Abstract
Background & Aims
E-cyclins control the transition of quiescent cells into the cell cycle. Two E-cyclins, CcnE1 and CcnE2, have been described, but their specific contributions to cell cycle re-entry in vivo are poorly understood. Liver regeneration following partial hepatectomy (PH) is an excellent in vivo model for the study of cell cycle re-entry of quiescent cells. We investigated the relevance of E-cyclins in directing resting hepatocytes into the cell cycle after PH using CcnE1 and CcnE2 knockout mice.
Methods
Partial hepatectomy (70%) was performed in CcnE1 (E1-/-) and CcnE2 (E2-/-) knockout and wild-type (WT) mice. Liver regeneration was monitored by cell cycle markers for G1/S-Phase, S-phase and M-phase as well as by determining the liver–body weight ratio after PH. Ploidy of hepatocytes was determined by fluorescence-activated cell sorting and fluorescent in situ hybridization.
Results
CcnE1 deletion resulted in normal liver regeneration with a slight delay of the G1/S-phase transition and a defect in endoreplication of otherwise polyploid hepatocytes. Surprisingly, E2-/- mice displayed accelerated and sustained DNA synthesis after PH, excessive endoreplication in hepatocytes, and a liver mass that was 45% greater than that of WT mice after termination of the regeneration process. CcnE2 depletion induced over-expression of CcnE1 and prolonged cdk2 kinase activity after PH.
Conclusions
CcnE2 has an unexpected role in repressing CcnE1; the phenotype of E2-/- mice appears to result from CcnE1 over-expression and cdk2 hyper-activation. CcnE1 and CcnE2 therefore have non-redundant functions for S-phase entry and endoreplication during liver regeneration.
Introduction
The cell cycle is an ordered set of events eventually leading to cell division and growth. Key regulators of the cell cycle machinery are cyclin-dependent kinases (cdk) and their regulatory subunits called cyclins (Ccn). In mammals, each phase of the cell cycle is characterized by its own set of cdks and cyclins1. Complexes of CcnD/cdk4 or cdk6 trigger G1 phase re-entry and initiate Retinoblastoma (Rb) protein phosphorylation. This leads to activation of cdk2 by E-cyclins thereby inducing S-phase transition. Formation of the CcnA/cdk2 complex is critical for DNA synthesis and pushes cells through G2 phase. CcnB/cdk1 interaction is finally responsible for mitosis.
E-type cyclins play an essential role in controlling transition of quiescent cells into cell cycle progression2. Two E-type cyclins – CcnE1 and CcnE2 - have been identified so far3-5, which show significant amino acid similarity6, 7. However, at present it is still unknown whether these two proteins have similar functions, or can perform different tasks during S-phase entry.
Mice deficient in CcnE1 or CcnE2 are viable and develop normally, except for testicular abnormalities seen in CcnE2 knock-out males2, 8. However, CcnE1/E2 double-knockout mice die during embryonic development at day 11.5, revealing that the two proteins perform overlapping functions during mouse development2. CcnE1/E2 deficient cells are also unable to re-enter cell cycle from quiescence2. However, a kinase-deficient CcnE1 mutant can specifically rescue the disability of CcnE1/E2 deficient cells to perform S-phase entry9. These results revealed a new kinase independent function of CcnE1 during cell-cycle progression.
Recent studies also demonstrated that E-type cyclins are indispensable for endoreplication8. Endoreplication results from duplication of genomic DNA without subsequent cell division and is preferentially found in differentiated cells with high metabolic activity10 such as salivary glands of Drosophila melanogaster11, trophoblast giant cells in mammals12 and - although to a lesser extent - in hepatocytes. In hepatocytes, multinucleation due to nuclear division lacking cytokinesis further increases the cellular DNA content13 and it was shown that partial 2/3 hepatectomy may further increase hepatic polyploidy14.
The aim of the present study was to characterise the role of E-type cyclins during G0-G1/S–transition in vivo; especially to define potential interdependent or redundant functions of CcnE1 and CcnE2. We thus used E1-/- and E2-/- mice and studied the phenotype of these animals during liver regeneration in the partial hepatectomy (PH) model15. In this model, resection of intact liver lobes induces a tightly regulated growth response resulting in synchronous replication of hepatocytes. After PH, DNA synthesis starts at 24 h and peaks after 36–40 h. The original liver mass is restored within 12-15 days, reflecting an average of 1.6 division cycles per hepatocyte16.
Materials and methods
Housing and breeding of mice
Animals were maintained in the animal facility of the University Hospital Aachen according to the German legal requirements. For our study we used previously described constitutive knock-out mice for CcnE1 (E1-/-) and CcnE2 (E2-/-) on a 129/Ola background2 of male gender and wildtype (WT) control animals.
Partial hepatectomy (PH) and tissue sampling
Partial hepatectomy was performed as described earlier17. For any indicated time point after PH, 5-10 mice at the age of 5-8 weeks from each cohort were analyzed. As a model of fatal hepatic failure we performed 90% hepatectomy with a subset of animals as described recently18. Mouse livers were analyzed for cell cycle markers by immunostaining and for gene expression of cell cycle-related genes by quantitative real-time PCR. In addition, immunoblots and in vitro kinase assays were performed from liver protein extracts. A detailed description of these techniques is provided as supplementary material.
Analysis of ploidy in hepatocytes
The DNA content of entire hepatocytes was measured using Fluorescence Activated Cell Sorting (FACS). For measuring the ploidy of individual nuclei, primary mouse hepatocytes were analyzed with Fluorescent In Situ Hybridization (FISH) techniques using a probe corresponding to the murine Y chromosome (Cambio, Cambridge, UK). The detailed procedures for FISH and FACS analyses are described as supplementary information.
Generation and in vivo use of recombinant adenoviruses
Detailed information about the construction of a recombinant CcnE2 adenovirus (adv-CcnE2) is given as supplementary information. The CcnE1 adenovirus used in this study (adv-CcnE1) has been described recently19. For in vivo application, a virus suspension containing 1.2 × 1010 plaque forming units (pfu) was injected i.v. via tail vein into mice 24 hours before hepatectomy. As control, an adenovirus expressing CMV-luciferase (adv-Luc) was used.
Evaluation of statistical Significance
All significant p-values were calculated and proven via Student's T-Test.
Results
CcnE1 – and CcnE2 deficient mice show different S-phase initiation and progression after partial hepatectomy
After PH, hepatocytes leave their quiescent, highly differentiated state and rapidly re-enter the cell cycle. The role of E-type cyclins for cell cycle progression was studied during liver regeneration in E1-/-, E2-/- and WT mice at 24 -168 hours (h) after surgery.
Proliferating Cell Nuclear Antigen (PCNA) is an essential factor initiating DNA synthesis. In WT livers, nuclear PCNA expression was first evident 36 h after PH and peaked at 40 h (Fig. 1A and supplementary Fig. 1A). E1-/- mice displayed only a slight delay in the onset of PCNA expression and a shift of the expression peak from 40 h to 48 h after surgery (Fig. 1A). Surprisingly, E2-/- mice showed an accelerated and amplified onset of PCNA expression along with continuous high expression levels 36-72 h after PH (Fig. 1B) indicating that depletion of CcnE1 or CcnE2 may have different consequences on G1/S phase transition.
Figure 1.

Aberrant G1/S phase transition in E1-/- and E2-/- mice. WT, E1-/- and E2-/- mice were subjected to PH and the remnant livers were analyzed at indicated time points. (A-B) G1/S phase was analyzed by nuclear PCNA staining in liver tissues of (A) WT compared to E1-/- hepatocytes or (B) WT compared to E2-/- hepatocytes. Each value represents the mean of 5-10 mice. (C) Determination of cdk2 kinase activity. Native cdk2 kinase complexes from mouse livers of WT, E1-/- and E2-/- mice after PH were subjected to in vitro histone H1 kinase assays. Representative data from three independent experiments is shown. (D) Protein extracts from WT, E1-/- and E2-/- mice after PH were depleted for CcnA/cdk2 complexes by immunoprecipitation with an anti-CcnA antibody where indicated and subjected to histone H1 kinase assays in comparison to untreated extracts representing total cdk2 activity. Depletion of CcnA was verified by CcnA western blot of the same lysates; GAPDH expression is shown as input control. (E) Whole liver cell extracts were investigated for RB phosphorylation and expression of cell cycle inhibitors p27 and p21, respectively. Two distinct signals for phospho-RB representing phosphorylated and hyper-phosphorylated RB were detected and highlighted by arrows. The expression levels of total RB and GAPDH are presented as internal loading controls. (F) Kinetics of E2F1 gene expression 0-72 h post PH measured by quantitative real-time PCR. The average of five independent experiments is shown. (*) p < 0.01; (#) p < 0.05.
A requirement for S-Phase initiation during liver regeneration is histone H1 phosphorylation through CcnE/cdk2 kinase complexes20, which is restricted to a short time frame of 40-48 h after PH in WT mice (Fig. 1C). In contrast, E1-/- mice show prolonged cdk2 activation until 72 h post PH (Fig. 1C) and in E2-/- mice strong cdk2 activity was evident between 36 h and 72 h after PH (Fig. 1C) and to some extend also 96 h after PH.
Cdk2 kinase activity can be mediated via CcnE/cdk2 or CcnA/cdk2. To discriminate between the two complexes we dissected both kinases by first depleting CcnA/cdk2 activities using an anti-CcnA antibody and subjected the remaining protein extracts to histone H1 kinase assays (Fig. 1D). In E1-/- mice, depletion of CcnA resulted in a substantial reduction of cdk2 kinase activity both 40 h and 72 h after PH, indicating that CcnE1 deficiency is predominantly substituted by CcnA. In contrast, deprivation of CcnA did not affect total cdk2 activity in E2-/- animals 40 h or 72 h post PH, suggesting that the prolonged S-phase and kinase activity seen in E2-/- mice is solely mediated by CcnE1/cdk2.
RB phosphorylation through CcnE/cdk2 kinase is a key event for G1/S-phase transition eventually leading to release and activation of E2F transcription factors. In WT - and E1-/- mice hyper-phosphorylation of RB peaked at 48 h after surgery (Fig. 1E). However, E1-/- animals showed a delayed onset of E2F1 expression (Fig. 1F) indicating, that not a lack of cdk2 kinase activity, but aberrant E2F1 expression may contribute to delayed S-phase onset in E1-/- mice.
In comparison to WT controls, E2-/- mice displayed accelerated and overall stronger RB hyper-phosphorylation beginning 24 h post PH reaching maximum levels 36-48 h post PH (Fig. 1E), which indicates that cdk2 kinase activity in these animals is persistent and overall stronger compared to WT or E1-/- mice. Accordingly, E2F1 expression in E2-/- mice was up-regulated between 36-48 h post PH compared to controls (Fig. 1F).
Cdk2 activity is negatively regulated by members of the Cip/Kip family of cdk inhibitors such as p27 and p21. During liver regeneration in WT mice following PH, p27 is continously expressed and only slightly up-regulated at the peak of DNA synthesis (Fig. 1E), which is consistent with earlier studies21. A similar expression profile was also found in E1-/- animals, although a significant down-regulation 72 h after PH was evident correlating with prolonged cdk2 kinase activity at this time point. Importantly, depletion of CcnE2 resulted in a strong down-regulation of p27 expression in both quiescent livers and throughout the whole time course of liver regeneration (Fig. 1E), thereby potentially contributing to accelerated and prolonged S-phase progression.
In WT mice, p21 is tightly regulated during liver regeneration with two peaks at 36 h and 72 h, respectively (Fig. 1E). E1-/- and E2-/- mice also showed increasing p21 expression after PH. However, no p21 was detected at 72 h in both strains which correlated with absence of p27 and exceeding cdk2 activity at this time point (compare Fig. 1C).
The observed differences in S-phase initiation are not due to differential hepatocyte priming by growth factors as CcnD1 expression profiles were almost identical in WT, E1-/- and E2-/- mice following PH (supplementary Fig. 1B). In contrast, CcnE mRNA expression profiles in E1-/- and E2-/- mice were significantly divergent and showed also strong differences compared to WT controls (Fig. 2A-B). In E1-/- mice, significantly lower CcnE2 levels were found at all time points investigated in comparison to WT animals and remarkably no CcnE2 expression was detected within the first 36 h after PH (Fig. 2A). In contrast, E2-/- mice expressed more CcnE1 mRNA compared to WT controls especially between 40 h and 72 h after PH (Fig. 2B) and additionally showed almost continuous CcnE1 protein expression between 24–96 h, whereas in control mice CcnE1 protein expression was restricted to 40–48 h after PH (Fig. 1C). CcnE2 protein expression was not analyzed as to our knowledge no commercial antibody is available specifically detecting the murine CcnE2 protein in immunoblots.
Figure 2.

Altered cell cycle progression in E1-/- and E2-/- mice is associated with modified expression profiles of E - and A-cyclins. (A-B) Relative real-time-PCR analysis of (A) CcnE2 in E1-/- mice and WT controls and (B) CcnE1 in E2-/- - and WT mice. (C) Western blot analysis for CcnE1 from WT, E1-/- and E2-/- mice at indicated time points after PH. (D) Absolute measurement of CcnE1 and E2 mRNA level in WT mice showing overall exceeding CcnE2 over CcnE1 expression. (E) Analysis of CcnA2 gene expression in WT, E1-/- and E2-/- mice by real-time PCR. (F) Western blot analysis of CcnA2 expression in E1-/-, E2-/- and WT control mice at indicated time points after PH. (*) p < 0.01; (#) p < 0.05.
Although the expression profiles of CcnE1 and CcnE2 during liver regeneration were similar in WT mice (compare Fig. 2A and 2B), absolute measurement of CcnE1 – and CcnE2 mRNA revealed continuous higher expression levels of CcnE2 (Fig. 2D). Untreated mice already had a 10fold higher basal CcnE2 expression and during liver regeneration the number of CcnE2 mRNA molecules always exceeded CcnE1 levels between 2-10 fold.
Consistent with the observed aberrant S-phase initiation in E1-/- and E2-/- mice, S-phase progression was also slightly delayed in E1-/- animals, whereas CcnE2 depletion resulted in accelerated and prolonged DNA synthesis as monitored by BrdU incorporation (supplementary Fig 1 C-E) and CcnA2 expression analysis (Fig. 2 E-F). Of notice, similar to their CcnE1 expression profile, E2-/- mice also showed some constitutive CcnA2 protein expression from 24-96 h post PH in sharp contrast to WT- or E1-/- animals, where CcnA2 expression was strictly up-regulated at distinct time points (Fig. 2F).
Ectopic over-expression of CcnE2 inhibits cell cycle progression in the regenerating liver
Our experiments indicated that CcnE2 might have antagonist properties for cell cycle progression by inhibiting CcnE1. To further support this hypothesis, adenoviruses over-expressing CcnE1 (adv-CcnE1) and CcnE2 (adv-CcnE2) were introduced in murine livers. As control, an adenovirus expressing luciferase was used (adv-Luc). WT - and E2-/- mice were injected with these adenoviruses followed by PH 24 h after virus application. In a first approach, E2-/- and WT mice were treated with adv-CcnE2. E2-/- mice were sacrificed 36 h after PH (strongest difference in S-phase compared to WT), whereas WT mice were investigated at the peak of S-phase (48 h after surgery). Adv-CcnE2 transduction induced CcnE2 expression comparable to WT baseline levels in E2-/- mice and an approximately 2fold increase of CcnE2 mRNA in WT animals 48 h after PH (Fig. 3A and supplementary figure 2 A-B). Adv-CcnE2 virus transduction showed a gradient with most efficient adenoviral infection in close proximity to portal venules and less evident in peripheral regions (Fig. 3B) and reverted the increased CcnE1 expression in E2-/- mice to almost WT levels (Fig. 3C).
Figure 3.

Ectopic over-expression of CcnE2 inhibits S-phase progression in hepatocytes after PH. E2-/- and WT mice were transduced with adv-CcnE2, adv-CcnE1 or adv-Luc adenovirus. 24 h after infection, the animals were subjected to PH and sacrificed at time points indicated. (A) Quantitative measurement of CcnE2 cDNA in WT – and E2-/- mice following adv-CcnE2 transduction and PH. CcnE2 gene expression was calculated as number of molecules/106 GAPDH. (B) Determination of adv-CcnE2 transduction efficiency. Liver cryosections of non-transduced (ctrl) or adv-CcnE2 injected (CcnE2) WT mice 48 h after PH were stained with an antibody directed against the adenoviral E1A protein (green). The area of maximal virus transduction around portal venules is highlighted. (C) Absolute measurement of CcnE1 in E2-/- livers 48 h after PH. Mice were either transduced with luciferase control (Luc) or CcnE2 (CcnE2) adenovirus before hepatectomy. The CcnE1 expression of WT animals (black bar) is indicated as a reference. (D) WT mice were transduced with adv-CcnE1 (CcnE1) or adv-Luc (Luc) where indicated. CcnE1 cDNA was measured before – and 36 h after PH. (E) Determination of BrdU incorporation following adenoviral over-expression of CcnE2 in WT - and E2-/- mice (green staining) or CcnE1 in WT animals (red staining) for time points post PH as indicated. Total nuclei are stained in blue with DAPI. As control, animals were transduced with adv-Luc (Luc). For each group a minimum of three animals was analyzed and quantitative data of five cryosections per animal is shown in the right part of the panel. (*) p < 0.01; (#) p < 0.05.
In a second approach, WT mice were transduced with adv-CcnE1 and analyzed before- and 36 h after PH (strongest effect of CcnE2 depletion) for CcnE1 expression. In quiescent livers, adenoviral CcnE1 delivery already resulted in a 10fold up-regulation of CcnE1 mRNA expression which further increased during liver regeneration (Fig. 3D).
The impact of ectopic CcnE2- and CcnE1 expression on cell cycle progression was determined by measuring BrdU incorporation (Fig. 3E). Adv-CcnE2 delivery significantly reduced the number of WT hepatocytes in S-phase from 19.5% to 10.6% (48 h post PH) and in E2-/- hepatocytes from 10.8% to 3.8% (36 h post PH). Of notice, the strongest inhibition of S-phase in tissue sections was found in periportal regions with maximal adenoviral infection (compare Fig. 3B and supplementary Fig. 2C-D). In sharp contrast, ectopic over-expression of CcnE1 in WT animals resulted in substantial higher DNA-synthesis already 36 h after PH (8.9% BrdU-positive cells compared to 3.1% in adv-Luc treated mice).
Increased DNA-synthesis in E2-/- mice results in augmented liver mass and improved survival in a model of fatal liver failure
To investigate the physiological relevance of earlier S-phase onset and enhanced DNA synthesis in E2-/- mice after PH, we determined the liver weight/body weight ratio at different time points after liver resection in WT, E1-/- and E2-/- mice. Of notice, untreated E2-/- mice displayed a slightly reduced relative liver mass, but 24 h after PH no differences in the liver weight index between the three groups were found (Fig. 4A). However, after seven days liver mass in E2-/- animals was 44% higher compared to WT mice (5.2% of body weight compared to 3.6% in WT, Fig. 4A) resulting in macroscopically visible increase of liver size (Fig. 4B).
Figure 4.

Pathophysiological effects of CcnE2 depletion. (A-B) CcnE2 ablation results in hepatomegaly after PH. (A) Liver weight index (%) at indicated time points after PH. (B) Representative livers from WT, E1-/- and E2-/- mice 168 h after PH. (C) Depletion of CcnE2 provides improved survival after 90% hepatectomy. Kaplan-Meyer survival curve shows time-dependent survival of WT mice (broken line, n=5) and E2-/- mice (solid line, n=5).
Accelerated and prolonged DNA synthesis in E2-/- mice was also correlated with a better prognosis in a model of fatal liver injury following 90% hepatectomy. In this model, all WT animals died within 24 h due to acute liver failure whereas 60% of E2-/- mice had a prolonged survival of 40 h after liver resection (Fig. 4C).
Amplified DNA synthesis in E2-/- mice is not correlated with increased mitotic activity
Next, we investigated if the observed hepatomegaly in E2-/- mice is caused by increased cell division following PH. Therefore, mitotic activity in the regenerating liver was analyzed by measuring histone H3 phosphorylation and CcnB1 expression. WT animals showed two peaks of histone H3 phosphorylation 48- and 84 h after surgery (Fig. 5A-B) which is consistent with previous findings, that normal hepatocytes undergo two rounds of mitosis following PH22. Accordingly, expression of the mitosis-related CcnB1 was also biphasic with expression maxima at 48 h and 84 h after PH (Fig. 5D-E). In contrast, neither E1-/- nor E2-/- mice showed this second round of mitosis as evidenced by reduced H3 phosphorylation (Fig. 5A-C) and lack of CcnB1 expression (Fig. 5D-E) during the late phase of liver regeneration (72-96 h post PH). Reduced mitosis and absence of a second wave of mitosis in E1-/- and E2-/- mice was also evident after direct determination of mitotic figures in liver histologies (supplementary figure 3A). However, the absolute numbers of mitotic figures were in general lower compared to phospho-H3 positive hepatocytes, as H3 is already phosphorylated at very early mitosis.
Figure 5.

Analysis of M-phase progression in E1-/- and E2-/- mice. (A-C) Liver cryosections from WT, E1-/- and E2-/- mice after PH were analysed for phosphorylation of histone H3. (A) Representative images at relevant time points are shown. Total nuclei are counterstained with DAPI (blue); green nuclei indicate H3 phosphorylation. (B-C) Percentage of histone H3-positive nuclei in liver sections from (B) WT and E1-/- mice and (C) WT and E2-/- mice. Each value represents the mean of 5-10 animals. (*) p < 0.01; (#) p < 0.05. (D) CcnB1 mRNA expression in response to PH at indicated time points in E1-/-, E2-/- and WT mice. (E) CcnB1 protein expression in E1-/-, E2-/- and WT mice between 0 h and 168 h after PH.
Consistently, computer assisted morphometric analysis of liver sections from E1-/- and E2-/- mice revealed an average reduced number of nuclei 96 h after PH in comparison to WT mice (supplementary Fig. 3B). These findings were in part unexpected and demonstrated that accelerated and amplified DNA synthesis in E2-/- mice did not initiate more mitosis after PH. Instead, depletion of CcnE1 or CcnE2 even results in slightly reduced nuclear division and mitosis. These data also raised the question, why E1-/- mice show normal liver regeneration (compare Fig. 4A) despite reduced mitosis. Measurement of cell size one week after PH revealed overall slightly enlarged E1-/- hepatocytes compared to WT- or E2-/- cells (supplementary Fig. 3C) indicating that E1-/- mice compensate liver resection both by hepatocyte proliferation and hyperplasia.
CcnE1 expression controls endoreplication in hepatocytes after PH
Although in E2-/- livers stronger DNA synthesis and hepatomegaly was found following PH, this was not associated with increased mitotic activity. These findings were unexpected and guided us to determine the replicated DNA in regenerating hepatocytes by FACS analysis. Following PH, propidium iodide-stained primary hepatocytes from WT, E1-/- and E2-/- animals were analyzed by FACS after 0 h (untreated controls), 48 h (maximum of S-phase), 72 h and 96 h (termination of liver regeneration). The cell populations were analysed via their DNA content and classified as diploid (2n), tetraploid (4n) and higher polyploid (>4n) hepatocytes.
Hepatocytes usually maintain high polyploidy due to their strong metabolic activity23 which further increases after PH14. Accordingly, after termination of the regeneration process, the majority of hepatocytes in WT controls were tetraploid (40%) or >4n (36%) with a minor subpopulation (24%) of diploid cells (Fig. 6A-B). However, under the same conditions the majority of E1-/- hepatocytes (45%) was diploid and only a small subpopulation (25%) was more than tetraploid (Fig. 6A-B). In clear contrast, more than 60% of E2-/- hepatocytes were highly polyploid (>4n, Fig. 6A-B) suggesting that E1-/- hepatocytes have a defect in endoreplication during liver regeneration, while enhanced CcnE1 expression and DNA-synthesis in E2-/- livers after PH triggers highly polyploid hepatocytes.
Figure 6.

Antagonistic roles of CcnE1 and CcnE2 for endoreplication in hepatocytes. (A-B) Primary hepatocytes from E1-/-, E2-/- and WT mice were isolated 96 h after PH. DNA content of propidium iodide (PI) stained hepatocytes was determined by FACS analysis. (A) Representative FACS histograms for WT, E1-/- and E2-/- hepatocytes. X-axis: PI intensity; Y-axis: cell number. (B) Distribution of diploid (2n), tetraploid (4n) and hyperploid (>4n) hepatocytes. (C-F) Mice were subjected to PH. 96 h after surgery primary hepatocytes were isolated and probed for the Y chromosome (red signals). (C) WT hepatocytes. (D) E1-/- hepatocytes. (E) E2-/- hepatocytes. (F) For each group, a minimum of 1000 nuclei were analyzed for the number of Y chromosomes. The data was calculated as the percentage of 2n, 4n and >4n nuclei per population.
Of notice, in untreated mice the distribution of polyploid hepatocytes in all three groups were similar and in all livers the majority of hepatocytes were higher than 4n (supplementary Fig. 3A). Between 48 h and 72 h after PH, in E1-/- mice the number of >4n cells already decreased, whereas no major differences in DNA distribution between WT and E2-/- mice were observed up to 72 h post PH (supplementary Fig. 3B-C), indicating that excessive endoreplication in E2-/- mice occurs between 72-96 h.
FACS analysis sorts and investigates entire hepatocytes. Therefore, it is unable to distinguish between binuclear cells (which may develop from nuclear division without cytokinesis) and mononuclear cells of higher ploidy (as a result of endoreplication). To differentiate between both events, primary hepatocytes were also subjected to FISH analysis 96 h post PH using a specific probe for the Y chromosome.
In WT cells, the majority of nuclei (approximately 70%) turned out to be diploid (Fig. 6C, 6F) and only minor subpopulations (26.2%) were tetraploid or octoploid and higher (7%). This is not in contrast to the FACS data but reflects the fact, that WT hepatocytes are frequently binuclear24, 25. Most strikingly, 90% of nuclei from E1-/- hepatocytes were diploid (Fig. 6D, 6F) whereas E2-/- hepatocytes showed an equal distribution of diploid, tetraploid and higher polyploid nuclei (Fig. 6E, F) reflecting overall higher polyploidy in E2-/- cells. These data demonstrate that CcnE1 is critical for restoring polyploidy after liver regeneration, whereas CcnE2 is antagonizing this effect.
Discussion
Recent data using CcnE1 and CcnE2 knockout mice demonstrated the need to redefine the role of E-type cyclins for S-phase entry in vivo. Therefore, the aim of the present study was to use the model of partial hepatectomy for re-evaluating the role of E-type cyclins for G0-G1/S-phase transition during cell cycle progression.
E1-/- mice display only marginal differences in cell cycle progression and show normal liver regeneration
CcnE1 depletion revealed only a minor effect on the course of liver regeneration involving a slight delay of S-phase entry, which was compensated by reinforced DNA synthesis at later time points eventually leading to normal liver mass restoration and liver function. A similar phenotype was recently described for cdk2-/- mice26 which is in agreement with earlier studies showing that neither CcnE1 or cdk2 are essential for cell proliferation2, 27. Accordingly, other cyclins may compensate for loss of CcnE1 function and potential candidates are CcnE2 and CcnA2 which both can activate cdk25. However, according to our data it seems unlikely that CcnE2 predominantly compensates for loss of CcnE1 as CcnE2 gene expression is strongly down-regulated in E1-/- mice implicating that CcnE1 positively controls CcnE2 expression. In contrast, the direct correlation between prolonged CcnA2 protein expression and the obvious contribution of CcnA for cdk2 kinase activity in E1-/- mice suggests that basically A-cyclins substitute CcnE1 in these animals.
Ablation of CcnE2 up-regulates CcnE1 expression and triggers excessive DNA synthesis during liver regeneration
Based on the initial hypothesis that CcnE1 and CcnE2 may share redundant functions, the phenotype found in E2-/- mice was unexpected. After PH, E2-/- mice show accelerated S-phase entry, persistent and strong DNA synthesis eventually leading to abnormal liver regeneration and hepatomegaly. This is associated with strong cdk2 kinase activity, higher and prolonged CcnE1- and CcnA2 expression and down-regulation of p27. In contrast, ectopic over-expression of CcnE2 in the liver inhibits S-phase progression and CcnE1 expression leading to the new hypothesis that CcnE2 has antagonistic cell cycle functions and is able to negatively control S-phase entry by down-regulating CcnE1 expression thereby restricting cdk2 kinase activity. This was further confirmed by ectopic over-expression of CcnE1 in WT mice 36 h after PH, which resulted in accelerated onset of S-phase reflecting the phenotype observed in E2-/- animals.
A suggested mechanism for the inhibition of S-phase entry and liver regeneration by CcnE2 is illustrated in Fig. 7. The predominance of CcnE2 over CcnE1 mRNA expression is preserved throughout the whole time course of liver regeneration, and from our data we further conclude that CcnE2 mediates weaker cdk2 kinase activity compared to CcnE1. Hence, cyclin E2-/- livers express increased levels of CcnE1 leading to overall stronger cdk2 activity eventually explaining excessive S-phase progression.
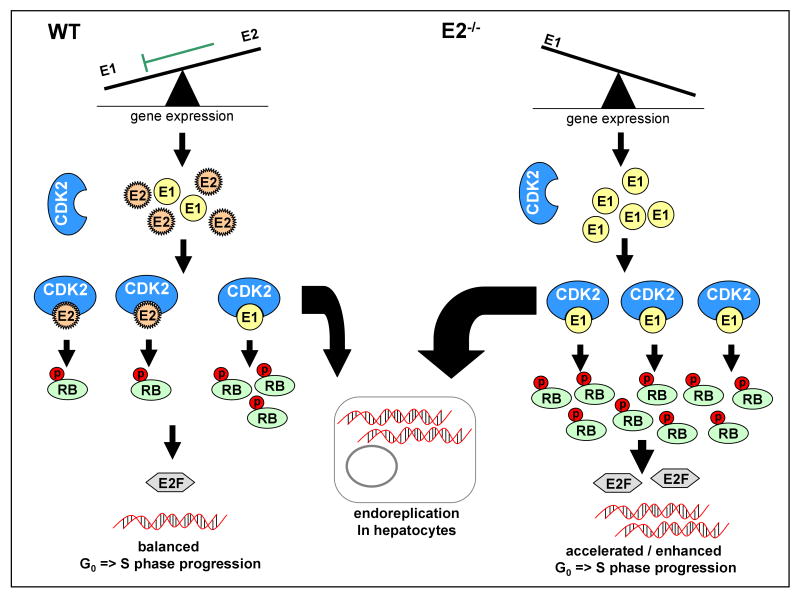
Figure 7.
Model explaining the phenotype of E2-/- mice as the result of stronger CcnE1 activation. Left: In quiescent WT livers, CcnE2 molecules are in excess over CcnE1 and CcnE2 competes with CcnE1 for binding to cdk2, whereas the kinase activity of CcnE2/cdk2 is lower compared to the CcnE1/cdk2 kinase leading to a balanced phosphorylation of S-phase related substrates such as RB. Moreover, CcnE1 - but not CcnE2, controls moderate endoreplication of the regenerating hepatocyte. Right: In E2-/- mice, total CcnE/cdk2 kinase activity is stronger and solely mediated via CcnE1 leading to earlier and stronger RB- and histone H1 phosphorylation. This may also result in prolonged kinase activity through an E2F-mediated feedback loop and excessive endoreplication.
The precise mechanism leading to transcriptional down-regulation of CcnE1 via CcnE2 is unknown yet. However, E-cyclins are tightly regulated by E2F transcription factors28, 29 consisting of activators (E2F1, 2 and 3a), which interact with pRB/p105 and repressors (E2F4 and 5) with high affinity to pRB2/p130 and pRB/p10730. Our results indicate that CcnE1/cdk2 and CcnE2/cdk2 may have differential affinities regarding RB phosphorylation and CcnE2/cdk2 complexes could have a preference for activating inhibitory E2Fs.
Differential roles of CcnE1 and CcnE2 for the control of endoreplication in hepatocytes
Earlier studies demonstrated that E-type cyclins are indispensable for endoreplication8. However, these studies did not discriminate between CcnE1 and CcnE2 as they were considered to be functionally redundant. Here, we demonstrated for the first time that CcnE1 and CcnE2 mediate non redundant and even antagonizing roles for endoreplication in hepatocytes following PH. CcnE1 turns out to be the key player for mediating endoreplication and can not be compensated by other cyclins such as CcnE2 or CcnA2. Therefore, CcnE1 depletion prevents polyploidization of hepatocytes following PH. In contrast, E2-/- hepatocytes show irregular strong CcnE1/cdk2 activity especially at late regeneration (72 h). As excessive endoreplication in these animals occurs between 72-96 h after PH, our data strongly suggests that this might directly be triggered by exceeding cdk2 activity.
Pathophysiological consequences of dysregulated CcnE activities
In E1-/- mice normal liver regeneration was evident and liver was fully restored within seven days although these animals displayed delayed S-phase onset and reduced mitosis. This partial defect in cell division seems to be compensated by hepatocyte hyperplasia, a mechanisms similarly described for Skp2-/- mice with reduced cell cycle activity due to p27 accumulation31.
In contrast, we found aberrant liver regeneration in E2-/- mice associated with hepatomegaly, which was not related to stronger hepatocyte proliferation, but rather with excessive polyploidization. We conclude that this abnormal increase in liver size and mass in E2-/- mice originates from exceeding DNA content and subsequent hepatocyte hypertrophy. However, hepatomegaly in E2-/- mice was not related to a specific phenotype, e.g. liver disease or impaired liver function at later time points (data not shown).
In a model of hepatic liver failure following 90% hepatectomy, CcnE2 deletion was even shown to evoke some benefits. WT mice died within 24 h from acute liver injury as reported earlier18, 32 whereas E2-/- mice showed improved survival, which might be related to accelerated onset of DNA synthesis. This could be of potential interest for future therapeutic approaches e.g. by using CcnE2 deficient hepatocytes for cell transplantation or bioartificial liver support.
In conclusion, we describe for the first time a phenotype for E2-/- mice apart from male sterility and provide evidence that CcnE2 negatively regulates CcnE1 activity and S-phase progression during liver regeneration.
Supplementary Material
Acknowledgments
We would like to thank Nisar P. Malek (Hanover Medical School, Germany) for providing us with the CcnE1 adenovirus.
Supported by Deutsche Forschungsgemeinschaft (DFG), grants LI1045/2-1 and LI1045/2-2 (C.L. and C.T.) and the National Cancer Institute (NCI), grant R01 CA108950 (P.S.).
Abbreviations used in this paper
- BrdU
5-bromo-2-deoxyuridine
- Ccn
cyclin
- Cdk
Cyclin-dependent kinase
- GAPDH
glycerinaldehyde-3-phosphate-dehydrogenase
- FACS
Fluorescence Activated Cell Sorting
- FISH
Fluorescent In Situ Hybridization
- MCM
minichromosome maintenance complex
- RT
reverse transcription
- PCNA
Proliferating Cell Nuclear Antigen
- PH
partial hepatectomy
- PI
propidium iodide
- RB
retinoblastoma
- WT
wildtype
Footnotes
The authors report no financial conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaldis P, Aleem E. Cell cycle sibling rivalry: Cdc2 vs. Cdk2. Cell Cycle. 2005;4:1491–4. doi: 10.4161/cc.4.11.2124. [DOI] [PubMed] [Google Scholar]
- 2.Geng Y, Yu Q, Sicinska E, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 3.Gudas JM, Payton M, Thukral S, et al. Cyclin E2, a novel G1 cyclin that binds Cdk2 and is aberrantly expressed in human cancers. Mol Cell Biol. 1999;19:612–22. doi: 10.1128/mcb.19.1.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koff A, Cross F, Fisher A, et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–28. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 5.Lauper N, Beck AR, Cariou S, et al. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–43. doi: 10.1038/sj.onc.1202477. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y, Yu Q, Sicinska E, et al. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc Natl Acad Sci U S A. 2001;98:194–9. doi: 10.1073/pnas.011522998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zariwala M, Liu J, Xiong Y. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene. 1998;17:2787–98. doi: 10.1038/sj.onc.1202505. [DOI] [PubMed] [Google Scholar]
- 8.Parisi T, Beck AR, Rougier N, et al. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. Embo J. 2003;22:4794–803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng Y, Lee YM, Welcker M, et al. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–39. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 11.Rudkin GT. Replication in polytene chromosomes. Results Probl Cell Differ. 1972;4:59–85. doi: 10.1007/978-3-540-37164-9_3. [DOI] [PubMed] [Google Scholar]
- 12.Barlow PW, Sherman MI. The biochemistry of differentiation of mouse trophoblast: studies on polyploidy. J Embryol Exp Morphol. 1972;27:447–65. [PubMed] [Google Scholar]
- 13.Gerlyng P, Abyholm A, Grotmol T, et al. Binucleation and polyploidization patterns in developmental and regenerative rat liver growth. Cell Prolif. 1993;26:557–65. doi: 10.1111/j.1365-2184.1993.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Sigal SH, Rajvanshi P, Gorla GR, et al. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol. 1999;276:G1260–72. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 16.Pistoi S, Morello D. Liver regeneration 7. Prometheus' myth revisited: transgenic mice as a powerful tool to study liver regeneration. Faseb J. 1996;10:819–28. doi: 10.1096/fasebj.10.8.8666158. [DOI] [PubMed] [Google Scholar]
- 17.Wustefeld T, Rakemann T, Kubicka S, et al. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514–22. doi: 10.1053/jhep.2000.16604. [DOI] [PubMed] [Google Scholar]
- 18.Makino H, Togo S, Kubota T, et al. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171–6. doi: 10.1016/j.jss.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 19.McEvoy JD, Kossatz U, Malek N, et al. Constitutive turnover of cyclin E by Cul3 maintains quiescence. Mol Cell Biol. 2007;27:3651–66. doi: 10.1128/MCB.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308(Pt 3):697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht JH, Poon RY, Ahonen CL, et al. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene. 1998;16:2141–50. doi: 10.1038/sj.onc.1201728. [DOI] [PubMed] [Google Scholar]
- 22.Trembley JH, Ebbert JO, Kren BT, et al. Differential regulation of cyclin B1 RNA and protein expression during hepatocyte growth in vivo. Cell Growth Differ. 1996;7:903–16. [PubMed] [Google Scholar]
- 23.Gupta S. Hepatic polyploidy and liver growth control. Semin Cancer Biol. 2000;10:161–71. doi: 10.1006/scbi.2000.0317. [DOI] [PubMed] [Google Scholar]
- 24.Mossin L, Blankson H, Huitfeldt H, et al. Ploidy-dependent growth and binucleation in cultured rat hepatocytes. Exp Cell Res. 1994;214:551–60. doi: 10.1006/excr.1994.1293. [DOI] [PubMed] [Google Scholar]
- 25.Wheatley DN. Binucleation in mammalian liver. Studies on the control of cytokinesis in vivo. Exp Cell Res. 1972;74:455–65. doi: 10.1016/0014-4827(72)90401-6. [DOI] [PubMed] [Google Scholar]
- 26.Satyanarayana A, Berthet C, Lopez-Molina J, et al. Genetic substitution of Cdk1 by Cdk2 leads to embryonic lethality and loss of meiotic function of Cdk2. Development. 2008 doi: 10.1242/dev.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega S, Prieto I, Odajima J, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 28.Botz J, Zerfass-Thome K, Spitkovsky D, et al. Cell cycle regulation of the murine cyclin E gene depends on an E2F binding site in the promoter. Mol Cell Biol. 1996;16:3401–9. doi: 10.1128/mcb.16.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995;92:12146–50. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun A, Bagella L, Tutton S, et al. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102:1400–4. doi: 10.1002/jcb.21609. [DOI] [PubMed] [Google Scholar]
- 31.Minamishima YA, Nakayama K, Nakayama K. Recovery of liver mass without proliferation of hepatocytes after partial hepatectomy in Skp2-deficient mice. Cancer Res. 2002;62:995–9. [PubMed] [Google Scholar]
- 32.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412–9. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.