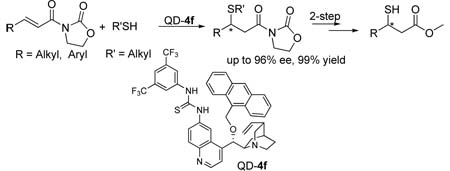
Abstract
In this communication, we describe an unprecedented highly enantioselective catalytic conjugate addition of simple alkyl thiols to α,β-unsaturated N-acylated oxazolidin-2-ones catalyzed by acid-base bifunctional catalysis. This reaction provides a useful catalytic method for the synthesis of optically active chiral sulfur compounds that are otherwise difficult to prepare by asymmetric catalysis. The successful development of this reaction resulted from a discovery that, upon proper modification, a cinchona alkaloid bearing a thiourea functionality at 6’ position can afford highly efficient catalysis for asymmetric conjugate additions.
Optically active chiral thiols and sulfides have seen broad applications as biologically interesting compounds,1 ligands for metallic catalysts,2 organic catalysts3 and chiral auxiliaries.4 The asymmetric conjugate additions with sulfur-nucleophile, or sulfa-Michael additions, constitute a direct and versatile approach towards optically active chiral sulfur compounds. This strategy is particular valuable, since enantioselective nucleophilic additions to carbon-sulfur double bond, unlike those to carbonyls and imines, are not synthetically viable.5 Accordingly considerable efforts have been devoted to the development of catalytic enantioselective sulfa-Michael reactions.
Several effective chiral metallic and organic catalysts have been developed for conjugate additions of aryl thiols to various Michael acceptors.6 However, the development of effective catalysts for asymmetric sulfa-Michael reactions involving the less active but synthetically more useful simple alkyl thiols remains a significant challenge.7 To date only iminium catalysis by chiral secondary amines has afforded high enantioselectivity for conjugate additions of simple alkyl thiols to α,β-unsaturated aldehydes and ketones.8 Although providing a significant breakthrough in the asymmetric synthesis of optically active sulfur compounds, due to the sensitive nature of thiols and sulfides toward oxidations, these reactions do not provide straightforward access to valuable chiral sulfur compounds such as β-mercapto acid derivatives,9 which contain functionalities of higher oxidation state. Currently, such thiol compounds are only accessible by chiral auxiliary-directed conjugate additions.10 Herein we report the use of acid-base bifunctional catalysts to realize a highly enantioselective sulfa-Michael reaction of simple alkyl thiols to α,β-unsaturated N-acylated oxazolidin-2-ones, an α,β-unsaturated carboxylic acid derivative. Being complementary in scope to existing catalytic asymmetric sulfa-Michael reactions of alkyl thiols, this reaction provides a uniquely valuable method for the enantioselective synthesis of chiral sulfur compounds.
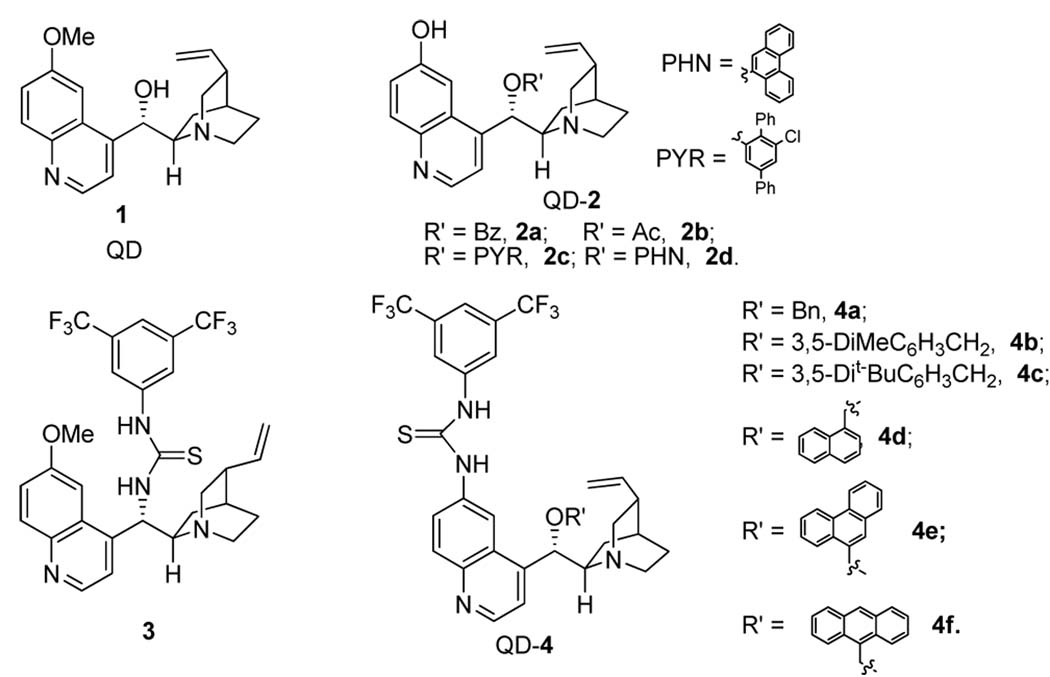
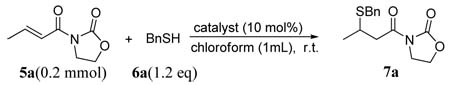
Cinchona alkaloids have a venerable history in the development of enantioselective catalytic sulfa-Michael reactions.11 However, it is until recently that a modified cinchona alkaloid were found to afford useful level of enantioselectivity for an asymmetric conjugate additions with aryl thiols.6c Later Acid-base bifunctional catalysis by cinchona alkaloids has also been extended to a sulfa-Michael reaction of aryl thiols to α,β-unsaturated carboxylic acid derivatives.12 However, our attempts to identify an effective catalyst from literature-reported cinchona alkaloid catalysts for the addition of benzyl thiol (6a) to α,β-unsaturated N-acylated oxazolidin-2-one 5a were unsuccessful. As summarized in Table 1, natural cinchona alkaloids such as quinidine failed to promote the conjugate addition (entry 1, Table 1). Other bifunctional cinchona alkaloids such as the 6’-OH cinchona alkaoids 2 and 9-thiourea cinchona alkaloid 3 afforded only modest enantioselectivity for the addition of 6a to 5a, although both have been shown to be highly effective catalysts for the promotion of numerous asymmetric conjugate additions involving a wide range of donors and acceptors.13,14,15
Table 1.
Conjugated addition of 6a to 5a with Cinchona Alkaloidsa
 | ||||
|---|---|---|---|---|
| entry | catalyst | time(h) | conv(%)b | ee(%)c |
| 1 | 1 | 24 | 0 | N.D. |
| 2 | 2a | 24 | 5 | 47 |
| 3 | 2b | 24 | 11 | 40 |
| 4 | 2c | 24 | 40 | 44 |
| 5 | 2d | 24 | 15 | 49 |
| 6 | 3 | 24 | 95 | 45 |
| 7 | 4a | 5 | >99 | 68 |
| 8 | 4b | 5 | >99 | 73 |
| 9 | 4c | 5 | >99 | 70 |
| 10 | 4d | 5 | >99 | 80 |
| 11 | 4e | 5 | >99 | 84 |
| 12 | 4f | 5 | >99 | 85 |
See Supporting Information (SI) for details,
Conversion was determined by 1H NMR.
Ee was determined by HPLC (column OD) analysis (see Supporting Information).
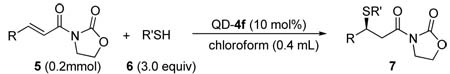
We noticed that the acidity of the hydrogen bond donor motifs in cinchona alkaloids 1–3 has a significant impact on their catalytic activity (entries 1–6, Table 1). The activity of the catalyst improves as the acidity of the hydrogen bond donor increases. Although the enantioselectivity obtained with cinchona alkaloids 1–3 were not promising, results from our initial studies suggested that further investigations should focus on cinchona alkaloids bearing a strong hydrogen bond donor and a readily tunable handle. Thus we began to synthesis and screen various 6’-thiourea cinchona alkaloids 4, although such catalysts had never been shown to be effective catalyst for asymmetric conjugate addition reactions.16 Indeed, the 6’-thiourea cinchona alkaloids 4 afforded considerably better activity than that by catalysts 1–3 (entries 7–12 vs 1–6). Furthermore, the structure of the 9-substituent was found to have a considerable influence on the enantioselectivity of 4. Our catalyst screening and tuning studies led to the discovery that 4f, a cinchona alkaloid not yet reported in the literature, could afford promising enantioselectivity at room temperature (entry 12). By lowering the reaction temperature to −20 °C, the 4f-catalyzed reaction of 6a and 5a generated the corresponding 1,4-adduct 7a in 94% ee and nearly quantitative yield (entry 1, Table 2).
Table 2.
Conjugated addition of 6 to 5 with 4fa
 | |||||
|---|---|---|---|---|---|
| entry | R | R' | t(°C) | yield(%)b | ee(%)c |
| 1 | Me (5a) | Bn (6a) | −20 | 98 | 94 |
| 2 | Me (5a) | 4-ClBn (6b) | −20 | 99 | 93 |
| 3 | Me (5a) | 4-OMeBn (6c) | −20 | 99 | 96 |
| 4 | Me (5a) | C6H5CH2CH2(6d) | −20 | 98 | 92 |
| 5 | Me (5a) | TBSOCH2CH2 (6e) | −20 | 97 | 93d |
| 6 | Me (5a) | TESOCH2CH2 (6f) | −20 | 95 | 92 |
| 7 | Me (5a) | TMSCH2CH2 (6g) | −20 | 92 | 92 |
| 8 | Me (5a) | i-Bu(6h) | −20 | 91 | 90 |
| 9 | Me (5a) | Cyclopentyl(6i) | −20 | 96 | 94 |
| 10 | Me (5a) | Allyl(6j) | −20 | 97 | 94 |
| 11 | Et (5b) | 4-OMeBn (6c) | −20 | 98 | 91 |
| 12 | n-Pr (5c) | 4-OMeBn (6c) | −20 | 96 | 93 |
| 13 | i-Pr(5d) | 4-OMeBn (6c) | −20 | 98 | 93 |
| 14 | n-penta (5e) | 4-OMeBn (6c) | −20 | 96 | 95 |
| 15 | n-hex (5f) | 4-OMeBn (6c) | −20 | 97 | 94 |
| 16 | n-hepta (5g) | 4-OMeBn (6c) | −20 | 99 | 93 |
| 17 | C6H5(5h)e | 4-OMeBn (6c) | −50 | 95 | 87 |
| 18 | C6H5(5h)e | Allyl(6j) | −50 | 93 | 94 |
| 19 | 4-ClC6H5(5i)e | Allyl(6j) | −50 | 84 | 93 |
| 20 | 3-FC6H5(5j)e | Allyl(6j) | −50 | 94 | 92 |
| 21 | 2-BrC6H5(5k)e | Allyl(6j) | −50 | 91 | 96 |
Unless noted, reactions were carried out at −20 °C for 72 hrs.
Isolate yield;
Determined by HPLC analysis (see Supporting Information);
Absolute configuration of the 1,4-adduct 7 was determined to be R, for details, see supporting information;
catalyst loading : 20 mol%.
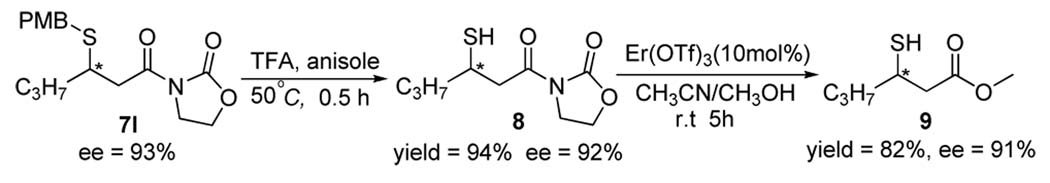
We were pleased to find that the high efficiency demonstrated by 4f for the model reaction could be sustained for reactions employing a broad range of alkyl thiols. As summarized in Table 2 (entries 1–10), in the presence of 4f, reactions with alkyl thiols containing either an aromatic or simple aliphatic group consistently proceeded in excellent enantioselectivity. Even secondary thiols, either acyclic (6h) or cyclic (6i), could be applied in the reaction. The presence of various functional groups in the alkyl thiols is well tolerated by the catalyst. Importantly, the catalyst also demonstrated a considerable latitude in accommodating steric as well as electronic variations of the β-substituent of the Michael acceptor 5 (entries 11–21). In particular the reaction accommodates both β-alkyl and –aryl substituent, and afforded similarly high enantioselectivity for γ-branched or –unbranched Michael acceptors (entry 13 vs 12). As demonstrated in the conversion of the 1,4-adduct 7l to a β-mercapto ester (9), this new catalytic enantioselective reaction provided a facile entry into chiral sulfur compounds bearing a functional group of high oxidation state such as carboxylic acid derivatives.
In summary we have developed an unprecedented catalytic enantioselective conjugate addition of simple alkyl thiols 6 to α,β-unsaturated N-acylated oxazolidin-2-ones 5. The reaction allows the employment of a wide range of alkyl thiols while tolerates a considerable degree of variations of the Michael acceptor. Consequently, the current reaction provides a useful catalytic method for the synthesis of optically active chiral sulfur compounds that are otherwise difficult to prepare by asymmetric catalysis. Notably, the current study also revealed that, upon suitable tuning, the 6’-thiourea cinchona alkaloids 4 could afford efficient catalysis for asymmetric conjugate additions that cannot be promoted in comparable efficiency with existing catalysts.
Supplementary Material
Experimental procedures and characterization of the products. This material is available free of charge via the internet at http://pubs.acs.org.
Figure 1.
Cinchona alkaloids 1–4
Scheme 1.
Asymmetric synthesis of β-mercapto ester 9
Acknowledgment
We are grateful for financial support from National Institute of Health (GM-61591). We acknowledge Brandeis University Mass Spectrometry Facility(BUMS) for MS analysis.
References
- 1.(a) Chatgilialoglu C, Asmus K-D. Sulfur-Centered Reactive Intermediates in Chemistry and Biology. New York: Springer; 1991. [Google Scholar]; (b) Fraústo da Silva JR, Williams RJP. The BiologicalChemistry of the Elements. New York: Oxford University Press; 2001. [Google Scholar]
- 2.(a) Zhou QL, Pfaltz A. Tetrahedron. 1994;50:4467. [Google Scholar]; (b) Kang J, Kim JB, Kim JW, Lee D. J . Chem. Soc., Perkin Trans. 1997;2:189. [Google Scholar]; (c) Jin M-J, Ahn S-J, Lee K-S. Tetrahedron Lett. 1996;37:8767. [Google Scholar]; (d) Anderson JC, Harding M. Chem. Commun. 1998:393. [Google Scholar]
- 3.Aroyan CE, Miller SJ. J. Am. Chem. Soc. 2007;129:256. doi: 10.1021/ja067139f. [DOI] [PubMed] [Google Scholar]
- 4.(a) Fanjul S, Hulme AN, White JW. Org. Lett. 2006;8:4219. doi: 10.1021/ol0614774. [DOI] [PubMed] [Google Scholar]; (b) Crimmins MT, King BW, Tabet EA, Chaudhary K. J. Org. Chem. 2001;66:894. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]
- 5.For approaches involving catalytic desymmetrization of meso compounds, see: Wu MH, Jacobsen EN. J. Org. Chem. 1998;63:5252. doi: 10.1021/jo981332d.
- 6.(a) Emori E, Arai T, Sasai H, Shibasaki M. J. Am. Chem.Soc. 1998;120:4043. [Google Scholar]; (b) Emori E, Iida T, Shibasaki M. J. Org. Chem. 1999;64:5318. doi: 10.1021/jo9904922. [DOI] [PubMed] [Google Scholar]; (c) McDaid P, Chen Y, Deng L. Angew. Chem. Int. Ed. 2002;41:338. doi: 10.1002/1521-3773(20020118)41:2<338::aid-anie338>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; (d) Wang W, Li H, Wang J, Zu L. J. Am. Chem. Soc. 2006;128:10354. doi: 10.1021/ja063328m. [DOI] [PubMed] [Google Scholar]; (e) Leow D, Lin S, Chittmalla SK, Fu X, Tan C-H. Angew. Chem. Int. Ed. 2008;47:5641. doi: 10.1002/anie.200801378. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kobayashi S, Ogawa C, Kawamura M, Sugiura M. Synlett. 2001:983. [Google Scholar]; (b) Kobayashi N, Iwai K. J. Org. Chem. 1981;46:1823. [Google Scholar]; (c) Abe AMM, Sauerland SJK, Koskinen AMP. J. Org. Chem. 2007;72:5411. doi: 10.1021/jo070492z. [DOI] [PubMed] [Google Scholar]; For a review, see: Enders D, Luttgen K, Narine A. Synthesis. 2007;7:959.
- 8.(a) Marigo M, Schulte T, Franzén J, Jørgensen KA. J. Am. Chem. Soc. 2005;127:15710. doi: 10.1021/ja055291w. [DOI] [PubMed] [Google Scholar]; (b) Brandau S, Maerten E, Jørgensen KA. J. Am. Chem. Soc. 2006;128:14986. doi: 10.1021/ja065507+. [DOI] [PubMed] [Google Scholar]; (c) Ricci P, Carlone A, Bartoli G, Bosco M, Sambri L, Melchiorre P. Adv. Synth. Catal. 2008;350:49. [Google Scholar]
- 9. Lee AHF, Chan Chan ASC, Li T. Tetrahedron. 2003;59:833. For applications of β-mercapto carboxylic acids in syntheses of nature products and bioactive peptide inhibitors, see: Beszant B, Bird John, Gaster LM, Harper GP, Hughes I, Karran EH, Markwell RE, Miles-Williams AJ, Smith SA. J. Med. Chem. 1993;36:4030. doi: 10.1021/jm00077a006. for applications in the synthesis of polymers, see: Tanaka S, Feng L, Inoue Y. Polymer Journal. 2004;36:570. Luetke-Eversloh T, Fischer A, Remminghorst U, Kawada J, Marchessault RH, Boegershausen A, Kalwei M, Eckert H, Reichelt R, Liu SJ, Steinbuechel A. Nature Materials. 2002;1:236. doi: 10.1038/nmat773.
- 10.(a) Palomo C, Oiarbide M, Dias F, Ortiz A, Linden A. J. Am. Chem. Soc. 2001;123:5602. doi: 10.1021/ja015860+. [DOI] [PubMed] [Google Scholar]; (b) Palomo C, Oiarbide M, Dias F, López R, Linden A. Angew. Chem. Int. Ed. 2004;43:3307. doi: 10.1002/anie.200453889. Angew. Chem. 2004, 116, 3369. [DOI] [PubMed] [Google Scholar]
- 11.(a) Hiemstra H, Wynberg H. J. Am. Chem. Soc. 1981;103:417. [Google Scholar]; (b) Wynberg H. Top. Stereochem. 1986;16:97. [Google Scholar]
- 12.Zou L, Wang J, Li H, Xie H, Jiang W, Wang W. J. Am. Chem. Soc. 2007;129:1036. doi: 10.1021/ja067781+. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Wang Y, Tang L, Deng L. J. Am. Chem. Soc. 2004;126:9906. doi: 10.1021/ja047281l. [DOI] [PubMed] [Google Scholar]; For a review, see: Marcelli T, van Maarseveen JH, Hiemstra H. Angew. Chem. Int. Ed. 2006;45:7496. doi: 10.1002/anie.200602318.
- 14.Vakulya B, Varga S, Csa´mpai A, Soo´s T. Org. Lett. 2005;7:1967. doi: 10.1021/ol050431s. [DOI] [PubMed] [Google Scholar]; For a review, see: Connon SJ. Chem. Commun. 2008:2499. doi: 10.1039/b719249e.
- 15.For a review, see: Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r.
- 16.Catalyst 4a was reported as an effective catalyst for asymmetric Henry reactions, see: Marcelli T, van der Haas RNS, van Maarseveen JH, Hiemstra H. Angew. Chem. Int. Ed. 2006;45:929. doi: 10.1002/anie.200503724.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and characterization of the products. This material is available free of charge via the internet at http://pubs.acs.org.