Abstract
Previous studies of the developing lens have shown that Notch signaling regulates differentiation of lens fiber cells by maintaining a proliferating precursor pool in the anterior epithelium. However, whether Notch signaling is further required after the onset of fiber cell differentiation is not clear. This work investigates the role of Notch2 and Jagged1 (Jag1) in secondary fiber cell differentiation using rat lens epithelial explants undergoing FGF-2 dependent differentiation in vitro. FGF induced Jag1 expression and Notch2 signaling (as judged by the appearance of activated Notch2 Intracellular Domain (N2ICD)) within 12–24 hours. These changes were correlated with induction of the Notch effector, Hes5, upregulation of N-cadherin (N-cad), and downregulation of E-cadherin (E-cad), a cadherin switch characteristic of fiber cell differentiation. Induction of Jag1 was efficiently blocked by U0126, a specific inhibitor of MAPK/ERK signaling, indicating a requirement for signaling through this pathway downstream of the FGF receptor. Other growth factors that activate MAPK/ERK signaling (EGF, PDGF, IGF) did not induce Jag1. Inhibition of Notch signaling using gamma secretase inhibitors DAPT and L-685, 458 or anti-Jag1 antibody markedly decreased FGF-dependent expression of Jag1 demonstrating Notch-dependent lateral induction. In addition, inhibition of Notch signaling reduced expression of N-cad, and the cyclin dependent kinase inhibitor, p57Kip2, indicating a direct role for Notch signaling in secondary fiber cell differentiation. These results demonstrate that Notch-mediated lateral induction of Jag1 is an essential component of FGF-dependent lens fiber cell differentiation.
Keywords: Notch signaling, Lens differentiation, Lens Fiber Cells, Jag1, Jagged, Notch2, lateral induction, FGF-2, NICD, p57Kip2, N-cadherin
Introduction
The vertebrate ocular lens is composed of two distinctive cell types: terminally differentiated fiber cells that make up the bulk of the lens and a monolayer of epithelial cells that covers the anterior surface. Fiber cells are constantly added to the bulk of the lens through the proliferation of epithelial cells in a region just above the lens equator known as the germinative zone. Following division, cells in the germinative zone migrate posteriorly where they enter a transition zone, withdraw from the cell cycle, and differentiate into fiber cells (McAvoy et al., 1999). Fiber cells formed in this way are referred to as secondary fibers, to distinguish them from the primary fiber cells found at the center of the lens, which form from the posterior cells of the lens vesicle during embryogenesis (McAvoy et al., 1999; Sue Menko, 2002). Secondary fiber differentiation continues throughout the lifetime of the organism, adding concentric layers of fibers around the primary fibers of the lens nucleus. Several morphological and molecular changes occur during differentiation, including cell cycle exit regulated by the cyclin dependent kinase inhibitors (CKI’s) p57Kip2 and p27Kip1 (Fromm and Overbeek, 1996; Lovicu and McAvoy, 1999; Zhang et al., 1998; Zhang et al., 1999), a cadherin switch from E-cadherin (E-cad) to N-cadherin (N-cad) (Xu et al., 2002) cell elongation, accumulation of fiber-specific proteins and the eventual loss of intracellular organelles and nuclei (McAvoy et al., 1999; Piatigorsky, 1981).
Proliferation of epithelial cells and their subsequent differentiation into fiber cells is controlled by growth factors from surrounding tissues of the eye, which reach the lens by diffusion through the aqueous and vitreous humors (Lang, 1999). A number of growth factors have been shown to promote lens epithelial cell proliferation in vivo and in vitro, including PDGF-A, PDGF-D, EGF, and IGF (Ray et al., 2005; Reddan and Wilson-Dziedzic, 1983; Reneker and Overbeek, 1996). However, only members of the FGF family can induce lens fiber differentiation, at least in mammals (Lovicu and Overbeek, 1998; Robinson, 2006; Robinson et al., 1995b; Schulz et al., 1993), as shown by a variety of model systems, including transgenic mice (Chow et al., 1995; Govindarajan and Overbeek, 2001; Robinson et al., 1995a; Robinson et al., 1998; Stolen and Griep, 2000; Stolen et al., 1997), conditional gene ablation (Zhao et al., 2008; Zhao et al., 2006), and in vitro studies of lens epithelial explants (Lovicu and McAvoy, 1989; Lovicu and Overbeek, 1998; McAvoy and Chamberlain, 1989; McAvoy et al., 1991). Although the signaling pathways that act downstream of FGF during differentiation are not fully understood, signaling via MAPK/ERK plays a central role and is required for expression of a number of fiber specific markers (Golestaneh et al., 2004; Lovicu and McAvoy, 2001). Nonetheless, other pathways, including PI3 kinase (Chandrasekher and Sailaja, 2003; Souttou et al., 1997; Wang et al., 2008; Weber and Menko, 2006; Zatechka and Lou, 2002), c-Jun NH(2)-terminal kinase (JNK) (Golestaneh et al., 2004), Jak/Stats (Ebong et al., 2004a; Ebong et al., 2004b; Potts et al., 1998) and Rho GTPases (Maddala et al., 2004) are also activated by FGF and seem to be required for certain aspects of differentiation.
Notch signaling is a highly conserved, cell-cell signaling pathway that is involved in determination of cell fate during development (Bray, 2006; Harper et al., 2003; Kadesch, 2004). Notch receptors (1–4) and their ligands (Delta, Serrate/Jagged) are transmembrane proteins with large extracellular domains. In canonical notch signaling, upon activation of Notch receptors by their ligands, the receptors undergo proteolytic cleavage, leading to the release of the Notch intracellular domain (NICD). The NICD translocates to the nucleus, where it forms a transcriptional complex with the DNA-binding protein RBP-J (or Rbp-J) and the coactivator Mastermind, leading to activation of target genes. Genes activated by Notch signaling include the Hes and Hey (Herp) family of transcription factors. The Notch pathway has a wide range of functions in both developing and adult tissues. These include creating mosaic patterns of alternating cell types, boundaries or oscillatory patterns of gene expression (Bray, 2006). Notch signaling is known to operate in three distinct modes: lateral inhibition, binary cell fate, and lateral induction. In lateral inhibition, signaling between Notch ligand and Notch receptor on an adjacent cell inhibits ligand production in the receiving cell through a negative feedback loop (Bray, 1998; Chitnis, 1995). During binary cell fate decisions, in contrast, distinct cell fates are determined by asymmetric distribution of Notch pathway components, such as the cytoplasmic Notch inhibitor, Numb. Finally, in lateral induction, which is the least well described mode of Notch action, signaling between Notch ligand and Notch receptor on adjacent cells results in a positive feedback, which promotes ligand expression and activation of Notch on both cells. This mechanism has been suggested to propagate Notch signals through a cell-to-cell relay mechanism (Ross and Kadesch, 2004). These various modes of signaling allow Notch to perform different functions within the same tissue in a spatially and temporally regulated manner.
In the developing lens, Notch signaling is required to maintain a population of proliferating epithelial precursor cells (Jia et al., 2007; Rowan et al., 2008). Loss of canonical Notch signaling due to conditional knockout of Rbp-J in the lens results in aberrant expression of the CKI, p57Kip2 in the germinative zone, resulting in premature exit from the cell cycle and decreasing the supply of proliferating precursor cells needed for secondary fiber cell differentiation (Jia et al., 2007). This regulation of p57Kip2 by Notch appears to be mediated by unidirectional Notch signaling from Jagged1-expressing fiber cells to the overlying epithelial cells of the germinative zone, as shown by the expression of the Notch effector Herp2 in these cells. Other cell cycle regulatory genes, including cyclins D1 and D2, and the cyclin-dependent kinase inhibitor p27Kip1 also act downstream of Notch signaling to maintain the progenitor pool (Rowan et al., 2008).
While these studies provide valuable insight into the role of Notch signaling in lens growth and development, a number of unanswered questions remain. It is unclear, for example, how Notch signaling is affected by differentiation cues, such as FGF, as cells enter the transition zone. Moreover, it has been difficult to determine whether Notch signaling has a specific role in secondary fiber cell differentiation distinct from its role in maintaining the precursor pool. The conditional knockout studies show an increase in the percentage of foxe3 negative cells at E14.5, suggesting that loss of Notch signaling promotes secondary fiber cell differentiation (Rowan et al., 2008). Nonetheless, the expression pattern of the lens fiber cell marker, beta-crystallin, was not altered in the Rbpj conditional knockouts, suggesting that loss of Notch signaling does not affect the differentiation process, per se (Jia et al., 2007). The results obtained from these studies are difficult to interpret because of the tight coupling of proliferation, migration, and differentiation. Since loss of Notch signaling causes cells in the germinative zone to cease proliferating, they fail to migrate and do not encounter the high concentrations of FGF present in the vitreous humor, which provide the differentiation cues. Thus, the effect of Notch signaling on differentiation cannot be definitively established using an in vivo model. To address these questions we have used the well established neonatal rat lens epithelial explant model (Lovicu et al., 1995; Lovicu and McAvoy, 1989; McAvoy, 1980; McAvoy and Chamberlain, 1989). This model provides a powerful tool to study secondary fiber cell differentiation in isolation, by inducing a cohort of epithelial cells to differentiate synchronously. Unlike in vivo mouse models, the explant system obviates the need to maintain a proliferating cell population, with the added advantage of being able to test the effect of individual growth factors. Our results demonstrate a role for Notch-dependent lateral induction of Jag1 in the proper expression of key proteins, such as p57Kip2 and N-cad, during FGF-induced secondary lens fiber cell differentiation.
Materials and Methods
Antibodies and reagents
Rabbit polyclonal Anti-Jag1 antibody (SC-8303), Rabbit Polyclonal Anti- p57Kip2 (SC-8298) were purchased from Santa Cruz Biotechnology Inc., Santa Cruz, CA. Rabbit Monoclonal Anti-GAPDH antibody (Cat#2118), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) were purchased from Cell Signaling technology, MA, USA. Rabbit polyclonal p57Kip2 (ab33169), Rabbit polyclonal anti-activated Notch2 (ab8926) were purchased from Abcam, Cambridge, MA. Mouse monoclonal anti-E-cadherin (Cat# 610182) and anti-N-cadherin (Cat# 610921) antibodies were purchased from BD, Biosciences, CA, USA. For blocking studies anti-rat Jagged1 antibody (AF-599) was purchased from R&D systems, Minneapolis, MN. For immunofluorescence secondary antibodies, Alexa Fluor 568 goat anti-mouse IgG (H+L) (2 mg/mL), Alexa Fluor 488 goat anti-mouse IgG (H+L), Alexa Fluor 568 goat anti-rabbit IgG (H+L), Alexa Fluor 488 goat anti-rabbit IgG (H+L), were purchased from Invitrogen (Molecular Probes), Carlsbad, CA. For immunoblotting, secondary antibodies ECL Rabbit IgG, HRP-Linked Whole Ab (from donkey), ECL Mouse IgG, HRP-Linked Whole Ab (from sheep) and ECL Plus Western Blotting Detection Reagents were purchased from GE Healthcare, Piscataway, NJ.
U0126 (a specific MEK1/2 inhibitor) was purchased from Cell Signaling Technology, Inc., Danvers, MA. ERK Activation Inhibitor Peptides (Cat#328000, 328005), DAPT {N- [N-(3, 5 -Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester}, L-658,458 were purchased from EMD Chemicals, Inc., (Calbiochem), Gibbstown, NJ. Growth factors IGF-1, EGF, PDGF were purchased from Peprotech Inc., Rocky Hill, NJ. Basic Fibroblast growth factor-2 (FGF-2) was purchased from Sigma, St Louis, MO.
Preparation of lens epithelial explants
All procedures involving animals conformed to the guidelines provided by the National Institutes of Health, Bethesda, MD and the Association for Research in Vision and Ophthalmology. Preparation of lens epithelial explants was adapted from methods described in (Lovicu and McAvoy, 2001). Neonatal (P2-P3) Wistar rats (Charles River Laboratories, Raleigh, NC) were euthanized, lenses were removed and placed in culture medium (M199+0.1%BSA, Streptomycin, Penicillin, Amphotericin B). The lens capsule (with the adherent epithelial monolayer) was removed from the fiber cell mass by microdissection and gently pinned out flat in the culture dish. Central epithelial (CE) explants were made by trimming away the peripheral epithelium (PE) with a sterile scalpel. Explants were washed 3 times with sterile phosphate buffered saline containing calcium and magnesium and once with 1mL of fresh, equilibrated (37°C, 5% CO2) medium. For differentiation assays, explants were cultured in medium containing 100 ng/mL FGF-2, 37°C in 5% CO2, for 1, 2 or 4 days (or as indicated).
Inhibitor and Growth factor treatments
Inhibitors were added 2 hours before addition of growth factors to yield the following final effective concentrations: U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene) − 50 μM (as used previously (Lovicu and McAvoy, 2001); DAPT ({N- [N-(3, 5 - Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester}) − 50 μM; L-685,458 (1S-Benzyl-4R-[1-(1S-carbamoyl-2-phenethylcarbamoyl)-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl} carbamic Acid tert-butyl Ester) − 20 μM. Concentrations of the gamma secretase inhibitors DAPT and L-685,458 used in this study are comparable to the concentrations used in previous studies on notch signaling (Dahlqvist et al., 2003; Daudet et al., 2009; Kanungo et al., 2008; Tang et al., 2006; Williams et al., 2006; Yao et al., 2007). Control dishes, lacking inhibitor, were supplemented with an equivalent volume of the vehicle, dimethylsulfoxide (DMSO). Growth factors FGF-2, IGF-1, EGF and PDGF were used at final concentrations of 100 ng/mL, 200 ng/mL, 20 ng/mL, 100 ng/mL respectively. These concentrations were comparable to the concentrations used by in a previous lens explant study (Iyengar et al., 2006; Lovicu and McAvoy, 2001). Control dishes for growth factor treatments were supplemented with 0.1% BSA. The inhibitor and growth factors were present for the duration of the culture.
Jag1 Blockade experiments
To block Jag1 functions, anti-rat Jagged1 antibody (AF-599, R&D systems) was added at a final concentration of 100 μg/mL (a concentration that is comparable to the concentration that can block binding of Jagged1 to Notch2 receptor in functional ELISA assays, suggested by the manufacturer) to the culture medium. After 24 hours of incubation an additional 100 μg/mL was added to bolster the blockade. Any unbound antibody was washed away at the end of the incubation by several washes of PBS.
Immunoblotting
Explants for each immunoblotting experiment were obtained from littermates and extracts prepared from pools of 5–10 explants. At the end of the experiment, explants to be used for SDS-PAGE and subsequent immunoblotting were rinsed in cold Phosphate Buffered Saline (PBS) and lens proteins extracted in RIPA buffer ((Tris-HCl (50 mM) pH 7.4, NaCl (150 mM), NP-40 (1%), sodium deoxycholate (0.5%), SDS (0.1%)) containing Complete Mini - protease inhibitor cocktail tablet (Roche, Indianapolis, IN) and Halt - phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). Protein levels were determined using the Biorad Dc protein assay (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. SDS-PAGE was peformed with NuPAGE Novex Bis-tris Gels and XCell Surelock minicell (both from Invitrogen) followed by western blotting using mini Western Blotting apparatus (Bio-Rad). Immunoblots were scanned using a Typhoon 9410 (GE Healthcare) variable mode scanner. Images were densitometrically analysed using Imagequant 5.2 (GE Healthcare) software for quantification of the signal intensities.
Immunofluorescence and confocal microscopy
Rat lens explants were fixed in 4% paraformaldehyde (Boston Bioproducts, Worcester, MA) for 30 minutes at room temperature, followed by a brief permeabilization with 0.25% Triton-X-100 in PBS. Slides were washed in PBS and blocked with 5% goat serum in PBS overnight. Primary antibodies (1:50 to 1:200 dilution) were incubated for overnight at 4°C with gentle shaking. Slides were washed in PBS to remove unbound antibody. Secondary antibodies conjugated to Alexa dyes were used as secondary antibody at a dilution of 1:250. Nuclei were stained with DAPI. Immunofluorescence was imaged using a Leica laser scanning confocal microscope. Images were collected with a confocal microscope (SP2; Leica, Exton, PA). All images were collected at a 1024 × 1024-pixel resolution and a depth of 8 bits per channel. Fluorescent signals for DAPI (400–500 nm), Alexa Fluor 488 (500–550 nm), and Alexa Fluor 568 (580–675 nm) were collected by using a sequential scan mode to reduce bleed-through. All fluorescence images that were to be compared were taken at the same time, using the same settings, using tissues that were stained together and explants for each immunostaining experiment were obtained from littermates.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Germantown, MD) from about 4–6 explants from each treatment/condition. Approximately 2–3 μg of total RNA with a concentration of 30–40 ng/μl was isolated from each sample. About 100–200 ng was used as a starting material to prepare cDNA using Omniscript RT kit (Qiagen). Reverse transcription was carried out at 37°C for 60 minutes. RT enzyme was inactivated at 95°C for 5 minutes. PCR was performed using PCR Platinum Super Mix (Invitrogen) in a total reaction volume of 50μl, comprising 45μl super mix, 2 μl cDNA and 2 μl (forward and reverse) primers. Primer sequences were as follows: Jag1 – Forward: 5′ AAA TAC ACG TGG CCA TTT CTG CCG 3′, Reverse: 5′ GCA CAT TGT TGG TGG TGT TGT CCT 3′; Hes5 - Forward: 5′ ACA GCA GCA TTG AGC AGC TGA AAC 3′, Reverse: 5′ TAA AGC AGC TTC ATC TGC GTG TCG 3′; Hes1- Forward 5′ ACA GCC TCT GAG CAC AGA AAG TCA3′, Reverse 5′ TGA GGA AAG CAA ATT GGC CGT CAG 3′. Thermocycling was as follows: 94°C for 30 seconds to 2 minutes; followed by 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 1 minute. PCR products were analysed by loading 10 to 20 μl of the reaction mix with 3 μl of 6X loading dye on agarose gel (1.0%).
Results
Jag1 and N2ICD are highly expressed in the peripheral lens epithelium
Previous studies have shown that Jag1 expression is localized in epithelial cells of the transition zone, which are in the early stages of differentiation, and in the anterior tips of the secondary fiber cells (Jia et al., 2007; Rowan et al., 2008). Since we planned to use rat lens epithelial explants for studies of Notch signaling during differentiation, we first examined the localization of Jag1 expression in isolated whole-mounted lens epithelia. Immunostaining lens epithelia from newborn rat lenses with Jag1 specific antibody showed a high level of Jag1 expression in the peripheral epithelium (PE). These cells were located in the transition zone (TZ) before the epithelium was peeled away from the intact lens (see Fig. 9 inset). In contrast, little or no Jag1 staining was seen in cells of the central epithelium (CE) (Fig. 1A). Co-immunostaining for the early differentiation marker N-cad, confirmed that the Jag1 expressing cells in the PE are, in fact, differentiating.
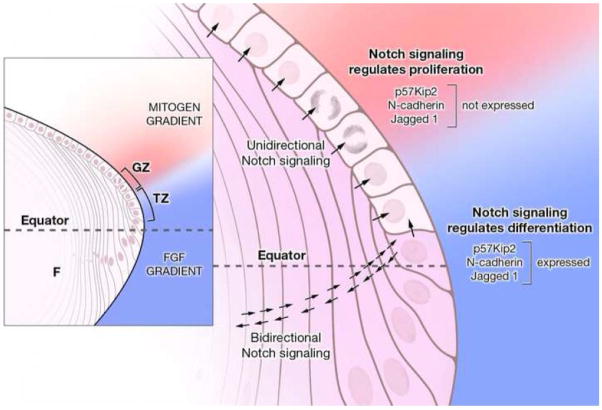
Fig. 9. A dual role for Notch signaling in the lens.
Epithelial cells in the germinative zone (GZ) and transition zone (TZ) are exposed to a cocktail of growth factors: FGF (blue gradient) diffusing from the vitreous humor and mitogenic growth factors (pink gradient) secreted into the aqueous humor by the ciliary body. Jag1 expression (shaded violet in the magnified view), which is restricted to fiber cells (F) and cells in the TZ in the early stages of differentiation, induces unidirectional Notch signaling (arrows) in the overlying epithelial cells and permits proliferation in the presence of appropriate mitogen concentration. Notch signaling in the GZ negatively regulates p57Kip2 (Jia et al., 2007; Rowan et al., 2008), thus maintaining a pool of precursor cells needed for secondary fiber cell production. As no differentiation occurs in this region, cells express E-cad, but not N-cad or Jag1. As the post-mitotic precursor cells migrate posteriorly they are exposed to increasing FGF concentration. At a critical, threshold concentration of FGF, an initial, Notch-independent induction of Jag1 occurs. This is rapidly amplified by lateral induction via positive feedback involving bidirectional Notch signaling between adjacent differentiating cells. A sharp boundary is thus established between non-differentiating and differentiating cells in the transition zone. In direct contrast to the role of Notch signaling in repressing p57Kip2 in the proliferating cells of the GZ, Notch signaling in the differentiating cells positively regulates p57Kip2. Moreover, Notch signaling regulates cadherin switching by augmenting N-cad expression, as E-cad is being lost from cell-cell junctions.
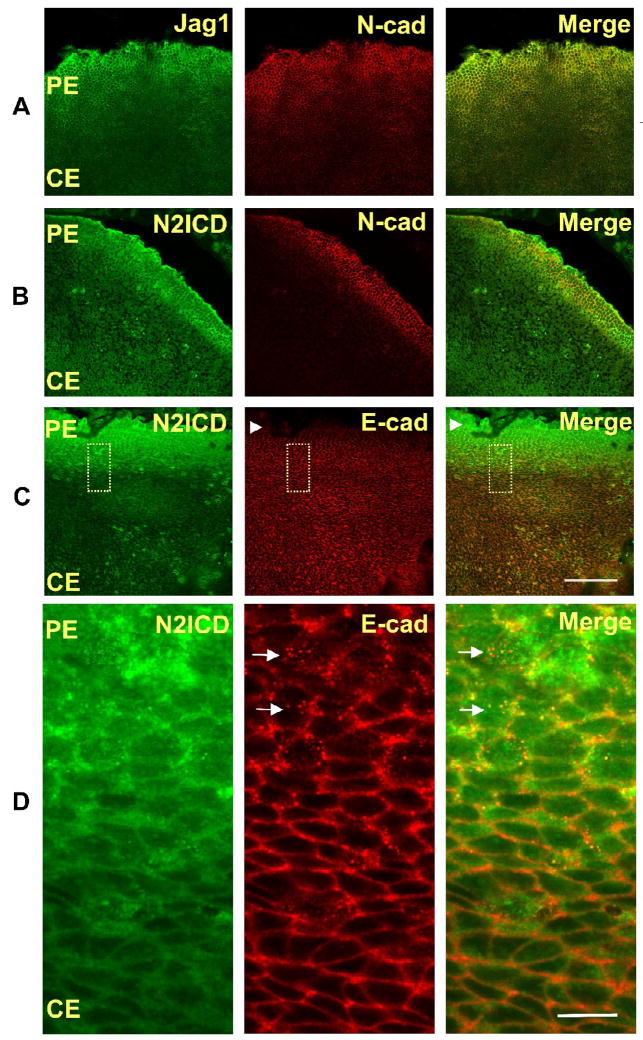
Fig. 1. Jag1 and N2ICD are highly expressed in the peripheral lens epithelium.
Representative immunofluorescence micrographs of whole mounts of freshly isolated postnatal day-2(PN2) rat lens epithelial explants stained with specific antibodies. (A,B) Jag1 (A) and N2ICD (B) expression colocalize with expression of N-cad, an early marker of differentiation, in the peripheral epithelium (PE). (C,D) The increase in the N2ICD corresponds to decreased immunostaining of Ecad (refer to arrowheads in panel C). Higher magnification (D), shown as an inset (dotted line box) in panel C, shows that the increased staining of N2ICD coincides with the appearance of intracellular vesicles (refer to arrows in panel D) that immunostain for E-cad, suggesting E-cad degradation (D). Scale bar on panel C represents 100 μm length and is applicable for all images of panels A, B and C. Scale bar on panel D represents 10 μm and is applicable for all images of panel D.
Localization of Notch signaling in the lens epithelium has been less well characterized, since it has been examined only by expression of the Notch effectors, Herp2(Hey1) (Jia et al., 2007) and Hes1(Rowan et al., 2008) These studies have localized Notch signaling to the anterior lens epithelium, particularly proliferating cells of the germinative zone. In order to gain a better understanding of Notch signaling, we immunostained whole-mounted lens epithelium for the N2ICD, a marker for active Notch2-dependent signaling (Fig. 1B,C). The results indicate that N2ICD, like Jag1, is highly localized in cells of the PE, rather than in the germinative zone, as expected. Co-immunostaining for N-Cad and N2ICD confirmed that N2ICD is localized to cells of the transition zone. In addition, N2ICD expression coincided with decreased E-cad immunostaining, loss of E-cad from cell-cell boundaries (Fig. 1C), and appearance of E-cad on intracellular vesicles (Fig. 1D), suggesting that both Jag1 expression and Notch2 signaling are correlated with the cadherin switch, which marks the onset of the fiber cell differentiation. Together these findings suggest a potential role for Notch signaling in secondary fiber cell differentiation, in addition to its previously identified role in maintaining a proliferating precursor pool.
FGF induces Jag1 and activates Notch2 signaling in cultured explants
The ability of FGF to induce differentiation of lens epithelial explants (Lovicu and McAvoy, 2001; Lovicu and McAvoy, 2005; McAvoy and Chamberlain, 1989; McAvoy and Chamberlain, 1990) provided a possible means of testing the role of Notch signaling in secondary fiber cell differentiation. The CE and PE can be separated by microdissection as previously described (Lovicu and McAvoy, 2001). The explanted CE has a distinct protein profile, with reduced levels of N2ICD and little or no expression of Jag1, N-cad, and p57Kip2 (Fig. 2). Explants of CE were cultured in the presence or absence of a concentration of FGF known to produce differentiation (100 ng/mL) and incubated for various times from 2 to 120 hours. Cell lysates were then immunoblotted for Jag1 and N2ICD (Fig. 3A). Anti-Jag1 antibody (H114; Santa Cruz Biotechnology, Santa Cruz, CA) has been previously used (Mancini et al., 2005), to specifically detect Jag1 and we confirmed the specificity of the N2ICD by blocking with the immunogenic peptide (not shown). FGF induced Jag1 expression between 24 hours and 48hours. Immunostaining of the explants after 4 days confirmed that Jag1 was expressed uniformly throughout the explant and was localized along cell-cell boundaries (Fig. 3B). To determine if induction of Jag1 was also seen at a transcriptional level, we isolated total RNA from explants cultured in the presence or absence of FGF for 24 hours and performed a RT-PCR using specific primers for Jag1. Results revealed a strong induction of Jag1 mRNA by FGF within 24hours (Fig. 3C). Induction of Jag1 was closely paralleled by an increase in N2ICD levels above the basal level seen in the control explants (Fig. 3A), suggesting that signaling arises from the interaction between Jag1 and Notch2 on adjacent differentiating cells.
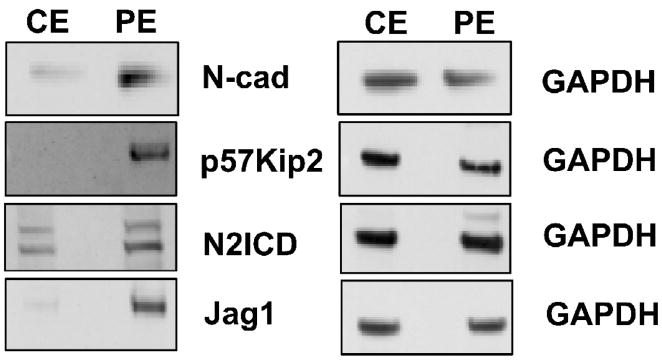
Fig. 2.
The protein profiles of microdissected peripheral epithelium (PE) and central epithelium (CE) with respect to N-cad, p57Kip2, Jag1 and N2ICD are compared in representative immunoblots. Near absence of N-cad, p57Kip2, and Jag1 in the CE demonstrates that microdissection of central explants efficiently separates cells of the CE from differentiating cells in the PE.
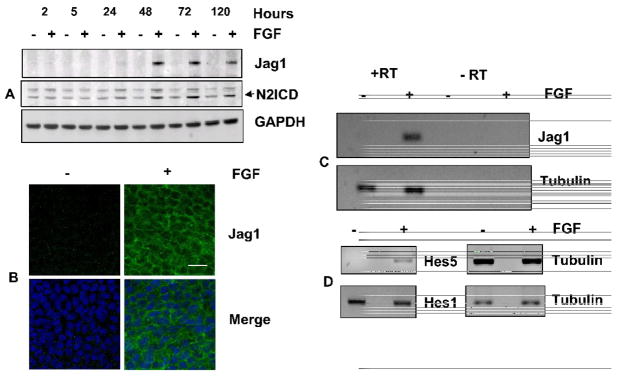
Fig. 3. FGF induces Jag1 and activates Notch signaling.
(A) Representative immunoblot of Jag1 and N2ICD from lens epithelial explants exposed to 100ng/mL FGF (+) or 0.1% BSA (−) for indicated times (hours). GAPDH has been used as a loading control. The two bands recognized by the N2ICD antibody likely represent sequential cleavage products of Notch2. Lower band represents the N2ICD (B) Representative immunofluorescence of Jag1 in the central region of rat lens epithelia cultured in the presence or absence of FGF for 4 days shows that FGF induces uniform expression of Jag1 in all cells in this region and demonstrates that Jag1(green) is localized along cell-cell boundaries. Nuclei have been stained with DAPI (blue). Scale bar is applicable to all images of panel B and represents 20 μm length (C) FGF induction of Jag1 mRNA was demonstrated by RT-PCR using total RNA isolated from CE explants cultured in the presence or absence of FGF (100 ng/mL) for 24 hours. Tubulin RT-PCR was performed as a positive control. Reactions lacking reverse transcriptase (RT) served as negative controls. Analyses of at least three different replicates provided similar results. (D) The ability of FGF to activate Notch signaling was examined by RT-PCR for Notch effectors Hes5 and Hes1, using total RNA isolated from CE explants cultured in the presence or absence of FGF (100 ng/mL) for 24 hours. Tubulin RT-PCR was performed as a positive control. Negative controls lacking reverse transcriptase (RT) showed no amplified products (not shown). Analyses of at least three different replicates provided similar results.
To determine whether FGF-dependent Jag1 induction and Notch2 activation results in canonical Notch signaling, we examined the transcription of two known Notch effectors, Hes5 and Hes1. FGF induced Hes5 expression within 24 hours (Fig. 3D). Although Hes1was also detected, its expression was not significantly affected by FGF within this time period. The activation of Notch dependent target gene Hes5 confirms that FGF induces canonical Notch signaling during fiber cells differentiation.
FGF-2 induction of Jag1 is dependent on MAPK/ERK1/2 signaling
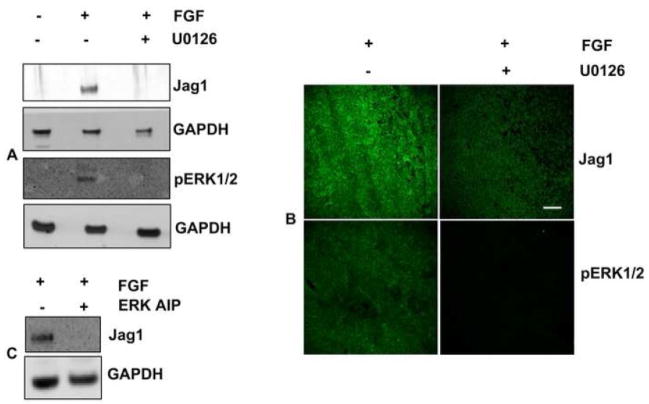
Activation of MAPK/ERK1/2 signaling has previously been shown to be required for FGF- dependent differentiation of rat explants (Lovicu and McAvoy, 2001; Wang et al., 2008; Weber and Menko, 2006). To address the importance of this pathway in Jag1 induction by FGF-2, we used the selective pharmacological inhibitor of MEK 1 and MEK 2, U0126 (1, 4-diamino-2, 3-dicyano-1, 4-bis [2-aminophenylthio] butadiene). Explants exposed to U0126 for 48 hours showed a complete suppression of Jag1 under conditions that blocked pERK1/2, an indicator of the level of MAPK/ERK1/2 signaling (Fig. 4A). Immunofluorescence of the explants treated with U0126 confirmed that induction of Jag1 and phosphorylation of ERK1/2 by FGF was impaired compared to the control explants (Fig. 4B). Moreover, Jag1 induction by FGF was also inhibited by inactivating ERK1/2 using ERK activation inhibitor peptides (AIP), as an alternative means of blocking MAPK/ERK1/2 signaling (Fig. 4C). These findings demonstrate that active MAPK/ERK1/2 signaling is required for the induction of Jag1 by FGF in the explants.
Fig. 4. Jag1 induction is regulated by MAPK/ERK pathway.
(A) Representative immunoblots of Jag1 and pERK1/2 expression in CE explants treated with the MEK inhibitor U0126. GAPDH expression is shown as loading control. (B) Representative whole mounts micrographs of rat lens CE explants cultured in the presence or absence of FGF and MAPK/ERK 1/2 pathway inhibitor U0126 (50 μM) for 48 hours and immunolabelled for Jag1 and pERK1/2. Scale bar is applicable to all images of panel B and represents 100 μm length. (C) Representative immunoblots of Jag1 expression in CE explants treated with the ERK Activation Inhibitor Peptide (ERK AIP). GAPDH expression is shown as loading control.
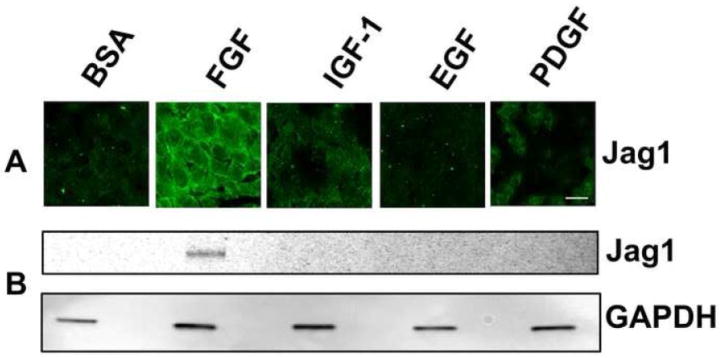
Although other growth factors also activate signaling via MAPK/ERK1/2 in rat lens explants, they are unable to induce differentiation (Lovicu et al., 1995; Lovicu and McAvoy, 2001; Zhou et al., 2003). To determine whether these growth factors also have the ability to induce Jag1, explants were cultured for 48 hours in the presence of appropriate concentrations of each growth factor. Immunofluorescence (Fig. 5A) and immunoblotting (Fig. 5B) showed that only FGF is competent to induce Jag1. These results suggest that induction of Jag1 may be an integral part of the differentiation process.
Fig. 5. Jag1 expression is not induced by IGF-1, EGF, or PDGF.
(A) Representative immunofluorescence micrographs of explants of central epithelium (CE) treated with BSA (control), FGF-2, IGF-1, EGF, or PDGF for 48 hours and immunostained for Jag1. Scale bar is applicable to all images of panel A and represents 10 μm length. (B) Corresponding immunoblots of lysates of treated explants from the same experiment immunoblotted for Jag1.
Inhibition of Jag1-Notch signaling reduces expression of differentiation markers N-cad and p57Kip2
To test whether the Notch signaling induced by FGF has a direct role in secondary fiber cell differentiation, we examined the effect of blocking Notch signaling on the expression of two genes activated early in the differentiation process, N-cad and p57Kip2. Anti-Jag1 antibody was added to the culture 3 hours prior to the addition of FGF to prevent productive engagement of surface expressed Jag1 with Notch receptors. After 48 hours incubation, lysates were immunoblotted with an antibody specific for N2ICD. As expected, FGF treatment increased levels of N2ICD above the basal level seen in untreated explants. This increase was inhibited (approximately 50%) in the presence of anti-Jag1 antibody, indicating effective blockade of Jag1-dependent Notch signaling (Fig. 6A,B). Under these conditions, expression of p57Kip2 and N-cad was also inhibited (Fig. 6C). Inhibition of these differentiation markers was confirmed by immunofluorescence staining (Fig. 6D). The gamma secretase inhibitors DAPT (50μM) and L-685,458 (20 μM), which have been widely used to suppress Notch signaling, also reduced the FGF-dependent increase in N2ICD, N-cad and p57Kip2 (Fig. 7A,B) confirming the results obtained with the anti-Jag1 antibody. These findings demonstrate that FGF-dependent Jag1-Notch signaling regulates expression of genes involved in secondary fiber cell differentiation.
Fig. 6. Blockade of Notch signaling with anti-Jag1 antibody reduces expression of p57Kip2 and N-cad.
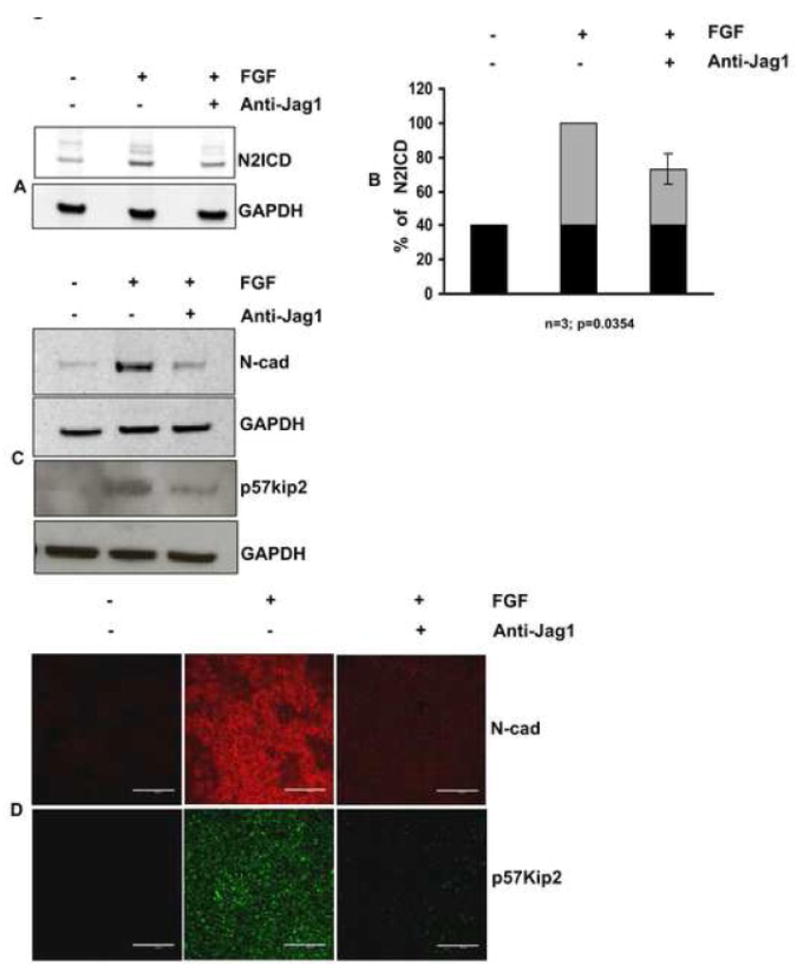
CE explants were cultured in the presence of FGF for 4 days in the presence or absence of anti- Jag1 antibody and analyzed for expression of N2ICD, p5Kip2, and N-cad. (A) Effectiveness of the blockade by anti-Jag1 was evaluated by immunoblotting for N2ICD. FGF induced an increase in N2ICD above the basal level (indicated by black in the bar graphs), as seen in control explants treated with only BSA. This FGF-dependent increase (indicated by gray areas of the bar graphs) was reduced by incubation with anti-Jag1. (B) Quantification of N2ICD expression by densitometric scanning of replicate immunoblots (n=3), demonstrated approximately a 50% decrease +/− 5.14 (s.e.m) (p=0.0354). (C) Immunoblotting lysates of the cultured explants confirmed the reduction in expression of p57Kip2 and N-cad. (D) Incubation with anti-Jag1 antibody sharply reduced immunofluorescence of p57Kip2 and N-cad in all cells of the explant. Scale bar is applicable to all images of panel D and represents 100 μm length.
Fig. 7. Inhibition of Notch signaling with gamma secretase inhibitors reduces expression of N-cad and p57Kip2.
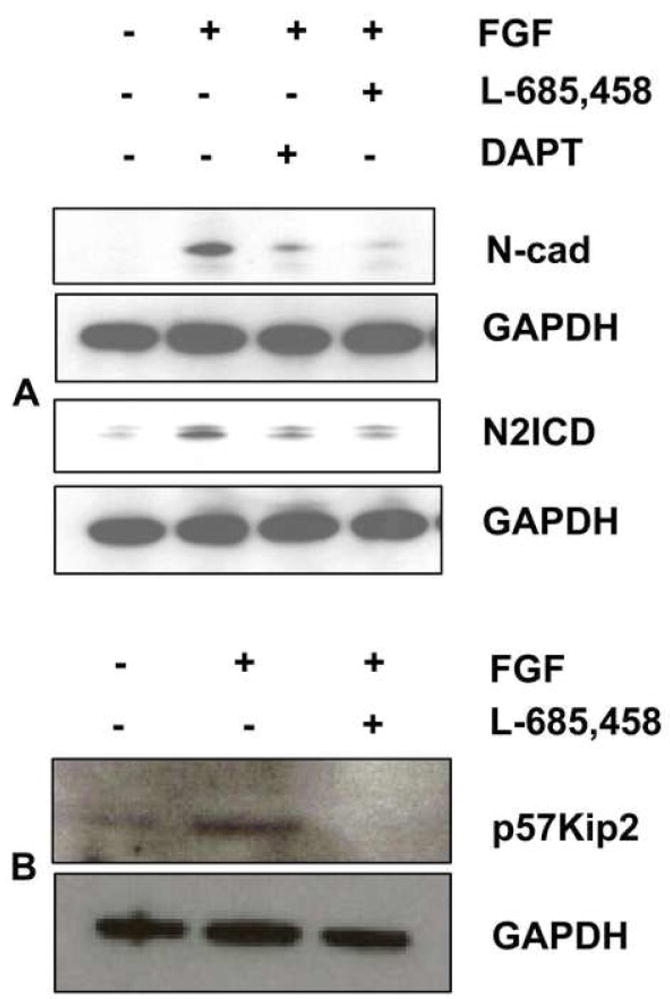
(A) CE explants were cultured in the presence of FGF for 4 days in the presence or absence of the gamma secretase inhibitors, DAPT (50 μM) and L-685,458 (20 μM), and immunoblotted to determine expression of N2ICD and N-cad. Reduction of N2ICD with these pharmacological inhibitors was paralleled by reduction in N-cad expression, confirming the results obtained with anti-Jag1 antibody. (B) Lysates from CE explants cultured in the presence or absence of FGF and/or L-685,458 were immunoblotted for p57Kip2, representative immunoblot shows reduction in the expression of p57Kip2 during suppression of notch signaling by L-685,458.
Jag1-Notch signaling regulates Jag1 expression via lateral induction
Previous studies have suggested that Jag1 itself may be a target of Notch signaling (Daudet et al., 2007; Ross and Kadesch, 2004). To test whether such a mechanism contributes to the expression of Jag1 during lens fiber cell differentiation, we examined the effect of Notch signaling blockade on the FGF-induced expression of Jag1. The results indicate blocking Notch signaling either by anti-Jag1 antibody or by gamma secretase inhibitors dramatically reduces expression of Jag1 (Fig. 8A, B, C). This demonstrates that Notch signaling is required for robust, sustained expression of Jag1 during differentiation. Since we observe a uniform expression of both the ligand Jag1 (Fig. 3B) and the N2ICD in all cells of the differentiating explants, the lateral induction of Jag1 in the signal-receiving cell apparently generates a reciprocal induction of Jag1 in the adjacent, signal-sending cell, thus creating a positive feedback amplification of Jag1 expression, similar to that observed in the developing inner ear (Daudet et al., 2007)
Fig. 8. Jag1-Notch signaling regulates Jag1 induction.

(A) CE explants were cultured in the presence of FGF for 4 days in the presence or absence of anti- Jag1 antibody and analyzed for expression of Jag1. Immunoblotting demonstrated a marked reduction of Jag1 protein in the presence of anti-Jag1 antibody. (B) Quantification of Jag1expression by densitometric scanning of replicate immunoblots (n=3), demonstrated approximately a 70% decrease +/− 10.9 (s.e.m) (p=0.0238). (C) CE explants were cultured in the presence of FGF for 4 days in the presence or absence of the gamma secretase inhibitors, DAPT (50μM) and L-685,458 (20μM). Inhibition of Notch signaling with these pharmacological inhibitors confirmed that Notch signaling regulates induction of Jag1.
Discussion
FGF induces Jag1 during fiber cell differentiation
FGF is known to induce a number of differentiation-specific genes during lens fiber cell differentiation, including cyclin dependent kinase inhibitors, N-cad, crystallins, and lens-specific intermediate filament proteins. The present study provides the first demonstration that FGF is also responsible for induction of Jag1 mRNA and protein during differentiation. Our data demonstrate that FGF signaling through MAPK/ERK1/2 is required for Jag1 induction, although other growth factors, such as EGF, PDGF, and IGF-1, which are known to activate this pathway in proliferating lens epithelial cells (Lovicu and McAvoy, 2001; Zhou et al., 2003) are not sufficient. Interestingly, Jag1 has also been shown to be a target of Toll Like receptor (TLR) signaling via ERK1/2 activation in human and murine macrophages (Goh et al., 2008), thus strengthening the link between Jag1 and the ERK1/2 pathway shown in the present study. While the transcription factors involved in Jag1 induction by FGF are not yet known, one potential candidate is Tcf/Lef (T cell-specific factor/lymphoid enhancer binding factor), a downstream effector of the Wnt/beta-catenin pathway. Jag1 has been shown to be a target of beta-catenin during hair follicle formation, and Tcf/Lef binding sites have been identified within the promoter region of human, mouse and rat Jag1 (Estrach et al., 2006; Katoh and Katoh, 2006). Moreover, Wnts and proteins in the Wnt signaling pathway are expressed in the lens throughout its development (Ang et al., 2004; Cain et al., 2008; Chen et al., 2006; de Iongh et al., 2006; Kreslova et al., 2007) and have been shown to promote morphological aspects of FGF induced differentiation (Lyu and Joo, 2004).
Notch-dependent lateral induction of Jag1
The results of the present study indicate that Notch-dependent lateral induction further amplifies the initial FGF-dependent induction of Jag1 in differentiating lens fiber cells. Induction of Jag1 in rat lens epithelial explants between 24 and 48 hours after FGF treatment was accompanied by Notch2 mediated signaling, as shown by the increased production of the N2ICD and the induction of the Notch effector, Hes5. By microarray analysis, induction of Jag1 and Hes5 has been detected in differentiating lens epithelial explants as early as 12 hr after addition of FGF (Ales Cvekl, personal communication). Immunostaining revealed that all cells in the explant expressed both the Jag1 ligand and the N2ICD. Moreover, a similar co-localization of Jag1 and N2ICD was seen in cells of the peripheral lens epithelium, suggesting that this pattern of Notch activation also occurs in vivo. To test whether Notch signaling is directly involved in Jag1 expression, we inhibited Notch signaling using pharmacological inhibitors of gamma secretase or anti-Jag1 antibody. The inefficiency of DNA delivery to the primary explants hampered the use of other strategies for blocking Notch signaling, such as siRNA. Our results showed that blocking Notch signaling markedly inhibited expression of Jag1, an indication of Notch-dependent lateral induction. Inhibition of Notch signaling with anti-Jag1 antibody was also shown to inhibit production of the N2ICD, confirming that the antibody effectively blocks FGF-dependent Notch signaling. While our data indicate a role for Notch2 and Hes5 in this process, we do not rule out the possibility that Notch1 and other effectors of Notch signaling, including Hey1 (Herp2) (Jia et al., 2007) and Hes1 (Rowan et al., 2008) may also be involved. Although the initial induction of Jag1 is clearly Notch-independent, as shown by the residual expression of Jag1 in lenses of RbpJ conditional knockouts (Jia et al., 2007; Rowan et al., 2008), a robust expression of Jag1 requires Notch-dependent lateral induction via positive feedback through Jag1-Notch signaling. This closely resembles the mechanism of Serrate1 (Jagged1) induction involved in the formation of the prosensory patches in the chick inner ear (Daudet et al., 2007). The similarity between these two developmental systems suggests positive feedback via Jag1-Notch may be a general mechanism for producing a uniform field of extended Notch signaling.
Notch effector, Hes5, is specifically induced by FGF
Our data indicate that Hes5 may be the relevant Notch effector involved in secondary lens fiber cell differentiation. Although the high level of N2ICD in the differentiating explants would be expected to induce other classical Notch effectors as well, we did not observe an increase in Hes1, which is also expressed in these cells. The inability of Notch signaling to increase Hes1 expression in our experiments is consistent with published studies indicating that Hes1 is, in fact, down-regulated following differentiation (Rowan et al., 2008). This suggests that Notch-independent mechanisms may regulate Hes1 during differentiation, restricting its Notch-dependent role to the regulation of epithelial cell proliferation in the germinative zone. Although the decrease in Hes1 expression seen in fiber cells in vivo did not occur in the differentiating explants within the first 24 hours, detecting such an effect in explants may require longer incubation times. The rat lens explant system will be a valuable tool for exploring the spatial and temporal dynamics of these and other Notch effectors, such as Hey1, at various stages of secondary fiber cell differentiation.
Notch signaling regulates cadherin switching during differentiation
N-cad is the principal fiber cell cadherin, replacing E-cad at the onset of differentiation (Pontoriero et al., 2008). Loss of N-cad interferes with fiber cell elongation and blocks the normal morphological development of the lens, leading ultimately to fiber cell vacuolization and degeneration. The present evidence for Notch-dependent regulation of this gene demonstrates that Notch signaling, indeed, has a specific role in secondary fiber cell differentiation. Moreover, regulation of N-cad by Notch is of particular interest, since the coupling of E-cad downregulation and N-cad upregulation is also seen during other developmental events and during epithelial to mesenchymal transitions (EMT), in general reviewed by (Katoh and Katoh, 2008). Previous work indicates that Notch signaling plays a role in this process by inducing Snail, a repressor of E-cad, which in turn leads to the loss of E-cad (Leong et al., 2007; Tiezzi et al., 2007; Zavadil et al., 2004). However, cadherin switching also involves the subsequent replacement of E-cad by N-cad (or other cadherins). Whether Notch signaling is also involved in this step has not been clear, although a recent study reported that loss of the Notch targets Hes1 and Prop1 is correlated with N-cad downregulation in pituitary cells (Himes and Raetzman, 2009). Thus, the present demonstration that inhibition of Notch-Jag1 signaling represses N-cad expression provides direct evidence linking Notch to N-cad expression and confirms the central role of Notch signaling in cadherin switching during fiber cell differentiation.
FGF reverses the mode of Notch signaling during differentiation
Differentiation of lens epithelial cells to form lens fiber cells requires proper withdrawal from the cell cycle at the lens equator, which is regulated by the cyclin dependent kinase inhibitors, p27Kip1 and p57Kip2 (Zhang et al., 1998; Zhang et al., 1999). Two independent studies using different promoters to direct Cre-mediated deletion of RbpJ in the lens observed a small lens phenotype, coupled with reduced epithelial cell proliferation, premature cell cycle withdrawal in the peripheral anterior epithelium, and aberrant expression of p57Kip2 and p27Kip1 (Jia et al., 2007; Rowan et al., 2008). Moreover, the small lens phenotype in the RbpJ conditional knockout was at least partially reversed by deletion of p57Kip2, indicating that Notch-dependent repression of this cell cycle inhibitor is important for maintaining a pool of proliferation-competent precursor cells. In RbpJ conditional mutants, Jag1 is also inappropriately induced in cells located in what would otherwise be the germinative zone (Jia et al., 2007; Rowan et al., 2008). In further confirmation of a role for Notch in repressing differentiation in the epithelium, overexpression of Notch1 Intracellular Domain (N1ICD) in the lens shifted the zone of cells expressing p57Kip2 and p27Kip1, toward the posterior (Rowan et al., 2008). Notch signaling has also been shown to repress these cell cycle inhibitors in other cell types using a variety of techniques (Georgia et al., 2006; Jia et al., 2007; Riccio et al., 2008). Notch effectors Hes1 (Georgia et al., 2006) and Hey1/Herp2 (Jia et al., 2007) can both repress p57Kip2. Thus, in general, previous studies support the idea that Notch signaling inhibits differentiation of lens epithelial cells in the germinative zone by repressing expression of cyclin-dependent kinase inhibitors, p57Kip2 and p27Kip1, and the Notch ligand, Jag1.
In contrast, the present study indicates that certain differentiation specific genes, such as Jag1, N-cad, and p57Kip2 are positively regulated by Notch signaling in the presence of differentiation-inducing concentrations of FGF. Thus, Notch signaling has a dual role in lens differentiation (Fig. 9). On the one hand, unidirectional Notch signaling from the fiber cells to the overlying epithelial cells of the germinative zone inhibits differentiation and permits proliferation. On the other hand, as cells migrate posteriorly and are exposed to increasing concentrations of FGF, signaling through the FGF receptor converts the inhibitory effect of Notch to an inductive effect. Expression is then further amplified by Notch-Jag1 signaling between adjacent cells once the tipping point is reached, to produce a uniform field of Jag1 expressing cells, as seen in secondary fiber cells and the differentiating explants. This powerful feedback amplification of expression defines a sharp boundary between non-differentiating and differentiating cells in the transition. This ability of FGF to switch the mode of Notch signaling from lateral inhibition to lateral induction exemplifies the versatility of Notch signaling and represents a novel interaction between these two developmentally important signaling pathways.
Acknowledgments
We thank Drs. Nadean Brown, Stanislav Tomarev, Joram Piatigorsky and Pushpa Pandiyan for valuable discussions and critical reading of this manuscript and general support, and the NEI Biological Imaging Core for confocal microscopy. This work was supported by the National Eye Institute Intramural Research Program Z01-EY000238-20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–95. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol. 1998;9:591–7. doi: 10.1006/scdb.1998.0262. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008;321:420–33. doi: 10.1016/j.ydbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Chandrasekher G, Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:4400–11. doi: 10.1167/iovs.03-0136. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin Cell Dev Biol. 2006;17:712–25. doi: 10.1016/j.semcdb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB. The role of Notch in lateral inhibition and cell fate specification. Mol Cell Neurosci. 1995;6:311–21. [PubMed] [Google Scholar]
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–93. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–99. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–78. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Abud HE, Hime GR. WNT/Frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–64. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- Ebong S, Chepelinsky AB, Robinson ML, Zhao H, Yu CR, Egwuagu CE. Characterization of the roles of STAT1 and STAT3 signal transduction pathways in mammalian lens development. Mol Vis. 2004;10:122–31. [PubMed] [Google Scholar]
- Ebong S, Yu CR, Carper DA, Chepelinsky AB, Egwuagu CE. Activation of STAT signaling pathways and induction of suppressors of cytokine signaling (SOCS) proteins in mammalian lens by growth factors. Invest Ophthalmol Vis Sci. 2004;45:872–8. doi: 10.1167/iovs.03-0311. [DOI] [PubMed] [Google Scholar]
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–38. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- Fromm L, Overbeek PA. Regulation of cyclin and cyclin-dependent kinase gene expression during lens differentiation requires the retinoblastoma protein. Oncogene. 1996;12:69–75. [PubMed] [Google Scholar]
- Georgia S, Soliz R, Li M, Zhang P, Bhushan A. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol. 2006;298:22–31. doi: 10.1016/j.ydbio.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Goh F, Irvine KM, Lovelace E, Donnelly S, Jones MK, Brion K, Hume DA, Kotze AC, Dalton JP, Ingham A, Sweet MJ. Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Fan J, Fariss RN, Lo WK, Zelenka PS, Chepelinsky AB. Lens major intrinsic protein (MIP)/aquaporin 0 expression in rat lens epithelia explants requires fibroblast growth factor-induced ERK and JNK signaling. J Biol Chem. 2004;279:31813–22. doi: 10.1074/jbc.M403473200. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–27. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin Genet. 2003;64:461–72. doi: 10.1046/j.1399-0004.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol. 2009;325:151–61. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JE, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83:667–78. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Jia J, Lin M, Zhang L, York JP, Zhang P. The Notch signaling pathway controls the size of the ocular lens by directly suppressing p57Kip2 expression. Mol Cell Biol. 2007;27:7236–47. doi: 10.1128/MCB.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506–12. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Zheng YL, Amin ND, Pant HC. The Notch signaling inhibitor DAPT down-regulates cdk5 activity and modulates the distribution of neuronal cytoskeletal proteins. J Neurochem. 2008;106:2236–48. doi: 10.1111/j.1471-4159.2008.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17:681–5. [PubMed] [Google Scholar]
- Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review) Int J Mol Med. 2008;22:271–5. [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–68. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Lang RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci. 1999;40:3075–8. [PubMed] [Google Scholar]
- Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–48. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Chamberlain CG, McAvoy JW. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest Ophthalmol Vis Sci. 1995;36:1459–69. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF) Exp Eye Res. 1989;49:479–94. doi: 10.1016/0014-4835(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Spatial and temporal expression of p57(KIP2) during murine lens development. Mech Dev. 1999;86:165–9. doi: 10.1016/s0925-4773(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–84. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–24. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84:679–92. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- McAvoy JW. Beta- and gamma-crystallin synthesis in rat lens epithelium explanted with neural retinal. Differentiation. 1980;17:85–91. doi: 10.1111/j.1432-0436.1980.tb01084.x. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–8. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Growth factors in the eye. Prog Growth Factor Res. 1990;2:29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13(Pt 3b):425–37. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG, de Iongh RU, Richardson NA, Lovicu FJ. The role of fibroblast growth factor in eye lens development. Ann N Y Acad Sci. 1991;638:256–74. doi: 10.1111/j.1749-6632.1991.tb49036.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JD, Kornacker S, Beebe DC. Activation of the Jak-STAT-signaling pathway in embryonic lens cells. Dev Biol. 1998;204:277–92. doi: 10.1006/dbio.1998.9077. [DOI] [PubMed] [Google Scholar]
- Ray S, Gao C, Wyatt K, Fariss RN, Bundek A, Zelenka P, Wistow G. Platelet-derived growth factor D, tissue-specific expression in the eye, and a key role in control of lens epithelial cell proliferation. J Biol Chem. 2005;280:8494–502. doi: 10.1074/jbc.M413570200. [DOI] [PubMed] [Google Scholar]
- Reddan JR, Wilson-Dziedzic D. Insulin growth factor and epidermal growth factor trigger mitosis in lenses cultured in a serum-free medium. 1983;24:409–416. [PubMed] [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol. 1996;180:554–65. doi: 10.1006/dbio.1996.0328. [DOI] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–40. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995;121:3959–67. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–14. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Ross DA, Kadesch T. Consequences of Notch-mediated induction of Jagged1. Exp Cell Res. 2004;296:173–82. doi: 10.1016/j.yexcr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–22. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–26. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Souttou B, Ahmad S, Riegel AT, Wellstein A. Signal transduction pathways involved in the mitogenic activity of pleiotrophin. Implication of mitogen-activated protein kinase and phosphoinositide 3-kinase pathways. J Biol Chem. 1997;272:19588–93. doi: 10.1074/jbc.272.31.19588. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Griep AE. Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev Biol. 2000;217:205–20. doi: 10.1006/dbio.1999.9557. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Jackson MW, Griep AE. Overexpression of FGF-2 modulates fiber cell differentiation and survival in the mouse lens. Development. 1997;124:4009–17. doi: 10.1242/dev.124.20.4009. [DOI] [PubMed] [Google Scholar]
- Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–90. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Tang LS, Alger HM, Pereira FA. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development. 2006;133:3683–93. doi: 10.1242/dev.02536. [DOI] [PubMed] [Google Scholar]
- Tiezzi DG, Fernandez SV, Russo J. Epithelial mesenchymal transition during the neoplastic transformation of human breast epithelial cells by estrogen. Int J Oncol. 2007;31:823–7. [PubMed] [Google Scholar]
- Wang Q, Stump R, McAvoy JW, Lovicu FJ. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47:4490–9. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–9. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–60. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Yao J, Duan L, Fan M, Wu X. Gamma-secretase inhibitors exerts antitumor activity via down-regulation of Notch and Nuclear factor kappa B in human tongue carcinoma cells. Oral Dis. 2007;13:555–63. doi: 10.1111/j.1601-0825.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- Zatechka SD, Jr, Lou MF. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp Eye Res. 2002;74:703–17. doi: 10.1006/exer.2002.1168. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. Embo J. 2004;23:1155–65. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–7. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–24. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–88. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang Y, Partanen J, Ciruna BG, Rossant J, Robinson ML. Fibroblast growth factor receptor 1 (Fgfr1) is not essential for lens fiber differentiation in mice. Mol Vis. 2006;12:15–25. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Fariss RN, Zelenka PS. Synergy of epidermal growth factor and 12(S)-hydroxyeicosatetraenoate on protein kinase C activation in lens epithelial cells. J Biol Chem. 2003;278:5388–98. doi: 10.1074/jbc.M209695200. [DOI] [PubMed] [Google Scholar]