Abstract
Sensitivity to interaural time difference (ITD) in constant-amplitude pulse trains was measured in four sequentially implanted bilateral cochlear implant (CI) subjects. The sensitivity measurements were made as a function of time beginning directly after the second ear was implanted, continued for periods of months before subjects began wearing bilateral sound processors, and extended for months while the subjects used bilateral sound processors in day-to-day listening. Measurements were also made as a function of the relative position of the left∕right electrodes. The two subjects with the shortest duration of binaural deprivation before implantation demonstrated ITD sensitivity soon after second-ear implantation (before receiving the second sound processor), while the other two did not demonstrate sensitivity until after months of daily experience using bilateral processors. The interaural mismatch in electrode position required to decrease ITD sensitivity by a factor of 2 (half-width) for CI subjects was five times greater than the half-width for interaural carrier-frequency disparity in normal-hearing subjects listening to sinusoidally amplitude-modulated high-frequency tones. This large half-width is likely to contribute to poor binaural performance in CI users, especially in environments with multiple broadband sound sources.
INTRODUCTION
The advantages of binaural over monaural listening for normal-hearing subjects include more accurate sound-source localization (Oldfield and Parker, 1986) and superior reception of a target sound when spatially separated from competing sound sources (Zurek, 1993). It is not, therefore, surprising that hearing-impaired patients have undergone bilateral implantation in an effort to restore some measure of these binaural advantages.
Multiple investigators have tested the ability of cochlear implant (CI) users to localize sound sources using their commercial sound-processing systems (e.g., van Hoesel and Tyler, 2003; Nopp et al., 2004; Litovsky et al., 2006b; Grantham et al., 2007) and to receive speech in the presence of spatially separated interfering sounds (e.g., Schleich et al., 2004; Litovsky et al., 2006a; Ricketts et al., 2006; Peters et al., 2007). While these studies show better performance for bilateral than monolateral listening, the level of bilateral advantage does not typically reach that of normal-hearing listeners. Furthermore, there is evidence that almost all of the benefits for binaural CI users are due to interaural level differences (ILDs), while little or no functional benefit has been shown from use of interaural time difference (ITD).
For example, two studies measured the total root-mean-squared error for localizing sound sources in the frontal horizontal plane for both CI users (listening with commercial sound-processing systems) and normal-hearing subjects using the same methods (Poon, 2006; Grantham et al., 2007). The mean error scores for the CI users were 28° and 30°; substantially larger (worse) than the 7° and 0° measured for the normal-hearing listeners. Several lines of evidence suggest that CI patients use ILD as the main cue for localization. van Hoesel (2004) pointed out the correspondence between localization error magnitude and ILD cue ambiguity as a function of azimuth. Grantham et al. (2007) demonstrated that the impact of a sound’s spectral content on CI users’ ability to localize was consistent with ILD being the principal cue. Poon (2006) and Grantham et al. (2008) used a single acoustic signal to measure both localization error and sensitivity to ITD and ILD in the same subjects and analyzed the degree to which the variation in ITD and ILD sensitivity could account for the variation in localization error. Both concluded that localization performance was based primarily on ILD cues. Seeber and Fastl (2008) studied two subjects with relatively good localization ability to assess the impact of several signal manipulations (spectral∕temporal composition, presence∕absence of the head-shadow cue, and virtual shifting of ITD∕ILD cues) on the subjects’ abilities to localize with their commercial sound processors; they concluded that both subjects relied predominantly on ILD cues, with little evidence of any contribution from ITD cues.
Poor ITD sensitivity is also implicated as a factor limiting the binaural advantage associated with speech reception in the presence of spatially separated speech-shaped noise sources. Zurek’s (1993) model analysis of this advantage measured in normal-hearing listeners concluded that it can largely be accounted for by two components: improvement in the signal-to-noise ratio at one ear due to the purely acoustic head-shadow effect and the binaural unmasking predicted solely from ITD. A third, relatively small, component of binaural advantage is the effect of listening to the better signal-to-noise ratio signal with both ears rather than just the better ear alone. This is called the “diotic effect” because the same signal is delivered to both ears. When measured in terms of speech-reception threshold in normal-hearing subjects and when the target is straight ahead and the masker is on the sides, the head-shadow effect provides an advantage of about 8 dB, the binaural unmasking ranges from approximately 2.5 to 5 dB, and the diotic effect ranges from 1.5 to 2.5 dB (Bronkhorst and Plomp, 1988; Arsenault and Punch, 1999). In the case of adult CI users listening through their sound processors, the advantages are smaller. Measurements of the head-shadow advantage range from 5.6 to 6.8 dB, and the “squelch” advantage, which combines the benefit from ITD and diotic listening, ranges from approximately 0.9 to 1.9 dB (Schleich et al., 2004; Litovsky et al., 2006a). van Hoesel et al. (2008) measured diotic benefit for CI users to be about 1.0–1.5 dB but found no binaural unmasking. The absence of binaural unmasking and the close agreement between the van Hoesel et al. (2008) measures of diotic benefit and measures of squelch benefit (Schleich et al., 2004; Litovsky et al., 2006a) are consistent with CI users not being able to access the information normally carried by ITD. The results of the study of van Hoesel et al. (2008) found similar results using both commercial sound-processing strategies that discard fine-timing information and a research processor that explicitly coded fine-timing cues.
Psychophysical measures of implanted subjects’ sensitivities to ITD and ILD in conditions that eliminate the possible adverse impact of commercial sound-processing strategies and multichannel stimulation are also consistent with the hypothesis that poor ITD sensitivity largely accounts for the abnormally small localization and binaural squelch advantages seen in CI users. Just noticeable differences (JNDs) for electric ILD measured using unmodulated pulse trains stimulating single interaural electrode pairs are as small as 0.2 dB (e.g., van Hoesel and Tyler, 2003). Acoustic (through-processor) ILD thresholds are near normal, with JNDs of 1–2 dB SPL (sound pressure level) measured for the best users (Laback et al., 2004). In contrast, ITD JNDs measured in CI subjects are substantially worse than normal. ITD sensitivity is best for low-rate (40–100 pps) pulse trains where ITD JNDs of 50 μs or less have been measured for some interaural electrode pairs in the best subjects but typically range between 100 and 500 μs, with JNDs for a few subjects extending beyond 700 μs (van Hoesel et al., 1993; van Hoesel and Clark, 1997; Lawson et al., 1998; van Hoesel et al., 2002; van Hoesel and Tyler, 2003; Wilson et al., 2003; van Hoesel, 2004; Laback et al., 2007; van Hoesel, 2007). As the pulse repetition rate increases, ITD sensitivity rapidly decreases, with JNDs typically increasing to 400 μs or greater by 600 pps (van Hoesel and Tyler, 2003; Majdak et al., 2006; van Hoesel, 2007). This effect of rate on ITD is similar to that for normal-hearing subjects listening to high-frequency, bandpass-filtered acoustic clicks (Hafter and Dye, 1983), but unlike normal-hearing subjects listening to pure tones, when the ITD JND decreases from approximately 75 μs at 90 Hz to 11 μs at 1000 Hz (Klumpp and Eady, 1956).
In this paper, results are presented that further characterize the ITD sensitivity of single-interaural electrode pairs stimulated with unmodulated pulse trains in bilaterally implanted CI subjects. Though only four CI listeners participated in the current study, their results provide data over extended periods of time and are relevant for several questions that have not been fully addressed and that are of current interest. The first set of results addresses whether the factors of (adult-onset) binaural deprivation and listening experience with bilateral CIs impact ITD sensitivity in adult CI users. Several studies suggest that deprivation and listening experience influence adult monolateral (Tyler and Summerfield, 1996; Pelizzone et al., 1999) and childhood bilateral (Peters et al., 2007) performance as well as the development of both cortical (Sharma et al., 2005) and brainstem (Gordon et al., 2007) potentials in young children. Except for a report showing that localization performance did not improve for most adult subjects between 5 and 15 months of post-activation (Grantham et al., 2007), the impact of deprivation and listening experience on binaural advantages or on ITD and ILD sensitivity has not been studied. While the results presented in this paper were not generated in a study explicitly designed to directly address the impact of deprivation and listening experience on ITD sensitivity in postlingually deafened CI users and are not, therefore, optimal in terms of some study parameters (see Sec. 2), they are of interest as the first data of their kind.
While the potential desirability of stimulating interaurally place-matched electrodes was recognized at the beginning of bilateral cochlear implantation (e.g., van Hoesel et al., 1993), published reports studying the effect of interaural place disparity on ITD sensitivity are few (van Hoesel and Clark, 1997; Long et al., 2003; Wilson et al., 2003; van Hoesel, 2004) and include data from a total of only five subjects. The authors report results for an additional four subjects and relate them to the impact of interaural carrier-frequency disparity on the modulator ITD JND measured in normal-hearing listeners and to the effect of interaural place disparity on the bilateral interaction component of the electrically evoked auditory brainstem response in cats.
METHODS
The Institutional Review Boards of the Massachusetts Eye and Ear Infirmary and the Massachusetts Institute of Technology reviewed and approved the methods used in the studies reported here.
Subjects, implants, and sound processors
Results are reported from four subjects with bilateral Advanced Bionics CII CIs who have been followed for a period of 2–4 years. They are part of an ongoing effort to understand how basic ITD and ILD sensitivity limits functional capabilities such as lateralization and speech reception in the presence of a spatially separated interferer. Our approach has been to more completely characterize ITD and ILD sensitivity using a wider range of stimuli and conditions for each of a smaller number of subjects than is practical for larger subject populations. The results reported here are extracted from this accumulating data set and are not from single studies, each designed to address the specific issues discussed in this report.
Table 1 lists the etiologies of the subjects and the age of each subject at the onset of: hearing loss, hearing-aid use, profound hearing impairment, and CI use. Subject C105’s idiopathic loss was sudden onset (never wore hearing aids), and C120’s autoimmune loss was rapidly progressive. All subjects were postlingually deafened, and all reports are consistent with our presumption that they had normal binaural hearing before the onset of their hearing loss.
Table 1.
Subject characteristics.
| Subject | Etiology | Age at onset of hearing loss (years) | Age at onset of hearing-aid use (years) | Age at profound loss (years) | Age at onset of CI use (years) | Durationa of bilateral deprivation (years) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | |||
| C105 | Idiopathic | 65 | 74 | N∕A | N∕A | 65 | 74 | 76 | 74 | 11 |
| C109 | Genetic | 30 | 30 | 37 | 34 | 44 | 44 | 48 | 50 | 6 |
| C120 | Autoimmune | 34 | 34 | 36 | 36 | 39 | 39 | 40 | 43 | 4 |
| C128 | Genetic | 11 | 11 | 17 | 17 | 25 | 25 | 36 | 39 | 14 |
Age at onset of CI use for second sound processor minus the age at profound loss for the earlier of the two ears.
The two ears of each subject were implanted sequentially with the first-implanted cochlea receiving an Advanced Bionics HiFocus electrode array with a “positioner” designed to place the array in a modiolar position. The second-implanted ears also received HiFocus electrode arrays: C105 and C109 with a positioner and C120 and C128 without a positioner. The age at which each ear began receiving daily stimulation is given in Table 1.
The authors partition the subjects’ listening experience with CIs into three periods that were part of the original study design and to which the subjects consented prior to implantation of the second device. Period I began with the implantation of their first ear, lasted for at least 18 months, and ended with the implantation of their second ear. During this monolateral listening period, the authors assume that the subjects developed monolateral listening strategies for speech reception (in quiet and in noise) and localization.
Period II began with implantation of their second implant. During this listening period, the subjects continued monolateral use of their first implant system for at least 8 months (C105: 347 days, C109: 272 days, C120: 329 days, and C128: 271 days). The only time their second implant produced stimulation was during laboratory testing sessions designed to (1) identify interaural electrodes matched in cochleotopic position to be paired in bilateral sound-processing strategies and (2) evaluate localization and speech-reception capabilities using monolateral and bilateral sound processors during a period when they presumably continued to employ a monolateral listening strategy in their day-to-day monolateral implant system use.
Listening period III began at least 8 months after implantation of the second ear when the second sound processor was fitted for daily use. Since then, subjects have used two sound processors that implement a version of the continuous interleaved sampling (CIS) strategy (Wilson et al., 1991). Because the two sound processors are not synchronized, the ITD between the carrier pulses generated by the output signals of the two processors is not controlled and can drift with time. The envelope ITD between the two processors accurately reflects the ITD at the subject’s microphones.
Laboratory testing sessions during listening period III were designed to (1) characterize ITD and ILD sensitivity and (2) evaluate localization and speech-reception capabilities using monolateral and bilateral sound processors during a period when subjects would be expected to use the bilateral listening strategy they develop during day-to-day use of their bilateral implant system use. The results presented in this report focus on measures of ITD sensitivity made using single-interaural electrode pairs stimulated with pulse trains during listening periods II and III.
The processor controlling the first implant in each subject mapped CIS analysis channels 1 (lowest frequency band) through 16 (highest frequency band) to electrodes 1 (most apical) through 16 (most basal). After implantation of the second implant and during listening period II, a combination of measures (e.g., binaural fusion, interaural pitch ranking, and interaural time sensitivity) was used to identify spatially matched electrodes across the two ears and to guide the mapping of analysis channel to electrode in the second implant system (Eddington et al., 2002; Eddington et al., 2003). Table 2 summarizes the implants, the sound-processing strategies, and the latest monolateral speech-reception performance. The column “Channel-electrode map” identifies the channel-to-electrode map used for each subject. These maps are specified in Table 3.
Table 2.
Devices and performance.
| Subject | Implant model (R and L) | Electrode model (R and L) | Electrode positioner | Strategy (R and L) | Channel-electrode map (Table 3) | Pulse rate (pps) | Pulse width (μs) | Latest NU-6 word score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R (%) | L (%) | |||||||
| C105 | CII | HiFocus | Yes | Yes | CIS-16 | 2 | 1450 | 21.6 | 28 | 38 |
| C109 | CII | HiFocus | Yes | Yes | CIS-16 | 1 | 1450 | 21.6 | 94 | 86 |
| C120 | CII | HiFocus | Yes | No | CIS-16 | 1 and 3 | 2320 | 13.5 | 84 | 70 |
| C128 | CII | HiFocus | Yes | No | CIS-16 | 1 and 4 | 2320 | 13.5 | 84 | 86 |
Table 3.
Mapping of channels to electrodes.
| Analysis channel | Map 1 | Map 2 | Map 3 | Map 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Left EL | Right EL | Left EL | Right EL | Left EL | Right EL | Left EL | Right EL | |
| 1 | 1 | 1 | 1 | 1a | 2 | 1 | 1b | 1 |
| 2 | 2 | 2 | 2 | 1a | 3 | 2 | 1b | 2 |
| 3 | 3 | 3 | 3 | 2 | 4 | 3 | 1b | 3 |
| 4 | 4 | 4 | 4 | 3 | 5 | 4 | 2 | 4 |
| 5 | 5 | 5 | 5 | 4 | 6 | 5 | 3 | 5 |
| 6 | 6 | 6 | 6 | 5 | 7 | 6 | 4 | 6 |
| 7 | 7 | 7 | 7 | 6 | 8 | 7 | 5 | 7 |
| 8 | 8 | 8 | 8 | 7 | 9 | 8 | 6 | 8 |
| 9 | 9 | 9 | 9 | 8 | 10 | 9 | 7,8 | 9 |
| 10 | 10 | 10 | 10 | 9 | 11 | 10 | 9 | 10 |
| 11 | 11 | 11 | 11 | 10 | 12 | 11 | 10 | 11 |
| 12 | 12 | 12 | 12 | 11 | 13 | 12 | 11 | 12 |
| 13 | 13 | 13 | 13 | 12 | 14 | 13 | 12 | 13 |
| 14 | 14 | 14 | 14 | 13 | 15 | 14 | 13 | 14 |
| 15 | 15 | 15 | 15 | 14 | 16c | 15 | 14 | 15 |
| 16 | 16 | 16 | 16 | 15,16 | 16c | 16 | 15 | 16 |
These electrodes were driven by a custom analysis channel with the combined bandwidth of the map 1 analysis channels 1 and 2.
These electrodes were driven by a custom analysis channel with the combined bandwidth of the map 1 analysis channels 1–3.
These electrodes were driven by a custom analysis channel with the combined bandwidth of the map 1 analysis channels 15 and 16.
The preliminary measures used to evaluate interaural electrode-array offset suggested minor differences for subjects C105, C109, and C120. The fusion and pitch ranking measures for C128 indicated an interaural electrode-array offset of approximately two electrodes for the most apical electrodes, tapering to a single-electrode offset for the more basal electrodes. In the cases of C120 and C128, two programs were downloaded to the second sound processor: one to implement channel-to-electrode map 1 and another to implement either map 3 (C120) or map 4 (C128). C120 and C128 have been switching between the two programs approximately every two days since they began wearing two sound processors. They both report not being able to distinguish between the two maps, and the authors are not able to measure significant differences in localization or speech reception in quiet or noise between the two programs.
The results reported below were measured using single interaural electrode pairs. Whether or not these interaural pairs were also paired by analysis channel in the channel-to-electrode map(s) used by the subject in listening period III is documented in the text and figure captions.
Stimulus generation for psychophysical studies
ITD sensitivity was measured using single pulses and fixed-amplitude pulse trains. All pulses were biphasic (typically 27 μs∕phase 1). Stimuli were delivered monopolarly (with the receiver∕stimulator cases serving as extracochlear return electrodes) to two intracochlear electrodes, one in each cochlea (an interaural pair). Custom software and a bilateral Clarion Research Interface (CRI2 manufactured by Advanced Bionics Corporation) were used to control the subjects’ bilaterally implanted receivers∕stimulators and to synchronize stimuli across ears to within 1 μs. ITD resolution of the controller was 13.5 μs. ITDs were generated by delaying the stimulus at one electrode relative to the other, producing an onset ITD and ongoing ITD that are equal.
All tests were conducted at stimulation levels adjusted to produce a comfortable sensation level in each ear using the following three-step procedure. Prior to each run, the monolateral-left stimulus amplitude was adjusted to elicit a most comfortable sensation level. The right stimulus level was then adjusted to match the left sensation level during repeated sequential left-right stimulation. Finally, the “level-matched” right∕left stimuli were played simultaneously (ITD=0 μs), and, when needed, the right-ear amplitude was adjusted to center the resulting sound image. If bilateral stimulation did not elicit a single sound image (e.g., when the individual electrodes of an interaural electrode pair were widely separated in cochlear place), the level-matched amplitudes were used. The “centered” or level-matched amplitudes were considered the “zero ILD” condition even though the actual stimulation levels in each ear were often different (typically by less than 1 dB).
For each subject, interaural electrode pairs mapped to the same analysis channel in the two asynchronous sound processors used in the field are referred to as processor-paired electrodes. Electrode pairs are denoted by the subject ID followed by the left and right electrode number (e.g., C109:L3R3).
Adaptive lateralization test
ITD sensitivity was measured using an adaptive (two-down, one-up, 14 reversals), two-alternative forced-choice procedure that targeted the 70.7% level on the psychometric function (Levitt, 1971). The first-interval stimulus was always the reference (ITD=0 μs). The adaptively determined ITD magnitude was applied to the second-interval stimulus with the leading side randomly selected. This procedure is sometimes referred to as a center-side or reminder task, in contrast to the two-interval, two-alternative forced-choice task, and it typically estimates a JND about √2 less than a two-cue, two-interval task where an opposite ITD is applied during each interval (Hartmann and Rakerd, 1989). The subject used a keyboard to indicate whether the probe was lateralized to the left or the right of the reference position. Correct-answer feedback was given after each trial.
Informal testing at the beginning of each run estimated the ITD JND and determined a starting ITD that the subject could easily lateralize. In cases where the subject was not able to perform above chance at the procedure’s maximum ITD (2 ms), the JND could not be measured (CNM in Fig. 1). A starting step size was chosen and then was reduced by half after the first peak reversal and further reduced (by half again) after the second peak reversal in the adaptive track. Based on the informal testing, the starting step size was typically set at 108 or 54 μs (resulting in a final step size of 27 or 13.5 μs for stimuli associated with the last ten reversals of the adaptive track) unless the informal testing revealed very poor ITD sensitivity, in which case a larger starting step size was sometimes used. After about 80% of the data reported here had been collected, the authors switched to a constant scaling factor of 2 dB (multiplying or dividing by a factor of 1.26 since 10(2∕20)∼1.26) for the adaptive runs (Saberi, 1995).
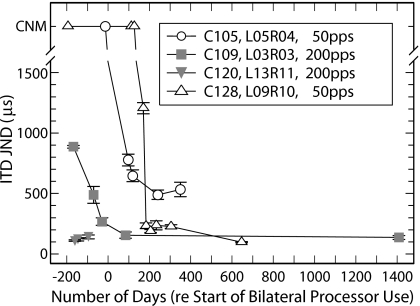
Figure 1.
ITD JND plotted as a function of time (relative to the day each subject began wearing their second sound processor). Stimuli were constant-amplitude, biphasic pulse trains (300-ms duration) with the repetition rate, subject, and interaural electrode pair identified in the legend. Symbols located above the axis break mark days when an ITD JND could not be measured (CNM) because the subject could not distinguish zero ITD from ITDs greater than 1500 μs or because stimulation of the interaural pair elicited multiple (non-fused) sound images. In cases where multiple measures were made using the same stimulus waveform on the same day, those runs were combined to determine the JND (see Sec. 2). Error bars represent the standard error of these multiple measurements.
A psychometric function was estimated from the run data using a generalized linear model (binomial distribution function and probit link function). The JND was defined as the ITD corresponding to a 70.7% correct response rate. In cases where multiple runs were conducted for the same stimulus configuration and parameter set, the trials from all runs were combined and the psychometric function estimated from the combined data set. The combined set of the last eight reversals of each run was used to compute an estimate of the standard error.
Measures of ILD sensitivity used the same methods as those used to measure the ITD JND. The adaptively determined ILD was applied to the second-interval stimulus, and the side incremented in level was randomly selected. Starting ILDs were selected that elicited easily lateralized sound images (typically 2 dB), and the beginning step size was 0.4 dB.
RESULTS
ITD sensitivity as a function of time
Figure 1 plots ITD JNDs measured with unmodulated pulse trains as a function of time for the four example subject∕stimulus-parameter combinations identified in the legend. Time is referenced to the day each subject began wearing his∕her second sound processor. As noted in Sec. 2, during the period between implantation of the second ear and the daily use of their second processor (i.e., before day 0 in the figure), the subjects continued monolateral listening in the field with the same sound processor used before their second surgery. The only time they experienced bilateral stimulation in this period was during laboratory testing sessions.
The data represented by gray-filled symbols are examples from subjects C109 and C120 showing that their sensitivity to ITD was apparent early in their testing and before daily use of their second sound processor The interaural pairs selected for plotting are among the first demonstrating ITD sensitivity. The JNDs were measured using the 200-pps rate of the stimulus used to search for ITD sensitivity. These two subjects also demonstrated early ILD sensitivity with JNDs of 0.22 dB for C109 and 0.28 dB for C120 by 180 days before beginning bilateral sound-processor use (day −180).
In the case of C105 and C128 (open symbols), ITD sensitivity did not develop until well into their daily experience with bilateral CI listening. By this time, the authors had added a search stimulus of 50 pps because of its greater ITD sensitivity. They expected that the emergence of ITD sensitivity would have occurred at an even later point in time using the 200-pps search stimulus because ITD JNDs could often not be measured at this higher rate when sensitivity was first detected using the 50-pps search stimulus. For instance, at day +98, an ITD JND of 777 μs was measured for C105 using the 50-pps stimulus but could not be measured (JND>1500 μs) using the 200-pps stimulus.
Like subjects C109 and C120, subjects C105 and C128 demonstrated relatively early ILD sensitivity with ILD JNDs measured before day −130 of 0.50 dB for C105 and 0.18 dB for C128. These results indicate that it is unlikely that the measurement of early ITD sensitivity for subjects C105 and C128 was limited by the subjects’ ability to perform the lateralization task. The early ILD-based lateralization does not demonstrate that binaural processing for ILD cues developed before ITD cues because the authors cannot rule out the use of monaural level cues.
The ITD JND measured in subjects C105, C109, and C128 generally improved for several months after ITD sensitivity was first observed. The improvements in C109’s and C128’s sensitivity are dramatic with ITD JNDs below 200 μs while C105’s is more modest with best JNDs of approximately 500 μs. C120’s sensitivity started out better than that C105 could achieve after more than a year of bilateral listening experience. The results marked by gray-filled squares illustrate the general trend for C109 of ITD sensitivity improving with time before she began using her second sound processor even though her exposure to bilateral stimuli consisted of only 2- or 3-hour testing sessions once or twice each month during this period. The data presented in subsequent sections of this report were measured after the ITD sensitivity had stabilized.
ITD JND as a function of interaural electrode separation
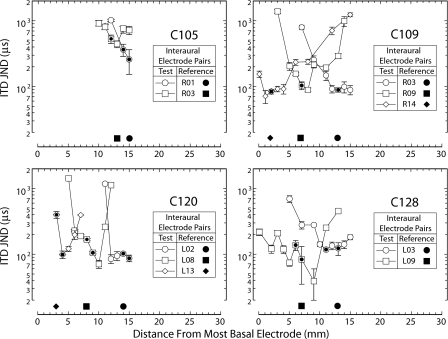
Figure 2 plots measures of ITD JND made in four subjects as a function of the contralateral test electrode used to form a single-interaural pair with the reference electrodes identified in the legends. Stimuli were 300-ms pulse trains at 50 pps for C105, C109, and C128 and at 200 pps for C120 (because C120 was lost to study before the 50-pps measures could be made). In the case of C105, only measures for apical references were made because the ITD JNDs for the most sensitive interaural pairs of the more basally placed reference electrodes (distance from most basal electrode less than 8 mm) were greater than 700 μs, and when the test electrode was changed from this most sensitive position, ITD JND was typically not measurable. Measures using a basal reference were not made in C128 because of time constraints.
Figure 2.
ITD JND plotted as a function of the position of the test electrode (open symbols) paired with the contralateral reference electrodes (filled symbols near the abscissa) identified in each legend. Each panel shows data from one subject as identified above the legend. Both the test and reference electrode arrays consisted of 16 electrodes (∼1-mm spacing, numbered from most apical to most basal). Reference electrodes are identified by side (R=right; L=left) and electrode number in the legend. Position of the reference electrode in its electrode array is shown by the filled symbols along the bottom of each panel. Open symbols represent test electrodes that are paired with the reference electrode identified by the same filled symbol. Open symbols enclosing a filled circle mark the test electrode(s) paired with the corresponding reference electrode in the subject’s sound-processing system. Because subjects C120 and C128 alternated between two channel-to-electrode maps (see Table 3), two test electrodes are marked for each reference electrode. Two test electrodes are also marked for subject C105 to identify the two left electrodes paired with electrode R01 in the subject’s sound-processing system (see map 2 of Table 3). Stimuli were 300-ms pulse trains at 50 pps for C105, C109, and C128 and at 200 pps for C120.
The electrode combination showing the greatest ITD sensitivity for a specific reference was often not the pair judged most similar in cochleotopic position. The symbols enclosing filled circles mark test electrodes that were paired with the specified reference electrode in the subject’s sound processors based on the combined measures described in Sec. 2. In only four of the ten reference conditions tested did the processor-paired electrodes correspond to the interaural pair with lowest ITD JND.
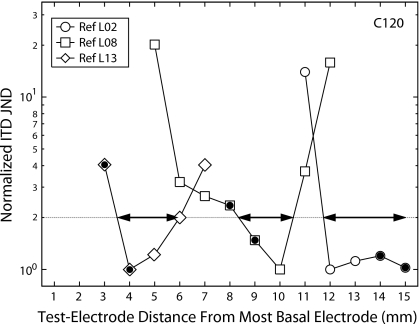
In order to compare the degree to which test electrode influences ITD sensitivity across reference conditions and subjects, the authors normalized the measures associated with each reference condition by the minimum ITD JND for that condition and, as illustrated in Fig. 3, determined the range (half-width) over the length of the electrode array within which the ITD JND is expected to be within a factor of 2 of the minimum for each reference electrode.
Figure 3.
Normalized ITD JND for three reference electrodes (see legend) plotted as a function of the contralateral test electrode position for subject C120 to illustrate the half-width metric. The horizontal dotted line marks the normalized ITD JND that is a factor of 2 greater than the minimum. The horizontal arrows identify the ranges (half-widths) defined by the apical end of the electrode array and the intersections of the dotted line with the line segments associated with each reference condition.
The half-widths computed from the Fig. 3 data are listed in Table 4 and range from 2.1 to 5.7 mm. The ANOVA-computed effects of subject and electrode position (apical, middle, or basal) on half-width were not significant
Table 4.
ITD JND half-widths.
| Subject | Reference electrode | ITD JND half-width (mm) |
|---|---|---|
| C105 | R01 | 0.8 |
| C105 | R03 | 4.6 |
| C109 | R03 | 4.7 |
| C109 | R09 | 3.3 |
| C109 | R14 | 4.8 |
| C120 | L02 | 3.3 |
| C120 | L08 | 2.1 |
| C120 | L13 | 2.5 |
| C128 | L03 | 5.7 |
| C128 | L09 | 2.5 |
DISCUSSION
Like most studies of ITD sensitivity in CI users, our measures of ITD JND show a substantial range across subjects. Figures 12 illustrate the poorer ITD sensitivity of C105 compared to the other subjects. C105 is also the oldest of the subjects (Table 1), and her speech-reception performance is modest with a maximum NU6 word score of 38% correct compared to the others who scored at least 84%.
ITD sensitivity as a function of time
The results plotted in Fig. 1 show that the point at which subjects demonstrate stable ITD sensitivity varies greatly across subjects. Three of the four subjects showed substantial improvements in ITD sensitivity over time frames of 6 months or more. Two of these three subjects did not begin improving until after the onset of daily bilateral stimulation. Only C120 showed relatively good ITD sensitivity directly after implantation. Another example of early sensitivity is subject CI3 of Laback et al. (2007) who suffered a symmetric, bilateral deafness for 2 months before implantation. After only 1 month of bilateral implant listening experience, an ITD JND of 30 μs was measured using four-pulse, 100-pps unmodulated pulse trains.
The right column of Table 1 shows that subjects C109 and C120, who demonstrated ITD sensitivity before beginning daily bilateral CI use, experienced relatively short durations of bilateral deprivation (6 and 4 years, respectively). The two subjects experiencing longer periods of deprivation (11 years for C105 and 14 years for C128) showed minimal improvement in testing before day-to-day binaural listening was available, and they required considerable bilateral listening experience before ITD sensitivity could be measured. Table 5 shows the subjects ordered by duration of bilateral deprivation and lists two metrics: (1) the day (day 700) when ITD JNDs below 700 μs were first measured (this day was referenced to the day each subject began wearing his∕her second sound processor) and the estimated hours of bilateral CI listening experienced by each subject before day 700. The listening experience metric splits the subjects into two widely separated groups: those exhibiting ITD JNDs less than 700 μs before and after beginning daily use of bilateral sound processors. While the correspondence of this delayed emergence of ITD sensitivity with the duration of bilateral deprivation in the small number of subjects studied does not establish an association, it does suggest the hypothesis that bilateral deprivation influences the emergence of bilateral sensitivity in CI subjects. Such a hypothesis is consistent with the deafness-induced plasticity observed in the structural and functional changes in auditory brainstem and midbrain reported in animals (reviews: Shepherd and Hardie, 2001; Hartmann and Kral, 2004; Moore and King, 2004) and warrants further study.
Table 5.
Deprivation and bilateral listening experience. Three metrics are listed for each subject: (1) the duration of bilateral deprivation in years (see Table 1 for definition), (2) the first day (referenced to when each subject began bilateral CI use) that ITD JNDs less than 700 μs were measured, and (3) estimated bilateral listening experience each subject experienced up to the day when ITD JNDs less than 700 μs were first measured. Bilateral listening experience before start of bilateral CI use was restricted to laboratory testing sessions. Bilateral listening experience after the start of bilateral CI use was estimated to be 14 h∕day.
| Subject | Duration of bilateral deprivation (years) | Day when ITD JND<700 μs (re start of bilateral CI use) | Bilateral listening experience when ITD JND<700 μs (estimated hours) |
|---|---|---|---|
| C120 | 4 | −161 | 8 |
| C109 | 6 | −70 | 14 |
| C105 | 11 | 119 | 1666 |
| C128 | 14 | 184 | 2576 |
The variability in the emergence of ITD sensitivity and the amount of bilateral listening experience some subjects require before ITD sensitivity stabilizes complicates the comparison of across-subject results when some subjects are studied within the first 6 months of bilateral implantation and others after significantly longer use. If within-subject ITD-sensitivity measures for different stimulus parameters∕conditions are also distributed within and beyond the first 6 months of bilateral sound-processor use, the comparison of conditions within and across subjects can be problematic.
ITD sensitivity as a function of electrode separation
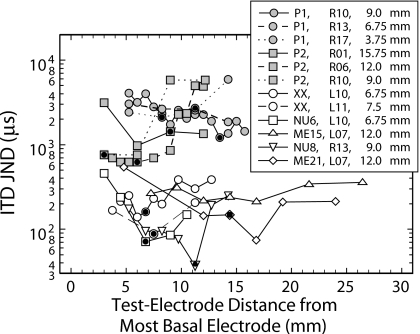
In order to compare the half-widths computed for our subjects (Table 4) with those for subjects from other studies, the authors reviewed the published and unpublished reports that used unmodulated pulse trains to measure ITD JNDs for a reference electrode (the electrode of an interaural pair that is held constant) as a function of the location of the contralateral test electrode. The authors identified the measures from 12 reference electrodes in seven subjects that are plotted in Fig. 4 (van Hoesel and Clark, 1997; Lawson et al., 2002; Wolford et al., 2003; van Hoesel, 2004). Even though half of the reference conditions tested were in P1 and P2 who exhibited very poor ITD sensitivity (ITD JNDs>600 μs) and substantial interaural offset (>4.5 mm) of their electrode arrays (see van Hoesel and Clark, 1997), this body of data is consistent with the results of Fig. 2 in suggesting that the test electrode paired with the reference can make a substantial difference in the pair’s sensitivity to ITD.
Figure 4.
ITD JND measured using single interaural electrode pairs and plotted as a function of position of the contralateral test electrode paired with the reference electrodes identified in the legend (R=right and L=left). The legend also specifies the subject and position (distance from most basal electrode of the reference electrode array) for each reference electrode. Test electrode position is given in distance from the most basal electrode of its electrode array to facilitate comparison between different arrays: Cochlear Corp. (22 electrodes, 0.75-mm spacing; subjects P1 and P2 with apical-to-basal numbering and subjects XX, NU6, and NU8 with basal-to-apical numbering) and Med-El Corp. (12 electrodes, ∼2.4-mm spacing; subjects ME15 and ME21). Monopolar electrode configurations were used in all subjects except for P1 and P2 where electrode numbers identify the more apical of the BP+1 bipolar pair. Symbols enclosing a filled circle mark test electrodes reported to be place matched with their respective reference electrode based on a pitch criterion. In the case of ME15, a place match could not be obtained: reference electrode L07 was judged lower in pitch than R07 and higher than R06 (Lawson et al., 2002). It is not always clear whether the bilateral sound processors used by these subjects paired the place-matched electrodes by analysis channel. Stimuli were constant-amplitude pulse trains with repetition rates varying from 50 to 200 pps depending on the study. Data taken from the original publications∕reports: NU6 and ME15 (Lawson et al., 2002); P1 and P2 (van Hoesel and Clark, 1997); XX (van Hoesel, 2004); NU8 and ME21 (Wolford et al., 2003).
In Figs. 24, the electrode combination showing the greatest ITD sensitivity for a specific reference was often not the pair judged most similar in cochleotopic position. The symbols enclosing filled circles (dark dots) in Figs. 24 mark the test electrode that elicited a pitch sensation matching that elicited by stimulation of the reference electrode. In only 10 of the 22 reference conditions of the combined results did the pitch-matched pair exhibit the smallest ITD JND.
The distribution of the half-width for the combined Figs. 24 data sets suggested that the half-widths computed for subjects P1 and P2 were larger (mean: 6.3 mm; range: 4.0–7.9 mm) than the others (mean: 3.7 mm; range: 0.8–6.75 mm). A t-test confirmed the significance of this difference (t=−3.1, df=18, and p<0.001). When results from P1 and P2 were excluded from the combined results, ANOVA found only nonsignificant effects on the half-widths computed from the results of Figs. 24 for the factors subject, electrode type, and electrode-pair position along the electrode array (apical, middle, or basal).
The results shown in Figs. 24 illustrate the impact of spatial disparity on ITD sensitivity. Given that the tonotopic organization of the auditory system includes neurons of the brainstem (e.g., Guinan et al., 1972; Yin and Chan, 1990) and midbrain (e.g., Kuwada et al., 1984), it is not surprising that ITD sensitivity decreases as the spatial disparity between stimulation electrodes of an interaural pair increases. The impact of this organization was also seen by Smith and Delgutte (2007) in their measures of the binaural interaction component (BIC) of electrically evoked auditory brainstem responses in cats. Based on their mean BIC data plotted as a function of interaural electrode offset [their Fig. 4(B)], the authors estimated the BIC half-width to be approximately 4.5 mm, well within the range of ITD JND half-widths found in the human CI subjects. The ITD JND and BIC half-widths are also consistent with the 3–5 mm spatial spread of excitation estimated by Long et al. (2003) from the monaural forward masking results reported by Cohen et al. (2001).
Nuetzel and Hafter (1981) measured the impact of interaural carrier-frequency disparity on the modulator ITD JND measured in normal-hearing listeners using sinusoidally amplitude-modulated high-frequency tones. The authors transformed their Figs. 23 results (ITD JND as a function of left-ear carrier frequency for a reference right-ear carrier) into ITD JND as a function of offset in cochlear place using Liberman’s (1982) cochlear frequency map adjusted for humans (Greenwood, 1990). The mean ITD JND half-width computed from these acoustically measured data was 0.7 mm (range: 0.4–1.1 mm). This is on the order of a critical band and significantly smaller than the ITD JND half-width (mean: 3.7 mm) the authors measured in implantees (t=5.0, df=18, and p<0.001).
In every day life, CI users will experience ITD information distributed across multiple sound-processing channels because the natural listening environment often includes multiple broadband sound sources. The ability of CI systems to elicit patterns of spike activity that accurately represent such environments is likely to be compromised by half-widths for electric stimulation that the authors estimate to be five times greater than those for normal hearing.
Selecting interaural pairs for bilateral sound processors
One challenge facing clinicians configuring sound processors for bilateral implantees is establishing a mapping of filter-bank channels to interaural electrodes that ensures that the ITD of a within-band signal is presented to a pair of electrodes that is most sensitive to the ITD. Our results demonstrate that some implantees exhibit ITD sensitivity at the time of fitting. In these cases, using direct measures of ITD sensitivity to guide the pairing of interaural electrodes is possible. This technique will probably provide the best chance of a functional binaural benefit. Our results also show that some subjects do not have sensitivity at the time of fitting, forcing clinicians to rely on more indirect techniques.
Interaural pitch comparisons are sometimes used to estimate relative cochleotopic position to aid in the interaural pairing of electrodes. Our data and others (e.g., Long et al., 2001) show that this technique does not guarantee identification of electrode pairs with optimal ITD sensitivity. Typical current practice is to ignore the issue and program each sound processor as if the implants were monolateral. The relatively large half-widths (mean: 3.7 mm) described in this study indicate that close but imperfect matching, while not optimum, may retain some useful ITD sensitivity when using these approaches.
Pelizzone et al. (1990) demonstrated that the BIC can be recorded in a bilaterally implanted human subject and, as noted in the previous section, the results of Smith and Delgutte (2007) measured in cats demonstrate that the BIC is sensitive to interaural electrode offset. If the BIC is present before ITD sensitivity can be measured psychophysically, it would be a valuable tool for pairing interaural electrodes and invites further investigation.
ACKNOWLEDGMENTS
We are especially indebted to the research subjects for their generous contribution of time and effort. We thank Bertrand Delgutte and two anonymous reviewers for valuable comments on the manuscript and recognize the support of Advanced Bionics Corp. for their donation of the second-ear implant systems. NIH Grant Nos. R01 DC005775 and R01 DC007528 provided major support. B.B.P. was partially supported by NIH Grant No. T32 DC00038.
Footnotes
In order to reach comfortable listening levels at reasonable stimulus amplitudes using single-pulse stimuli, the phase duration used in the psychophysical experiments was longer than the 13.5 and 21.6 μs phase durations used by the subjects’ sound-processing systems (see Table 3).
References
- Arsenault, M. D., and Punch, J. L. (1999). “Nonsense-syllable recognition in noise using monaural and binaural listening strategies,” J. Acoust. Soc. Am. 105, 1821–1830. 10.1121/1.426720 [DOI] [PubMed] [Google Scholar]
- Bronkhorst, A. W., and Plomp, R. (1988). “The effect of head-induced interaural time and level differences on speech intelligibility in noise,” J. Acoust. Soc. Am. 83, 1508–1516. 10.1121/1.395906 [DOI] [PubMed] [Google Scholar]
- Cohen, L. T., Saunders, E., and Clark, G. M. (2001). “Psycohphysics of a prototype peri-modiolar cochlear implant electrode array,” Hear. Res. 155, 63–81. 10.1016/S0378-5955(01)00248-9 [DOI] [PubMed] [Google Scholar]
- Eddington, D. K., Poon, B. B., Colburn, H. S., Noel, V., Herrmann, B., Tierney, J., and Whearty, M. (2003). “Speech processors for auditory prostheses: Fifth quarterly progress report,” Massachusetts Institute of Technology, Cambridge.
- Eddington, D. K., Tierney, J., Noel, V., Herrmann, B., Whearty, M., and Finley, C. C. (2002). “Speech processors for auditory prostheses: Third quarterly progress report,” Massachusetts Institute of Technology, Cambridge.
- Gordon, K. A., Valero, J., and Papsin, B. C. (2007). “Auditory brainstem activity in children with 9–30 months of bilateral cochlear implant use,” Hear. Res. 233, 97–107. 10.1016/j.heares.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Grantham, D. W., Ashmead, D. H., Ricketts, T. A., Haynes, D. S., and Labadie, R. F. (2008). “Interaural time and level difference thresholds for acoustically presented signals in post-lingually deafened adults fitted with bilateral cochlear implants using CIS+processing,” Ear Hear. 29, 33–44. [DOI] [PubMed] [Google Scholar]
- Grantham, D. W., Ashmead, D. H., Ricketts, T. A., Labadie, R. F., and Haynes, D. S. (2007). “Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants,” Ear Hear. 28, 524–541. 10.1097/AUD.0b013e31806dc21a [DOI] [PubMed] [Google Scholar]
- Greenwood, D. D. (1990). “A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Norris, B. E., and Guinan, S. S. (1972). “Single auditory units in the superior olivary complex II: Locations of unit categories and tonotopic organization,” Int. J. Neurosci. 4, 147–166. 10.3109/00207457209147165 [DOI] [Google Scholar]
- Hafter, E. R., and Dye, R. H., Jr. (1983). “Detection of interaural differences of time in trains of high-frequency clicks as a function of interclick interval and number,” J. Acoust. Soc. Am. 73, 644–651. 10.1121/1.388956 [DOI] [PubMed] [Google Scholar]
- Hartmann, R., and Kral, A. (2004). “Central responses to electrical stimulation,” in Cochlear Implants: Auditory Prostheses and Electric Hearing, edited by Zeng F. -G., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ), pp. 213–285. [Google Scholar]
- Hartmann, W. M., and Rakerd, B. (1989). “On the minimum audible angle—A decision theory approach,” J. Acoust. Soc. Am. 85, 2031–2041. 10.1121/1.397855 [DOI] [PubMed] [Google Scholar]
- Klumpp, R. G., and Eady, E. R. (1956). “Some measurements of interaural time difference thresholds,” J. Acoust. Soc. Am. 28, 859–860. 10.1121/1.1908493 [DOI] [Google Scholar]
- Kuwada, S., Yin, T. C. T., Syka, J., Buunen, T. J. F., and Wickesberg, R. E. (1984). “Binaural interaction in low-frequency neurons in inferior colliculus of the cat. IV. Comparison of monaural and binaural response properties,” J. Neurophysiol. 51, 1306–1325. [DOI] [PubMed] [Google Scholar]
- Laback, B., Majdak, P., and Baumgartner, W. -D. (2007). “Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing,” J. Acoust. Soc. Am. 121, 2182–2191. 10.1121/1.2642280 [DOI] [PubMed] [Google Scholar]
- Laback, B., Pok, S. M., Baumgartner, W. D., Deutsch, W. A., and Schmid, K. (2004). “Sensitivity to interaural level and envelope time differences of two bilateral cochlear implant listeners using clinical sound processors,” Ear Hear. 25, 488–500. 10.1097/01.aud.0000145124.85517.e8 [DOI] [PubMed] [Google Scholar]
- Lawson, D. T., Wilson, B. S., Zerbi, M., van den Honert, C., Finley, C. C., Farmer, J. C., Jr., McElveen, J. T., Jr., and Roush, P. A. (1998). “Bilateral cochlear implants controlled by a single speech processor,” Am. J. Otol. 19, 758–761. [PubMed] [Google Scholar]
- Lawson, D. T., Wolford, R., Wilson, B. S., and Schatzer, R. (2002). “Speech processors for auditory prostheses: First quarterly progress report,” Research Triangle Institute, Research Triangle Park.
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Liberman, M. C. (1982). “The cochlear frequency map for the cat: Labeling auditory-nerve fibers of known characteristic frequency,” J. Acoust. Soc. Am. 72, 1441–1449. 10.1121/1.388677 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Johnstone, P. M., Godar, S., Agrawal, S., Parkinson, A., Peters, R., and Lake, J. (2006b). “Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle,” Ear Hear. 27, 43–59. 10.1097/01.aud.0000194515.28023.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006a). “Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2001). “Sensitivity to interaural time difference as a function of interaural electrode position in a cochlear implant user,” in 2001 Conference on Implantable Auditory Prostheses, Pacific Grove, CA, p. 20.
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2003). “Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- Majdak, P., Laback, B., and Baumgartner, W. D. (2006). “Effects of interaural time differences in fine structure and envelope on lateral discrimination in electric hearing,” J. Acoust. Soc. Am. 120, 2190–2201. 10.1121/1.2258390 [DOI] [PubMed] [Google Scholar]
- Moore, D. R., and King, A. J. (2004). “Plasticity of binaural systems,” in Plasticity of the Auditory System, edited by Parks T. N., Rubel E. W., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ), pp. 96–172. [Google Scholar]
- Nopp, P., Schleich, P., and D’Haese, P. (2004). “Sound localization in bilateral users of MED-EL COMBI 40∕40+ cochlear implants,” Ear Hear. 25, 205–214. 10.1097/01.AUD.0000130793.20444.50 [DOI] [PubMed] [Google Scholar]
- Nuetzel, J. M., and Hafter, E. R. (1981). “Discrimination of interaural delays in complex waveforms: Spectral effects,” J. Acoust. Soc. Am. 69, 1112–1118. 10.1121/1.385690 [DOI] [Google Scholar]
- Oldfield, S. R., and Parker, S. P. A. (1986). “Acuity of sound localisation: A topography of auditory space. III. Monaural hearing conditions,” Perception 15, 67–81. 10.1068/p150067 [DOI] [PubMed] [Google Scholar]
- Pelizzone, M., Cosendai, G., and Tinembart, J. (1999). “Within-patient longitudinal speech reception measures with continuous interleaved sampling processors for ineraid implanted subjects,” Ear Hear. 20, 228–237. 10.1097/00003446-199906000-00005 [DOI] [PubMed] [Google Scholar]
- Pelizzone, M., Kasper, A., and Montandon, P. (1990). “Binaural interaction in a cochlear implant patient,” Hear. Res. 48, 287–290. 10.1016/0378-5955(90)90069-2 [DOI] [PubMed] [Google Scholar]
- Peters, B. R., Litovsky, R., Parkinson, A., and Lake, J. (2007). “Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants,” Otol. Neurotol. 28, 649–657. 10.1097/01.mao.0000281807.89938.60 [DOI] [PubMed] [Google Scholar]
- Poon, B. B. (2006). “Sound localization and interaural time sensitivity with bilateral cochlear implants,” Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, p. 170. [Google Scholar]
- Ricketts, T. A., Grantham, D. W., Ashmead, D. H., Haynes, D. S., and Labadie, R. F. (2006). “Speech recognition for unilateral and bilateral cochlear implant modes in the presence of uncorrelated noise sources,” Ear Hear. 27, 763–773. 10.1097/01.aud.0000240814.27151.b9 [DOI] [PubMed] [Google Scholar]
- Saberi, K. (1995). “Some considerations on the use of adaptive methods for estimating interaural-delay thresholds,” J. Acoust. Soc. Am. 98, 1803–1806. 10.1121/1.413379 [DOI] [PubMed] [Google Scholar]
- Schleich, P., Nopp, P., and D’Haese, P. (2004). “Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBIN 40∕40+ cochlear implant,” Ear Hear. 25, 197–204. 10.1097/01.AUD.0000130792.43315.97 [DOI] [PubMed] [Google Scholar]
- Seeber, B. U., and Fastl, H. (2008). “Localization cues with bilateral cochlear implants,” J. Acoust. Soc. Am. 123, 1030–1042. 10.1121/1.2821965 [DOI] [PubMed] [Google Scholar]
- Sharma, A., Dorman, M. F., and Kra, A. (2005). “The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants,” Hear. Res. 203, 134–143. 10.1016/j.heares.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Shepherd, R. K., and Hardie, N. A. (2001). “Deafness-induced changes in the auditory pathway: Implications for cochlear implants,” Audiol. Neuro-Otol. 6, 305–318. 10.1159/000046843 [DOI] [PubMed] [Google Scholar]
- Smith, Z. M., and Delgutte, B. (2007). “Using evoked potential to match interaural electrode pairs with bilateral cochlear implants,” J. Assoc. Res. Otolaryngol. 8, 134–151. 10.1007/s10162-006-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, R. S., and Summerfield, A. Q. (1996). “Cochlear implantation: Relationships with research on auditory deprivation and acclimatization,” Ear Hear. 17, 38S–51S. 10.1097/00003446-199617031-00005 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M. (2004). “Exploring the benefits of bilateral cochlear implants,” Audiol. Neuro-Otol. 9, 234–246. 10.1159/000078393 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M. (2007). “Sensitivity to binaural timing in bilateral cochlear implant users,” J. Acoust. Soc. Am. 121, 2192–2206. 10.1121/1.2537300 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M., Böhm, M., Pesch, J., Vandali, A., Battmer, R. D., and Lenarz, T. (2008). “Binaural speech unmasking and localization in noise with bilateral cochlear implants using envelope and fine-timing based strategies,” J. Acoust. Soc. Am. 123, 2249–2263. 10.1121/1.2875229 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., and Clark, G. M. (1997). “Psychophysical studies with two binaural cochlear implant subjects,” J. Acoust. Soc. Am. 102, 495–507. 10.1121/1.419611 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M., Ramsden, R., and O’Driscoll, M. (2002). “Sound-direction identification, interaural time delay discrimination and speech intelligibility advantages in noise for a bilateral cochlear implant user,” Ear Hear. 23, 137–149. 10.1097/00003446-200204000-00006 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., Tong, Y. C., Hollow, R. D., and Clark, G. M. (1993). “Psychophysical and speech perception studies: A case report on a binaural cochlear implant subject,” J. Acoust. Soc. Am. 94, 3178–3189. 10.1121/1.407223 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M., and Tyler, R. S. (2003). “Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., Finley, C. C., Lawson, D. T., Wolford, R. D., Eddington, D. K., and Rabinowitz, W. M. (1991). “Better speech recognition with cochlear implants,” Nature (London) 352, 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., Lawson, D. T., Muller, J. M., Tyler, R. S., and Kiefer, J. (2003). “Cochlear implants: Some likely next steps,” Annu. Rev. Biomed. Eng. 5, 207–249. 10.1146/annurev.bioeng.5.040202.121645 [DOI] [PubMed] [Google Scholar]
- Wolford, R., Lawson, D. T., Schatzer, R., Sun, X., and Wilson, B. S. (2003). “Speech processors for auditory prostheses: Fourth quarterly progress report; NIH Contract N01-DC-2-1002,” Research Triangle Institute, Research Triangle.
- Yin, T. C. T., and Chan, J. C. K. (1990). “Interaural time sensitivity in medial superior olive of cat,” J. Neurophysiol. 64, 465–488. [DOI] [PubMed] [Google Scholar]
- Zurek, P. M. (1993). “Binaural advantages and directional effects in speech intelligibility,” in Acoustical Factors Affecting Hearing Aid Performance, edited by Studebaker G. A. and Hochberg I. (Allyn and Bacon, Boston: ), pp. 255–276. [Google Scholar]