Abstract
Objectives
To examine the effects of chronic amiodarone on the electrophysiology of canine pulmonary vein (PV) sleeve preparations and left ventricular wedge preparation.
Background
Amiodarone is commonly used for the treatment of ventricular and supraventricular arrhythmias. Ectopic activity arising from the PV plays a prominent role in the development of atrial fibrillation (AF).
Methods
Standard microelectrode techniques were used to evaluate the electrophysiological characteristics of superfused PV sleeve (left superior or inferior) and arterially perfused left ventricular (LV) wedge preparations isolated from untreated and chronic amiodarone-treated dogs (amiodarone, 40 mg/kg daily for 6 weeks).
Results
In PV sleeves, chronic amiodarone (n = 6) induced a significant increase in action potential duration at 90% repolarization (APD90) and a significant use-dependent reduction in Vmax leading to 1:1 activation failure at long cycle lengths (basic cycle length of 124 ± 15 ms in control vs 420 ± 320 ms after chronic amiodarone [P < 0.01]). Diastolic threshold of excitation increased from 0.3 ± 0.2 to 1.8 ± 0.7 mA (P < 0.01). Delayed and late phase 3 early afterdepolarizations and triggered activity could be induced in PV sleeve preparations using acetylcholine (ACh, 1 μM), high calcium ([Ca2+]o = 5.4 mM), isoproterenol (Iso, 1 μM), or their combination in 6 of 6 untreated PV sleeves, but in only 1 of 5 chronic amiodarone-treated PV sleeve preparations. Vmax, conduction velocity, and 1:1 activation failure were much more affected in PV sleeves versus LV wedge preparations isolated from amiodarone-treated animals.
Conclusions
The results point to potent effects of chronic amiodarone to preferentially suppress arrhythmogenic substrates and triggers arising from the PV sleeves of the dog.
Keywords: atrial fibrillation, antiarrhythmic drugs, sodium channel blocker, amiodarone, pulmonary vein
Introduction
Amiodarone is an antiarrhythmic agent widely used in the treatment of both ventricular and supraventricular arrhythmias, atrial fibrillation (AF) in particular. The antiarrhythmic efficacy of amiodarone is thought to be due to multiple actions at the cellular level. Amiodarone has been shown to inhibit a number of cardiac ionic currents (IKr, IKs, INa, late INa, Ito, ICa-L, ICa-T, IK1, IK(ACh), IK(ATP)) as well as to possess α- and β-adrenoceptor blocking activity. Although possessing properties of all 4 Vaughan Williams classes of antiarrhythmic agents, amiodarone is often classified as a Class III antiarrhythmic.1,2
The recent work from our laboratory has shown that chronic amiodarone treatment exerts potent atrial-predominant effects to depress INa-dependent parameters and that this action of the drug is greatly potentiated by its ability to preferentially prolong action potential duration (APD) in the right atrium, thus contributing to its effectiveness to suppress AF.3
Ectopic activity arising from the pulmonary veins (PV) has been shown to play a prominent role in the development of AF.4 PV isolation is a procedure frequently used to eliminate the triggers arising from the PV. Late phase 3 early afterdepolarizations (EADs) as well as delayed afterdepolarizations (DADs)-induced triggered activity, originating from PV sleeves following parasympathetic and/or sympathetic stimulation, have been proposed as potential triggers in the initiation of AF.5-9 Few data are available concerning the effects of chronic amiodarone on the electrophysiology of PV sleeves.
The objective of the present study was to determine the effect of chronic amiodarone treatment on the electrophysiologic characteristics and its potential to inhibit the development of triggered activity in canine-isolated PV sleeve preparations, and to test the hypothesis that chronic amiodarone preferentially depresses excitability and conduction in PV sleeves, acting to suppress the triggers and substrate for the development of atrial fibrillation, with relatively little effect on these parameters in ventricular myocardium.
Methods
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, Revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Adult mongrel dogs weighing 20-35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left thoracotomy and the heart excised and placed in a cold cardioplegic solution ([K+]0 = 8 mmol/L, 4°C).
Superfused Pulmonary Vein Sleeve Preparation
PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from left canine atria. The thickness of the preparation was approximately 2 mm. Left superior PVs were used preferentially in most experiments. The preparations were placed in a small tissue bath and superfused with Tyrode’s solution of the following composition (mM): 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, 5.5 glucose, buffered with 95% O2/5% CO2 (35 ± 0.5°C). The PV preparations were stimulated at a basic cycle length (BCL) of 1,000 ms during the equilibration period (1 hour) using electrical stimulation (1-3 ms duration, 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded (at a sampling rate of 41 kHz) using glass microelectrodes filled with 3 M KCl (10-20 MΩ DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT, USA). The following parameters were measured: diastolic threshold of excitability, take-off potential (TOP), action potential amplitude (APA), action potential duration at 50 and 90% repolarization (APD50 and APD90), and maximal rate of rise of action potential upstroke (Vmax). The TOP was used instead of the resting membrane potential because of the slow phase 3 of the action potential of the PV sleeve preparation, which does not return to the resting potential at the shortest BCLs. The changes in conduction time were measured between 2 microelectrodes placed along the length of the PV sleeve. Acetylcholine (ACh, 1 μM), isoproterenol (1 μM), high [Ca2+]0 (5.4 mM) or their combination were used to induce late phase 3 EADs, DADs and triggered activity. The combination of parasympathetic and sympathetic stimulation or increased extracellular calcium has been shown to facilitate the development of late phase 3 EADs in PV sleeve preparations,5-7 whereas sympathetic stimulation is known to lead to calcium overload, a condition responsible for the development of DADs.10,11 DADs or EADs were elicited using stimulation trains of 20 beats introduced at progressively faster rates followed by a pause.
Left Ventricular Wedge Preparation
In vitro experiments were performed using left ventricular (LV) arterially perfused wedge preparations (≈3.0 × 1.2 × 1.2 cm). The methods used for isolation and perfusion of these preparations have been described elsewhere.12 Briefly, the preparations were dissected from hearts removed from anesthetized (sodium pentobarbital) adult mongrel dogs (20-25 kg), untreated or treated with chronic amiodarone. The LV wedge was perfused through a branch of the left anterior descending coronary artery. Unperfused tissue was removed with a razor blade or scissors. The cut ventricular branches were ligated using silk thread. After these procedures (performed in a cold cardioplegic solution, 4-8°C), the preparations were transferred to a temperature-controlled bath and arterially perfused with Tyrode’s solution by use of a roller pump. The composition of Tyrode’s solution was (in mM) NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5, buffered with 95% O2 and 5% CO2 (37 ± 0.5 °C, pH = 7.35). Transmembrane action potential (AP) recordings were obtained using standard or floating glass microelectrodes. A pseudoelectrocardiogram (ECG) was recorded using 2 electrodes consisting of Ag/AgCl half cells placed in Tyrode’s solution bathing the preparation, 1.0-1.2 cm from the 2 opposite sides of the atrial or ventricular coronary-perfused preparations. Stable action potential recordings and Vmax measurements could not be readily obtained in vigorously contracting perfused preparations. Due to a substantial interpreparation variability, Vmax values were normalized for each experiment and then averaged. The change in conduction time was approximated by measuring the duration of the “QRS” complex of the wedge preparation.
Drugs
Amiodarone (Cordarone®, 200 mg TAB) was obtained from Wyeth Pharmaceuticals (Vonore, TN, USA) and was chronically administered orally at a dose of 40 mg/kg/day for a period of 6 weeks.
Statistics
Results are presented as means ± SD. Statistical analysis was performed using one-way repeated measures analysis of variance (ANOVA) followed by Bonferoni’s test. Mean values were considered to be different at a P-value of < 0.05.
Results
Six weeks of amiodarone treatment significantly slowed heart rate from 140 ± 21 to 102 ± 16, prolonged the QT from 208 ± 20 to 248 ± 16 ms, and prolonged the QTc interval from 297 ± 17 to 314 ± 17 ms in conscious dogs. The QRS duration remained unchanged, but P wave duration increased from 42 ± 6 to 49 ± 7 ms and PR interval prolonged from 113 ± 4 to 140 ± 3 ms (n = 12, P < 0.01). The same animals were used in a parallel study evaluating the effect of chronic amiodarone in the coronary-perfused right atrial preparation.3
Effects of Chronic Amiodarone Treatment in PV Sleeve Preparations
Figure 1 shows a representative example of the rate-dependent effects of chronic amiodarone treatment on the action potential of a PV sleeve preparation (panels A and B). Chronic amiodarone induced a prolongation of the action potential duration, a marked decrease in Vmax and a depolarization of TOP in the PV sleeve preparation. This effect was more accentuated at short cycle lengths. Composite data of the cycle length-dependent effects of chronic amiodarone on APD50, APD90, TOP, APA, and Vmax recorded under steady-state conditions in a total of 12 PV sleeve preparations isolated from 6 untreated and 6 chronic-amiodarone treated animals are shown in Figure 2. At a BCL of 1,000 ms, chronic amiodarone induced a significant (P < 0.05) increase in APD90 (from 201 ± 25 to 289 ± 47 ms, Fig. 2A), a significant cycle length-dependent decrease in Vmax (from 314 ± 79 to 115 ± 89 ms, Fig. 2C,D) and APA (from 114 ± 3 to 100 ± 6 mV, Fig. 2E), and a significant depolarization of TOP (from -85 ± 2 to -79 ± 4 mV, Fig. 2F). Chronic amiodarone treatment significantly accentuated the cycle length-dependent depression of Vmax (Fig. 2D). Diastolic threshold of excitation increased from 0.3 ± 0.2 mA to 1.8 ± 0.7 mA in preparations from untreated and chronic amiodarone treated dogs, respectively (P < 0.01).
Figure 1.
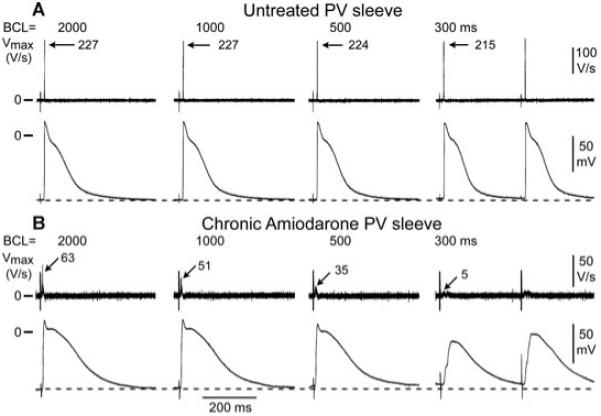
Rate-dependent effects of chronic amiodarone on the maximum rate of rise of action potential upstroke (Vmax) and action potential characteristics in a pulmonary vein (PV) sleeve preparation. A-B: Action potentials recorded at different basic cycle lengths (BCLs) from PV sleeves isolated from untreated (panel A) and chronic amiodarone-treated dogs (panel B). Chronic amiodarone induced a prolongation of the action potential duration, a marked decrease in Vmax, and a cycle length-dependent depolarization of the take-off potential.
Figure 2.
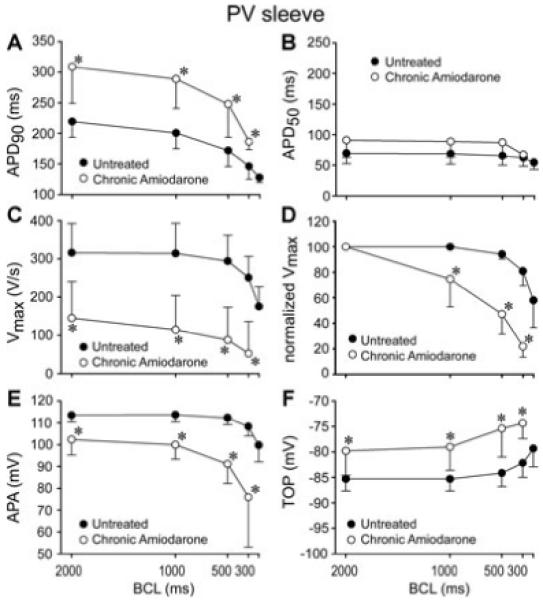
Cycle length-dependent effects of chronic amiodarone on action potential duration measured at 90 and 50% repolarization (APD50 and APD90, panels A and B), maximum rate of rise of action potential upstroke (Vmax), expressed in absolute as well as normalized values (panels C and D), action potential amplitude (APA, panel E), and take-off potential (TOP, panel F) recorded from pulmonary vein (PV) sleeve preparations isolated from untreated (n = 6) or chronic amiodarone-treated dogs (n = 6). Chronic amiodarone induced a significant prolongation of APD90, a significant decrease in Vmax and APA, and a significant depolarization of TOP. Data are expressed as mean ± SD. *P < 0.05 chronic amiodarone versus control.
Effects of Chronic Amiodarone on Vmax and Conduction: PV Sleeves versus Left Ventricular Wedge
In another series of experiments, we evaluated the effects of chronic amiodarone in the left ventricular (LV) wedge preparation. Figure 3 summarizes a comparison of the effects of Vmax and conduction time between PV (left, n = 6) and LV wedge (right, n = 4) preparations. The upper left panel shows action potentials recorded simultaneously from 2 sites of a PV sleeve preparation and the upper right panel displays action potentials recorded simultaneously from Endo, Epi, and M regions, together with a pseudo-ECG in a left ventricular wedge preparation. The lower right panel compares the effects on Vmax, normalized to the values at the longest cycle length (BCL = 2,000 ms). Conduction time was measured between electrodes placed along the PV sleeve or approximated by the duration of the “QRS” complex in the pseudo-ECG recorded in the LV wedge preparation. These data show that the effect of chronic amiodarone to increase conduction time and reduce Vmax is much more pronounced in PV sleeve as compared with the LV wedge preparation. Chronic amiodarone increased conduction time following an increase in stimulation rate. In the experiment shown in Figure 3 (upper panel), reducing BCL from 2,000 to 300 ms increased conduction time from 10.6 to 23.6 ms (130%) in the PV sleeve preparation and from 46.3 to 51.6 ms in the LV wedge preparation (11.5%). Composite data from 4 PV sleeve and 4 LV wedge preparations showed an increase in conduction time from 8.1 ± 2 to 16.3 ± 6 ms in PV sleeve (101%) and from 48.3 ± 3 to 54.6 ± 2 ms (13%) in LV wedge preparations. A change in BCL from 2,000 to 300 ms induced a 78.1% and 16.1% decrease in Vmax in PV and LV wedge preparations, respectively (Fig. 3, lower panel). The effects on Vmax were more pronounced at the fastest rates in both PV and ventricular tissues (P < 0.05 chronic amiodarone vs untreated preparations). At a BCL of 200 ms, 1:1 conduction was observed in 3 of 4 LV wedge preparations, but in 0 of 6 PV sleeve preparations, isolated from amiodarone-treated dogs.
Figure 3.
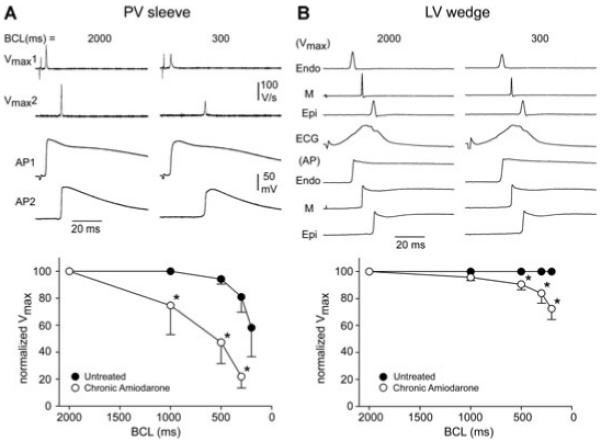
Comparison of the effects of chronic amiodarone on maximum rate of rise of action potential upstroke (Vmax) and conduction time between pulmonary vein (PV) (left, n = 6) and left ventricular (LV) wedge (right, n = 4) preparations. A: Action potentials (APs) recorded simultaneously from 2 sites of a PV sleeve preparation. B: APs recorded simultaneously from endocardial (Endo), epicardial (Epi), and midmyocardial (M) regions together with a pseudo-ECG in a LV wedge preparation. Lower panels show the rate dependence of Vmax normalized to the value at a basic cycle length (BCL) of 2,000 ms. Data are expressed as mean ± SD. *P < 0.05, chronic amiodarone versus control.
The marked use-dependent depression of INa, as reflected by the cycle length-dependent decrease in Vmax, led to activation failure in chronic amiodarone-treated animals (Fig. 3, lower left panel). Activation failure was observed at much longer cycle lengths in PV preparations (n = 5), as compared with LV wedge (n = 4) preparations. In PV preparations, activation failure occurred at a BCL of 124 ± 15 ms versus 420 ± 320 ms in preparations isolated from untreated and amiodarone treated dogs (P < 0.05), whereas in the LV wedge preparation activation failure occurred at a BCL of 150 ± 4 ms in preparations from untreated dogs versus 220 ± 42 ms in preparations isolated from amiodarone-treated dogs (Fig. 4, P < 0.05).
Figure 4.
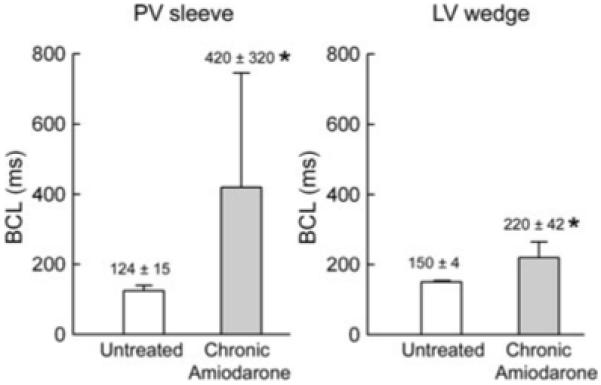
Composite data of the effect of chronic amiodarone on basic cycle length (BCL) at which 1:1 activation failure occurred in pulmonary vein (PV) sleeve (n = 5) and left ventricular (LV) wedge (n = 4) preparations isolated from untreated and chronic amiodarone-treated dogs. Chronic amiodarone induced a significant increase in the BCL at which activation failure occurred in PV sleeve and LV wedge preparations, but the effect was much more pronounced in PV sleeves. Data are expressed as mean ± SD. *P < 0.05, chronic amiodarone versus control.
Effects of Chronic Amiodarone on Early and Delayed After depolarization (EAD and DAD)-Induced Triggered Activity
In another series of experiments, we determined the effects of chronic amiodarone on DADs and late phase 3-EADs-induced triggered activity elicited by exposure of the PV sleeve preparations to acetylcholine (ACh, 1 μM), isoproterenol (1 μM), or high [Ca2+]o (5.4 mM) or their combination when stimulated at short cycle lengths (BCL < 300 ms). In the example illustrated in Figure 5 (upper panel, untreated PV), isoproterenol induced prominent rate-dependent DAD activity at BCLs of 300 and 200 ms and DAD-induced triggered response at a BCL of 120 ms. In the lower panel of Figure 5 (PV preparation from chronic amiodarone treated dog), isoproterenol is shown to induce no DADs at 300 or 200 ms and activation failure was observed at a BCL of 120 ms. Figure 6 illustrates the effect of combined ACh and high extracellular calcium to markedly shorten the action potential during fast pacing (BCL of 200 ms) and induce late phase 3 EAD and triggered activity following fast pacing (2-second pause) in a preparation from an untreated dog (panel A) but not in a preparation (panel B) from a chronic amiodarone-treated dog. DAD- and EAD-induced triggered activity following ACh, high extracellular calcium and isoproterenol or their combination occurred in 6 of 6 and 1 of 5 PV preparations from untreated versus chronic amiodarone-treated dogs. The lack of shortening of AP during fast rates following ACh was a constant feature in PV sleeves preparations isolated from dogs chronically treated with amiodarone. This effect may play an important role in the antiarrhythmic effect of chronic amiodarone in supraventricular arrhythmias, AF in particular.
Figure 5.
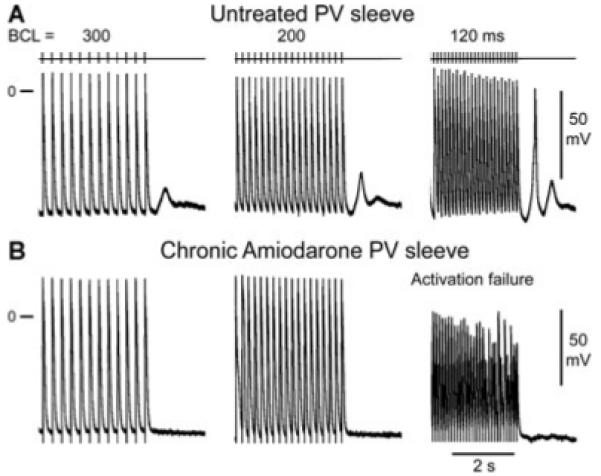
Chronic amiodarone prevents the development of isoproterenol-induced delayed afterdepolarizations (DADs) and triggered activity in a pulmonary vein (PV) sleeve preparation. A: PV sleeve preparation from untreated dog: isoproterenol induces prominent DAD activity at basic cycle lengths (BCL) of 300 and 200 ms. DAD reaches threshold and induces a triggered beat followed by a DAD at a BCL of 120 ms. B: PV sleeve from chronic amiodarone-treated dog: isoproterenol fails to induce DAD activity at 300 and 200 ms and 1:1 activation failure occurs at BCL 120 ms.
Figure 6.
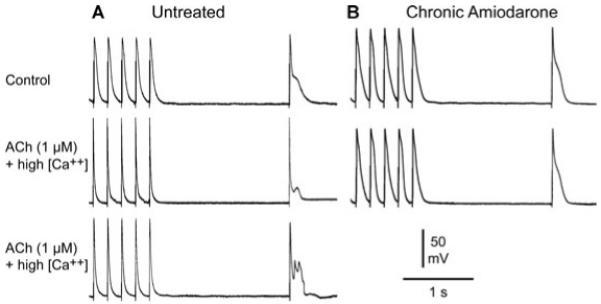
Chronic amiodarone prevents the development of late phase 3 early afterdepolarizations (EADs) and triggers activity in a pulmonary vein (PV) sleeve preparation. A: PV sleeve preparation from untreated dog: acetylcholine (ACh) and high calcium combined markedly shorten action potential (AP) during fast pacing (basic cycle length, BCL = 200 ms) and induce late phase 3 EAD (middle panel) and triggered activity (lower panel) upon return to a BCL of 2,000 ms. B: PV sleeve from chronic amiodarone-treated dog. ACh and high extracellular calcium do not shorten AP at fast rates and fail to induce late phase 3 EAD and triggered activity.
Discussion
Ectopic activity arising from the PV sleeves is thought to be an important source of triggers and in some cases substrate for the development of AF.13-15 Over the past decade, radiofrequency ablation has become the treatment of choice for drug-resistant AF. Segmental PV isolation and circumferential PV ablation are surgical procedures now commonly used to suppress refractory atrial arrhythmias including AF.16
The results of the present study indicate that in PV sleeve preparations, chronic amiodarone treatment induces rate-dependent depression of Vmax and excitability, prolongation of the action potential duration, slowing of conduction, and 1:1 activation failure at rapid rates of stimulation. In addition, chronic amiodarone induced a significant prolongation of PR interval. The data also show that atrial PV preparations are much more sensitive than ventricular preparations to the effects of chronic amiodarone to depress excitability and conduction. Moreover, our results indicate that chronic amiodarone is capable of preventing the appearance of DADs, late phase 3 EADs, and triggered activity induced in PV sleeves. Additionally, chronic amiodarone was observed to prevent ACh-induced marked shortening of the action potential.
Effects of Chronic Amiodarone: PV Sleeves versus Right Atrial Preparation
In a parallel study, we evaluated the effects of chronic amiodarone in coronary-perfused isolated canine right atrial preparations.3 Chronic amiodarone produced qualitatively similar but less accentuated use-dependent depression of sodium channel parameters in the coronary-perfused isolated canine right atrial preparations when compared to the results reported here for PV sleeves.3 At BCLs of 500 and 300 ms, Vmax was 58 and 65% smaller in right atrial preparations isolated from chronic amiodarone-treated versus untreated dogs. In contrast, in PV sleeves, Vmax was 71% (87 vs 294 V/s) and 79% (52 vs 251 V/s) smaller in preparations isolated from chronic amiodarone-treated versus untreated dogs at BCls of 500 and 300 ms, respectively. As the BCL was reduced from 500 to 300 ms, conduction time (measured between 2 microelectrodes in PV sleeves and approximated by the duration of the “P wave” in the coronary-perfused right atrium) was 26% longer (45 vs 35.7 ms) in right atrial preparations, but 39% longer (16.3 vs 11.7 ms) in PV sleeves preparations isolated from dogs treated with chronic amiodarone (P < 0.05). The shortest BCL permitting 1:1 activation was 72% longer (222 vs 129 ms) in right atrial preparations but 238% longer (420 vs 124 ms) in PV sleeves preparations isolated from chronic amiodarone-treated versus untreated animals (P < 0.05). The effects of chronic amiodarone on the action potential duration were qualitatively similar although less accentuated in right atrial preparations. At a BCL of 500 ms, APD90 was 44% longer (248 vs 172 ms) in PV sleeves but only 14% longer (256 vs 225 ms) in cristae terminalis and 17.5% longer (245 vs 202 ms) in right atrial pectinate muscle isolated from chronic amiodarone-treated versus untreated dogs.3
The effects of chronic amiodarone treatment to depress sodium-channel activity, reduce excitability, increase conduction time and to induce conduction block in PV sleeve preparations are qualitatively similar to those recently reported for the effects of ranolazine in canine atrial tissues.17,18
Effects of Chronic Amiodarone on EAD and DAD-Induced Triggered Activity in PV Sleeves
Late phase 3 EAD and DAD activity has been reported by Chen and coworkers in canine- and rabbit-isolated single PV myocytes,10,11 by Patterson and coworkers in isolated canine PV sleeve preparations,6,7 and by Burashnikov and coworkers in canine coronary-perfused right atrial preparations.5,19 In isolated rabbit and dog myocytes, isoproterenol was shown to increase automaticity and induce spontaneous as well as EAD or DAD-induced triggered activity.10,11 The present study shows that late phase 3 EADs and DADs, although readily induced in PV sleeve preparations from untreated dogs following the addition of isoproterenol, ACh, high extracellular calcium alone or in combination, are only rarely observed in preparations from chronic amiodarone-treated animals.
Ionic Mechanisms and Pharmacologic Profile of Amiodarone
Although possessing properties of all 4 Vaughan Williams classes of antiarrhythmic agents, amiodarone is often classified as a Class III antiarrhythmic.1,2 It is a predominantly inactivated-state sodium channel blocker with rapid binding and unbinding kinetics (similar to a Class IB agent).1 Acute amiodarone has been shown to inhibit IKr, whereas chronic amiodarone also leads to a reduction of IKs.20 The effects of acute amiodarone on ventricular APD are controversial, given that prolongation, abbreviation, and no effect have been reported.1 Chronic amiodarone, on the other hand, consistently prolongs ventricular APD and QT intervals.1,21 Intravenous administration of amiodarone generally produces more conduction delay and less prolongation of refractoriness than chronic therapy.22,23 Both acute and chronic amiodarone prolong the effective refractory period more than APD, due to development of postrepolarization refractoriness.24,25 Whereas acute amiodarone reduces Vmax and blocks INa in practically all studies (for review see1), chronic amiodarone was reported to either depress Vmax26,27 or, in most studies, to cause no or little changes in Vmax in superfused ventricular muscle and Purkinje fibers.1,21,28 Ventricular conduction velocity is slowed and QRS duration is prolonged in both acute and chronic amiodarone-treated humans and animals in vivo.28-30 However, the duration of the QRS complex was significantly prolonged only at relatively rapid pacing rates (e.g., at CL of ≤ 500 ms in humans), displaying a nonstatistically significant prolongation at normal or slow heart rates,21,30 consistent with our results in the canine heart. In rabbit atrial superfused tissue preparations, acute amiodarone produces an abbreviation of APD and a reduction in Vmax, whereas chronic amiodarone has been reported to prolong APD and cause little change in Vmax.31
Chronic administration of amiodarone has also been shown to decrease transmural dispersion of repolarization (TDR) in canine multicellular preparations.27 Acute amiodarone as well as the new antiarrhythmic agent dronedarone has been shown to reduce transmural dispersion of repolarization and eliminate (acute amiodarone) or reduce (dronedarone) EADs and triggered activity originating in M cells of the canine ventricle.32
The effect of chronic amiodarone treatment to prevent DAD and late phase 3 EAD activity is best explained by its actions to reduce late INa and intracellular calcium loading secondary to its effects to inhibit INa and ICa, as well as its sympatholytic action. It is noteworthy that acute amiodarone has been shown to prolong APD, induce EADs, and increase transmural dispersion of repolarization under conditions in which late INa is enhanced. Inhibition of late INa under these conditions suppresses the development of EADs, reduces TDR, and prevents the development of Torsade de Pointes arrhythmias.33
The effect of chronic amiodarone to prevent marked shortening of AP following ACh may be due to the effect of amiodarone to inhibit IK-ACh in atrial tissues1,34 and thereby contribute to the antifibrillatory effect of chronic amiodarone. The lack of ACh-induced shortening of AP in PV sleeves suggests the possibility of altered distribution/density of G-protein-gated inward rectifier potassium channel subunits, including Kir3.1 and Kir3.4.
Atrial Selective Effects of Chronic Amiodarone on Sodium Channel Activity
Our data indicate that chronic amiodarone treatment produces use-dependent depression of Vmax, excitability and conduction velocity preferentially in PV compared to ventricular preparations (Figs. 4 and 5). The effects of chronic amiodarone that we describe in PV sleeves preparations appear to be more prominent than those observed in arterially perfused right atrial preparations.3
The atrial selective effects of chronic amiodarone to depress peak INa and associated parameters, such as impulse conduction velocity, excitability, and postrepolarization refractoriness,35-37 have been shown to result from a more negative steady-state inactivation relationship for INa as well as the much slower action potential phase 3 in atrial versus ventricular cells.17 The slow phase 3 repolarization in atrial cells potentiates the effect of chronic amiodarone at rapid activation rates by abolishing the diastolic interval and preventing full repolarization, which leads to a more positive take-off potential for the next beat and reduced availability of sodium channels. Another factor that contributes to the reduced availability of peak INa in atria is the more depolarized resting membrane potential of atrial versus ventricular cells.17 Intrinsic atrioventricular differences in the resting membrane potential slope of late repolarization are largely explained by a smaller IK1 in atrial versus ventricular cells.38
Clinical Implications
In the clinic, acute and chronic amiodarone are widely used in the management of atrial and ventricular arrhythmias.2,39,40 Chronic amiodarone is still considered the most effective pharmacological agent currently available for the maintenance of sinus rhythm following termination of AF.2 The antiarrhythmic efficacy of chronic amiodarone has been related to the multiplicity of effects on ion channel activity (Class I, II, III and IV actions), resulting in the reduction of dispersion of repolarization, induction of postrepolarization refractoriness, prolongation of the excitable gap, suppression of triggered activity and attenuation of atrial remodeling.2,25,27,39,41,42
Clinical and experimental studies have highlighted the role of PV in the triggering of atrial arrhythmias, AF in particular.4,11 The results of the present study suggest that the PV-selective depression of INa-related parameters contributes prominently to the action of chronic amiodarone to suppress the development of AF and that the effect of chronic amiodarone to prolong the duration of the PV sleeve action potential potentiates this effect of the drug. These effects of chronic amiodarone suppress the development of arrhythmogenic substrates and triggers in the PV sleeves. The actions of chronic amiodarone to produce potent block of the sodium channels in the atria are similar to Class IC antiarrhythmic agents such as propafenone and flecainide. However, unlike the IC antiarrhythmics, the effects of chronic amiodarone are atrial selective,3 similar to the actions of ranolazine.17 Our data also point to an anticholinergic effect of chronic amiodarone, which can contribute to its effectiveness in the management of AF. Finally, the data confirm an atrialpredominant effect of chronic amiodarone,3 in this case for the PV sleeves.
Study Limitations
Experiments were performed in canine PV sleeves but not in other part of the canine left atrium; consequently, we are unable to contrast changes in PV versus left atrium electrophysiology. The oral dose of amiodarone used in our study (40 mg/kg/day) is larger than that used in the clinic, where the loading dose of amiodarone ranges from 800 to 1,600 mg/day (which is up to 20-25 mg/kg/day). Similar dosage regimens have been used in previous studies reflecting the relatively lower sensitivity of dogs to amiodarone.43 It should be noted, however, that loading doses are not given to patients for 6 weeks duration, but typically for 1-2 weeks. Plasma or tissue concentrations of amiodarone were not measured in this study. However, tissue concentrations were measured in a previous study using similar dose of chronic amiodarone in a dog experimental model.27 The tissue concentration of amiodarone in ventricular epicardium (17-22 ng/mL) persisted over a 6-hour period and was smaller that the tissue concentration of amiodarone found in humans following chronic amiodarone treatment (40 ng/mL);44 amiodarone treatment in this study was 600 mg for 1 week, followed by 200 mg for a period of 7 weeks.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Judy Hefferon and Robert Goodrow Jr.
This work was supported by grant HL47678 from NHLBI (CA) and NYS and Florida Grand Lodges F. & A.M.
Dr. Antzelevitch received research support from AstraZeneca and CV Therapeutics. He reports receiving honoraria for lectures including these data and serving on the advisory board of CV Therapeutics. Dr. Belardinelli is an employee of CV Therapeutics and Dr. Carlsson is an employee of AstraZeneca.
References
- 1.Kodama I, Kamiya K, Toyama J. Amiodarone: Ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;9A:20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 2.Singh BN. Amiodarone: A multifaceted antiarrhythmic drug. Curr Cardiol Rep. 2006;5:349–355. doi: 10.1007/s11886-006-0074-2. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;12:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;10:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;3:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;6:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: An arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;6:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Wongcharoen W, Chen YC, Chen YJ, Chen SY, Yeh HI, Lin CI, Chen SA. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;10:1338–1349. doi: 10.1016/j.hrthm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Lo LW, Chen YC, Chen YJ, Wongcharoen W, Lin CI, Chen SA. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007;2-3:197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: Implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;2:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;2:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: A novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;12:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 14.Nattel S, Allessie MA, Haissaguerre M. Spotlight on atrial fibrillation-the “complete arrhythmia.”. Cardiovasc Res. 2002;2:197–203. doi: 10.1016/s0008-6363(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 15.Nattel S. Combined parasympathetic-sympathetic nerve discharge and pulmonary vein after depolarizations: A new unifying concept with basic and clinical relevance. Heart Rhythm. 2005;6:632–633. doi: 10.1016/j.hrthm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;3:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 17.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: Differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;13:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;7:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early after depolarization-induced triggered activity. Circulation. 2003;18:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya K, Nishiyama A, Yasui K, Hojo M, Sanguinetti MC, Kodama I. Short- and long-term effects of amiodarone on the two components of cardiac delayed rectifier K+ current. Circulation. 2001;9:1317–1324. doi: 10.1161/01.cir.103.9.1317. [DOI] [PubMed] [Google Scholar]
- 21.Sosunov EA, Anyukhovsky EP, Rosen MR. Chronic in vivo and in vitro effects of amiodarone on guinea pig hearts. J Pharmacol Exp Ther. 1996:906–912. [PubMed] [Google Scholar]
- 22.Gallagher JD, Bianchi J, Gessman LJ. A comparison of the electrophysiologic effects of acute and chronic amiodarone administration on canine Purkinje fibers. J Cardiovasc Pharmacol. 1989;5:723–729. [PubMed] [Google Scholar]
- 23.Yabek SM, Kato R, Singh BN. Acute effects of amiodarone on the electrophysiologic properties of isolated neonatal and adult cardiac fibers. J Am Coll Cardiol. 1985;5:1109–1115. doi: 10.1016/s0735-1097(85)80012-7. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama T, Richardson LC, Sun W, McCarthy JJ, Gettes LS. Acute effects of amiodarone on membrane properties, refractoriness, and conduction in guinea pig papillary muscles. Heart Vessels. 1995;2:78–86. doi: 10.1007/BF01744498. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof P, Degen H, Franz MR, Eckardt L, Fabritz L, Milberg P, Laer S, Neumann J, Breithardt G, Haverkamp W. Amiodarone-induced postrepolarization refractoriness suppresses induction of ventricular fibrillation. J Pharmacol Exp Ther. 2003;1:257–263. doi: 10.1124/jpet.102.046755. [DOI] [PubMed] [Google Scholar]
- 26.Mason JW, Hondeghem LM, Katzung BG. Block of inactived sodium channels and of depolarization-induced automaticity in guinea pig papillary muscle by amiodarone. Circ Res. 1984:277–285. doi: 10.1161/01.res.55.3.278. [DOI] [PubMed] [Google Scholar]
- 27.Sicouri S, Moro S, Litovsky SH, Elizari MV, Antzelevitch C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 28.Levine JH, Moore EN, Kadish AH, Weisman HF, Balke CW, Hanich RF, Spear JF. Mechanisms of depressed conduction from long-term amiodarone therapy in canine myocardium. Circulation. 1988:684–691. doi: 10.1161/01.cir.78.3.684. [DOI] [PubMed] [Google Scholar]
- 29.Nanas JN, Mason JW. Pharmacokinetics and regional electrophysiological effects of intracoronary amiodarone administration. Circulation. 1995;2:451–461. doi: 10.1161/01.cir.91.2.451. [DOI] [PubMed] [Google Scholar]
- 30.Morady F, Dicarlo LA, Krol RB, Baerman JM, De Buitleir M. Acute and chronic effects of amiodarone on ventricular refractoriness, intraventricular conduction and ventricular tachycardia induction. J Am Coll Cardiol. 1986:148–157. doi: 10.1016/s0735-1097(86)80273-x. [DOI] [PubMed] [Google Scholar]
- 31.Sun W, Sarma JS, Singh BN. Chronic and acute effects of dronedarone on the action potential of rabbit atrial muscle preparations: Comparison with amiodarone. J Cardiovasc Pharmacol. 2002;5:677–684. doi: 10.1097/00005344-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Moro S, Ferreiro M, Celestino D, Medei E, Elizari MV, Sicouri S. In vitro effects of acute amiodarone and dronedarone on epicardial, endocardial, and M cells of the canine ventricle. J Cardiovasc Pharmacol Ther. 2007;4:314–321. doi: 10.1177/1074248407306906. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Rajamani S, Shryock JC, Li H, Ruskin J, Antzelevitch C, Belardinelli L. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008;3:481–488. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe Y, Hara Y, Tamagawa M, Nakaya H. Inhibitory effect of amiodarone on the muscarinic acetylcholine receptor-operated potassium current in guinea pig atrial cells. J Pharmacol Exp Ther. 1996;2:617–624. [PubMed] [Google Scholar]
- 35.Kirchhof PF, Fabritz CL, Franz MR. Postrepolarization refractoriness versus conduction slowing caused by class I antiarrhythmic drugs: Antiarrhythmic and proarrhythmic effects. Circulation. 1998;25:2567–2574. doi: 10.1161/01.cir.97.25.2567. [DOI] [PubMed] [Google Scholar]
- 36.Costard-Jackle A, Franz MR. Frequency-dependent antiarrhythmic drug effects on postrepolarization refractoriness and ventricular conduction time in canine ventricular myocardium in vivo. J Pharmacol Exp Ther. 1989;1:39–46. [PubMed] [Google Scholar]
- 37.Lee RJ, Liem LB, Cohen TJ, Franz MR. Relation between repolarization and refractoriness in the human ventricle: Cycle length dependence and effect of procainamide. J Am Coll Cardiol. 1992;3:614–618. doi: 10.1016/s0735-1097(10)80281-5. [DOI] [PubMed] [Google Scholar]
- 38.Golod DA, Kumar R, Joyner RW. Determinants of action potential initiation in isolated rabbit atrial and ventricular myocytes. Am J Physiol. 1998;6(Pt 2):H1902–H1913. doi: 10.1152/ajpheart.1998.274.6.H1902. [DOI] [PubMed] [Google Scholar]
- 39.Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;9:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 40.Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, Murphy E. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;9:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: Insights into the mechanism of the superior efficacy of amiodarone. Circulation. 2003;10:1440–1446. doi: 10.1161/01.cir.0000055316.35552.74. [DOI] [PubMed] [Google Scholar]
- 42.Maury P, Zimmermann M. Effect of chronic amiodarone therapy on excitable gap during typical human atrial flutter. J Cardiovasc Electrophysiol. 2004;12:1416–1423. doi: 10.1046/j.1540-8167.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 43.van Opstal JM, Schoenmakers M, Verduyn SC, De Groot SH, Leunissen JD, Der Hulst FF, Molenschot MM, Wellens HJ, Vos MA. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001;22:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- 44.Holt DW, Tucker GT, Jackson PR, McKenna WJ. Amiodarone pharmacokinetics. Br J Clin Pract Suppl. 1986:109–114. [PubMed] [Google Scholar]


