Abstract
S100A8 and S100A9 and their heterocomplex calprotectin (S100A8/A9) are abundant cytosolic constituents in human neutrophils previously shown to possess antifungal activity. This study was designed to investigate mechanisms involved in the modulation of the antifungal properties of S100A8/A9. S100A8, S100A9 and site-directed mutants of both proteins were tested for their antifungal effect against Candida albicans in microplate dilution assays. Whereas S100A8 alone did not inhibit fungal growth, S100A9 by itself had a moderate antifungal effect. Combining both proteins had the strongest effect. Supporting a potential role for oxidation in S100A8/A9, substitution of methionine 63 or 83 of S100A9 resulted in the loss of antifungal activity. Additionally, the substitution to alanine of cysteine 42 of S100A8 also caused a loss of S100A8’s ability to enhance S100A9’s antifungal effect. Overall, our data indicates that both S100A8 and S100A9 are required for their fully active antifungal effect and that oxidation regulates S100A8/A9 antifungal activity through mechanisms that remain to be elucidated and evaluated. Finally, together with our previous work describing the oxidation sensitive anti-inflammatory effects of S100A8/A9, we propose that S100A8/A9 exerts an anti-inflammatory activity in healthy state and that conditions associated with oxidative stress activate the antifungal activity of S100A8/A9.
Keywords: Candida albicans, antifungal, calprotectin, S100A8, S100A9, oxidation, innate immunity
INTRODUCTION
Candida albicans (C. albicans) is the most common fungal pathogen in humans (15, 16). Overgrowth or invasion of the host by this commensal organism may initiate diseases ranging from superficial mucosal infections such as oral thrush to life-threatening systemic illness. Serious infections with this opportunistic pathogen are increasingly observed in immunocompromised patients (1). The role of innate immunity in control of candidiasis has been extensively studied (3).
Neutrophils, key cellular components of the innate immune system, can exert antimicrobial activity by oxidative and non-oxidative mechanisms (13). The former is characterized by the respiratory burst, a process whereby molecular oxygen is used to produce reactive oxygen species with direct antimicrobial effect. The non-oxidative mechanisms include the production and release of proteins with antimicrobial activity. The most abundant cytoplasmic protein in neutrophils is calprotectin (referred in this manuscript as S100A8/A9). The antimicrobial activity of S100A8/A9 has been confirmed in many pathogenic microorganisms (21), including C. albicans (14).
S100A8/A9, an important component of the innate immune response is a complex of two small, acidic calcium-binding proteins designated as S100A8 and S100A9. S100A8/A9 is produced by neutrophils, monocytes and epithelial cells and it is found in inflammatory exudates and saliva. Epithelial expression of the two S100 proteins is observed in response to stress while myeloid cells constitutively express the S100 proteins.
Its interactions with leukocytes at sites of inflammation and infection are not completely understood.
Other than its antimicrobial activity (21), the immunologically relevant activities of S100A8/A9 include regulation of leukocyte trafficking (11, 17, 19, 20), the transport of arachidonic acid (7) and the induction of NADPH oxidase (2). S100A8/A9 and its components are functionally regulated by their quaternary structure (12), their oxidation (11, 19, 20) and/or divalent cations binding (24).
A consensus HXXXH domain shared by S100A8 and S100A9 (10) allow both proteins to bind divalent cations. The pro-apoptotic activity of S100A8/A9 (4), its interaction with arachidonic acid (8), and its growth-inhibitory effect in C. albicans are all influenced by divalent metal cations. For instance, zinc has been shown to reverse the fungistatic effect of S100A8/A9 and zinc deprivation may be the mechanism of action of S100A8/A9’s fungistatic effect (18) (14).
While the domain involved in zinc chelation (HXXXH) is present at the carboxyl tail of both S100A8 and S100A9, work done by others (14, 18) has shown that both proteins are required for a maximal fungistatic effect. It has been suggested that the formation of tetramers of S100A8 and S100A9 lead to enhanced zinc binding capacity (10) and in fact may be required for S100A8/A9- driven fungistasis. Whether S100A8 and S100A9 possess fungistatic activity independent of each other or not remains unclear. Murthy et al (14) have shown that HPLC fractions of native S100A8/A9 containing S100A9 exhibit fungistatic activity, while fractions depleted of S100A9 but enriched for S100A8 lacked fungistatic activity. Conversely, Sohnle et al (18) did not detect any fungistatic activity with either protein on their own.
Other than the binding of divalent cations and the formation of higher order heterocomplexes (12), oxidation has also been shown to functionally regulate immune modulation by S100A8/A9. Data previously published by others (5) and confirmed in our laboratory (19) indicate that oxidation of an interspecies-conserved residue on S100A8 (cysteine 42) regulates the effect of this protein on leukocyte trafficking. The effect of S100A9 on neutrophil migration also is regulated by oxidation (20). Specifically, our previous work has shown that oxidation of inter-species conserved methionine 63 or 83 but not 81 inhibits the fugetactic effect of S100A9 on neutrophils in-vitro.
In the present study, the effect of oxidation on the antifungal activity of S100A8/A9 was evaluated. We hypothesized that oxidation regulates the antifungal effect of S100A8/A9 and is required for S100A8/A9 antifungal activity in-vitro. First, the antifungal effect of S100A8 and S100A9 alone or in combination was tested. Next, the antifungal effect of S100A8/A9 was examined after the substitution to alanine of methionine 63 or 83 and 61 (on S100A9) and of cysteine 42 (on S100A8). Finally, to test the potential direct effect of the above site-directed substitutions on the quaternary complex of S100A8/A9, we investigated formation of S100A8/A9 heterocomplexes by wild type and mutated protein.
MATERIALS AND METHODS
Recombinant proteins: expression, production and purification
Expression, production and purification of S100A8, S100A9 and site-directed mutants of both proteins have been described elsewhere (20, 22). Briefly, top10F’ E-coli cells (Invitrogen, Carlsbad, CA) transfected with the different pGEX2T constructs were used for production of recombinant proteins. Standard GST protocols from Amersham (Amersham, Piscataway, NJ) were followed. Recombinant proteins were cleaved from their GST tag with Thrombin (Amersham, Piscataway, NJ).
Microdilution plate growth inhibition assays
C. albicans strain SC5314 was used in all experiments. SC5314 was propagated in YPD or YNB+CSM+2% glucose. Growth inhibition of C. albicans by S100A8/A9 or individual S100A8/A9 peptides was assayed in microdilution plates. The assay was adapted from previously published NCCLS methods (9). RPMI1640 without sodium bicarbonate and with L-glutamine (INVITROGEN, Carlsbad, CA), was buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS), adjusted to a glucose concentration of 2% and prepared as sterile filtered 2X solution. 100 μl were added to each microdilution well, then the assay medium was diluted to 1x by addition of C. albicans cells (1000 cells/ml) and S100 proteins in a combined volume of 100 μl sterile, pyrogen-free H2O. Candida cells were inoculated from stationary phase cultures in YNB+CSM+2% glucose. Negative controls did not contain proteins or fungal cells. Fungal growth was assayed overnight. Cell counts were determined through optical density reading at OD600 after 24hrs of growth at 30°C. To determine the minimal inhibitory concentration (MIC), a dilution series of S100 proteins in water was prepared.
Formation of heterocomplex
1 μg of S100A8, S100A9 or mutant proteins were mixed in PBS. The protein mixture was heated to 40°C for five minutes and left to cool at air temperature. The protein mixture was separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and stained with coomassie blue stain. Confirmation of the heterocomplex band identity was done by standard western blot with a monoclonal antibody for S100A8/A9 (27E10 Bachem, Torrance, CA) complex which recognizes an epitope not found in individual S100 subunits. Immunoprecipitation of the S100A8/A9 heterocomplex was conducted by adding 1 μl of the monoclonal antibody 27E10 to 5 μg each of S100A8 and S100A9 protein which were incubated together at 4C overnight. Precipitation was conducted with protein G plus agarose (Santa Cruz Biotechnology, Santa Cruz, CA) according to manufacturer instructions.
Statistical Analyses
Single sample t-tests were calculated to test the S100A8, S100A9 and combined proteins in comparison to the control wells with alpha set to ≤ 0.05 to determine statistical significance.
RESULTS
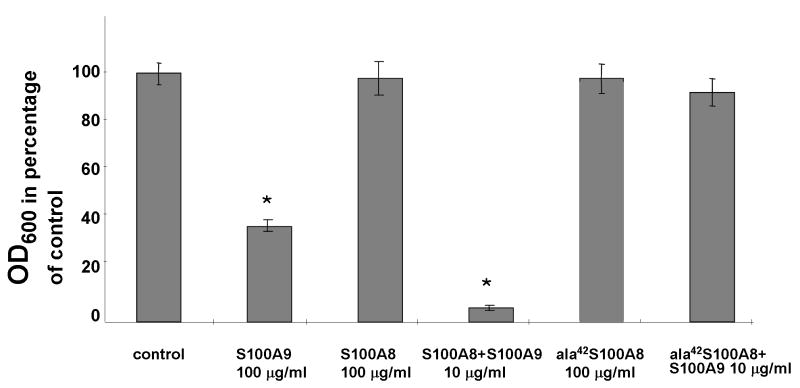
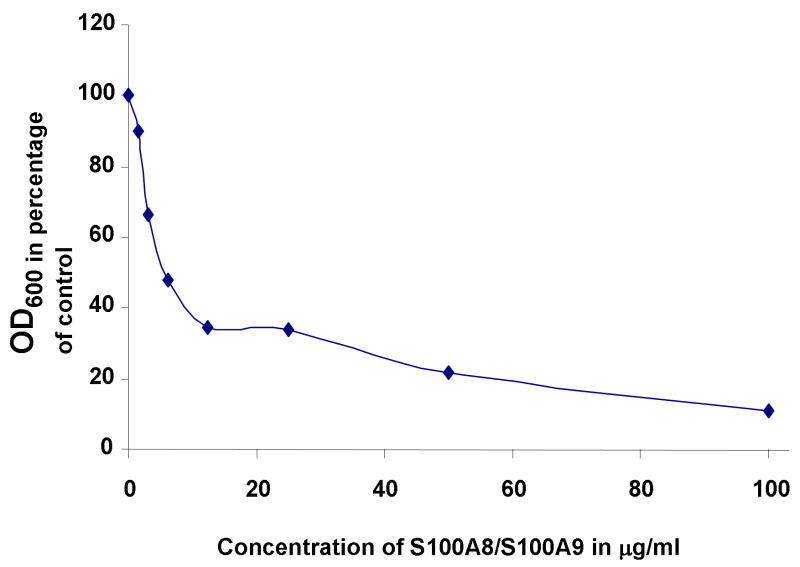
To address the question whether both components of S100A8/A9 are required to inhibit fungal growth, S100A8, S100A9 and both proteins in combination were tested in a microdilution plate assay. Neither S100A8 nor S100A9 significantly inhibited fungal growth at concentrations below 100 μg/ml (data not shown). At 100 μg/ml, S100A9 inhibited fungal growth (tdf2 = −23.019, p < 0.01) while S100A8 alone was inactive at all concentrations tested. (Fig. 1) In contrast, S100A8 and S100A9 together were highly active at inhibiting fungal growth at 10 μg/ml (tdf2 = −62.844, p < 0.001 at 10 μg/ml). Dose response analysis of the antifungal effect of S100A8 and S100A9 added in combination inhibited fungal growth at a concentration of 20 μg/ml with a MIC50 of 6.5 μg/ml as calculated from microplate dilution assays (Fig. 2)
Figure 1. Inhibition of Candida albicans growth by S100A8, S100A9 and S100A8/A9 complex.
Recombinant S100A8, S100A9 and ala42S100A8 were added separately and in combination to 96-well plates in order to test their ability to inhibit the growth of Candida albicans. The plates were incubated at 30°C overnight. 100 μg/ml of S100A9 demonstrate growth inhibition whereas the same concentration of S100A8 shows no effect. S100A8 and S100A9 together exert a strong growth inhibition at 10 μg/ml. Ala42S100A8 on it own or in combination with S100A9 had no effect on yeast growth. *p<0.01 (Single sample t-test, compared with untreated control). The data is representative of experiments conducted in triplicate a minimum of four times.
Figure 2. Dose response to the inhibition of Candida albicans growth by S100A8/S100A9.
Recombinant S100A8 and S100A9 were added at different concentration. The dose response curve allowed for the calculation of a MIC50 of 6.5 μg/ml of combined S100A8 and S100A9 proteins.
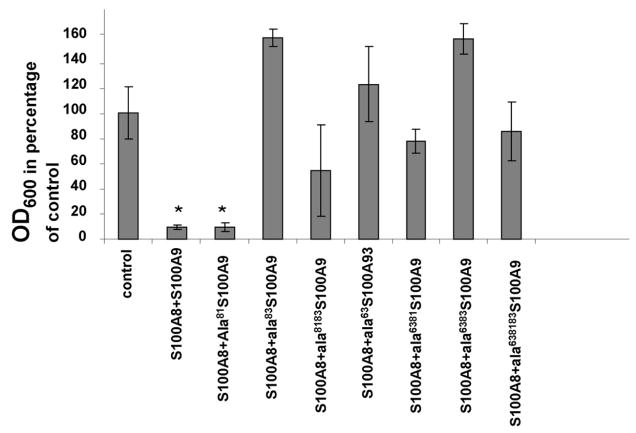
Next, we tested the hypothesis that oxidation regulated the fungistatic effect of S100A8/A9. Based on our previous work, specific residues previously shown to regulate the S100A8 and S100A9 effect on leukocyte migration were substituted with alanine (19, 20). First, the cysteine 42 of S100A8 was substituted with alanine. When tested in microdilution assays, ala42S100A8 did not enhance the fungistatic effect of S100A9 alone (Fig. 1) so that the substitution of cysteine 42 to alanine abrogated the enhancement of S100A9 fungistatic effect by WTS100A8.
Methionine residues conserved in mouse, rat and human S100A9 at positions 63, 81 and 83 were replaced with alanine. The growth of C. albicans was tested in microdilution plates in the presence of WTS100A8 and different site- directed mutants of S100A9. Except for the mutant of S100A9 in which methionine 81 was substituted to alanine, all S100A9 mutants displayed a loss of antifungal activity (Fig. 3). The data showed that substitution to alanine of either methionine 63 or 83 resulted in a significant decreased or in a loss of the antifungal effect (Figure 3).
Figure 3. Inhibition of Candida albicans growth by S100A8, S100A9 and S100A8/A9 complex.
Recombinant S100A8, S100A9 and site-directed mutants of S100A9 were tested for their fungal growth. 10 μg/ml of S100A8 added to 10 μg/ml of wild type or mutant S100A9 was tested for their ability to inhibit fungal growth. The data indicate that substitution to alanine of either methionine 63 or 83 results in a loss of antifungal effect whereas substitution of methionine 81 does not result in the same loss of function. *p<0.01 (Single sample t-test, compared with untreated control). The data is representative of experiments conducted in triplicate a minimum of four times.
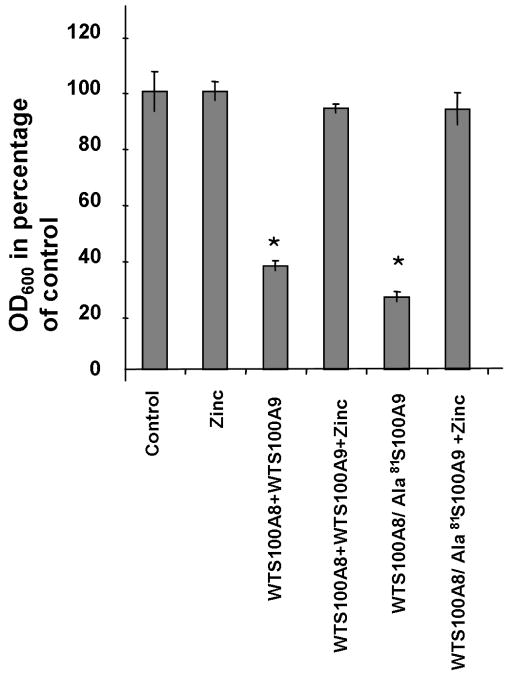
Previous reports have indicated that zinc inhibits the antifungal activity of S100A8/A9 (18). We confirmed this finding by testing the effect of zinc chloride (25 μM) on the antifungal activity of WT S100A9 and met81S100A9 together with WT S100A8. The data showed (Figure 4) that both proteins lost their antifungal activity with the addition of zinc chloride.
Figure 4. Inhibition by zinc Chloride of the antifungal effect of S100A8/A9 on Candida albicans.
Recombinant S100A8, S100A9 and Ala81S100A9 were tested in combination in the presence or absence of 25 μM zinc chloride supplementation. The data indicate that the addition of zinc on its own had no effect on fungal growth but that zinc inhibited the observed antifungal effect of WT and ala81S100A9 in combination with WT S100A8. The data is representative of experiments conducted in triplicate a minimum of three times.
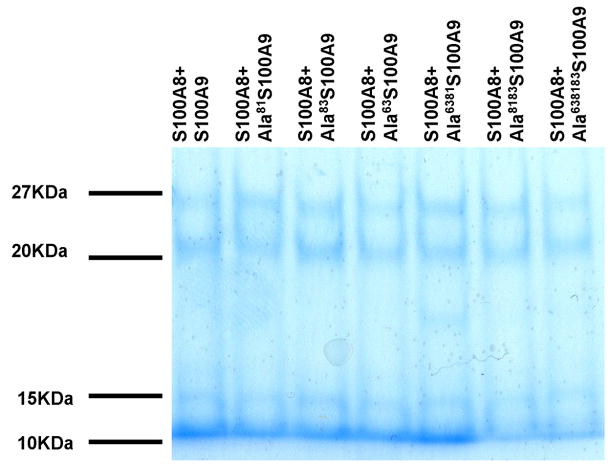
Finally, we compared the ability of mutant and wild type S100A8 and S100A9 to form heterocomplexes. We first found that WTS100A8 and WTS100A9 did not form heteromers after simple mixing of equimolar amounts of the proteins and independently of calcium concentration (data not shown). The two proteins, however, did form heterodimers after a short denaturing heat treatment at 40°C. The data showed that mutation of methionine residues, which resulted in a loss of antifungal activity, did not affect the ability of the mutated S100A9 protein to form heterodimers with S100A8 (Fig. 5A). Conversely, substitution of alanine 42 in S100A8 did inhibit the formation of S100A8/S100A9 heterodimers. Identity of the heterodimer of S100A8/A9 was confirmed by western blot analysis (Figure 5B). To confirm the ability of S100A9 mutated protein to form complexes with S100A8, we immuno-precipitated WT and mutated S100A9 mixed 1:1 with S100A8 with an antibody previously shown to recognize an epitope formed in the heteromer of S100A8/A9 not present in S100A8 or S100A9 alone (6). The data showed that WT and mutated S100A9 could in the presence of S100A8 be precipitated by a S100A8/A9 heterocomplex antibody (Figure 5C). S100A8 or S100A9 alone could not be precipitated (data not shown).
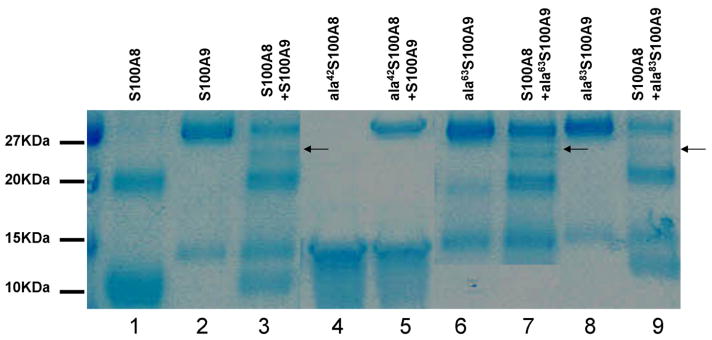
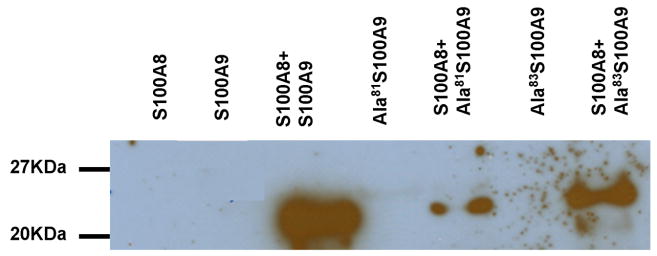
Figure 5. Coomassie stain SDS polyacrylamide gel and western blot of recombinant S100A8 and S100A9 proteins.
A. S100A8 and S100A9 form monomers and dimers. When together S100A8 and S100A9 for an additional quaternary structure (lane 3 marked with arrow). Ala42S100A8 does not form dimers (lane 4) or heterocomplexes with S100A9 (lane 5). Ala63S100A9 and ala83S100A9 form heterocomplexes with S100A8 (lane 7 and 9 respectively) similar to the ones form by WTS100A9 (lane 3) B. An antibody which recognizes an epitope present in heterocomplexes of S100A8 and S100A9 demonstrate that WTS100A9, ala81S100A9 and Ala83S100A9 form heterocomplexes of S100A8/A9 when mixed with WTS100A8. C. Immunoprecipitation of S100A8/S100A9 heterocomplexes with a monoclonal antibody against an epitope formed by the heterocomplex. S100A9 and mutant S100A9 precipitate in the presence of S100A8. Both S100A8 and S100A9 form monomers and dimers as in panel A.
DISCUSSION
The present study was undertaken to investigate the molecular mechanisms that regulate the anti-fungal properties of S100A8/A9 and its constituent proteins. The data indicated that S100A8 has no antifungal affect on its own at the concentrations tested but that S100A9 has an antifungal effect that is further enhanced by S100A8. Therefore, S100A8/A9 is likely regulated by the formation of specific heteromers in which S100A9 would be potentiated by stabilization afforded by S100A8 as has been suggested of the tetramer of S100A8 and S100A9 (10).
Our data indicated that substitution to alanine of either methionine 63 or 83 in S100A9 or cysteine 42 in S100A8 resulted in a loss on antifungal activity. Substitution of methionine 81 did not caused the same functional loss. Our previous work indicated that substitution to alanine of methionine 63 and 83 (but not 81) in S100A9 or cysteine 42 in S100A8 resulted in a fugetactic molecule with decreased functional sensitivity to oxidation. The data presented herein indicate that the same substitutions result in loss of the antifungal effect of S100A8/A9, suggesting a role for oxidation in regulating S100A8/A9’s fungal activity.
WTS100A8 and WTS100A9 did not form heterocomplexes unless first denatured by heat. This finding is in accordance with data published by others (23). Vogl et al have first reported the need for a denaturing and renaturing steps for the formation of S100A8/A9 in-vitro. This finding is also remarkable since heterocomplex formation in-vitro may not reflect accurately the complexity of the regulation of quaternary structure in-vivo. Nevertheless, the data we present indicate that ala42S100A8 does not form heterocomplexes with S100A9 under conditions in which WTS100A8 would. Deficiency in heterocomplex formation on its own or together with oxidation could explain the non antifungal phenotype of ala42S100A8. Conversely, methionine mutants of S100A9 formed heterocomplexes with S100A8 similar to what was observed with WTS100A9 supporting a direct regulatory function for oxidation on S100A8/A9 antifungal activity. Without knowing which quaternary S100A8/A9 structures are antifungal, it is premature to derive any definitive conclusions from this study of quaternary structure formed by the mutant S100 proteins. The data we present is limited to a crude SDS-PAGE study and it does not exclude the possibility that oxidation of the residues we substituted to alanine affect the formation of active antifungal species of S100A8/A9 (tetramers or others). The position of methionine 63 and 83 in alpha helices is crucial to the stability of S100A8/A9 tetramers (10). This would indicate that their oxidation may affect the quaternary structure of S100A8/A9. It is remarkable that methionine 83 and not 81 which are both in helix IV of S100A9 affect so differently S100A8/A9’s functions. This could be explained by the hydrophobic pocket in which methionine 83 is found (10) so that its oxidation may result in a more significant structural alteration. It is evident that further work is needed first to determine which quaternary structure of S100A8/A9 is the active antifungal species and, then, to assess the effect of oxidation and the above substitutions on the formation of those species.
In summary, the concept of S100A8/A9 as a simple metal chelator with growth-inhibitory activity should be expanded to encompass the complexity and versatility of regulatory mechanisms involving oxidation and quaternary structure formation uncovered in the present studies.
Finally, we propose that in the healthy state, S100A8/A9 subunits would have no antifungal effect. However, conditions associated with oxidative stress, including infection and overgrowth of opportunistic pathogens in sanctuaries such as the oral cavity may; activate the antifungal functions of S100A8/A9. Together with our previous work (19, 20), the data presented in this paper support a model in which S100A8/A9 and its components act either as a chemo-repulsive anti-inflammatory agent under reduced conditions or as an antimicrobial pro-inflammatory agent under oxidative conditions.
Acknowledgments
This work was supported by NIH grant support from PO1 DE 07946, K16 DE 00386, T32 DE07204, K22DE017161-02.
References
- 1.Berrouane YF, Herwaldt LA, Pfaller MA. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J Clin Microbiol. 1999;37:531–7. doi: 10.1128/jcm.37.3.531-537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doussiere J, Bouzidi F, Vignais PV. The S100A8/A9 protein as a partner for the cytosolic factors of NADPH oxidase activation in neutrophils. Eur J Biochem. 2002;269:3246–55. doi: 10.1046/j.1432-1033.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- 3.Fidel PL., Jr History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 2004;12:220–7. doi: 10.1016/j.tim.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–75. doi: 10.1189/jlb.0903435. Epub 2004 Apr 1. [DOI] [PubMed] [Google Scholar]
- 5.Harrison CA, Raftery MJ, Walsh J, Alewood P, Iismaa SE, Thliveris S, Geczy CL. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J Biol Chem. 1999;274:8561–9. doi: 10.1074/jbc.274.13.8561. [DOI] [PubMed] [Google Scholar]
- 6.Hessian PA, Fisher L. The heterodimeric complex of MRP-8 (S100A8) and MRP-14 (S100A9). Antibody recognition, epitope definition and the implications for structure. Eur J Biochem. 2001;268:353–63. doi: 10.1046/j.1432-1033.2001.01894.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40:241–8. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhoff C, Vogl T, Nacken W, Sopalla C, Sorg C. Zinc binding reverses the calcium-induced arachidonic acid-binding capacity of the S100A8/A9 protein complex. FEBS Lett. 1999;460:134–8. doi: 10.1016/s0014-5793(99)01322-8. [DOI] [PubMed] [Google Scholar]
- 9.Kohler GA, White TC, Agabian N. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J Bacteriol. 1997;179:2331–8. doi: 10.1128/jb.179.7.2331-2338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korndorfer IP, Brueckner F, Skerra A. The Crystal Structure of the Human (S100A8/S100A9)(2) Heterotetramer, Calprotectin, Illustrates how Conformational Changes of Interacting alpha-Helices Can Determine Specific Association of Two EF-hand Proteins. J Mol Biol. 2007;370:887–98. doi: 10.1016/j.jmb.2007.04.065. Epub 2007 May 3. [DOI] [PubMed] [Google Scholar]
- 11.Lackmann M, Rajasekariah P, Iismaa SE, Jones G, Cornish CJ, Hu S, Simpson RJ, Moritz RL, Geczy CL. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP-10. J Immunol. 1993;150:2981–91. [PubMed] [Google Scholar]
- 12.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–72. doi: 10.1016/j.jmb.2006.04.009. Epub 2006 Apr 21. [DOI] [PubMed] [Google Scholar]
- 13.Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect Immun. 2000;68:6257–64. doi: 10.1128/iai.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–301. [PubMed] [Google Scholar]
- 15.Odds FC. Pathogenic fungi in the 21st century. Trends Microbiol. 2000;8:200–1. doi: 10.1016/s0966-842x(00)01752-2. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn Microbiol Infect Dis. 1998;30:121–9. doi: 10.1016/s0732-8893(97)00192-2. [DOI] [PubMed] [Google Scholar]
- 17.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory Activities of S100: Proteins S100A8, S100A9, and S100A8/A9 Induce Neutrophil Chemotaxis and Adhesion. J Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 18.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–5. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 19.Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 Triggers Oxidation-sensitive Repulsion of Neutrophils. J Dent Res. 2006;85:829–33. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–24. doi: 10.1189/jlb.0706433. Epub 2006 Nov 30. [DOI] [PubMed] [Google Scholar]
- 21.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–5. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 22.Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/14 protein complex. J Virol. 2005;79:1099–112. doi: 10.1128/JVI.79.2.1099-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogl T, Leukert N, Barczyk K, Strupat K, Roth J. Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations. Biochim Biophys Acta. 2006;1763:1298–306. doi: 10.1016/j.bbamcr.2006.08.028. Epub 2006 Aug 25. [DOI] [PubMed] [Google Scholar]
- 24.Yui S, Mikami M, Tsurumaki K, Yamazaki M. Growth-inhibitory and apoptosis-inducing activities of calprotectin derived from inflammatory exudate cells on normal fibroblasts: regulation by metal ions. J Leukoc Biol. 1997;61:50–7. [PubMed] [Google Scholar]