Abstract
Background
Cerebrospinal fluid (CSF) findings are often used to diagnose meningitis in neonates given antibiotics before the lumbar puncture is performed. Traumatic lumbar punctures are common and complicate interpretation of CSF white blood cell counts. The purpose of this study is to evaluate the diagnostic utility of adjusting CSF white blood cell counts based on CSF and peripheral red blood cell counts.
Methods
Cohort study of lumbar punctures performed between 1997 and 2004 at 150 neonatal intensive care units managed by the Pediatrix Medical group. Traumatic lumbar punctures were defined as CSF specimens with ≥500 red blood cells/mm3. CSF white blood cell counts were adjusted downward for traumatic lumbar punctures using several commonly used methods. We calculated sensitivity, specificity, likelihood ratios, and area under the receiver operating characteristic curve of unadjusted and adjusted CSF white blood cell counts for predicting meningitis in neonates with traumatic lumbar punctures.
Results
Of 6,374 lumbar punctures, 2,519 (39.5%) were traumatic. 114/6,374 (1.8%) were positive for meningitis; 50 neonates with traumatic lumbar punctures had meningitis. The areas under the receiver operating characteristic curve for white blood cell count unadjusted and adjusted by all methods were similar.
Conclusions
Adjustment of CSF white blood cell counts to account for increased red cells does not improve diagnostic utility. Adjustment can result in loss of sensitivity with marginal gain in specificity. Adjustment of WBC counts in the setting of a traumatic lumbar puncture does not aid in the diagnosis of bacterial and fungal meningitis in neonates.
Keywords: diagnosis, meningitis, neonate
INTRODUCTION
Meningitis occurs more commonly in the first month of life than at any other age and results in high rates of mortality and morbidity.1–4 Diagnosis of meningitis is complicated in the neonatal population because the signs are often subtle, and neonates are often exposed to empiric or intrapartum antibiotics before a lumbar puncture (LP) is performed. In this situation, clinicians rely heavily on interpretation of cerebrospinal fluid (CSF) white blood cell (WBC) count.1,5,6
Relying on CSF WBC count to make the diagnosis of meningitis, determine antibiotic duration, and influence prognosis has significant limitations. The CSF WBC count can be altered by the presence of peripheral blood in the CSF after a traumatic LP. Traumatic LPs are common in the neonatal population, with reported incidences ranging from 35–46%.7,8 Erroneous interpretation of the WBC count can lead to the under- or overdiagnosis of meningitis.
In older pediatric patients and adults, physicians have evaluated several methods to account for the extra WBCs from peripheral blood in traumatic LPs.9–15 Some authors have assessed the use of an established average ratio of 500 or 1000 red blood cells (RBCs) to 1 WBC in the CSF.9 The number of WBCs that can be accounted for by the number of RBCs in the specimen is simply subtracted from the number of WBCs observed in the CSF. The number of observed WBCs in the CSF can also be corrected by subtracting the expected number of WBCs calculated using the ratio of peripheral blood WBCs:RBCs.10–12 An alternative method for interpretation of CSF WBC count after traumatic taps is to calculate the observed to predicted (O:P) ratio by dividing the number of observed CSF WBCs by the number of predicted CSF WBCs, as determined by the peripheral blood WBC:RBC ratio.13–15
Although literature exists describing methods for interpreting the CSF WBC count after traumatic LPs in adult and pediatric populations,9–15 previous reports in neonates are scarce. Only one study to date has reported results separately for neonates, and that study investigated the validity of correction in only 13 neonates: 9 without meningitis and 4 with likely viral meningitis.10 In this study, we examine historical methods of correcting WBC counts in a large cohort of neonates with traumatic LPs by evaluating their ability to accurately identify neonates with culture proven meningitis and to approximate the true number of WBCs in the CSF.
MATERIALS AND METHODS
Study population
We evaluated the first LP from all neonates ≤ 30 days of age and discharged from one of 150 neonatal intensive care units managed by the Pediatrix Medical Group from 1997 through 2004. The data were obtained from an administrative database as described previously.16 The CSF parameter data were checked independently by three individuals for accuracy. We identified 6502 neonates with values recorded for the number of RBCs in the CSF. We excluded 46 neonates with CSF reservoirs and ventriculoperitoneal shunts, 65 neonates with likely contaminated CSF specimens (51 coagulase-negative Staphylococcus, 8 Gram positive rods, 6 viridans streptococci), 6 neonates positive for mixed species not otherwise identified, and 11 neonates positive for viral meningitis by culture or polymerase chain reaction.
Statistical Analysis
The primary outcome variable, meningitis, was defined as a positive CSF culture or Gram stain for bacteria or fungi. Traumatic LPs were defined as CSF specimens with 500 RBCs/mm3.9,15 Due to the variance in the RBC cut-off values for traumatic LP used in previous studies,13,17 we also performed several analyses on CSF specimens with ≥ 10,000 RBCs/mm3.
We evaluated unadjusted and adjusted CSF WBC counts for predicting meningitis. The CSF WBC count was adjusted by several methods. Using a simple ratio method, the number of CSF WBCs observed (WBCobserved) was modified by subtracting the CSF RBC count/500 (500:1 ratio method) and the CSF RBC count/1000 (1000:1 ratio method). For example, if the CSF RBC count was 2,000 and the CSF WBC count was 50, then correcting by the 500:1 ratio method resulted in an adjusted CSF WBC count of 46 (50 – 2000/500), while the 1000:1 method resulted in an adjusted CSF WBC count of 48 (50 – 2000/1000).
The predicted WBC count (WBCpredicted) as determined by the ratio of RBCs to WBCs in the peripheral blood was calculated using the formula WBCpredicted = CSF RBC count × [(peripheral WBC count)/(peripheral RBC count)]. The CSF WBC count was adjusted by subtracting WBCpredicted from WBCobserved. In the previous example, if the peripheral RBC count was 4×1012 cells/mm3 and the peripheral WBC count was 20×109 cells/mm3, then WBCpredicted = 2000 × [(20×109)/(4×1012)] = 10. Therefore, the adjusted CSF WBC count = 50 – 10 = 40. Finally, the observed:predicted (O:P) ratio was calculated by the formula (WBCobserved)/(WBCpredicted).
We used nonparametric methods to produce receiver operator characteristic (ROC) curves for unadjusted WBC count and all methods of adjustment for neonates with traumatic LPs. For neonates with LPs with < 500 RBC/mm3, we calculated the ROC curve for the unadjusted WBC count. To calculate the sensitivity, specificity, positive likelihood ratio (+LR), and negative likelihood ratio (−LR), we used unadjusted and adjusted WBC count cut-off values of 10, 20, and 100 cells/mm3 and O:P ratio cut-off values of 1, 10, and 100. Finally, we evaluated the ability of adjustment methods to approximate the true number of WBCs in the CSF by comparing the adjusted values and the true CSF WBC count in patients with traumatic LPs and without meningitis using a Wilcoxon signed-rank test.
We conducted the analysis with STATA 9 (College Station, TX) and used the Wilcoxon rank sum test and Fisher’s exact test where appropriate to compare values between groups. Reported p-values are two-tailed, and p-values < 0.05 were considered significant. The Duke University Institutional Review Board provided permission to conduct this analysis.
RESULTS
Table 1 shows the demographic characteristics of the 6,374 neonates included in the analysis. The median [interquartile range (IQR)] gestational age was 37 [32–39] weeks for neonates with traumatic LPs and 37 [32–39] weeks for neonates with non-traumatic LPs (p=0.81). The median birth weight was 2.8 [1.8–3.4] kg for neonates with traumatic LPs and 2.8 [1.6–3.4] kg for neonates with non-traumatic LPs (p=0.05). The median age at LP was 2 [0–6] days for neonates with traumatic LPs and 2 [1–11] days for neonates with non-traumatic LPs (p<0.0001).
Table 1.
Demographics of the cohort.
| Demographics | Non-traumatic N (%) | Traumatic N (%) | Total N (%) |
|---|---|---|---|
| Total | 3,855 (60.5) | 2,519 (39.5) | 6,374 (100) |
| Gestational age (weeks) | |||
| <30 | 652 (16.9) | 415 (16.4) | 1,067 (16.7) |
| 30–36 | 951 (24.7) | 623 (24.7) | 1,574 (24.7) |
| >36 | 2,252 (58.4) | 1,481 (58.8) | 3,733 (58.6) |
| Gender | |||
| Female | 1,677 (43.5) | 1,059 (42.0) | 2,736 (42.9) |
| Male | 2,178 (56.5) | 1,460 (58.0) | 3,638 (57.1) |
| Race/Ethnicity | |||
| White | 1,842 (47.8) | 1,214 (48.2) | 3,056 (47.9) |
| Black | 611 (15.9) | 394 (15.6) | 1,005 (15.8) |
| Hispanic | 1,139 (29.6) | 719 (28.5) | 1,858 (29.2) |
| Other | 263 (6.8) | 192 (7.6) | 455 (7.1) |
| Birth weight (g) | |||
| <750 | 128 (3.3) | 106 (4.2) | 234 (3.7) |
| 750–999 | 248 (6.4) | 144 (5.7) | 392 (6.2) |
| 1000–1499 | 476 (12.4) | 258 (10.2) | 734 (11.5) |
| 1500–2499 | 778 (20.2) | 490 (19.5) | 1,268 (19.9) |
| >2499 | 2,185 (56.7) | 1,506 (59.8) | 3,691 (57.9) |
| Day of life at time of LP | |||
| 0–3 | 2,193 (56.9) | 1,709 (67.8) | 3,902 (61.2) |
| 4–7 | 403 (10.5) | 241 (9.6) | 644 (10.1) |
| 8–14 | 559 (14.5) | 257 (10.2) | 816 (12.8) |
| 15–30 | 700 (18.2) | 312 (12.4) | 1,012 (15.9) |
Of the 2519 neonates with traumatic LPs, 50 (2.0%) had meningitis (47 culture-proven, 3 sterile culture but Gram stain positive), and 64 (1.7%) of the 3,855 neonates with non-traumatic LPs had meningitis (61 culture-proven, 3 sterile culture but Gram stain positive) (p=0.34). Overall, 49% of the identified pathogens were Gram-positive bacteria, 40% were Gram-negative bacteria, and 11% were Candida species. The most common pathogens in both traumatic and non-traumatic LP groups were group B Streptococcus and Escherichia coli.
The median WBC count was 13 [4–53] for neonates with traumatic LPs and 4 [2–9] for neonates with non-traumatic LPs (p<0.0001). The median RBC count was 4,625 [1,365–23,000] for neonates with traumatic LPs, 17 [3–99] for neonates without traumatic LPs, and 167 [9–2,420] overall.
Among neonates with traumatic LPs, the unadjusted number of WBCs was significantly higher in neonates with meningitis, 292 [28–2,800], than without meningitis, 13 [4–50], (p<.0001). Similarly, the O:P ratio was higher in neonates with meningitis, 24 [2–879], than without meningitis, 0.8 [0.2–2.6], (p<0.0001).
The sensitivity, specificity, and positive and negative likelihood ratios for the prediction of meningitis at various cut-offs for those with non-traumatic and those with traumatic taps are presented in Table 2. There was little difference in the areas under the ROC curves (AUC) between unadjusted WBC counts and adjusted WBC counts (Table 3). Because the majority of traumatic LPs occurred in neonates born at term (59%) and in the first week of life (77%), we analyzed the AUC for each of these groups separately. For term neonates, the AUC of the WBC count adjusted by the peripheral RBC:WBC ratio, 0.82 (0.70–0.94), was slightly higher than the AUC of the unadjusted WBC count, 0.75 (0.59–0.90), while for preterm neonates, the AUC for the adjusted WBC count, 0.75 (0.63–0.88), was slightly lower than the AUC of unadjusted WBC count, 0.78 (0.68–0.88). For neonates in the first week of life, the AUC of the WBC count adjusted by the peripheral RBC:WBC ratio, 0.80 (0.69–0.91), was slightly higher than the AUC of the unadjusted WBC count, 0.74 (0.60–0.87). For neonates after the first week of life, the AUC of the WBC count adjusted by the peripheral RBC:WBC ratio, 0.73 (0.58–0.88), was lower than the AUC of the unadjusted WBC count, 0.78 (0.69–0.88).
Table 2.
Utility of unadjusted and adjusted white blood cell values to predict meningitis.
| Predictor | RBCs in CSF (/mm3) | Cut-off* | Sensitivity (%) | Specificity (%) | + LR | − LR |
|---|---|---|---|---|---|---|
| WBC, unadjusted | <500 | WBC ≥ 10 | 70 | 77 | 3.0 | 0.39 |
| WBC ≥ 20 | 61 | 90 | 6.3 | 0.43 | ||
| WBC ≥ 100 | 44 | 98 | 25.0 | 0.57 | ||
| WBC, unadjusted | ≥500 | WBC ≥ 10 | 86 | 42 | 1.5 | 0.34 |
| WBC ≥ 20 | 82 | 57 | 1.9 | 0.31 | ||
| WBC ≥ 100 | 64 | 82 | 3.5 | 0.44 | ||
| WBC, adjusted by 500:1 ratio | ≥500 | WBC ≥ 10 | 82 | 67 | 2.5 | 0.27 |
| WBC ≥ 20 | 74 | 75 | 3.0 | 0.35 | ||
| WBC ≥ 100 | 56 | 89 | 5.2 | 0.49 | ||
| WBC, adjusted by 1000:1 ratio | ≥500 | WBC ≥ 10 | 82 | 58 | 2.0 | 0.31 |
| WBC ≥ 20 | 76 | 69 | 2.4 | 0.35 | ||
| WBC ≥ 100 | 58 | 86 | 4.3 | 0.49 | ||
| WBCobserved − WBCpredicted | ≥500 | WBC ≥ 10 | 75 | 75 | 3.0 | 0.33 |
| WBC ≥ 20 | 73 | 82 | 4.0 | 0.33 | ||
| WBC ≥ 100 | 55 | 92 | 6.8 | 0.49 | ||
| Observed:predicted ratio | ≥500 | O:P ≥ 1 | 75 | 55 | 1.7 | 0.45 |
| O:P ≥ 10 | 57 | 93 | 7.6 | 0.47 | ||
| O:P ≥ 100 | 36 | 99 | 42.3 | 0.64 |
LR (likelihood ratio).
Table 3.
Area under the ROC curve.
| Predictor | RBCs in CSF (/mm3) | Area Under the Curve (95% CI) |
|---|---|---|
| WBC, unadjusted | <500 | 0.78 (0.71–0.86) |
| WBC, unadjusted | ≥500 | 0.77 (0.68–0.85) |
| WBC, adjusted by 500:1 ratio | ≥500 | 0.81 (0.73–0.88) |
| WBC, adjusted by 1000:1 ratio | ≥500 | 0.79 (0.71–0.86) |
| WBCobserved − WBCpredicted | ≥500 | 0.78 (0.70–0.87) |
| Observed to Predicted ratio of WBC | ≥500 | 0.78 (0.68–0.87) |
CI (confidence interval)
When the AUC were calculated for only the CSF specimens with ≥10,000 RBCs, the AUC for the WBC count adjusted by the peripheral RBC:WBC ratio, 0.85 (0.86–1.00), was similar to the AUC for the unadjusted WBC count, 0.88 (0.77–0.98).
For neonates with traumatic LPs and without meningitis, all of the WBCs found in the CSF specimen should be due to peripheral blood contamination. Therefore, if the correction methods are accurate, they should approximate the true number of WBCs found in this group. When the CSF WBC count of neonates with traumatic LPs and without meningitis was compared with predicted WBC count based on the peripheral RBC:WBC ratio, the 500:1 CSF RBC:WBC ratio, and the 1000:1 CSF RBC:WBC ratio, these methods were poor estimates of the true CSF WBC count in this group. The median CSF WBC count predicted by the peripheral RBC:WBC ratio, 15 [5–77], was higher than the true WBC count in this group, 12 [4–48], (p<0.0001). In contrast, the 500:1 and 1000:1 ratios underestimated the true WBC count with predicted WBC counts of 9 [3–46] and 5 [1–23], respectively (p<0.0001).
We also calculated the 95th percentile of the unadjusted and adjusted WBC count to determine whether correcting traumatic specimens leads to values similar to those of non-traumatic specimens. For neonates without meningitis, the 95th percentile of the unadjusted WBC count for non-traumatic specimens was 30, while the 95th percentile of unadjusted WBC count for traumatic specimens was 800. Correcting traumatic specimens by the peripheral RBC:WBC, 500:1, and 1000:1 ratios led to 95th percentiles of WBC counts of 299, 440, and 220, respectively. For neonates with meningitis, the 95th percentile of unadjusted WBC count for non-traumatic specimens was 3,930, while the 95th percentile of unadjusted WBC count for traumatic specimens was 13,350. Correcting traumatic specimens by the peripheral RBC:WBC, 500:1, and 1000:1 ratios led to 95th percentiles of WBC counts of 13,346, 120, and 60, respectively.
DISCUSSION
Interpretation of the CSF WBC count is difficult in neonates without traumatic taps and further complicated in the presence of traumatic taps.16 We evaluated the diagnostic utility of adjusting the WBC in neonates after traumatic taps using previously described methods. The prevalence of traumatic LPs in our study, 39.5%, was higher than the 10–20% reported in previous studies of older patients9,17 but consistent with rates observed in prior studies of neonates.7,8 The prevalence of meningitis in our study, 1.8%, was lower than the 8–33% reported in previous studies in older patients describing CSF RBC counts13–15 but consistent with prior studies conducted in neonates.18,19
Although normal values for CSFWBC count vary according to postnatal age and gestational age, we chose our main WBC cut-off of 20 cells/mm3 in accordance with published reference values for term infants.20,21 In our cohort, the 95th percentile CSF WBC count was 21 cells/mm3 in LPs where the CSF RBC count was <10 cells/mm3. We also provided results for additional WBC cut-offs of 10 and 100 cells/mm3.
We found that the AUC for each of the methods of adjusting WBC counts were similar (Table 3). In a study of 104 traumatic LPs (>500 but <10,000 RBC/mm3) in children 1 month to 18 years of age with presumed viral or bacterial meningitis, the AUC using the 500:1 ratio method (0.81) was not significantly better than the use of the unadjusted WBC value (0.79).9 No prior studies have evaluated the prediction of meningitis using the peripheral blood RBC:WBC ratio correction in children or neonates, but several studies of adults and children > 1 month of age have examined the O:P ratio method. O:P ratios were found to be helpful in predicting meningitis, with sensitivities varying between 80–100% and specificities varying between 63–97% depending on the exact ratio used.13–15 The highest sensitivity obtained in this study (at a cut-off for the ratio of 1), however, was only 75%, and the specificity was low (55%). Increasing the cut-off to 10 further decreased the sensitivity (57%) while increasing the specificity to a value comparable to prior studies (93%).
We excluded from our analysis neonates positive for coagulase-negative Staphylococcus. Although this organism is sometimes considered a contaminant, it can be an pathogen in neonates, frequently occurring without abnormal CSF indices.22 To determine whether this exclusion made a significant impact on the results, we repeated the analysis with the 51 neonates with coagulase-negative Staphylococcus categorized as positive for meningitis. The median CSF WBC count was lower in this group, 6 [2–26], compared to neonates positive for meningitis with other organisms, 106 [12–1310]; however, the difference between the groups was not significant (p=0.77). When neonates with coagulase-negative Staphylococcus were included in the estimates of test performance, WBC count was a less effective diagnostic test (AUC = 0.69 (0.64–0.74)). In the traumatic LP group, adjustment of WBC count by the peripheral RBC:WBC ratio (AUC = 0.70 (0.62–0.77)) did not offer an advantage over the unadjusted value (AUC = 0.70 (0.63–0.77)).
Adjusting the WBC count to account for traumatic taps increases the specificity and positive likelihood ratio at the expense of sensitivity. Thus, WBC count adjustments lead to a reduction in false positives (patients without meningitis who unnecessarily receive treatment) but an increase in false negatives (missed cases of meningitis). The clinical implications of the results of our study can be illustrated by extrapolating to a hypothetical population of 1000 neonates with traumatic LPs and the same (1.8%) prevalence of bacterial or fungal meningitis as our cohort. If a WBC cut-off of 20 cells/mm3 is used, the unadjusted method results in 422 false positives, compared with 246 false positives with the 500:1 correction and 177 false positives with the peripheral RBC:WBC ratio correction. This improvement in specificity must be weighed against the fact that only 3 of the 18 cases of meningitis would be missed when the WBC count is not adjusted, compared with 5 missed cases with both the 500:1 and peripheral blood corrections.
It is possible that adjustment of the WBC count does not increase the AUC because none of the corrections led to an accurate approximation of the WBC count if the LP had not been traumatic. In our study, the 95th percentiles of adjusted WBC count values for infants with traumatic LPs failed to approximate the 95th percentiles of the unadjusted values for infants without traumatic LPs. A previous study of 682 traumatic LPs in children without meningitis found that the CSF WBCs predicted by 500:1 WBC:RBC ratio poorly matched the observed number of WBCs (r2=0.11).9 We found that both 500:1 and 1000:1 ratio methods led to an underestimation of the true number of WBCs present in the CSF from the peripheral blood. Therefore, correcting CSF WBC counts in patients with traumatic LPs using these methods would lead to an inflated estimate of the WBC count, resulting in more false positive cases than if the tap had not been traumatic.
Alternatively, in our study and in previous reports, correction of the CSF WBC count by the peripheral RBC:WBC overestimates the WBCs from the peripheral blood, leading to an underestimation of the true number of WBCs in the CSF.10–12 Using a WBC value corrected by this method could result in the masking of a true leukocytosis, and, consequently, in missed cases of meningitis. The degree of overcorrection is dependent on the number of RBCs present in the CSF: In patients with a CSF RBC count greater than 500 but less than 5000 cells/mm3, the estimated WBC count was actually slightly lower (median 4.7) than the true WBC count (median 8.0), but in patients with a CSF RBC count greater than 5000, the estimated WBC count (median 82) was substantially greater than the true WBC count (median 29). Thus, if the peripheral blood cell count correction is used, patients with very large amounts of blood in the CSF (>5000 RBCs/mm3) are at the greatest risk for having a missed case of meningitis.
The strengths of this study include the large sample size, our focus on the neonatal population, and the comparison of several methods of adjusting the CSF WBC count. This study is limited in that the source of our data is an administrative dataset and antibiotic exposure of the neonates could not be accurately determined. Including neonates with negative CSF findings as a result of prior antibiotic exposure in the meningitis negative group could have falsely decreased the performance of the diagnostic test. However, this effect would be present for both the unadjusted and adjusted tests and should not influence the difference between them. In addition, information regarding xanthochromic specimens and intracranial hemorrhages was incomplete. A higher prevalence of intracranial hemorrhage could have contributed to the higher rate of traumatic LPs observed in neonates < 3 days old.
Neonatal meningitis is a significant cause of morbidity and mortality. When CSF cultures are sterile, clinicians often rely on CSF indices such as WBC count for diagnosis. Although WBC counts are significantly higher in neonates with meningitis compared with those without meningitis, using the WBC count as a screening test is not useful. This emphasizes the need to perform a LP at the time meningitis is suspected in stable neonates, prior to initiation of antibiotic therapy, to improve the reliability of culture results. The results of this study indicate that adjustment of WBC counts in the setting of a traumatic LP does not aid in the diagnosis of bacterial and fungal meningitis in neonates.
Figure 1.
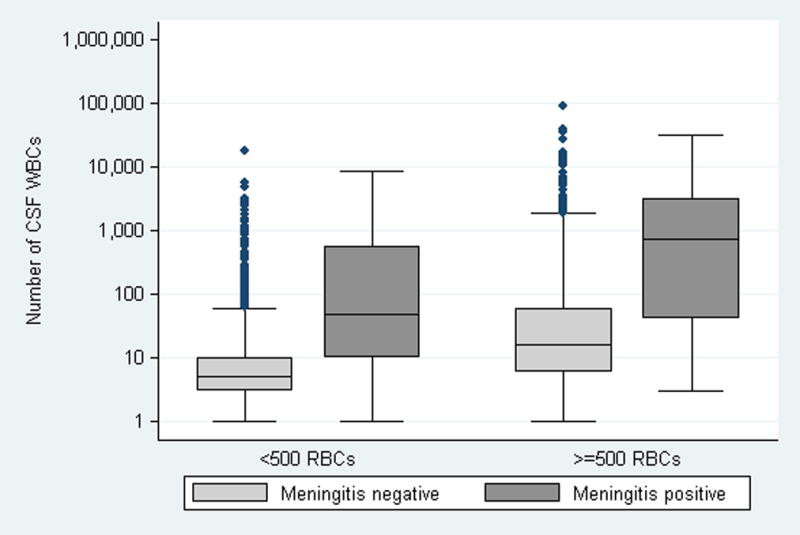
Number of WBCs in the CSF according to the presence or absence of meningitis and traumatic LP.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113(5):1181–6. doi: 10.1542/peds.113.5.1181. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Wenger JD, Hightower AW, Facklam RR, Gaventa S, Broome CV. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. The Bacterial Meningitis Study Group. J Infect Dis. 1990;162(6):1316–23. doi: 10.1093/infdis/162.6.1316. [DOI] [PubMed] [Google Scholar]
- 4.Klinger G, Chin CN, Beyene J, Perlman M. Predicting the outcome of neonatal bacterial meningitis. Pediatrics. 2000;106(3):477–82. doi: 10.1542/peds.106.3.477. [DOI] [PubMed] [Google Scholar]
- 5.Haworth JC. The diagnosis of acute meningitis in infancy. Lancet. 1953;1(19):911–4. doi: 10.1016/s0140-6736(53)92058-3. [DOI] [PubMed] [Google Scholar]
- 6.Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics. 2001;108(5):1169–74. [PubMed] [Google Scholar]
- 7.Pinheiro JM, Furdon S, Ochoa LF. Role of local anesthesia during lumbar puncture in neonates. Pediatrics. 1993;91(2):379–82. [PubMed] [Google Scholar]
- 8.Schreiner RL, Kleiman MB. Incidence and effect of traumatic lumbar puncture in the neonate. Dev Med Child Neurol. 1979;21(4):483–7. doi: 10.1111/j.1469-8749.1979.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonsu BK, Harper MB. Corrections for leukocytes and percent of neutrophils do not match observations in blood-contaminated cerebrospinal fluid and have no value over uncorrected cells for diagnosis. Pediatr Infect Dis J. 2006;25(1):8–11. doi: 10.1097/01.inf.0000195624.34981.36. [DOI] [PubMed] [Google Scholar]
- 10.Osborne JP, Pizer B. Effect on the white cell count of contaminating cerebrospinal fluid with blood. Arch Dis Child. 1981;56(5):400–1. doi: 10.1136/adc.56.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak RW. Lack of validity of standard corrections for white blood cell counts of blood-contaminated cerebrospinal fluid in infants. Am J Clin Pathol. 1984;82(1):95–7. doi: 10.1093/ajcp/82.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein JS, Yogev R. What represents pleocytosis in blood-contaminated (“traumatic tap”) cerebrospinal fluid in children? J Pediatr. 1985;107(2):249–51. doi: 10.1016/s0022-3476(85)80137-2. [DOI] [PubMed] [Google Scholar]
- 13.Mayefsky JH, Roghmann KJ. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med. 1987;82(6):1175–81. doi: 10.1016/0002-9343(87)90221-x. [DOI] [PubMed] [Google Scholar]
- 14.Bonadio WA, Smith DS, Goddard S, Burroughs J, Khaja G. Distinguishing cerebrospinal fluid abnormalities in children with bacterial meningitis and traumatic lumbar puncture. J Infect Dis. 1990;162(1):251–4. doi: 10.1093/infdis/162.1.251. [DOI] [PubMed] [Google Scholar]
- 15.Mazor SS, McNulty JE, Roosevelt GE. Interpretation of traumatic lumbar punctures: who can go home? Pediatrics. 2003;111(3):525–8. [PubMed] [Google Scholar]
- 16.Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094–100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 17.Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. Jama. 2002;288(16):2001–7. doi: 10.1001/jama.288.16.2001. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CE, Whitwell JK, Pethe K, Saxena K, Super DM. Term newborns who are at risk for sepsis: are lumbar punctures necessary? Pediatrics. 1997;99(4):E10. doi: 10.1542/peds.99.4.e10. [DOI] [PubMed] [Google Scholar]
- 19.Fielkow S, Reuter S, Gotoff SP. Cerebrospinal fluid examination in symptom-free infants with risk factors for infection. J Pediatr. 1991;119(6):971–3. doi: 10.1016/s0022-3476(05)83058-6. [DOI] [PubMed] [Google Scholar]
- 20.Gunn VL, Nechyba C Johns Hopkins Hospital. Children’s Medical and Surgical Center. The Harriet Lane handbook: a manual for pediatric house officers. 16. xiv. Philadelphia: Mosby; 2002. p. 1036. [1] folded leaf p. [Google Scholar]
- 21.Ahmed A, Hickey SM, Ehrett S, et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J. 1996;15(4):298–303. doi: 10.1097/00006454-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gruskay J, Harris MC, Costarino AT, Polin RA, Baumgart S. Neonatal Staphylococcus epidermidis meningitis with unremarkable CSF examination results. Am J Dis Child. 1989;143(5):580–2. doi: 10.1001/archpedi.1989.02150170082027. [DOI] [PubMed] [Google Scholar]


