Summary
Asthma is a highly prevalent chronic respiratory disease affecting 300 million people world‐wide. A significant fraction of the cost and morbidity of asthma derives from acute care for asthma exacerbations. In the United States alone, there are approximately 15 million outpatient visits, 2 million emergency room visits, and 500 000 hospitalizations each year for management of acute asthma. Common respiratory viruses, especially rhinoviruses, cause the majority of exacerbations in children and adults. Infection of airway epithelial cells with rhinovirus causes the release of pro‐inflammatory cytokines and chemokines, as well as recruitment of inflammatory cells, particularly neutrophils, lymphocytes, and eosinophils. The host response to viral infection is likely to influence susceptibility to asthma exacerbation. Having had at least one exacerbation is an important risk factor for recurrent exacerbations suggesting an ‘exacerbation‐prone’ subset of asthmatics. Factors underlying the ‘exacerbation‐prone’ phenotype are incompletely understood but include extrinsic factors: cigarette smoking, medication non‐compliance, psychosocial factors, and co‐morbidities such as gastroesophageal reflux disease, rhinosinusitis, obesity, and intolerance to non‐steroidal anti‐inflammatory medications; as well as intrinsic factors such as deficient epithelial cell production of the anti‐viral type I interferons (IFN‐α and IFN‐β). A better understanding of the biologic mechanisms of host susceptibility to recurrent exacerbations will be important for developing more effective preventions and treatments aimed at reducing the significant cost and morbidity associated with this important global health problem.
Introduction: the problem
Asthma is a highly prevalent, chronic respiratory condition characterized by reversible airflow obstruction, airway hyperresponsiveness (AHR), and airway inflammation producing symptoms of shortness of breath, cough, wheezing, and chest tightness. While there is no clear consensus definition for asthma exacerbation, clinical trials usually define a severe exacerbation as the need for treatment with systemic corticosteroids, hospital admission, or emergency treatment for worsening asthma, or a decrease in morning peak flow>25% baseline on 2 consecutive days [1].
The public health burden of chronic asthma has increased over the past two decades, and acute exacerbations of asthma are a particularly important and costly problem.
There are 300 million people affected by asthma world‐wide [2]. According to the Centers for Disease Control and Prevention, National Center for Health Statistics 2005 estimates, the US prevalence of asthma in the United States is 22.2 million (or 7.7% of the population) [3]. The highest prevalence rates have been reported in the United Kingdom (>15%) and New Zealand (15.1%) [2]. In 2004, asthma exacerbations resulted in 14.7 million outpatient visits, 1.8 million emergency room visits, 497 000 hospitalizations and 4055 deaths in the United States alone [3]. Case‐fatality from asthma in the United States is estimated at 5.2 per 100 000, with wide variations across Europe (e.g. 1.6 per 100 000 in Finland and 9.3 per 100 000 in Denmark) [2]. While only 20% of asthmatics have had exacerbations requiring treatment in the emergency department or hospitalization, these patients account for more than 80% of total direct costs [4]. This is in large part because the average cost of a hospital admission for asthma treatment in the United States in 2010 was US$9000 [5].
The purpose of this review is to examine the epidemiologic features of acute asthma exacerbations and to summarize recent advances in our understanding of the clinical and biological features of asthma exacerbations. We will also review the potential mechanisms underlying the susceptibility of a subgroup of asthmatics to recurrent and severe exacerbations.
Epidemiology
According to the latest NIH National Asthma Education and Prevention Guidelines, asthma exacerbations are acute or subacute episodes of progressively worsening shortness of breath, cough, wheezing, and chest tightness, or some combination of these symptoms, characterized by decreases in expiratory airflow and objective measures of lung function (spirometry and peak flow) [6]. These episodes are distressing to patients and result in considerable utilization of health care resources and loss of work productivity and school attendance. Most exacerbations are managed in the outpatient setting. In the United States in 2004 there were 14.7 million visits to physician offices and hospital outpatient departments for acute asthma, but that still left 1.4 million patients who required emergency room management of their asthma exacerbation.
More severe exacerbations result in hospitalization, which constitutes about one‐third of the total $14.7 billion in US annual asthma‐related health care expenditures [7]. The Agency for Healthcare Research and Quality sponsors the Nationwide Inpatient Sample (NIS), the largest all‐payer source of data on hospitalized patients in the United States, and data from 2000 shows that there were 65 381 admissions for asthma (ages>5 years) [5]. Among these, there were 2770 intubations for respiratory failure associated with acute severe asthma (Table 1). Further data from the NIS demonstrates that all age groups experienced hospitalization at similar rates, with a slight predominance of the 35–54‐year age range, representing 31.7% of admissions for asthma. However, the NIS shows that mortality increases dramatically with increasing age. Children and adolescents have the lowest mortality rate (0.02%) and the elderly have the highest mortality from asthma (1.9% for ages>75). Notably, among the approximately 4210 asthmatic patients who die from acute asthma annually in the United States, the majority (approximately 2/3) still occurs outside the hospital [8].
Table 1.
Epidemiology of asthma exacerbations in the United States
| Hospital admissions (age>5) | 65 381 |
| Intubations (no., %) | 2770 (4.2%) |
| Hospital mortality | 0.5 |
| Mean hospital length of stay | 2.7 days |
| Mean hospital charge | US$9078 |
| Total hospital days | 1.1 million |
| Total costs | US$2.9 billion |
Data taken from the Agency for Healthcare Research and Quality sponsored Nationwide Inpatient Sample (NIS) for the United States in 2000 [5].
Asthma exacerbations are more common in females than in males, and females are twice as likely as males to be hospitalized for asthma [5]. However, asthma prevalence is higher in post‐pubertal females than post‐pubertal males and this fact is a large part of the explanation for the higher numbers of adult females seeking care for acute asthma [9, 10]. The higher prevalence of asthma in adult females contrasts with the higher prevalence of asthma in male children [11]. This difference in gender predisposition for asthma in adulthood vs. childhood likely reflects the complicated effects of sex hormones in asthma pathogenesis. Evidence for a role of estrogen in asthma comes from observations of increased asthma exacerbations during menses [12], a higher incidence of adult‐onset asthma in women taking hormone replacement therapy [13], and from animal models [14].
Race and ethnicity also play an important role in risk of asthma exacerbation. African American and Hispanic patients with asthma are at higher risk than Caucasians to be admitted to the hospital for management of an exacerbation [5, 15]. These associations are not explained by differences in socio‐economic status, asthma severity, or differences in asthma therapy. Although overall mortality rates from asthma are considerably higher among African Americans than Caucasians, this is explained by factors related to out‐of‐hospital mortality because in‐hospital mortality is comparable between these groups [5]. These out of hospital factors may include differences in access to health care, poor preventive management, and delays in seeking medical attention [16, 17, 18, 19].
In the Unites States, the prevalence of self‐reported asthma exacerbation is particularly high among Americans of Puerto‐Ricans ancestry which is in contrast to a relatively low frequency of asthma exacerbation in other Hispanic subgroups such as Mexican Americans [8]. The reasons for increased exacerbation risk are unclear and could include social, environmental, or genetic factors. While significant progress has been made in identifying potential genetic factors associated with the development of asthma in Puerto‐Ricans [20], genetic susceptibility factors for exacerbation risk have not been explored.
Rates of hospitalization for asthma are comparable across seasons [5]. While respiratory viruses cause most exacerbations, there are several different viruses that can cause exacerbation (including rhinovirus, influenza, and coronavirus), each with different seasonal variation. For example, exacerbations precipitated by rhinovirus can be documented throughout the year, with a predilection for late spring (April–May) and fall months (September–November) [21]. Interestingly, hospital mortality from asthma is highest in the winter months (January–March) perhaps related to higher rates of influenza infection [22].
Longitudinal adult prospective studies demonstrate a relationship between obesity and asthma, although this relationship may be confounded, in part by age, gender, and co‐morbidities such as gastroesophageal reflux disease (GERD) and sleep‐disordered breathing [23]. Data from longitudinal studies suggest that there may also be a link between obesity and risk of asthma exacerbations, including exacerbations severe enough to warrant treatment in the emergency department or in the hospital [24]. The mechanisms by which obesity could be a risk factor for asthma exacerbation are not known, but it is interesting that obesity is associated with the production of pro‐inflammatory factors and chemokines both in animal models and humans. These factors include eotaxin, an eosinophil chemoattractant, that is increased in the sputum during acute asthma [25, 26] as well as leptin, IL‐6, and TNF‐α [27]. However, there does not seem to be a relationship between BMI and sputum eosinophil counts suggesting that alternative inflammatory mechanisms may be contributing in obese asthmatics [28].
Exacerbation‐prone asthma
While some asthmatics may have rare or intermittent exacerbations, there is evidence for a subset of asthmatics that are ‘exacerbation‐prone’. For example, among 3151 patients presenting to 83 US emergency departments with acute asthma, 73% reported at least one visit for asthma in the prior year, with 21% reporting six or more visits [29]. Factors such as non‐white race, Medicaid insurance, other public or no insurance, and markers of asthma severity predicted risk of frequent visits. These data suggest that patients presenting with recurrent exacerbations are prone because of risks that relate to social factors, poor access to care, and inadequate chronic asthma control. It is also possible that some of these exacerbations presenting to emergency departments may be from causes other than asthma, such as vocal cord dysfunction.
However, data from epidemiologic studies in the ‘difficult‐to‐control’ asthmatic population provides evidence for a more intrinsic phenotype of ‘exacerbation‐prone’ asthma. The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens Study is a 3‐year, multi‐centre, observational study of the natural history, treatment regimens, and outcomes of severe, or difficult‐to‐treat asthmatics in the United States. In a 1.5‐year, prospective analysis of 2780 adult patients, those with a history of recent severe exacerbation requiring an emergency room visit or hospitalization in the past 3 months were at significantly increased risk of having recurrent, future exacerbations (odds ratio 6.33, 95% confidence interval 4.57–8.76) [24]. This risk was independent of demographic and clinical factors, asthma severity and asthma control. Thus, it is likely that there is a subgroup of asthmatics whose susceptibility to exacerbation is independent of traditional measures of asthma control.
Several other important epidemiologic studies provide further evidence for ‘exacerbation‐prone’ asthma. The National Heart, Lung, and Blood Institute's Severe Asthma Research Program identified 438 asthmatics with mild, moderate, and severe disease based on criteria that included inhaled and oral corticosteroid use, controller medication use, airflow obstruction, and history of near‐fatal events [30]. Notably, a significant percentage of asthmatics had multiple exacerbations requiring three or more bursts of oral corticosteroids per year ranging across all levels of severity: mild (5%), moderate (13%), and severe (54%). Near fatal events were also not infrequent among these groups: mild (4%), moderate (6%), and severe (23%). In addition, case–control studies demonstrate that asthmatics with multiple exacerbations compared with those with single exacerbations are at increased risk for severe exacerbation, defined as initiation or escalation of systemic corticosteroids, despite more intensive maintenance anti‐inflammatory treatment [31]. Characteristic features of the exacerbation‐prone subjects include irreversible airflow limitation, chronic sinusitis, psychological dysfunction, and intolerance to non‐steroidal anti‐inflammatory medications [31, 32].
Some ‘exacerbation‐prone’ asthmatics belong to a distinct group who experience near‐fatal episodes of asthma characterized by acute respiratory failure requiring mechanical ventilation and treatment in the intensive care unit. The European Network for Understanding Mechanisms of Severe Asthma characterized subjects with severe asthma and found that the subgroup with prior near‐fatal asthma (NFA) differed from asthmatics of all severity groups with respect to reduced compliance with prescribed asthma medications and less corticosteroid use [33]. Interestingly, the NFA group was more similar to mild‐to‐moderate, rather than severe, asthmatics with regard to clinical and inflammatory parameters. Other studies have confirmed that asthmatics who suffer near fatal exacerbations are not distinguished by lung function, AHR, duration of asthma, gender, smoking status, ethnicity, and prevalence of atopy, but are distinguished by medication non‐compliance, poor asthma control, and less corticosteroid use [34, 35, 36]. Asthmatics who experience near fatal exacerbations are an important subgroup of asthma, because better interventions for education, compliance, and use of inhaled corticosteroids might reduce the incidence of recurrent near fatal events. In addition, there is an association between admission to an intensive care unit and skin test sensitivity to fungal allergens, including Alternaria and Cladosporium [37]. The results of this cross‐sectional study need to be replicated in further studies, but it is possible that environmental intervention to reduce exposure to moulds could help reduce the frequency of asthma exacerbations in certain asthmatics.
Exacerbations and accelerated loss of lung function
Asthma is associated with accelerated loss of lung function, although it is likely that a subgroup of asthmatics drives this association [38, 39], and a minority of asthmatics (27% in one cohort study of difficult‐to‐control asthmatics [40]) develops some degree of irreversible airflow obstruction. This subgroup of asthmatic patients with chronic airflow obstruction is at increased risk for acute asthma exacerbation, because reduced forced expiratory volume in 1 s (FEV1) has been shown to be an important risk factor for multiple asthma exacerbations requiring either hospital care or systemic corticosteroids [31, 41]. The relationship between FEV1 and risk of asthma exacerbation could be because exacerbations cause or promote long‐term reductions in lung function or alternatively, the relationship may be a result of specific types of airway inflammation or specific host factors, such as smoking, that increase the risk of both exacerbation and lung function decline. A recent retrospective cohort study provided evidence in favour of exacerbation as an independent risk for accelerated loss of lung function. Specifically, the effect of severe exacerbations, defined by hospitalization or a decrease in FEV120% and 500 mL, on the progression of airway obstruction was evaluated in 93 non‐smoking asthmatics with moderate‐to‐severe disease before treatment with inhaled corticosteroids [42]. Those asthmatics who experienced at least one severe exacerbation had accelerated loss of lung function compared with those who did not have any exacerbations. The relationship between exacerbations and accelerated loss of lung function was independent of airflow obstruction at baseline. Indeed, one severe exacerbation per year was associated with a 30.2 mL greater annual decline in FEV1 and more severe airflow obstruction after 11 years of follow‐up. A possible explanation is that the multiple inflammatory pathways activated during acute exacerbation, including elevation of matrix metalloproteinases [43], may have long‐term consequences for airway remodelling [44] that result in airway narrowing. In this way asthma exacerbation may become self‐perpetuating with one exacerbation causing changes in lung function that promote a subsequent exacerbation. This cycle may help explain the ‘exacerbation‐prone’ phenotype (Fig. 1).
Figure 1.

A cycle of exacerbations and accelerated loss of lung function in asthma: Acute severe exacerbations in susceptible asthmatics activate pathways of inflammation and remodelling resulting in deterioration of lung function. Accelerated loss of lung function in turn puts these patients at increased risk of recurrent exacerbation resulting in a vicious cycle that may promote the exacerbation‐prone phenotype.
Causes of asthma exacerbation
Airflow obstruction in asthma exacerbations occurs because of concentric smooth muscle contraction, airway wall oedema, and luminal obstruction with mucus [45, 46]. The role of mucus plugs is emphasized by pathologic studies in fatal cases of asthma which show that the airways are invariably impacted with mucus [47, 48]. NFA is also frequently associated with segmental or subsegmental lung collapse because of mucus plugs (2, 3).
Figure 2.
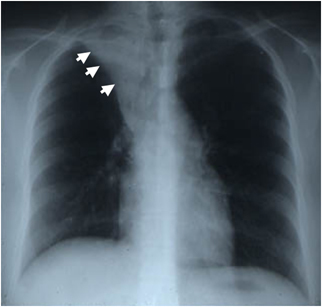
Chest radiograph from a 20‐year‐old woman admitted to the intensive care unit for management of acute severe asthma. The chest radiograph shows collapse of the right upper lobe secondary to mucus impaction. The abnormality resolved completely within 24 h of treatment with mechanical ventilation, corticosteroids, and bronchodilators. Reproduced, with permission, from Fahy [102].
Figure 3.
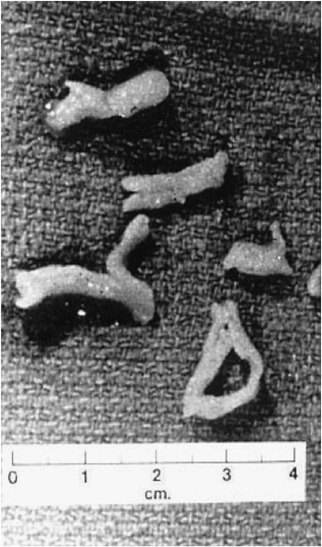
Airway casts recovered from bronchoalveolar lavage from an asthmatic subject in acute exacerbation. Reproduced, with permission, from Lang et al. [47].
The precipitants of acute asthma exacerbations are numerous and include viruses, allergens (dust mite, pollen, animal dander), occupational exposures (grains, flours, cleaning agents, metals, irritants, woods), hormones (menstrual asthma), drugs [ASA, non‐steroidal anti‐inflammatory drugs (NSAIDs), β‐blockers], exercise, stress, and air pollutants. Asthma is a heterogeneous disease and it is likely that these different extrinsic precipitants for exacerbation are more or less important in different individuals. While the environmental causes of asthma exacerbation are heterogeneous, common upper respiratory tract viruses, especially rhinoviruses, are the most common and important causes of exacerbation in both children and adults [49, 50, 51, 52, 53, 54]. With the development of PCR techniques for viral detection, multiple community‐based studies have consistently shown this. For example, in a study of children experiencing asthma exacerbations with decreased peak flow rates, viruses were detected in airway secretions in 80% to 85% of the cases, with rhinoviruses being the most commonly detected virus [54]. Other studies report rates of viral infection in acute asthma of between 55% and 78%, and rhinoviruses and coronaviruses are the most common culprits [52, 53]. Other viral causes include influenza, parainfluenza, respiratory syncytial virus, Chlamydia pneumoniae, and Mycoplasma pneumoniae [49, 53, 55, 56]. Newer virus detections methods such as DNA microarray‐based viral detection methods show that multiple serotypes of rhinoviruses are detectable in airway secretions during respiratory infection in asthmatics [57].
Interactions between viruses and allergens
While respiratory viral infection is commonly associated with asthma exacerbation, there are probably multiple additive factors that contribute to airway inflammation resulting in acute asthma. For example, there appears to be a synergistic interaction between exposure to high levels of allergen and viral infection in causing a severe deterioration of asthma. For example, a case–control study of 60 adult patients compared those hospitalized with acute asthma to two control groups – patients with stable asthma and patients hospitalized for non‐asthma conditions [55]. Compared with controls, a significantly higher proportion of acute asthmatics were both sensitized and exposed to allergens, including dust mite, cat, and dog allergen (66% vs. 37% and 15%, respectively). Most interestingly, the combination of sensitization, high exposure to one or more allergens, and viral detection significantly increased the risk of hospitalization for asthma compared with controls with stable asthma.
Further evidence for interaction between viruses and allergens comes from allergen challenge experiments. Subjects challenged with allergen before and after intranasal inoculation of rhinovirus had increases in the late‐phase allergen responses to histamine and ragweed during their colds [58, 59]. These findings are corroborated by studies using segmental airway challenge before, during, and after infection with rhinovirus. Allergic rhinitis subjects infected with rhinovirus, and then challenged with allergen during their colds had greater histamine release (both during and 48 h after allergen challenge), as well as greater recruitment of eosinophils to the airway 48 h afterwards [60]. These effects persisted up to 1 month following the infection and were not observed in non‐atopic subjects. Viral infection is likely to cause inflammation that primes the airway for enhanced responses to allergen exposure. It is likely that viral and allergic mechanisms act cooperatively to create an acute, severe asthmatic response.
Mechanisms of viral‐induced asthma
Most of our understanding of the role of viruses in acute asthma is from studying the biology of rhinovirus infection, as this is the predominant virus causing exacerbations. Rhinovirus affects both the upper and lower airways, and primarily infects the epithelial cells lining the airway in close contact with the environment [61]. Infection of airway epithelial cells produces a variety of pro‐inflammatory mediators including IL‐1, IL‐6, IL‐8, IFN‐inducible protein of 10 kDa (IP‐10), regulated on activation normal T cell expressed (RANTES), granulocyte macrophage‐colony stimulating factor, and eotaxin (Fig. 4) [62, 63, 64]. These cytokines and chemokines recruit inflammatory cells, such as neutrophils, lymphocytes, and eosinophils to the airway. Neutrophils are the predominant inflammatory cell detected in airway secretions during acute exacerbation [65, 66, 67]. Subjects who report having a respiratory tract infection as the trigger for their exacerbation have significantly higher percentages of neutrophils in their sputum [65]. This is probably caused by increases in epithelial IL‐8 production, which is a potent chemoattractant for neutrophils. Although corticosteroids can reduce epithelial cell expression of several chemokines, such as RANTES and IL‐8, they may not be effective at inhibiting the other virus‐induced pathways of inflammation, and their effectiveness at preventing virus‐induced exacerbation remains controversial [68].
Figure 4.
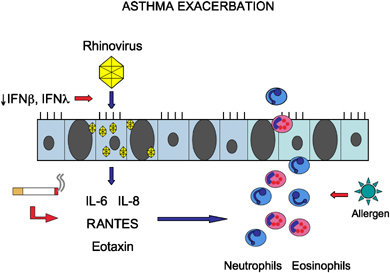
Model for asthma exacerbation. Viral infection of the epithelium is an important trigger for exacerbation, resulting in the production of pro‐inflammatory cytokines and chemokines, and the recruitment of inflammatory cells to the airway. This inflammatory process may be amplified by extrinsic or intrinsic host factors, such as cigarette smoking, epithelial cell deficiency of IFN‐β or IFN‐λ, or exposure to allergen.
Besides elaborating inflammatory chemokines that recruit inflammatory cells, infection of epithelial cells by rhinovirus also activates several important anti‐viral pathways as part of the innate immune response. After rhinovirus binds to its receptor, intercellular adhesion molecule (ICAM)‐1, on the surface of the epithelial cell, the virus is internalized, and undergoes replication [69]. Double‐stranded RNA, a byproduct of viral replication then binds to Toll‐like receptor and activates intracellular signalling through the nuclear factor‐κ B pathway, resulting in elaboration of the type I interferons (IFN‐α and IFN‐β) [70]. The IFNs activate a programme of anti‐viral genes that act to limit viral replication and viral spread to neighbouring epithelial cells [71].
The adaptive immune response may also be important for limiting viral replication. Rhinovirus infection stimulates the production of T‐helper type 1 (Th1) cytokines, including IFN‐γ and IL‐12, by peripheral blood mononuclear cells [72]. IFN‐γ has similar anti‐viral activities as IFN‐α and IFN‐β. These include up‐regulation of class I MHC, inhibition of viral replication, and the induction of several IFN‐sensitive genes [73]. Clinical studies show that decreased generation of IFN‐γ is associated with more severe colds and increased severity of acute asthma [74, 75].
The Th1 cytokines induced by rhinovirus infection during acute asthma differ from the production of Th2 cytokines more typical of chronic asthma (IL‐4, IL‐5, IL‐13) [72]. The balance of Th1 and Th2 cytokines may be an important determinant of the severity of exacerbation. For example, during experimental infection of asthmatic subjects with rhinovirus, the ratio of sputum IFN‐γ (Th1) to IL‐4 (Th2) correlated inversely with peak cold symptoms and time to virus clearance [75]. This suggests that generation of robust Th1 responses may be important for limiting the effect of viral airway infection and that failure to generate such responses may increase the risk of virus‐associated asthma exacerbations (Fig. 4).
Aetiologies of exacerbation‐prone asthma
It is clear that a subset of asthmatics is particularly susceptible to recurrent exacerbations. Risk of exacerbation is a function of the complex interplay between pathogenicity of specific respiratory viruses such as rhinoviruses, host airway susceptibility factors, and environmental modifiers such as exposure to cigarette smoke. The susceptibility factors can be divided into causes that are ‘extrinsic’ or ‘intrinsic’ to the asthmatic airway (Fig. 4).
An example of an extrinsic risk factor is cigarette smoking. Among patients hospitalized with acute asthma, those infected with rhinovirus are more likely to be current smokers [21], and there is a high prevalence of current smoking (35%) in adults presenting with acute asthma to emergency departments [76]. In addition, smoking was found to be the most powerful independent modifiable risk factor for multiple exacerbations requiring hospital care in a prospective HMO‐based study [41]. Another important modifiable risk factor in this study was exposure and allergic sensitivity to cat or dog, further underscoring the interaction between allergic and viral causes. Cigarette smoke has several effects on the airway that could promote asthma exacerbations. For example, cigarette smoking is associated with airway neutrophilia [77] and with changes in airway epithelial mucin stores that could affect viral clearance from the airway [78] (Fig. 4). Other important extrinsic susceptibility factors include co‐morbidities such as chronic rhinosinusitis, history of pneumonia, obesity, and GERD, and intolerance to NSAIDs [24, 31, 79]. Psychosocial factors such as depression, poor social support, public insurance, and unemployment play an important role as well [29, 80].
Recently, exciting advances have been made in our understanding of susceptibility factors that are intrinsic to the asthmatic airway and that could promote exacerbations in a subset of asthmatics. Specifically, experimental models which infect primary bronchial epithelial cells from asthmatic and healthy subjects with rhinovirus have shown that epithelial cells from healthy subjects are quite resistant to infection but that cells from asthmatics have enhanced viral replication and cell lysis [81]. In addition, apoptosis, rather than direct lysis, of an infected cell was important for controlling viral production and release of inflammatory mediators, and early induction of apoptosis was dramatically impaired in asthmatic vs. healthy cells [81]. Furthermore, asthmatic cells were deficient in the production of IFN‐β, an early important anti‐viral cytokine, and exogenous IFN‐β restored the apoptosis defect in asthmatic cells and inhibited viral replication, suggesting that anti‐viral pathways are intact in asthmatic cells but there is a deficiency in activating key anti‐viral cytokines (Fig. 4). Similar results were also discovered for IFN‐λ, a novel type III IFN with similar anti‐viral properties [82]. This deficiency was observed whether or not asthmatics were taking inhaled corticosteroids. These defects in asthmatic epithelial cells also extended to macrophages from asthmatics, which were also deficient in IFN production. These data are important because a deficient anti‐viral response to rhinovirus infection could enhance viral replication, cell damage, and production of pro‐inflammatory cytokines resulting in exacerbation of asthma. At this time the mechanism of this suboptimal anti‐viral response is poorly understood and specific interventions to remedy it are not available.
Further evidence for intrinsic host defects comes from genetic studies. IL‐4 is an important Th2 cytokine that promotes the production of IgE by B cells. Two polymorphisms in the IL‐4 receptor gene were associated with increased risk of severe exacerbations and lower lung function in two independent cohorts [83]. These polymorphisms were more common in African Americans, but conferred a similar risk in both African Americans and Caucasians. One of these polymorphisms was also associated with increased tissue mast cells, and higher levels of IgE bound to mast cells.
Prevention of asthma exacerbations
Pharmacologic interventions shown to be successful at reducing asthma exacerbations include inhaled corticosteroids, long‐acting β‐agonists, leukotriene modifiers, and anti‐IgE therapy. A recent systematic review and meta‐analysis comparing these strategies demonstrates that inhaled corticosteroids are the most successful, reducing exacerbations by 55% compared with placebo or short‐acting β‐agonists alone [84]. Long‐acting β‐agonists reduced exacerbations by 25% compared with placebo and also further reduced exacerbations by 26% when added to inhaled corticosteroids. Leukotriene modifiers have important anti‐inflammatory effects [85] and may reduce exacerbations by as much as 41% compared with placebo. However, they are inferior to inhaled corticosteroids at reducing exacerbation and less effective than long‐acting β‐agonists as add‐on therapy for those already taking inhaled corticosteroids [86].
While inhaled corticosteroids are quite effective at reducing exacerbations, poor compliance with inhaled corticosteroids is a risk factor for recurrent exacerbations as well as NFA. Corticosteroids have been shown to enhance innate immunity and epithelial host defense while reducing pro‐inflammatory cytokine production, which may explain their role in reducing asthma exacerbation [68]. Furthermore, rhinovirus infection of epithelial cells leads to up‐regulation of its own viral receptor, ICAM‐1, whereas, corticosteroids can inhibit the induction of ICAM‐1 by rhinovirus [87]. Corticosteroids also reduce AHR, a strategy that might lead to better control of exacerbations [88]. Despite their clear benefits, asthmatics at risk for exacerbation are generally under‐treated with inhaled corticosteroids. An emphasis on asthma exacerbations as a specific outcome in asthma therapy in the recent guidelines of the NIH/NHLBI National Asthma Education and Prevention Program should help to educate providers about the need for corticosteroid use in patients with fewer daily symptoms, but recurrent exacerbations [6]. Recognizing that exacerbations occur even in some asthmatics using chronic systemic corticosteroids highlights the need to continue working towards more effective, mechanism‐targeted therapies.
Omalizumab is a humanized monoclonal antibody to IgE that has shown promise in preventing asthma exacerbations. Treatment with omalizumab significantly reduces levels of circulating IgE as well as tissue and sputum eosinophils [89, 90]. Interestingly, omalizumab reduces exacerbations by approximately 50% even in patients taking high doses of inhaled corticosteroids despite having only modest effects on lung function and AHR [91]. The effectiveness of this therapy underscores the importance of developing therapies specifically aimed at reducing exacerbations as a primary outcome in clinical trials. However, protein therapeutics such as omalizumab are expensive, and the cost effectiveness of omalizumab is hotly debated [92, 93, 94, 95, 96, 97].
Levels of sputum eosinophils can predict an increased risk of exacerbation [98] and strategies for targeting sputum eosinophils with anti‐inflammatory therapy can reduce the rate of exacerbation [99]. In this context the measurement of exhaled nitric oxide to optimize anti‐inflammatory therapy is relevant. Levels of exhaled nitric oxide (FENO) correlate with numbers of sputum eosinophils and can be measured less invasively [100]. Clinical studies have now shown that FENO can be used as a biomarker to guide reduction of the corticosteroid dose without an increase in the rate of exacerbation [101]. Whether or not FENO measures will ultimately prove beneficial in reducing asthma exacerbations remains to be seen.
Conclusions
Asthma is a highly prevalent global health problem with significant morbidity and mortality. Cost is largely driven by acute care visits for the disease including clinic, emergency room visits, and hospitalizations. A minority of asthmatics, approximately 20%, is responsible for the majority of health care costs associated with exacerbations. A subset of asthmatics is ‘exacerbation‐prone’ and the clinical characteristics of this subgroup include a history of cigarette smoking, psychosocial factors, medication non‐compliance, and co‐morbidities such as rhinosinusitis, obesity, GERD, and NSAID intolerance. Intrinsic host factors for this asthma subgroup are beginning to be identified, such as deficient epithelial cell production of anti‐viral type I IFNs. Mechanistic studies in humans focusing on the ‘exacerbation‐prone’ asthmatic will be important for better understanding how we can develop new preventions and treatments aimed at reducing the significant cost and morbidity associated with asthma.
Acknowledgements
Funding: Research support was from NIH AI077439 (J. V. F.), HL080414 (J. V. F.), 1F32HL093999‐01 (R. H. D.), and generous support from the Will Rogers Institute (R. H. D.).
References
- 1. O'Byrne PM, Barnes PJ, Rodriguez‐Roisin R et al Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164 (Part 1):1392–7. [DOI] [PubMed] [Google Scholar]
- 2. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–78. [DOI] [PubMed] [Google Scholar]
- 3. CDC National Center for Health Statistics. Asthma prevalence, health care use and mortality: United States, 2003–05 [cited]; available from: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/ashtma03-05/asthma03-05.htm#fig1
- 4. Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest 2004; 125:1081–102. [DOI] [PubMed] [Google Scholar]
- 5. Krishnan V, Diette GB, Rand CS et al Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med 2006; 174:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busse WW. Expert Panel Report 3 (EPR‐3): guidelines for the diagnosis and management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120 (Suppl.):S94–138. [DOI] [PubMed] [Google Scholar]
- 7. American Lung Association. Trends in asthma morbidity and mortality [cited]; available from: http://www.lungusa.org/site/pp.asp?c=dvLUK9O0E&b=33347
- 8. Centers for Disease Control. National surveillance for asthma – United States, 1980–2004. 2007 [updated 2007; cited]; available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5608a1.htm
- 9. Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med 2003; 70:215–24. [PubMed] [Google Scholar]
- 10. Redline S, Gold D. Challenges in interpreting gender differences in asthma. Am J Respir Crit Care Med 1994; 150 (Part 1):1219–21. [DOI] [PubMed] [Google Scholar]
- 11. Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med 2000; 3:57–61. [PubMed] [Google Scholar]
- 12. Martinez‐Moragon E, Plaza V, Serrano J et al Near‐fatal asthma related to menstruation. J Allergy Clin Immunol 2004; 113:242–4. [DOI] [PubMed] [Google Scholar]
- 13. Barr RG, Wentowski CC, Grodstein F et al Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med 2004; 164:379–86. [DOI] [PubMed] [Google Scholar]
- 14. Melgert BN, Postma DS, Kuipers I et al Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy 2005; 35:1496–503. [DOI] [PubMed] [Google Scholar]
- 15. Erickson SE, Iribarren C, Tolstykh IV, Blanc PD, Eisner MD. Effect of race on asthma management and outcomes in a large, integrated managed care organization. Arch Intern Med 2007; 167:1846–52. [DOI] [PubMed] [Google Scholar]
- 16. Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med 2001; 161:1660–8. [DOI] [PubMed] [Google Scholar]
- 17. Haas JS, Cleary PD, Guadagnoli E, Fanta C, Epstein AM. The impact of socioeconomic status on the intensity of ambulatory treatment and health outcomes after hospital discharge for adults with asthma. J Gen Intern Med 1994; 9:121–6. [DOI] [PubMed] [Google Scholar]
- 18. Forester JP, Ong BA, Fallot A. Can equal access to care eliminate racial disparities in pediatric asthma outcomes? J Asthma 2008; 45:211–4. [DOI] [PubMed] [Google Scholar]
- 19. Persky V, Turyk M, Piorkowski J et al Inner‐city asthma: the role of the community. Chest 2007; 132 (Suppl.):831S–9S. [DOI] [PubMed] [Google Scholar]
- 20. Choudhry S, Taub M, Mei R et al Genome‐wide screen for asthma in Puerto Ricans: evidence for association with 5q23 region. Hum Genet 2008; 123:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venarske DL, Busse WW, Griffin MR et al The relationship of rhinovirus‐associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis 2006; 193:1536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCoy L, Redelings M, Sorvillo F, Simon P. A multiple cause‐of‐death analysis of asthma mortality in the United States, 1990–2001. J Asthma 2005; 42:757–63. [DOI] [PubMed] [Google Scholar]
- 23. Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 2006; 174:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller MK, Lee JH, Miller DP, Wenzel SE. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med 2007; 101:481–9. [DOI] [PubMed] [Google Scholar]
- 25. Vasudevan AR, Wu H, Xydakis AM et al Eotaxin and obesity. J Clin Endocrinol Metab 2006; 91:256–61. [DOI] [PubMed] [Google Scholar]
- 26. Park SW, Kim DJ, Chang HS et al Association of interleukin‐5 and eotaxin with acute exacerbation of asthma. Int Arch Allergy Immunol 2003; 131:283–90. [DOI] [PubMed] [Google Scholar]
- 27. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol 2008; 121:1087–93; quiz 94–5. [DOI] [PubMed] [Google Scholar]
- 28. Todd DC, Armstrong S, D'Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy 2007; 37:1049–54. [DOI] [PubMed] [Google Scholar]
- 29. Griswold SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA. Jr Asthma exacerbations in North American adults: who are the “frequent fliers” in the emergency department? Chest 2005; 127:1579–86. [DOI] [PubMed] [Google Scholar]
- 30. Moore WC, Bleecker ER, Curran‐Everett D et al Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koga T, Oshita Y, Kamimura T, Koga H, Aizawa H. Characterisation of patients with frequent exacerbation of asthma. Respir Med 2006; 100:273–8. [DOI] [PubMed] [Google Scholar]
- 32. Ten Brinke A, Sterk PJ, Masclee AA et al Risk factors of frequent exacerbations in difficult‐to‐treat asthma. Eur Respir J 2005; 26:812–8. [DOI] [PubMed] [Google Scholar]
- 33. Romagnoli M, Caramori G, Braccioni F et al Near‐fatal asthma phenotype in the ENFUMOSA Cohort. Clin Exp Allergy 2007; 37:552–7. [DOI] [PubMed] [Google Scholar]
- 34. Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near‐fatal asthma. A case–control study in hospitalized patients with asthma. Am J Respir Crit Care Med 1998; 157 (Part 1):1804–9. [DOI] [PubMed] [Google Scholar]
- 35. Boulet LP, Deschesnes F, Turcotte H, Gignac F. Near‐fatal asthma: clinical and physiologic features, perception of bronchoconstriction, and psychologic profile. J Allergy Clin Immunol 1991; 88:838–46. [DOI] [PubMed] [Google Scholar]
- 36. Alvarez GG, Schulzer M, Jung D, Fitzgerald JM. A systematic review of risk factors associated with near‐fatal and fatal asthma. Can Respir J 2005; 12:265–70. [DOI] [PubMed] [Google Scholar]
- 37. Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life‐threatening asthma. Allergy 2000; 55:501–4. [DOI] [PubMed] [Google Scholar]
- 38. Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis 1987; 70:171–9. [PubMed] [Google Scholar]
- 39. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15‐year follow‐up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–200. [DOI] [PubMed] [Google Scholar]
- 40. Aburuz S, McElnay J, Gamble J, Millership J, Heaney L. Relationship between lung function and asthma symptoms in patients with difficult to control asthma. J Asthma 2005; 42:859–64. [DOI] [PubMed] [Google Scholar]
- 41. Osborne ML, Pedula KL, O'Hollaren M et al Assessing future need for acute care in adult asthmatics: the Profile of Asthma Risk Study: a prospective health maintenance organization-based study. Chest 2007; 132:1151–61. [DOI] [PubMed] [Google Scholar]
- 42. Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007; 30:452–6. [DOI] [PubMed] [Google Scholar]
- 43. Oshita Y, Koga T, Kamimura T, Matsuo K, Rikimaru T, Aizawa H. Increased circulating 92 kDa matrix metalloproteinase (MMP‐9) activity in exacerbations of asthma. Thorax 2003; 58:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 2007; 120:1233–44; quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 45. Hogg JC. The pathology of asthma. APMIS 1997; 105:735–45. [DOI] [PubMed] [Google Scholar]
- 46. Kuyper LM, Pare PD, Hogg JC et al Characterization of airway plugging in fatal asthma. Am J Med 2003; 115:6–11. [DOI] [PubMed] [Google Scholar]
- 47. Lang DM, Simon RA, Mathison DA, Timms RM, Stevenson DD. Safety and possible efficacy of fiberoptic bronchoscopy with lavage in the management of refractory asthma with mucous impaction. Ann Allergy 1991; 67:324–30. [PubMed] [Google Scholar]
- 48. Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960; 13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993; 307:982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heymann PW, Carper HT, Murphy DD et al Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004; 114:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus‐induced asthma. Eur Respir J 2002; 19:68–75. [DOI] [PubMed] [Google Scholar]
- 52. Grissell TV, Powell H, Shafren DR et al Interleukin‐10 gene expression in acute virus‐induced asthma. Am J Respir Crit Care Med 2005; 172:433–9. [DOI] [PubMed] [Google Scholar]
- 53. Atmar RL, Guy E, Guntupalli KK et al Respiratory tract viral infections in inner‐city asthmatic adults. Arch Intern Med 1998; 158:2453–9. [DOI] [PubMed] [Google Scholar]
- 54. Johnston SL, Pattemore PK, Sanderson G et al Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995; 310:1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case–control study. BMJ 2002; 324:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lieberman D, Printz S, Ben‐Yaakov M et al Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003; 167:406–10. [DOI] [PubMed] [Google Scholar]
- 57. Kistler A, Avila PC, Rouskin S et al Pan‐viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis 2007; 196:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lemanske RF Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest 1989; 83:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calhoun WJ, Swenson CA, Dick EC, Schwartz LB, Lemanske RF Jr, Busse WW. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis 1991; 144:1267–73. [DOI] [PubMed] [Google Scholar]
- 60. Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest 1994; 94:2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Papadopoulos NG, Bates PJ, Bardin PG et al Rhinoviruses infect the lower airways. J Infect Dis 2000; 181:1875–84. [DOI] [PubMed] [Google Scholar]
- 62. Chen Y, Hamati E, Lee PK et al Rhinovirus induces airway epithelial gene expression through double‐stranded RNA and IFN‐dependent pathways. Am J Respir Cell Mol Biol 2006; 34:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP‐10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 2005; 289:L85–95. [DOI] [PubMed] [Google Scholar]
- 64. Corne JM, Holgate ST. Mechanisms of virus induced exacerbations of asthma. Thorax 1997; 52:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995; 95:843–52. [DOI] [PubMed] [Google Scholar]
- 66. Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL‐8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med 2000; 161 (Part 1):1185–90. [DOI] [PubMed] [Google Scholar]
- 67. Lamblin C, Gosset P, Tillie‐Leblond I et al Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med 1998; 157:394–402. [DOI] [PubMed] [Google Scholar]
- 68. Zhang N, Truong‐Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol 2007; 179:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greve JM, Davis G, Meyer AM et al The major human rhinovirus receptor is ICAM‐1. Cell 1989; 56:839–47. [DOI] [PubMed] [Google Scholar]
- 70. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double‐stranded RNA and activation of NF‐kappaB by Toll‐like receptor 3. Nature 2001; 413:732–8. [DOI] [PubMed] [Google Scholar]
- 71. Takaoka A, Hayakawa S, Yanai H et al Integration of interferon‐alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 2003; 424:516–23. [DOI] [PubMed] [Google Scholar]
- 72. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax 2002; 57:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67:227–64. [DOI] [PubMed] [Google Scholar]
- 74. Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus‐induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol 2000; 105:692–8. [DOI] [PubMed] [Google Scholar]
- 75. Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med 2000; 162:2226–31. [DOI] [PubMed] [Google Scholar]
- 76. Silverman RA, Boudreaux ED, Woodruff PG, Clark S, Camargo CA. Jr Cigarette smoking among asthmatic adults presenting to 64 emergency departments. Chest 2003; 123:1472–9. [DOI] [PubMed] [Google Scholar]
- 77. Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1304–9. [DOI] [PubMed] [Google Scholar]
- 78. Innes AL, Woodruff PG, Ferrando RE et al Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 2006; 130:1102–8. [DOI] [PubMed] [Google Scholar]
- 79. Ng TP, Lim TK, Abisheganaden J, Eng P, Sin FL. Factors associated with acute health care use in a national adult asthma management program. Ann Allergy Asthma Immunol 2006; 97:784–93. [DOI] [PubMed] [Google Scholar]
- 80. Eisner MD, Katz PP, Lactao G, Iribarren C. Impact of depressive symptoms on adult asthma outcomes. Ann Allergy Asthma Immunol 2005; 94:566–74. [DOI] [PubMed] [Google Scholar]
- 81. Wark PA, Johnston SL, Bucchieri F et al Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005; 201:937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Contoli M, Message SD, Laza‐Stanca V et al Role of deficient type III interferon‐lambda production in asthma exacerbations. Nat Med 2006; 12:1023–6. [DOI] [PubMed] [Google Scholar]
- 83. Wenzel SE, Balzar S, Ampleford E et al IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med 2007; 175:570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sin DD, Man J, Sharpe H, Gan WQ, Man SF. Pharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysis. JAMA 2004; 292:367–76. [DOI] [PubMed] [Google Scholar]
- 85. Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti‐inflammatory effects of leukotriene‐modifying drugs and their use in asthma. Chest 2001; 119:1533–46. [DOI] [PubMed] [Google Scholar]
- 86. Ringdal N, Eliraz A, Pruzinec R et al The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respir Med 2003; 97:234–41. [DOI] [PubMed] [Google Scholar]
- 87. Suzuki T, Yamaya M, Sekizawa K et al Effects of dexamethasone on rhinovirus infection in cultured human tracheal epithelial cells. Am J Physiol Lung Cell Mol Physiol 2000; 278:L560–71. [DOI] [PubMed] [Google Scholar]
- 88. Sont JK, Willems LN, Bel EH, Van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long‐term treatment. The AMPUL Study Group. Am J Respir Crit Care Med 1999; 159 (Part 1):1043–51. [DOI] [PubMed] [Google Scholar]
- 89. Djukanovic R, Wilson SJ, Kraft M et al Effects of treatment with anti‐immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2004; 170:583–93. [DOI] [PubMed] [Google Scholar]
- 90. Holgate ST. The role of mast cells and basophils in inflammation. Clin Exp Allergy 2000; 30 (Suppl. 1):28–32. [DOI] [PubMed] [Google Scholar]
- 91. Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti‐IgE for chronic asthma in adults and children. Cochrane Database Syst Rev 2006; CD003559. [DOI] [PubMed] [Google Scholar]
- 92. Brown R, Turk F, Dale P, Bousquet J. Response to Campbell, Spackman, Sullivan letter to Allergy. Allergy 2008; 63:783. [DOI] [PubMed] [Google Scholar]
- 93. Sullivan SD, Turk F. An evaluation of the cost‐effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy 2008; 63:670–84. [DOI] [PubMed] [Google Scholar]
- 94. Cheng JW, Arnold RJ. Pharmacoeconomic review of medical management of persistent asthma. Allergy Asthma Proc 2008; 29:109–22. [DOI] [PubMed] [Google Scholar]
- 95. Revicki D, Brown R, Dale P. Questioning the economic evaluation of omalizumab. J Allergy Clin Immunol 2008; 121:1514; author reply ‐5. [DOI] [PubMed] [Google Scholar]
- 96. Wu AC, Paltiel AD, Kuntz KM, Weiss ST, Fuhlbrigge AL. Cost‐effectiveness of omalizumab in adults with severe asthma: results from the Asthma Policy Model. J Allergy Clin Immunol 2007; 120:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Krishnan JA, Gould M. Omalizumab for severe allergic asthma: dollars and sense. J Allergy Clin Immunol 2007; 120:1015–7. [DOI] [PubMed] [Google Scholar]
- 98. Belda J, Giner J, Casan P, Sanchis J. Mild exacerbations and eosinophilic inflammation in patients with stable, well‐controlled asthma after 1 year of follow‐up. Chest 2001; 119:1011–7. [DOI] [PubMed] [Google Scholar]
- 99. Green RH, Brightling CE, McKenna S et al Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360:1715–21. [DOI] [PubMed] [Google Scholar]
- 100. Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001; 164 (Part 1):1376–81. [DOI] [PubMed] [Google Scholar]
- 101. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005; 352:2163–73. [DOI] [PubMed] [Google Scholar]
- 102. Fahy JV. Airway mucus and the mucociliary system In: Middleton E, Jr, Reed CE, Ellis EF, Adkinson NF, Jr, Yunginger JW, Busse WW, eds. Allergy principles & practice, 5th Edn St Louis: Mosby‐Year Book Inc., 1998, pp. 520–31. [Google Scholar]


