Abstract
OBJECTIVE
The purpose of this study was to determine whether the intrauterine contraceptive device (IUD) is effective and safe among women who are infected with the human immunodeficiency virus (HIV).
STUDY DESIGN
We randomly assigned 599 postpartum, HIV-infected women in Zambia to receive either a copper IUD or hormonal contraception and followed them for at least 2 years.
RESULTS
Women who were assigned randomly to hormonal contraception were more likely to become pregnant than those who were assigned randomly to receive an IUD (rate, 4.6/100 vs 2.0/100 woman-years; hazards ratio, 2.4; 95% CI, 1.3–4.7). One woman who was assigned to the IUD experienced pelvic inflammatory disease (crude rate, 0.16/100 woman-years; 95% CI, 0.004–868); there was no pelvic inflammatory disease among those women who were assigned to hormonal contraception. Clinical disease progression (death or CD4+ lymphocyte count dropping below 200 cells/μL) was more common in women who were allocated to hormonal contraception (13.2/100 woman-years) than in women who were allocated to the IUD (8.6/100 woman-years; hazard ratio, 1.5; 95% CI, 1.04–2.1).
CONCLUSION
The IUD is effective and safe in HIV-infected women. The unexpected observation that hormonal contraception was associated with more rapid HIV disease progression requires urgent further study.
Keywords: AIDS, contraception, HIV, hormonal contraception, intrauterine contraceptive device (IUD), sub-Saharan Africa
Pregnancy and acquired immunodeficiency syndrome (AIDS) are among the most important health risks faced by sexually active women in sub-Saharan Africa.1–3 In Zambia, as many as 1 in 5 pregnant women is infected with human immunodeficiency virus (HIV),4 and a woman’s lifetime risk of dying from a pregnancy complication exceeds 5% (nearly 100 times the risk faced by women in Europe and North America).2 The provision of reliable contraception to HIV-infected women who desire it has been identified by the World Health Organization (WHO) as a primary strategy for the prevention of pediatric AIDS.5 Thus, safe and effective contraception for HIV-infected women represents a critical component of reproductive health, with benefits that range from averting obstetric catastrophe to preventing pediatric AIDS.
Even though there are approximately 18 million women living with HIV worldwide,3 surprisingly little data are available on contraception options for this large population of women. Among immunocompetent women, the intra-uterine contraceptive device (IUD) is known to be highly effective and safe6,7 and represents a particularly attractive choice in developing world settings, where inconsistent supply chains and imperfect patient follow-up can produce large discrepancies between “ideal effectiveness” and “user effectiveness” for hormonal methods.
Historically, there has been confusion around the use of the IUDs in HIV-infected women. The International Planned Parenthood Federation and the WHO have recommended against its use in this population, although more recently the WHO recommendations have been modified with a stated caveat that there is “limited evidence” of its safety.8 As a result, there is hardly any use of the IUD in HIV-infected women, anywhere.
Materials and Methods
Participants
This trial recruited women who had delivered recently from 2 government primary care clinics in Lusaka, Zambia, who had undergone HIV-1 serostatus testing in antenatal care as part of an ongoing program to prevent mother-to-child HIV transmission.9 Candidate participants were identified antenatally and assigned randomly after their 6-week postpartum visit. We offered enrollment to women who desired at least 2 years of continuous contraception and who reported ≤2 sexual partners in the previous year. We excluded women with advanced HIV disease (WHO stage III or IV), a history of a bleeding disorder, and/or a history of pelvic inflammatory disease (PID) within the previous 5 years, and/or who were <16 years old (the “age of majority” in Zambia).
Study procedures
We randomly assigned women to receive either the IUD or hormonal contraception using sequentially numbered opaque envelopes that were generated at the University of Alabama at Birmingham. A separate blocked randomization scheme with random block sizes of ≤20 was used for each clinical facility. Sealed envelopes were opened by the study nurses at the time of study enrollment and randomization. Neither practitioners nor patients were masked to intervention assignments.
All participants underwent pelvic examination at randomization. Women who were allocated to the IUD arm who did not have gross (visual) evidence of cervicovaginal infection had immediate sterile insertion of a copper-containing device (ParaGard TCu 380A, which known as the “Copper T Model TCu 380A” outside the United States; Duramed Pharmaceuticals, Inc, El Segundo, CA). Although swabs were taken for microscopy, the results of these investigations were not available immediately and did not affect the decision to place an IUD. Women who were allocated to the hormonal contraception arm were allowed to choose between oral contraceptive pills or depo-medroxy-progesterone acetate (DMPA) 150-mg injections. Each was provided at 3-month intervals. Breastfeeding women who chose oral contraceptives were prescribed progesterone-only pills (oral levonorgestrel 0.03 mg/d) for a period of 6 months, after which they were switched to a combined preparation (oral levonorgestrel 0.15 mg and ethinyl estradiol 0.03 mg/d).
Scheduled study visits occurred at postenrollment week 4 and thereafter at 6-month intervals. We encouraged participants to return to the clinic for care, especially if they had a gynecologic complaint. Participants were instructed specifically to return to the study clinic for urine pregnancy testing if (1) expected menses was delayed by ≥20 days; (2) they experienced excessive cramping, vaginal bleeding, or passage of tissue; or (3) they suspected pregnancy for any other reason.
At each study visit, whether scheduled or unscheduled, participants received a physical examination (with pelvic examination) and provided specimens for urine pregnancy testing and complete blood count. Cervicovaginal samples were taken for potassium hydroxide (KOH) and saline wet mount to evaluate the presence of candidiasis, bacterial vaginosis, trichomoniasis, and/or white blood cells. Participants who had evidence of a vaginal or cervical infection on wet mount were treated according to WHO guidelines for the management of sexually transmitted infections.10 We offered condoms to participants at each visit, but we did not monitor their reported use. Women who wished to discontinue contraceptive use or to change to another method were allowed to do so at any time and continued to be followed in the study. Women with suspected PID were evaluated by a consultant gynecologist. Women who met the PID criteria were treated with outpatient combination antibiotics.
Participants were followed until the most recently recruited woman had been followed for 24 months. In August 2003, antiretroviral therapy became available in the public sector in Lusaka.11 After this time, women who met clinical (WHO stage) or immunologic (CD4+ lymphocyte count) criteria for antiretro-viral therapy were referred for HIV care and treatment. Patients were classified as lost to follow-up if study nurses were unable to locate them after a minimum of 2 home visit attempts.
Laboratory testing
We performed CD4+ lymphocyte enumeration at our central laboratory with the FACSCount System (Becton Dickinson Biosciences, Inc, San Jose, CA). Complete blood counts were done with the Ac·T diff Hematology Analyzer (Beckman Coulter, Inc, Miami, FL). We diagnosed pregnancy with a rapid urine assay for human chorionic gonadotropin (QuickVue One-Step hCG; Quidel Corporation, San Diego, CA).
Outcomes and statistical analysis
Incident pregnancy was determined at the clinical sites by urine human chorionic gonadotropin testing. Incident PID was diagnosed by the criteria of Hager et al12: abdominal, uterine/cervical, and adnexal tenderness, with at least 1 of the following findings: (1) oral temperature of >38°C, (2) detection of pus in the peritoneal cavity by culdocentesis, (3) pelvic mass or suspected inflammatory complex on bimanual examination, or (4) mucopurulent cervicitis. Method discontinuation rates were measured as time to discontinuation of the randomized method.
Because breastfeeding in our setting is nearly universal, we expected lactational amenorrhea to mitigate the pregnancy rate in both study arms during the first year after delivery.13 Thus, we estimated a 2-year pregnancy rate of 9% in the hormonal arm14 and a 2-year pregnancy rate of 2% in the IUD arm.15 To be conservative in our estimates, we based the original sample size calculations on a proportional, rather than time-to-event, outcome. To have 80% power to demonstrate this difference (2-tailed alpha, .05), 249 patients would be required in each group. We elected to assign randomly 300 per arm to allow for an approximate 15% loss-to-follow-up rate. We also hypothesized that, given the low rates of PID that were observed in HIV-infected women who received IUD contraception,16 only a fairly large increase in risk relative to hormonal contraception would be clinically significant. Our sample size of 250 in each group would be adequate to detect an approximate 2.5-fold increased risk of PID that would be attributable to the IUD.
Clinical disease progression was evaluated separately as (1) death, (2) CD4+ lymphocyte count falling to <200 cells/μL, and (3) either of these outcomes. Analysis of CD4+ cell count decline was limited to those women who were at risk; thus, women with baseline values of <200 cells/μL (n = 60) were excluded from the analysis of this outcome.
Continuous covariates were compared across 2-level variables (participation or contraceptive method) with the use of unpaired, 2-tailed t-tests and evaluated the normality assumption with the Kolmogorov-Smirnov test. Dichotomous and categoric variables were compared with the use of the Pearson χ2 test statistic. Primary analyses were based on intention to treat. We used the Kaplan-Meier method to compare rates of incident pregnancy, PID, method discontinuation, and clinical disease progression. Treatment arms were compared for outcomes of interest with the use of Cox proportional hazards regression. Because there was a high rate of method cross-over observed in this trial, we also performed actual-use analyses for the outcomes of incident pregnancy and clinical disease progression. In these analyses, contraceptive method was treated as a time-varying exposure in an extended Cox proportional hazards regression. Data were analyzed with SAS software (version 9.1.3; SAS Institute, Cary, NC).
The trial was approved by the Research Ethics Committee of the University of Zambia and by the Institutional Review Board of the University of Alabama at Birmingham and underwent continuing review by both bodies for its duration.
Results
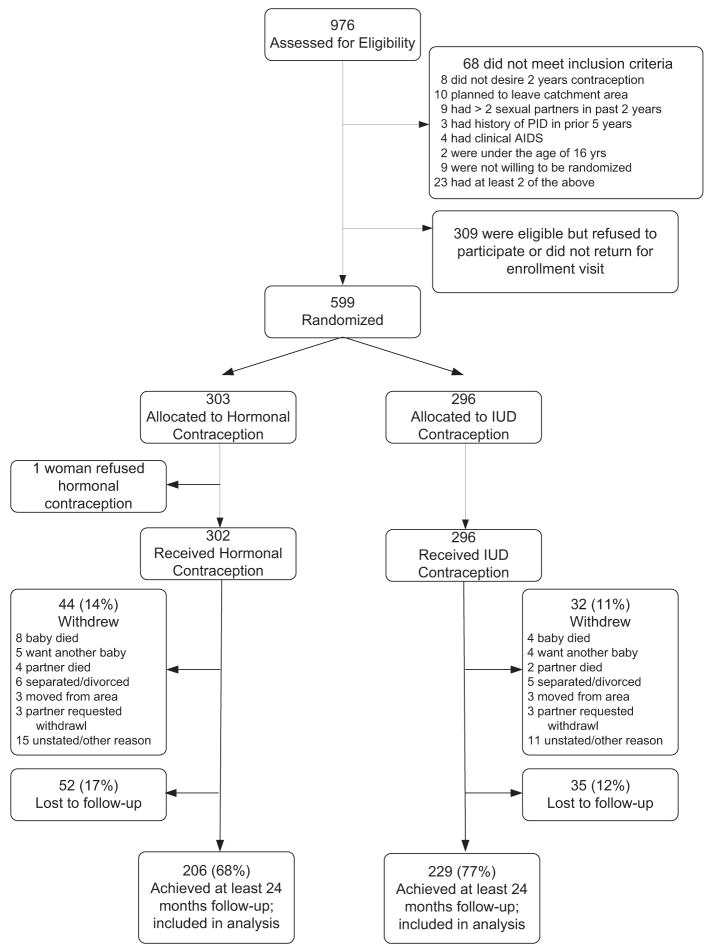
Between June 12, 2002, and Oct. 2, 2003, we approached 976 HIV-infected women to participate in the clinical trial; 68 women (7%) did not meet the inclusion criteria, and 309 women (32%) either refused participation or did not return for enrollment. Women who declined participation or who did not return for enrollment differed from those women who accepted with respect to age (25.5 y vs 26.5 y; P = .01), number of previous pregnancies (2.6 vs 2.9; P = .05), and previous infant death (74/309 [24%] vs 183/599 [31%]; P = .04). A total of 599 women were assigned randomly. Of the 599 HIV-1–infected women, 303 women were assigned randomly to the hormonal arm, and 296 women were assigned to the IUD arm (Figure 1).
FIGURE 1.
Trial profile
Baseline characteristics of the study population can be found in Table 1. A total of 269 women (45%) received antibiotics or antifungals for cervicovaginal conditions at enrollment. This included 10 women (2%) for candidiasis, 101 women (17%) for trichomoniasis, 62 women (10%) for bacterial vaginosis, and 96 women (16%) for unspecified cervicitis. There were no notable differences between randomization groups in any of the demographic or clinical indicators that we measured at baseline (Table 1).
TABLE 1.
Baseline characteristics of participants in a randomized trial of IUD vs hormonal contraception in HIV-infected women
| Variable | Total (n = 599) | Hormonal (n = 303) | IUD (n = 296) |
|---|---|---|---|
| Age (y)* | 26.1 ± 4.9 | 25.9 ± 4.9 | 26.3 ± 4.9 |
| Married (n) | 536 (90%) | 272 (90%) | 264 (89%) |
| Total pregnancies (n)† | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Total living children (n)† | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Report at least 1 child death (n) | 166 (28%) | 81 (27%) | 85 (29%) |
| Years of schooling (n)* | 7.3 ± 3.0 | 7.4 ± 3.0 | 7.3 ± 3.0 |
| Any previous contraceptive use (n) | 367 (61%) | 186 (61%) | 181 (61%) |
| Previous oral contraceptive use (n) | 286 (48%) | 142 (47%) | 144 (49%) |
| Previous injectable use (n) | 96 (16%) | 47 (15.5%) | 49 (17%) |
| Previous IUD use (n) | 7 (1.2%) | 6 (2.0%) | 1 (0.3%) |
| Enrollment CD4+ count (cells/μL)* | 503 ± 259 | 493 ± 251 | 514 ± 266 |
| CD4+ count <200 cells/μL (n) | 60 (10%) | 30 (10%) | 30 (10%) |
| Enrollment hemoglobin level (g/dL)* | 11.7 ± 1.9 | 11.6 ± 1.9 | 11.7 ± 1.9 |
| Body mass index at enrollment (kg/m2)* | 24.1 ± 4.0 | 24.1 ± 4.0 | 24.2 ± 3.9 |
| Antibiotics for any cervicovaginal infection (n) | 269 (45%) | 141 (47%) | 128 (43%) |
| Cervicitis (n) | 96 (16%) | 50 (17%) | 46 (15%) |
| Candidiasis (n) | 10 (2%) | 8 (3%) | 2 (1%) |
| Trichomoniasis (n) | 101 (17%) | 53 (17%) | 48 (16%) |
| Bacterial vaginosis (n) | 62 (10%) | 30 (10%) | 32 (11%) |
Data are given as mean ± SD.
Data are given as median (interquartile range).
Overall, 76 women (13%; 44 women randomly assigned to hormonal contraception and 32 women randomly assigned to the IUD) withdrew from the study (Figure 1). Reasons for withdrawal were death of infant (n = 12; 16%), death of partner (n = 6; 8%), decision to have another child (n = 9; 12%), separation or divorce from partner (n = 11; 14%), relocation away from Lusaka (n = 6; 8%), partner request to withdraw (n = 6, 8%), and unstated reasons (n = 26; 34%). Eighty-seven women (15%; 52 women assigned to hormonal contraception and 35 women assigned to the IUD) were lost to follow-up.
Pregnancy
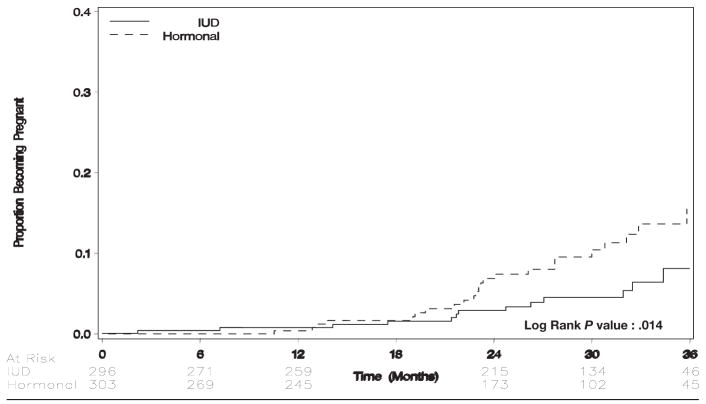
Women who were assigned to hormonal contraception were more likely to become pregnant than were women who were assigned to the IUD (4.62 pregnancies per 100 woman-years vs 2.17 pregnancies per 100 woman-years; hazards ratio, 2.2; 95% CI, 1.2–4.2; Table 2; Figure 2). Of 27 women who were assigned to hormonal contraception who became pregnant, 22 women stated that they were still taking hormonal contraception; 5 women had discontinued contraception altogether. Of 14 women who were assigned randomly to IUD contraception who became pregnant, 8 women had switched to hormonal contraception; 4 women had discontinued contraception altogether (including 1 women with IUD expulsion that was not reinserted), 1 woman became pregnant with an IUD in situ, and 1 woman underwent minilaparotomy for ectopic pregnancy (details later). In actual-use analysis, women who used hormonal contraception were considerably more likely to become pregnant than women who used the IUD (4.09 pregnancies per 100 woman-years vs 0.38 pregnancies per 100 woman-years; hazards ratio, 10.5; 95% CI, 2.5–44).
TABLE 2.
Study outcomes of a randomized trial of IUD vs hormonal contraception in HIV-infected women
| Outcome | Contraceptive method | |||||
|---|---|---|---|---|---|---|
| Hormonal | IUD | |||||
| n/N | Patient-years of follow-up | Rate/100 patient-years (95% CI) | n/N | Patient-years of follow-up | Rate/100 patient-years (95% CI) | |
| Pregnancy | 27/303 | 585 | 4.62 (3.17–6.73) | 14/296 | 645 | 2.17 (1.29–3.67) |
| Discontinuation of randomized method | 38/303 | 558 | 6.81 (4.95–9.35) | 146/296 | 510 | 28.6 (24.4–33.7) |
| Pelvic inflammatory disease | 0/303 | 588 | 0.00 (0.00–0.51)* | 1/296 | 642 | 0.16 (0.004–87)* |
| Death | 17/303 | 588 | 2.89 (1.80–4.65) | 13/296 | 645 | 2.01 (1.17–3.47) |
| CD4 count falling to <200 cells/μL† | 56/273 | 499 | 11.2 (8.64–14.6) | 41/265 | 548 | 7.48 (5.51–10.2) |
| CD4 count falling to <200 cells/μL or death | 72/303 | 547 | 13.2 (10.5–16.6) | 52/296 | 607 | 8.57 (6.53–11.2) |
Because of sparse numbers of events, CIs were calculated using exact methods based on Poisson count.
Restricted to women with baseline CD4+ count of >200 cell/μL (n = 538).
FIGURE 2.
Time to pregnancy by randomization arm
Discontinuation
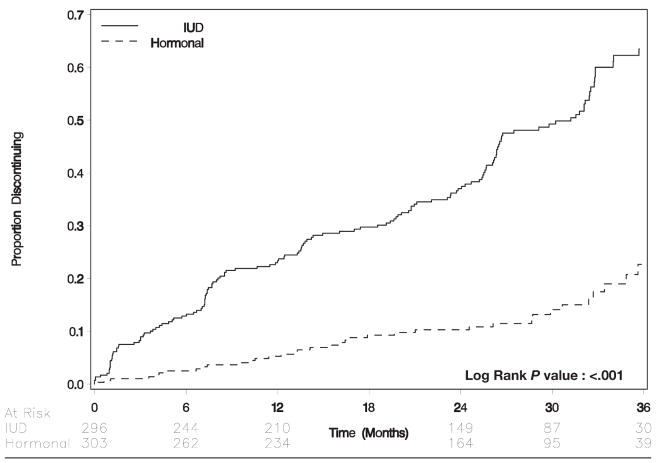
Overall, 184 patients (31%) discontinued their originally allocated form of contraception over the course of study follow-up. Women who were assigned to hormonal contraception were less likely to discontinue than were women who were assigned to the IUD (6.81 discontinuations per 100 woman-years vs 28.6 discontinuations per 100 woman-years; hazards ratio, 0.23; 95% CI, 0.16–0.33; Table 2; Figure 3). Among 38 patients (13%) who discontinued hormonal contraception, the median time to discontinuation was 475 days (interquartile range, 222–861); 6 of these 38 women (16%) switched to the IUD; 13 women (34%) switched to condoms, and 19 women (50%) discontinued contraception altogether. Among 146 women (49%) who discontinued the IUD, the median time to discontinuation was 408 days (interquartile range, 171–771). Seventy-three of these 146 women (50%) switched to DMPA; 38 women (26%) switched to combined oral contraceptive pills; 8 women (5.5%) switched to condoms, and 27 women (18%) discontinued contraception altogether.
FIGURE 3.
Time to discontinuation by randomization arm
Safety
We diagnosed 1 case of PID over the study period. Although there were no cases of PID among the women who were assigned to hormonal contraception, 1 woman who was assigned randomly to IUD contraception experienced PID over 642 woman-years of follow-up (crude PID rate: 0.16 cases per 100 woman-years; 95% CI, 0.004–868; Table 2). The single woman who was diagnosed with PID had with abdominal pain and vaginal discharge 29 days after the device inserted was inserted. The IUD was removed, and cultures were positive for Chlamydia trachomatis. Ten women had expulsions of their IUD (4 were partial expulsions that required medical attention, and 6 were complete expulsions). Only 1 woman with an expulsion elected to have the device reinserted.
Clinical disease progression
Death
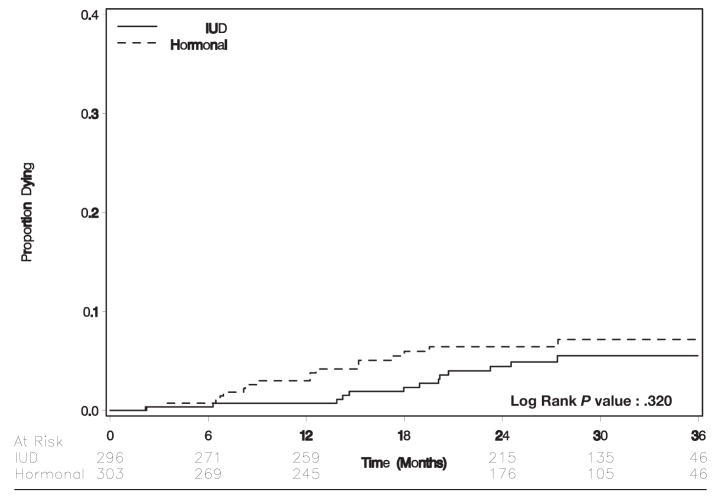
Thirty women died during follow-up. Univariate risk factors for death included death of index baby (relative risk, 2.1; 95% CI, 0.99–4.9), low CD4+ cell count (probability from t-test, < .0001), CD4+ cell count <200 cells/μL (relative risk, 6.0; 95% CI, 3.0–16), older age (probability from t-test, .10), and lower hemoglobin level (probability from t-test, .001). Seventeen deaths occurred in 588 person-years of follow-up time among women who were assigned to hormonal contraception; 13 deaths occurred in 645 person-years of follow-up among women who were assigned to the IUD (probability value for the Wilcoxon test, .32; Figure 4). In Cox proportional hazards regression, the hazards ratio for hormonal contraception was 1.4 (95% CI, 0.70–3.0; Table 2).
FIGURE 4.
Time to death by randomization arm
CD4+ count decline
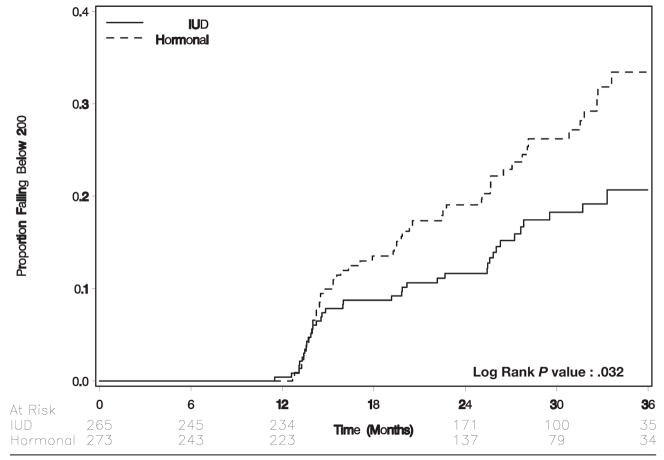
A Kaplan-Meier analysis that was limited to women whose initial CD4+ count was >200 cells/μL (n = 538) indicated that women who received hormonal contraception were more likely to experience a CD4+ count decline to <200 cells/μL than were women who received the IUD (log-rank test P = .03; Figure 5). In Cox proportional hazards regression, the hazards ratio for CD4+ count falling to <200 cells/μL among women who received hormonal contraception compared with those who received the IUD was 1.6 (95% CI, 1.04–2.3; Table 2).
FIGURE 5.
Time to CD4+ count falling to below 200 cells/uL by randomization arm
Death or CD4+ count decline
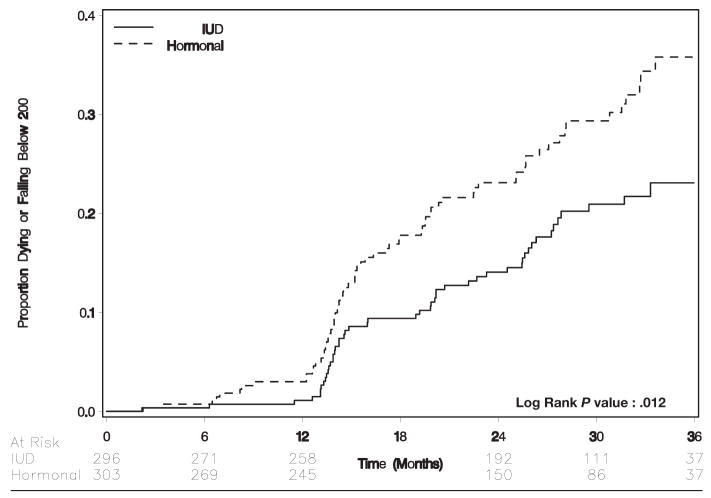
We also evaluated the composite outcome of either CD4+ count falling to <200 cells/μL or death by contraceptive method. In intent-to-treat analysis, women who received hormonal contraception were more likely to meet this composite criterion than were women who received the IUD (crude rate, 13.2 per 100 woman-years vs 8.57 per 100 woman-years; hazards ratio, 1.6; 95% CI, 1.1–2.3; Table 2; Figure 6). In actual-use analysis, 84 patients met the criteria over 633 woman-years of hormonal contraception exposure (crude rate, 11.9 per 100 woman-years); 36 patients met the composite criteria over 494 woman-years of IUD exposure (crude rate, 7.3 per 100 woman-years) to yield a hazards ratio of 1.7 (95% CI, 1.1–2.5).
FIGURE 6.
Time to either CD4+ cell count falling to below 200 cells/uL or death by randomization arm
Comment
This first-ever randomized trial of the IUD in HIV-infected women demonstrates that the IUD is more efficacious than hormonal contraception in pregnancy prevention and that it has a very low rate of associated pelvic infection in this population. Women who received the IUD were more likely to switch to another form of contraception than were women who received hormonal contraception. However, it should be noted that women in the hormonal arm were allowed to switch between oral contraceptives and DMPA (or vice versa)—and 34% did—without being categorized as having “discontinued” their allocated method. When these women are included in the overall switching comparison, the effect is attenuated considerably (hazard for switching from hormonal contraceptives compared with the IUD, 0.6; 95% CI, 0.46–0.78). Taken together, these data confirm, in HIV-infected women, what is already known about contraception in the general population: that women change contraceptive methods frequently17 and that the most important component of good contraceptive public health is availability of method choice.18 We believe this study demonstrates that, although the IUD may not be acceptable to all women, it certainly should figure prominently in the portfolio of contraceptive options that are available to HIV-infected women.
The theoretic concern that IUD use in HIV-infected women may lead to high rates of pelvic infection was not confirmed in this study. In fact, our observed incidence of pelvic inflammatory disease is even lower than that observed by Sinei et al,16 who described a 1.4% rate of postinsertion PID among HIV-infected women in Kenya. Reasons for the lower PID rates in the present study could be attributable to our postpartum population, who tend to delay sexual activity and may have fewer partners after delivery. Another potential explanation is our clinicians’ liberal use of empiric antibiotics, although this has not been shown to reduce postinsertion PID incidence in HIV-uninfected populations.19
Strengths of this study are its randomized design, high participation rate, reasonably large sample size, and long-term (at least 2 years for retained participants) follow-up evaluation. Although we designed the study with adequate power to detect what we believed to be a clinically significant difference in PID risk among women who use the IUD, the unexpectedly low rates of infection in both randomization arms made this impossible. In fact, a trial, which was powered to detect the difference that we observed in this study, would need >18,000 participants to be followed for 2 years. Principal limitations of this study include the relatively high rates of method switching or discontinuation and the withdrawal or loss of approximately one-third of our participants. Although our loss-to-follow-up rate was comparable to most other published contraceptive trials,20,21 it is possible that these women may have in some way been different from those who were retained. Other potential limitations include (1) that this was not a masked trial (there may have been provider bias in diagnosis of adverse events) and (2) that we made condoms available to all participants but did not collect information on their use.
Ensuring safe, woman-controlled contraceptive access to HIV-infected women who wish to delay or forgo child-bearing represents an important approach to the prevention of mother-to-child HIV transmission.22–24 It has been estimated that relatively modest investments in meeting the unmet contraceptive need of HIV-infected women worldwide could prevent more cases of pediatric AIDS per year than any current antiretroviral-based approach,24 which would make this a particularly cost-effective approach.25 To eradicate pediatric HIV, public health decision makers must be relentless in addressing the issue from all angles.
Women who used hormonal contraception were more likely to experience clinical disease progression than were women who used the IUD. Although this provocative finding is by no means definitive (it was not an a priori hypothesis), if borne out in further investigation, it could have large public health implications. Most HIV-infected women who seek care (in Zambia, approximately 80% in Prevention of Mother-to-Child Transmission of HIV programs) will not qualify for antiretroviral therapy immediately, on the basis of their clinical stage and CD4+ count. Thus, any contraceptive intervention that hastens the progression to AIDS could have real implications on patient survival and program costs.
The potential benefits of reliable, woman-controlled contraception for HIV-infected women are huge. Contraception prevents perinatal AIDS; prevents AIDS orphans; contributes to the health of existing children through birth spacing; and, in parts of the world where pregnancy is unsafe, can avert obstetric catastrophe. This study suggests that the IUD offers a safe and effective means of woman-controlled contraception that is particularly well-suited for HIV-infected women in stable relationships who are healthy enough to not yet require antiretroviral therapy. Further investigation into the effect of hormonal contraception on HIV-1 disease progression is a matter of particular urgency.
Acknowledgments
Supported by a grant from the Elizabeth Glaser Pediatric AIDS Foundation (PG-51161) with complementary resources from the US Agency for International Development (HRN-A-00-98-00020-00; CSA-04-395). Investigators receive salary or stipend support from the US National Institutes of Health (D43-TW01035, K23-AI01411, K01-TW05708, K01-TW06670, D43-TW010035).
References
- 1.World Health Organization. Maternal mortality in 2000: estimates developed by WHO, UNICEF and UNFPA. Geneva: WHO Press; 2004. [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. Joint United Nations Programme on HIV/AIDS: 2004 report on the global AIDS epidemic: 4th global report. Geneva: WHO Press; 2004. [Google Scholar]
- 4.Central Statistical Office [Zambia], Central Board of Health [Zambia], and ORC Macro. Zambia demographic and health survey 2001–2002. Calverton (MD): Central Statistical Office, Central Board of Health, and ORC Macro; 2003. [Google Scholar]
- 5.World Health Organization. Strategic approaches to the prevention of HIV infection in infants: report of a WHO meeting; Morges, Switzerland. 20–22 March 2002; Geneva: WHO Press; 2002. [Google Scholar]
- 6.Mishell D. Intrauterine devices: mechanisms of action, safety, and efficacy. Contraception. 1998;58:45S–53S. doi: 10.1016/s0010-7824(98)00082-1. [DOI] [PubMed] [Google Scholar]
- 7.Arias R. Compelling reasons for recommending IUDs to any woman of reproductive age. Int J Fertil Womens Med. 2002;47:87–95. [PubMed] [Google Scholar]
- 8.World Health Organization. World Health Organization’s second edition of improving access to quality care in family planning: medical eligibility criteria for contraceptive use. Geneva: WHO Press; 2000. [Google Scholar]
- 9.Stringer EM, Sinkala M, Stringer JSA, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17:1377–82. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines for the management of sexually transmitted infections. Geneva: WHO press; 2003. [PubMed] [Google Scholar]
- 11.Stringer JSA, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 12.Hager W, Eschenbach D, Spence M, Sweet R. Criteria for diagnosis and grading of salpingitis. Obstet Gynecol. 1983;61:113–4. [PubMed] [Google Scholar]
- 13.Kennedy K, Trussell J. Postpartum contraception and lactation. In: Hatcher RA, Trussel J, Stewart F, editors. Contraceptive technology. 18. New York: Ardent Media; 2004. [Google Scholar]
- 14.Allen S, Serufilira A, Gruber V, et al. Pregnancy and contraception use among urban Rwandan women after HIV testing and counseling. Am J Public Health. 1993;83:705–10. doi: 10.2105/ajph.83.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiery M, Van Kets H, Van der Pas H. Immediate postplacental IUD insertion: the expulsion problem. Contraception. 1985;31:331–49. doi: 10.1016/0010-7824(85)90002-2. [DOI] [PubMed] [Google Scholar]
- 16.Sinei SK, Morrison CS, Sekadde-Kigondu C, et al. Complications of use of intrauterine devices among HIV-1-infected women. Lancet. 1998;351:1238–41. doi: 10.1016/S0140-6736(97)10319-1. [DOI] [PubMed] [Google Scholar]
- 17.Steele F, Diamond I. Contraceptive switching in Bangladesh. Stud Fam Plann. 1999;30:315–28. doi: 10.1111/j.1728-4465.1999.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 18.Delbanco TL, Daley J. Through the patient’s eyes: strategies toward more successful contraception. Obstet Gynecol. 1996;88:S41–S7. doi: 10.1016/0029-7844(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 19.Grimes DA, Schulz KF. Prophylactic antibiotics for intrauterine device insertion: a meta-analysis of the randomized controlled trials. Contraception. 1999;60:57–63. doi: 10.1016/s0010-7824(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 20.Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. JAMA. 2001;285:2347–54. doi: 10.1001/jama.285.18.2347. [DOI] [PubMed] [Google Scholar]
- 21.Feldblum PJ, Caraway J, Bahamondes L, et al. Randomized assignment to copper IUD or depot-medroxyprogesterone acetate: a feasibility of enrollment, continuation, and disease ascertainment. Contraception. 2005;72:187–91. doi: 10.1016/j.contraception.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Abrams E. Prevention of mother-to-child transmission of HIV: successes, controversies and critical questions. AIDS Rev. 2004;6:131–43. [PubMed] [Google Scholar]
- 23.Preble EA. Perinatal transmission in developing countries: new developments, new challenges. AIDS Link. 1995;32:12–3. [PubMed] [Google Scholar]
- 24.Reynolds HW, Janowitz B, Homan R, Johnson L. The value of contraception to prevent perinatal HIV transmission. Sex Transm Dis. 2006;33:350–6. doi: 10.1097/01.olq.0000194602.01058.e1. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds HW, Steiner MJ, Cates W. Contraception’s proved potential to fight HIV. Sex Transm Infect. 2005;81:180–8. doi: 10.1136/sti.2004.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]