. Four hours after the ingestion of the labeled carbohydrate drink and directly following the 240 min blood sample, subjects were given an I.V. bolus (1.0 mg) of glucagon to stimulate glycogenolysis in an attempt to estimate the release of glucose from glycogen which was labeled from the ingestion of the labeled galactose (Protocol A), fructose (Protocol B) or fructose with unlabeled glucose (Protocol C). Additional blood samples were at 250, 260, 270, 285 and 300 min.
Analytical Methods
Plasma analyses
Glucose concentrations were determined using a Glucose and Lactate Analyzer, model 2700 Select (Yellow Springs Instruments, Yellow Springs, OH). Galactose concentrations were determined using a Glucose and Lactate Analyzer, model 2300 Stat+, retrofitted to read galactose (Yellow Springs Instruments). Plasma insulin concentrations were measured using commercially available RIA kits (Linco Research, St. Charles, MO).
The pentaacetate derivatives of glucose and galactose were prepared and the glucose and galactose isotopomers were determined by gas chromatography-mass spectrometry (GCMS) as previously described (17). The glucose was analyzed using positive chemical ionization with methane as the reactant gas with selective monitoring of m/z 331–337, reflecting unlabeled glucose and glucose molecules labeled with 13C in one to six of its carbons.
The acetyl-pentafluorobenzyl derivative of lactate was prepared as described previously (18). The method was modified as follows. The pentafluorobenzyl derivative was acetylated by the addition of a mole excess of 2:1 acetic anhydride/pyridine (Aldrich, Milwaukee, WI). The 13C enrichments in lactate were analyzed by GCMS (HP 6890 GC coupled with HP 5973 MSD Hewlett-Packard, Palo Alto, CA; an HP 5 column [25m × 0.25 mm × 0.25 µm]) was used. The conditions for the GCMS lactate analysis were: injector 250 degree (splitless injection); temperature program: initial temperature 70°C for 1 min, ramp at 15 °C/min up to 320°C followed by a 2-min hold. Negative chemical ionization with methane as the reagent gas and selective monitoring of m/z 131–134 was performed. The coefficient of variation (CV) of the tracer analysis methods (GC/MS, IR/MS, etc) in our laboratory is less than 2% and often < 1%. (19)
Calculations
The mole ratios of M+2, and M+6 of glucose were calculated from the mass to charge ratios using daily standards curves of [6,6-2H2]glucose; and [U-13C]glucose, respectively. Further, the M+2 isotopomer of glucose was corrected for the contribution from M+1 glucose using standard curve based on the M+2 molar ratio obtained from the [1-2H]glucose standard curve. The enrichment of each isotopomer of glucose (i.e. M+1, M+2, M+3 and M+6) was calculated as the isotopomer divided by the sum of all the glucose isotopomers (20).
The rates of appearance (Ra) of glucose into the systemic circulation were calculated using the average enrichments of M+2 between −45 to 0 min and at each time point throughout the study using the following equation:
| (Eq.) 1 |
where Ra is the rate of appearance, Ei and Ep are the isotopic enrichments in the infusate and the plasma, respectively, and I is the rate of infusion of [2H2]glucose.
Area Under the Curve
These studies were designed to determine relative metabolic pathways. True steady states were not achieved to utilize steady state equations. The number of subjects is small because of the tremendous cost of the isotopes. Therefore, to determine the relative contributions of galactose and fructose to glucose production, we analyzed the post ingestion data as area under the curve. The areas under the curve (AUC) were calculated using the trapezoidal method: AUC = (C1 + C2 / 2) (t2 − t1), where C1 and C2 represent the concentrations of the variable being calculated and t2 and t1 are the corresponding time points.
For each Protocol, the change from baseline (i.e. after subtraction of baseline values) in plasma glucose and insulin concentrations, plasma enrichments of [13C3] glucose and [13C6] glucose and the plasma concentrations of [13C3] and [13C6]glucose were calculated between 0 to 240 min (time of meal ingestion and absorption) and after glucagon injection, i.e. injection between 240 and 300 min (after subtraction of the 240 min values).
Direct and Indirect Pathways
Following the ingestion of the uniformly labeled galactose or fructose, the ratio of the area under the curve of the plasma [13C3] glucose / ([13C3] glucose + [13C6] glucose) enrichments or concentrations provides an estimate of the rates of entry of the labeled fructose or galactose into the plasma glucose pool via the indirect pathway. Conversely, the ratio of the area under the curve of the plasma [13C6] glucose / ([13C3] glucose + [13C6] glucose) enrichments or concentrations provides an estimate of the rates of entry of the labeled fructose or galactose into the plasma glucose pool via the direct pathway.
Similarly, the relative amounts of labeled carbon from the uniformly labeled galactose and fructose that were stored in glycogen via the direct and indirect pathways were calculated using the AUC data following the glucagon infusion.
Statistics
All data are presented as mean ± SE. Data were compared using ANOVA followed by Student-Newman-Keuls test as Post Hoc analysis, with a level of significance of p < 0.05.
Results
Baseline Glucose, Insulin and Glucose Ra
The mean (± SE) baseline (−60 to 0 min) plasma glucose concentrations were 4.8 ± 0.3 mM, 4.4 ± 0.1, and 4.4 ± 0.4 mM in Protocols A, B, and C, respectively, and the baseline plasma insulin concentrations were 5.3 ±1.2, 7.1 ± 2.1 and 6.9 ± 2.1 µU/ml respectively. The steady state baseline glucose Ra was 11.5 ± 0.9, 13.2 ± 1.2, and 12.3 ± 0.7 µmol·kg−1·min−1 in Protocol A, B, and C, respectively. No differences were observed among these values.
Plasma galactose concentrations
The plasma galactose concentrations were 0.07 ± 0.01, 2.57 ± 0.53, 0.73 ± 0.26, 0.16 ± 0.04, 0.10 ± 0.03, and 0.07 ± 0.01 mM at 0h, 1h, 2h, 3h, 4h, and 5h, respectively in Protocol A.
Plasma enrichments of [13C6] glucose and [13C3] glucose
The enrichment values of [13C6] glucose and [13C3] glucose for Protocol A, B, and C are shown in Table 2. Following ingestion of [U-13C6] galactose, the enrichment of both [13C6] glucose and [13C3] glucose increased. However, only the enrichment of [13C3] glucose increased after the ingestion of [U-13C6] fructose (Table 2).
Table 2.
Glucose isotopomers enrichment values (%).
| [13C6] glucose | |||||
| Protocol* | 1 h | 2 h | 3 h | 4 h | 5 h |
| A | 0.52 ± 0.05 | 0.82 ± 0.06 | 0.81 ± 0.01 | 0.65 ± 0.03 | 0.59 ± 0.03 |
| B | 0 | 0 | 0 | 0 | 0 |
| C | 0 | 0 | 0 | 0 | 0 |
| [13C3] glucose | |||||
| Protocol | 1 h | 2 h | 3 h | 4 h | 5 h |
| A | 0.36 ± 0.02 | 0.49 ± 0.03 | 0.59 ± 0.05 | 0.60 ± 0.01 | 0.51 ± 0.01 |
| B | 1.86 ± 0.03 | 2.38 ± 0.08 | 2.43 ± 0.18 | 1.87 ± 0.23 | 1.42 ± 0.29 |
| C | 0.49 ± 0.04 | 0.65 ± 0.01 | 0.82 ± 0.02 | 1.02 ± 0.09 | 1.09 ± 0.04 |
Mean ± SE.
Protocols: A, Galactose bolus; B, Fructose bolus; C Fructose + Glucose bolus.
Area Under the Curve Analysis
Glucose
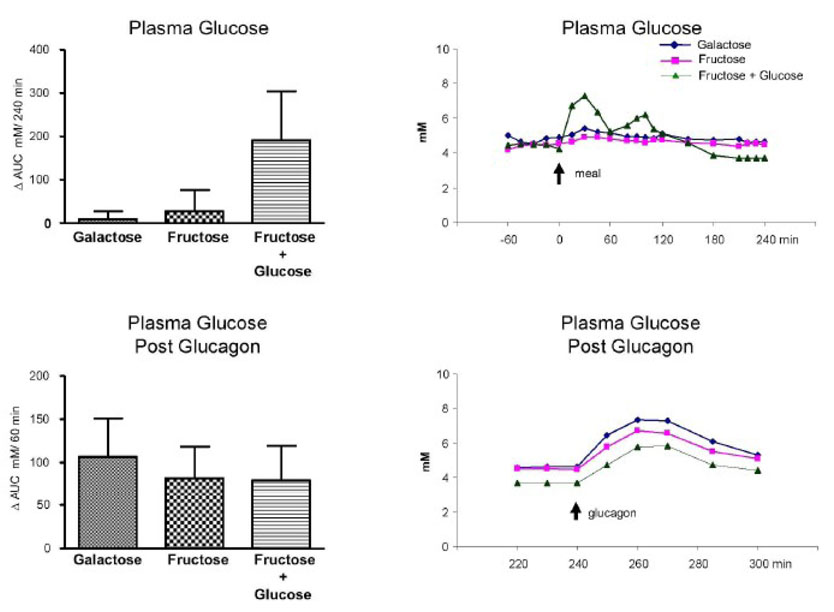
The Δ AUC (mean ± SE) for glucose for protocols A, B, And C were 10 ± 18, 27 ± 48 and 194 ± 113 mmole / l per 4 h, respectively. Following glucagon, the Δ AUC for glucose were 107 ± 44, 82 ± 37 and 80 ± 40 mmole / l per 1 h for protocols A, B, and C, respectively. No difference was observed among the groups for either value. (Fig. 1)
Fig. 1.
Plasma glucose concentration. Left upper panel: ⋗ AUC for plasma glucose concentrations (mM)/240 min (0 to 240 min after subtraction of baseline values). Left bottom panel: ⋗ AUC for plasma glucose concentrations (mM)/60 min after the glucagon infusion (240 to 300 min after subtraction of the pre-glucagon 240 min values). Right upper panel: Average values of the plasma glucose concentrations; 0 min represents the time of the drink ingestion. Right bottom panel: Average values of the plasma glucose concentrations after the glucagon infusion (240 to 300 min); 240 min represents the time of the glucagon administration. Values represent mean ± SE for n=3.
Insulin
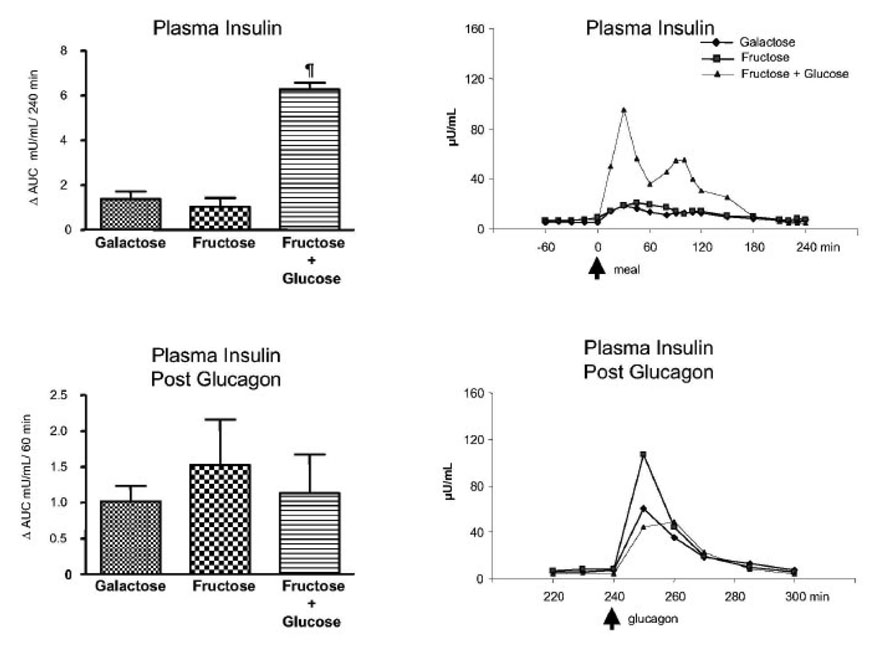
The Δ AUC for insulin for protocols, A, B, and C were 1.37 ± 0.34, 1.03 ± 0.40 and 6.28 ± 0.30 mUnits / ml per 4 h. No difference was observed between the galactose alone and fructose alone but fructose + glucose was greater (p < 0.05) than either of the other two. Following glucagon, the Δ AUC for insulin were similar among the three groups (1.02 ± 0.21, 1.52 ± 0.63 and 1.13 ± 0.54 mUnits / ml per 1 h for protocols A, B, and C, respectively, NS). (Fig. 2)
Fig. 2.
Plasma insulin concentration. Left upper panel: ⋗ AUC for plasma insulin concentrations (mU/mL)/240 min (0 to 240 min after subtraction of baseline values). ¶ p < 0.05 Fructose + glucose vs. galactose and fructose bolus by ANOVA. Left bottom panel: ⋗ AUC for plasma insulin concentrations (mU/mL)/60 min after the glucagon infusion (240 to 300 min after subtraction of the pre-glucagon 240 min value). Right upper panel: Average values of the plasma insulin concentration (µU/mL); 0 min represents the time of the drink ingestion. Right bottom panel: Average values of the plasma insulin concentration after the glucagon infusion (µU/mL) (240 to 300 min); 240 min represents the time of the glucagon administration. Values represent mean ± SE for n=3.
Glucose Ra
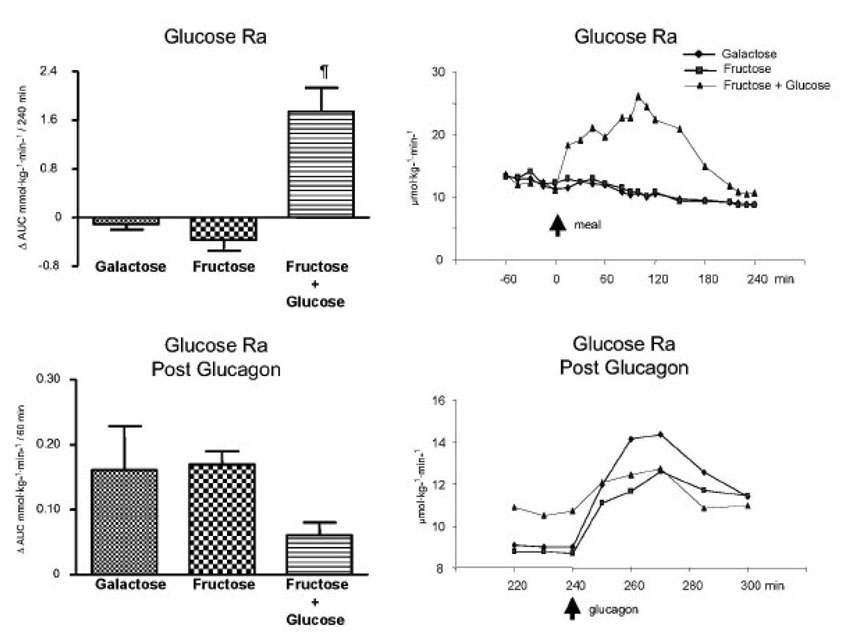
The Δ AUC for glucose Ra for protocols A, B, and C were −0.11 ± 0.09, −0.37 ± 0.18 and 1.74 ± 0.39 mmol·kg−1·min−1 per 4 h. No difference was observed between the galactose alone and fructose alone but fructose + glucose was greater (p < 0.05) than either of the other two. Following glucagon, the Δ AUC for glucose Ra were not different among the groups (0.16 ± 0.07, 0.17 ± 0.02 and 0.06 ± 0.02 mmol·kg−1·min−1 per 1 h for protocols A, B, and C, respectively). (Fig. 3)
Fig. 3.
Glucose rate of appearance. Left upper panel: ⋗ AUC for glucose rate of appearance (Ra) (mmol·kg−1·min−1) /240 min (0 to 240 min after subtraction of baseline values). ¶ p < 0.05 Fructose + glucose vs. galactose and fructose bolus by ANOVA. Left bottom panel: ⋗ AUC for glucose rate of appearance (Ra) (mmol·kg−1·min−1) /60 min after the glucagon infusion (240 to 300 min after subtraction of the pre-glucagon 240 min value). Right upper panel: Average values of the glucose rate of appearance (Ra) (µmol·kg−1·min−1); 0 min represents the time of the drink ingestion. Right bottom panel: Average values of glucose rate of appearance (Ra) (µmol·kg−1·min−1) after the glucagon infusion (240 to 300 min); 240 min represents the time of the glucagon administration. Values represent mean ± SE for n=3.
[13C6] glucose
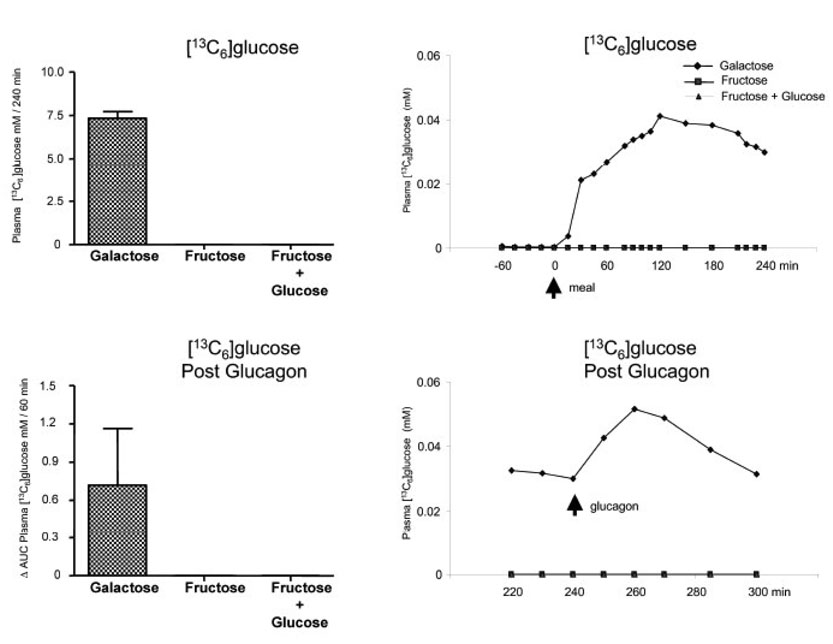
The Δ AUC for the plasma concentration of [13C6] glucose after the bolus ingestion of [13C6] galactose was 7.28 ± 0.39 mMoles / l per 4 h; whereas, that following [13C6] fructose was 0 mMole / l per 4 h whether glucose was included in the meal or not (Fig 4). Following glucagon, the Δ AUC for plasma concentrations of [13C6] glucose increased in 2 of the 3 subjects following galactose bolus ingestion with a mean value of 0.72 ± 0.44 mMoles / l per 1 h, but in one subject no change was observed. No [13C6] glucose was observed follow ingestion of [13C6] fructose, with or without glucose. The AUC values for the plasma enrichments of [13C6] glucose are included in Table 3.
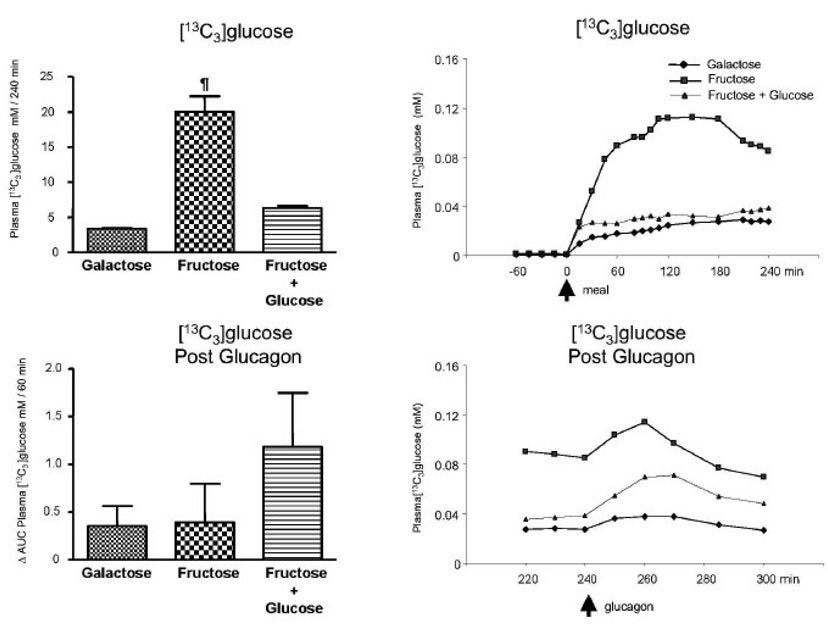
Fig. 4.
[13C6]glucose. Left upper panel: [13C6]glucose mM/240 min (0 to 240 min). Left bottom panel: ⋗ AUC for plasma [13C6]glucose mM/60 min after the glucagon infusion (240 to 300 min after subtraction of pre-glucagon 240 min value). Right upper panel: Average values of [13C6]glucose (mM); 0 represents the time of the meal ingestion. Right bottom panel: Average values of [13C6]glucose (mM) after the glucagon infusion (240 to 300 min); 240 min represents the time of the glucagon administration. Values represent mean ± SE for n=3.
Table 3.
AUC values for plasma enrichments of [13C6] glucose and [13C3] glucose.
| [13C6] glucose | ||
| Protocol* | ⊗0–240 min | ⊗240–300 min |
| A | 1.48 ± 0.05 | 0.007 ± 0.02 |
| B | 0 | 0 |
| C | 0 | 0 |
| [13C3] glucose | ||
| Protocol | ⊗ 0–240 min | ⊗ 240–300 min |
| A | 0.92 ± 0.16 | −0.044 ± 0.003 |
| B | 4.52 ± 0.11‡ | −0.17 ± 0.05‡ |
| C | 1.45 ± 0.04¶ | 0.08 ± 0.03¶ |
Mean ± SE.
Protocols: A, Galactose bolus; B, Fructose bolus; C Fructose + Glucose bolus.
p < 0.05 vs Galactose and Fructose bolus
p < 0.05 vs Galactose bolus.
[13C3] glucose
The Δ AUC for the plasma concentration of [13C3] glucose after the bolus ingestion of galactose was 3.52 ± 0.05 mMole /l per 4 h (Fig. 5) where as that following fructose alone (Protocol B) or fructose plus glucose (Protocol C) were 20.21 ± 2.41 and 6.25 ± 0.34 mMole / l per 4 h, respectively (p < 0.05). Differences (p < 0.05) existed for the fructose bolus group when compared to the others. Over the one hour following glucagon, the Δ AUC for the plasma concentration of [13C3] glucose were 0.35 ± 0.21, 0.39 ± 0.40 and 1.18 ± 0.56 mMole / l per 1 hr for Protocols A, B, and C, respectively. There were no significant differences among the groups. The AUC values for the plasma enrichments of [13C3] glucose are included in Table 3.
Fig. 5.
[13C3]glucose. Left upper panel: [13C3]glucose mM/240 min (0 to 240 min). ¶ p < 0.05 Fructose alone vs. Galactose or Fructose + glucose by ANOVA. Left bottom panel: ⋗ AUC for plasma [13C3]glucose mM/60 min after the glucagon (240 to 300 min after subtraction of the pre-glucagon 240 min value). Right upper panel: Average values of [13C3]glucose (mM); 0 min represents the time of the meal ingestion. Right bottom panel: Average values of [13C3]glucose (mM) after the glucagon injection (240 to 300 min); 240 min represents the time of the glucagon administration. Values represent mean ± SE for n=3.
Direct and Indirect pathways
The fraction of galactose entering the glucose pool by the direct pathway (13C6 / 13C6 + 13C3) was 0.67 and that by the indirect pathway (13C3 /13C6 + 13C3) was 0.33. Conversely all of the labeled fructose entered the plasma as 13C3 regardless of the co-ingestion of glucose.
Discussion
We previously demonstrated that during ingestion of galactose at 33 µmol·kg−1·min−1, the splanchnic uptake of galactose was saturable at about 15 µmol·kg−1·min−1 and glucose production was derived almost exclusively from the conversion of galactose to glucose without increase in the absolute rate of glucose production. In that study, only [1-13C]galactose was used and, therefore, we could not distinguish the contributions of galactose to glucose formation via the direct and indirect pathways respectively. The use of [U-13C]galactose in the current study enabled us to quantify both pathways. The results of study A (galactose alone) confirm our previous finding that the majority of ingested galactose is converted to glucose without increase in the rate of glucose production, indicating a compensatory reduction in glucose production from other substrates.
No enrichment of [13C6]glucose was observed in plasma after ingestion of [U-13C]fructose (essentially zero enrichment of glucose isotopomers M+6) (Fig. 4). Thus, the present study clearly demonstrates that no direct conversion of fructose into glucose (fructose-1-phosphate to fructose-1,6-bisphosphate) occurs. Only M+3 glucose was observed in both studies using [U-13C]fructose (ingestion of fructose alone and fructose + glucose, respectively) (Fig. 5). In addition, the ⊗ AUC for [13C3] glucose was significantly higher during ingestion of fructose alone (Fig. 5) compared to ingestion of fructose plus glucose. It was not possible to determine how much of the label was derived from direct conversion of the triose phosphates to glucose vs. via lactate and pyruvate because the fructose isotopomers (i.e. M+3 fructose) were too low to be accurately measured by GCMS.
Gopher et al. (13), addressed the metabolic pathways of fructose conversion to glucose using 13 C NMR measurement of plasma [13C]glucose isotopomers. Eight control infants and three children with hereditary fructose intolerance received a constant nasogastric infusion of D-[U-13C]fructose. The investigators reported that direct conversion of fructose by fructose 1-phosphate aldolase activity accounted for ≈ 50% and ≈ 30% of the total amount of hepatic fructose conversion to glucose in the control infants and children with hereditary fructose intolerance, respectively. These authors assumed that the probability of D-[U-13C]glucose forming as a result of recombination of two molecules of [U-13C]triose phosphates was negligible (13). These results imply a direct pathway converting fructose-1-phosphate to fructose 1,6-bisphosphate. The enzymatic activity in the liver needed to accomplish that conversion has not been reported. Finally, these authors did not provide precision or accuracy data regarding their NMR method.
Chandramouli et al. (11), reexamined the pathway of fructose conversion to glucose in healthy adults under similar experimental conditions as Gopher et al. (13). Six healthy adult subjects received [1-14C]lactate intravenously and unlabeled fructose with [6-14C]sorbitol or [6-14C]fructose either intravenously or intragastrically after an overnight fast. The authors measured the distribution of 14C in blood glucose and calculated the ratio of 14 C in C1 (carbon 1 of glucose) to C6 and in C3 to C4. The results showed that on average 94.9% of the fructose converted to glucose underwent cleavage of the carbon skeleton of the fructose with only minimal direct conversion to glucose. Thus, both these indirect results and our direct results would dispute those of Gopher et al. (13) thus supporting the main pathway of fructose to glucose conversion by an initial phosphorylation of fructose to fructose-1-phosphate, which enters the triose phosphates before ending up in glucose-6-phosphate and finally glucose.
Ingested fructose is known to be converted to glucose-6-phosphate and result in glycogen storage in the liver (21, 22). Several studies have investigated the effect of fructose ingestion on hepatic glucose metabolism in healthy human subjects (15, 16, 23). Nuttall et al. (15), demonstrated that in normal male volunteers receiving intravenous infusion of [3-3H]glucose, plus a meal consisting of 50 g fructose, only 20% of the ingested fructose could be accounted for as glucose appearing in the circulation. Presumably, the remaining fructose was stored as glycogen. Tounian et al. (16), reported in healthy subjects infused with [1-13C]fructose for 3 h under a pancreatic clamp that approximately 67% of the fructose infused underwent non-oxidative disposal (fructose infusion rate minus fructose oxidation rate) and concluded that this value should represent the actual amount of glycogen synthesized from fructose.
Using the change in [13C3]glucose and [13C6]glucose plasma concentrations following the glucagon stimulated release of hepatic glycogen as an indicator of uptake of labeled fructose ± glucose, our results demonstrate that a greater fraction of fructose was converted to glycogen when fructose was co-ingested with glucose as compared to fructose ingested alone. This indicates that during the ingestion of fructose plus glucose, fructose carbons labeled with 13C were deposited as glycogen in the liver and then released after the stimulation with glucagon. We believe this to be due to the glucose mediated increase in plasma insulin and insulin effect on increasing hepatic glycogen content. In contrast, ingestion of fructose or galactose boluses without co-ingestion of glucose yielded negative values of the AUC of the plasma enrichment of [13C3]glucose. These results reflect release of a higher number of unlabeled glucose carbons in proportion to labeled carbons derived from labeled fructose or galactose respectively, indicating that a smaller amount of labeled 13C carbons were coming from liver glycogen.
We are aware that the small sample size limits the power of the analysis. Nonetheless, we believe, our data allow us to answer the questions outlined in the study aims. Secondly, given that values of several variables measured have not returned to baseline conditions, we believe the interpretation of the data is still valid.
The present data demonstrate that in healthy human subjects: 1) the majority of ingested galactose is converted to glucose, ~ 65% of which is derived via the direct pathway and ~ 35% via the indirect pathway; 2) when fructose or fructose plus glucose are given as an oral bolus, there is no direct conversion of fructose to glucose; 3) a greater fraction of fructose carbon is converted to glycogen when fructose is co-ingested with glucose as compared to fructose or galactose alone.
Acknowledgments
This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the US Government. We would like to acknowledge and thank the technicians in our laboratory (Kathryn Louie, Patricia Langley, Lauren Loyal, Shaji Chacko and Daniel Donaldson), our nurse coordinators (Andrea Dotting Jones, R.N. and Amy Pontius, R.N.), the staff in the Metabolic Research Unit and Kitchen who greatly facilitated the execution of these studies.
Supported by Grant No. 6250-51000-048 from the United States Department of Agriculture (USDA) Cooperative Agreement and by Grant No. 5 RO1 DK 55478 from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Henderson JM, Kutner MH, Bain RP. First-order clearance of plasma galactose: The effect of liver disease. Gastroenterology. 1982;83:1090–1096. [PubMed] [Google Scholar]
- 2.Shreeve WW, Shoop JD, Ott DG, McInteer BB. Test for alcoholic cirrhosis by conversion of [14c]- or [13c]galactose to expired co2. Gastroenterology. 1976;71:98–101. [PubMed] [Google Scholar]
- 3.Segal S, Berry G. Disorders of galactose metabolism. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. 7th ed. New York: McGraw-Hill, Inc., Health Professions Division; 1995. pp. 967–1000. [Google Scholar]
- 4.Williams CA, Phillips T, Macdonald I. The influence of glucose on serum galactose levels in man. Metabolism. 1983;32:250–256. doi: 10.1016/0026-0495(83)90189-0. [DOI] [PubMed] [Google Scholar]
- 5.Sunehag AL, Haymond MW. Splanchnic galactose extraction is regulated by coingestion of glucose in humans. Metabolism. 2002;51:827–832. doi: 10.1053/meta.2002.33346. [DOI] [PubMed] [Google Scholar]
- 6.Campbell MJ, Williams J, Elwood PC. Sugar and health. Lancet. 1987;1:1311. doi: 10.1016/s0140-6736(87)90559-9. [DOI] [PubMed] [Google Scholar]
- 7.Park YK, Yetley EA. Intakes and food sources of fructose in the united states. Am J Clin Nutr. 1993;58:737S–747S. doi: 10.1093/ajcn/58.5.737S. [DOI] [PubMed] [Google Scholar]
- 8.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 9.Gitzelman R, Steinmann B, Van der Berghe G. Disorders of fructose metabolism. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill, Inc; 1995. pp. 905–934. [Google Scholar]
- 10.Truswell AS, Seach JM, Thorburn AW. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr. 1988;48:1424–1430. doi: 10.1093/ajcn/48.6.1424. [DOI] [PubMed] [Google Scholar]
- 11.Chandramouli V, Kumaran K, Ekberg K, Wahren J, Landau BR. Quantitation of the pathways followed in the conversion of fructose to glucose in liver. Metabolism. 1993;42:1420–1423. doi: 10.1016/0026-0495(93)90192-q. [DOI] [PubMed] [Google Scholar]
- 12.Delarue J, Normand S, Pachiaudi C, Beylot M, Lamisse F, Riou JP. The contribution of naturally labelled 13c fructose to glucose appearance in humans. Diabetologia. 1993;36:338–345. doi: 10.1007/BF00400238. [DOI] [PubMed] [Google Scholar]
- 13.Gopher A, Vaisman N, Mandel H, Lapidot A. Determination of fructose metabolic pathways in normal and fructose-intolerant children: A 13c nmr study using [u-13c]fructose. Proc Natl Acad Sci U S A. 1990;87:5449–5453. doi: 10.1073/pnas.87.14.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau BR, Marshall JS, Craig JW, Hostetler KY, Genuth SM. Quantitation of the pathways of fructose metabolism in normal and fructose-intolerant subjects. J Lab Clin Med. 1971;78:608–618. [PubMed] [Google Scholar]
- 15.Nuttall FQ, Khan MA, Gannon MC. Peripheral glucose appearance rate following fructose ingestion in normal subjects. Metabolism. 2000;49:1565–1571. doi: 10.1053/meta.2000.18553. [DOI] [PubMed] [Google Scholar]
- 16.Tounian P, Schneiter P, Henry S, Jequier E, Tappy L. Effects of infused fructose on endogenous glucose production, gluconeogenesis, and glycogen metabolism. Am J Physiol. 1994;267:E710–E717. doi: 10.1152/ajpendo.1994.267.5.E710. [DOI] [PubMed] [Google Scholar]
- 17.Argoud GM, Schade DS, Eaton RP. Underestimation of hepatic glucose production by radioactive and stable tracers. Am J Physiol. 1987;252:E606–E615. doi: 10.1152/ajpendo.1987.252.5.E606. [DOI] [PubMed] [Google Scholar]
- 18.Hachey DL, Patterson BW, Reeds PJ, Elsas LJ. Isotopic determination of organic keto acid pentafluorobenzyl esters in biological fluids by negative chemical ionization gas chromatography/mass spectrometry. Anal Chem. 1991;63:919–923. doi: 10.1021/ac00009a017. [DOI] [PubMed] [Google Scholar]
- 19.Sunehag AL, Treuth MS, Toffolo G, Butte NF, Cobelli C, Bier DM, Haymond MW. Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: An evaluation of their reproducibility. Pediatr Res. 2001;50:115–123. doi: 10.1203/00006450-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Haymond MW, Sunehag AL. The reciprocal pool model for the measurement of gluconeogenesis by use of [u-(13)c]glucose. Am J Physiol Endocrinol Metab. 2000;278:E140–E145. doi: 10.1152/ajpendo.2000.278.1.E140. [DOI] [PubMed] [Google Scholar]
- 21.Niewoehner CB. Metabolic effects of dietary versus parenteral fructose. J Am Coll Nutr. 1986;5:443–450. doi: 10.1080/07315724.1986.10720147. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson LH, Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974;33:5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- 23.Dirlewanger M, Schneiter P, Jequier E, Tappy L. Effects of fructose on hepatic glucose metabolism in humans. Am J Physiol Endocrinol Metab. 2000;279:E907–E911. doi: 10.1152/ajpendo.2000.279.4.E907. [DOI] [PubMed] [Google Scholar]