Abstract
Background
Unsafe practices during illegal plasma donation in the late 1980s and early 1990s spread blood-borne infections in central China.
Methods
A cross-sectional survey of a random sample of 538 adult residents of 12 villages in rural Shanxi Province, where there had been an illegal commercial plasma-collection center, was conducted in 2003. Structured questionnaires were administered, and blood samples were tested for hepatitis C virus (HCV) antibodies.
Results
HCV seroprevalence rates were 8.2% in all subjects, 27.7% in former commercial plasma/blood donors, and 2.6% in nondonors. Selling blood or plasma was the strongest independent predictor of HCV seropositivity (odds ratio [OR], 14.4 [95% confidence interval {CI}, 7.1–31.6]). A history of blood transfusion was also independently associated with HCV seropositivity (OR, 8.3 [95% CI, 2.1–32.0]). Plasma donors had a higher risk of being HCV seropositive than did whole-blood donors (OR, 7.6 [95% CI, 2.9–20.9]), and female donors had a lower risk than did male donors (OR, 0.32 [95% CI, 0.12–0.80]). The strength of the association between selling blood and HCV seropositivity was weaker when plasma donors were excluded (OR, 8.0 vs. 14.4).
Conclusions
Unsafe practices during illegal plasma donation led to a high risk of HCV seropositivity for donors during the 1980s and 1990s. Failure to screen for HCV increased the risk of seropositivity for transfusion recipients during this same period. China has taken steps to halt illegal plasma collection and to improve blood-banking methods. However, there will be an ongoing challenge to care for patients with HCV infection, even as its incidence decreases.
The global hepatitis C virus (HCV) seroprevalence rate varies widely, from <1% in Hong Kong and Sweden to >114% in Egypt and Cameroon [1]. An estimated 3.2% of persons in mainland China are infected with HCV [2]. Percutaneous exposures—including reuse of needles and syringes, injection drug use, and transfusion of unscreened blood products—are well-established risk factors. Two Chinese studies published in 2004 showed that >70% of injection drug users (IDUs) in Sichuan and Guangxi Provinces were seropositive for HCV antibodies [3, 4], compared with 60% of IDUs in the United States [5]. In most industrialized countries, the risk of acquiring HCV infection through blood transfusion is now significantly reduced, because transfused blood is screened and risk behaviors in donors are identified. However, in resource-limited developing countries, transmission of HCV by blood transfusion and the medical reuse of needles and syringes remain serious public-health problems [6, 7].
The first major HCV outbreak documented in China was reported in 1985 in plasma donors [8]. During the late 1980s and early 1990s, illegal commercial plasma-collection centers were common in selected rural areas of central China. At these centers, pooling blood and then reinfusing red blood cells of the compatible blood type into donors was performed, so that donors would not become anemic and therefore could donate more frequently. Several bloodborne diseases, including HCV and HIV infection, were thus transmitted efficiently. A high HCV seroprevalence rate was observed in plasma donors in Hebei, Shandong, Hunan, Hubei, Henan, and even in Beijing [8–12]. In the late 1990s, illegal plasma-collection practices were reduced markedly as a result of government legal action. We conducted a community-based epidemiological study to evaluate the HCV seroprevalence rate in residents of communities where illegal plasma-collection practices have been documented and to explore the risk factors associated with HCV seropositivity.
Subjects, Materials, and Methods
Study context
This epidemiological survey was conducted for planning purposes as part of a larger, ongoing project—the China Integrated Programs for Research on AIDS—led by the Chinese Center for Disease Control and Prevention. Our study estimated HCV seroprevalence rates and risk factors for HCV seropositivity in residents of communities in Shanxi Province in which illegal commercial plasma-collection centers had been present. The study protocol was approved by the Division of AIDS Prevention Science Review Committee of the National Institute of Allergy and Infectious Diseases and the institutional review boards of the Chinese National Center for AIDS/STD Prevention and Control and the University of Alabama, Birmingham. Written, informed consent was obtained from all subjects. The human-experimentation guidelines of the US Department of Health and Human Services and the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, were followed in the conduct of the research.
Study site and subjects
Shanxi Province is located in central China and, to the south, borders Henan Province, which had the most severe HIV epidemic caused by unhygienic practices during plasma collection. Of the 644 HIV infections reported during 1995–2003, 76% were associated with plasma/blood collection [13]. A township in the southern part of Shanxi Province was selected as the study site because an illegal commercial plasma-collection center had operated there in approximately 1995 and because local health officials had reported that many HIV-infected persons lived there. Twelve of 25 villages in the township were selected on the basis of their history of having illegal plasma-collection practices and the willingness of village leaders to collaborate. A roster of all residents of the study villages was obtained from local authorities. With the help of village leaders, this roster was updated to include new residents and exclude those who had died or had permanently moved out of the villages.
A roster of 660 adult villagers was selected randomly from a sampling of 9205 villagers. Inclusion criteria were as follows: (1) permanent residence, having lived in a target village continuously for at least 6 months; (2) 18–59 years old; (3) able and willing to provide informed consent; (4) able and willing to provide contact information. The study was conducted in November and December 2003.
Data collection
Standardized questionnaire–based interviews were administered by same-sex, trained interviewers who collected demographic and medical history and behavioral data, including blood/plasma donation history. Venous blood was collected for HCV antibody testing by use of an EIA (DiaSorin S.P.A.; Suyog Diagnostics). Individual pretest counseling was provided to all subjects by same-sex, trained counselors. After the study visit was completed, each subject received 15 Chinese yuan (approximately US$1.80) for travel expenses and to compensate for the time taken from work. Posttest counseling and referral-to-care services were provided to subjects. Questionnaire completion and laboratory testing were monitored by internal and external quality controls.
Statistical analysis
Original questionnaire and laboratory testing data were entered into and managed by a DataFax system (Clinical DataFax Systems), and then they were transferred to an SAS database (SAS Institute) for analysis. HCV seroprevalence rates were calculated for all subjects and for the subgroups of former plasma/blood donors and nondonors. Fisher's exact 95% confidence intervals (CIs) for point estimates were obtained, and binomial distributions were assumed to be present. Univariate logistic analysis was first used to explore associations with HCV seropositivity. Variables that were significant (P ≤ .05) in the univariate analysis were included in a multivariate logistic regression analysis. Variables that were not significant in the multivariate analysis were eliminated in a step-wise manner, so that variables independently associated with HCV seropositivity could be identified. To explore the association between HCV seropositivity and the characteristics of commercial plasma/blood donation activities, separate subgroup analyses were also performed for donors. All probability values were reported as being 2 sided.
Results
Recruitment rates
With the assistance of village leaders, 87.6% (578/660) of selected villagers were contacted successfully; 3 (0.5%) of those contacted were ineligible, and 35 (6.1%) refused to participate. Two persons (0.3%) refused to have blood drawn, such that 538 subjects were included in the analysis (figure 1). Nonparticipants were slightly more likely (71/122 [58.2%]) to be men than were participants (268/538 [49.8%]) (P =.091), and nonparticipants were younger than participants (59/122 [48.3%] vs. 110/538 [20.4%] were <30 years old) (P <.001).
Figure 1.
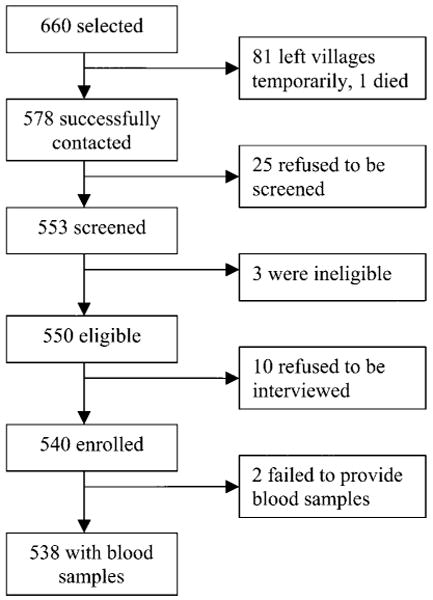
Screening and enrollment of subjects
Commercial blood/plasma donation
Of the 538 subjects, 119 (22.1%) had sold plasma/blood: 91 (16.9%) had sold whole blood only, 7 (1.3%) had sold plasma only, and 21 (3.9%) had sold both. The rates of commercial plasma/blood donation in the 12 villages ranged from 8.8% to 48.7%. The most common reasons for selling plasma/blood were a need for money (72/119 [60.5%]) and being convinced by other people (46/119 [38.7%]). Subjects sold blood as early as 1973 and as late as 1998; 60.5% of the 119 subjects donated during 1991–1995. No subject reported selling plasma/blood after 1998; the primary reasons for stopping paid donations were improved economic status (20/119 [16.8%]), concern about the harm to health caused by plasma/blood donation (20/119 [16.8%]), no longer being qualified to donate because of abnormal liver function or hepatitis (18/119 [15.1%]), and the closure of the illegal commercial plasma-collection centers (18/119 [15.1%]). A total of 46.2% of former plasma/blood donors had sold plasma/blood 2–9 times in their lifetimes, and 3.4% reported selling plasma/blood ≥ 100 times. The institutions where plasma/blood was sold included official plasma/blood-collection centers (79.8%), illegal commercial plasma-collection centers (23.5%), and government hospitals (17.6%). Of former plasma/blood donors, 82.4% sold plasma/blood outside Wenxi County, and 4.2% sold plasma/blood outside Shanxi Province.
HCV seroprevalence rates and risk factors
Anti-HCV antibodies were detected in 44 subjects, which gave a seroprevalence rate of 8.2% (95% CI, 4.4%–10.9%). Of former plasma/blood donors, 27.7% (33/119 [95% CI, 19.9%–29.3%]) were HCV seropositive; of nondonors, 2.6% (11/419 [95% CI, 1.3%–4.7%]) were HCV seropositive.
In the univariate analysis, subjects ≥ 35 years old had a higher HCV seroprevalence rate than did those <35 years old (table 1). There was a significant positive trend for an association between HCV seropositivity and age (P < .001). Subjects who had attended middle school or beyond had a lower HCV seroprevalence rate than did those who had attended elementary school only or had no education (P = .003). HCV seropositivity was also associated with selling plasma/blood, multiple lifetime sex partners, and a history of blood transfusion.
Table 1. Factors associated with hepatitis C virus (HCV) seropositivity in 538 residents of rural Shanxi Province, China, where there had been an illegal commercial plasma donation center.
| Factor | Subjects, no. | HCV-positive subjects, no. (%) | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P |
|---|---|---|---|---|---|---|
| Sex | .11 | … | ||||
| Male | 268 | 27 (10.1) | 1.0 | … | ||
| Female | 270 | 17 (6.3) | 0.60 (0.31–1.2) | … | ||
| Age | <.001 | … | ||||
| <35 years | 185 | 3 (1.6) | 1.0 | … | ||
| ≥35 years | 353 | 41 (11.6) | 8.0 (2.9–33.3) | … | ||
| Marital status | … | … | ||||
| Single | 21 | 0 (0.0) | 1.0 | … | ||
| Married | 517 | 44 (8.5) | … | … | ||
| Education | .003 | … | ||||
| Elementary school or less | 130 | 19 (14.6) | 1.0 | … | ||
| Middle school or beyond | 408 | 25 (6.1) | 0.38 (0.20–0.73) | … | ||
| Sold plasma/blood | <.001 | <.001 | ||||
| No | 419 | 11 (2.6) | 1.0 | 1.0 | ||
| Yes | 119 | 33 (27.7) | 14.2 (7.1–30.5) | 14.4 (7.1–31.6) | ||
| No. of lifetime sex partners | .027 | .058 | ||||
| ≤1 | 473 | 34 (7.2) | 1.0 | 1.0 | ||
| >1 | 65 | 10 (15.4) | 2.4 (1.05–4.9) | 2.4 (0.94–5.6) | ||
| Illicit drug use | … | … | ||||
| No | 536 | 44 (8.2) | 1.0 | … | ||
| Yes | 2 | 0 (0.0) | … | … | ||
| History of tooth extraction | .075 | … | ||||
| No | 403 | 28 (6.9) | 1.0 | … | ||
| Yes | 135 | 16 (11.9) | 1.8 (0.93–3.4) | … | ||
| History of acupuncture | .33 | … | ||||
| No | 507 | 40 (7.9) | 1.0 | … | ||
| Yes | 31 | 4 (12.9) | 1.7 (0.49–4.7) | … | ||
| History of surgery | .23 | … | ||||
| No | 501 | 39 (7.8) | 1.0 | … | ||
| Yes | 37 | 5 (13.5) | 1.9 (0.61–4.7) | … | ||
| History of blood transfusion | <.001 | .003 | ||||
| No | 523 | 38 (7.3) | 1.0 | 1.0 | ||
| Yes | 15 | 6 (40.0) | 8.5 (2.7–24.9) | 8.3 (2.1–32.0) | ||
| History of medical injectionb | .29 | … | ||||
| No | 120 | 7 (5.8) | 1.0 | … | ||
| Yes | 418 | 37 (8.9) | 1.6 (0.72–3.9) | … |
NOTE. CI, confidence interval; OR, odds ratio.
Multivariate logistic regression analysis using variables significant at the P ≤ .05 level in the univariate analysis.
Intravenous or intramuscular use of medicines for treating diseases.
Multivariate logistic regression analysis demonstrated that subjects who sold plasma/blood (OR, 14.4 [95% CI, 7.1–31.6]) or who had a history of blood transfusion (OR, 8.3 [95% CI, 2.1–32.0]) were far more likely than nondonors to be HCV seropositive. An association between HCV seropositivity and having multiple lifetime sex partners was suggested (OR, 2.4 [95% CI, 0.94–5.6]) (table 1).
In our subgroup analysis of 119 former plasma/blood donors, the univariate analysis suggested that HCV seropositivity was associated with male sex, selling plasma, selling plasma/blood in official blood-collection centers, multiple lifetime sex partners, and a history of blood transfusion (table 2). Only male sex and selling plasma were statistically significant in the multivariate logistic regression analysis. Female former plasma/blood donors were less likely than male donors to be HCV seropositive (OR, 0.32 [95% CI, 0.12–0.80]). Former plasma donors had a higher risk of being HCV seropositive than did those who had donated whole blood only (OR, 7.6 [95% CI, 2.9–20.9]).
Table 2. Factors associated with hepatitis C virus (HCV) seropositivity in 119 former commercial plasma/blood donors in rural Shanxi Province, China.
| Factor | Subjects, no. | HCV-positive subjects, no. (%) | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P |
|---|---|---|---|---|---|---|
| Sex | .015 | .018 | ||||
| Male | 61 | 23 (37.7) | 1.0 | 1.0 | ||
| Female | 58 | 10 (17.2) | 0.34 (0.14–0.79) | 0.32 (0.12–0.8) | ||
| Education | .13 | … | ||||
| Elementary school or less | 48 | 17 (35.4) | 1.0 | … | ||
| Middle school or beyond | 71 | 16 (22.5) | 0.53 (0.23–1.20) | … | ||
| Ever sold plasma | <.001 | |||||
| No | 91 | 16 (17.6) | 1.0 | <.001 | 1.0 | |
| Yes | 28 | 17 (60.7) | 7.2 (2.9–18.9) | 7.6 (2.9–20.9) | ||
| Sold plasma/blood during 1991–1995 | .67 | … | ||||
| No | 47 | 12 (25.5) | 1.0 | … | ||
| Yes | 72 | 21 (29.2) | 1.2 (0.53–2.8) | … | ||
| Frequency of selling plasma/blood | .29 | … | ||||
| <10 times | 74 | 18 (24.3) | 1.0 | … | ||
| ≥10 times | 45 | 15 (33.3) | 1.6 (0.68–3.5) | … | ||
| Sold to illegal commercial plasma-collection center | .71 | … | ||||
| No | 91 | 26 (28.6) | 1.0 | … | ||
| Yes | 28 | 7 (25.0) | 0.83 (0.30–2.1) | … | ||
| Sold to official blood-collection center | .03 | … | ||||
| No | 24 | 2 (8.3) | 1.0 | … | ||
| Yes | 95 | 31 (32.6) | 5.3 (1.4–34.6) | … | ||
| Sold to government hospital | .25 | … | ||||
| No | 98 | 25 (25.5) | 1.0 | … | ||
| Yes | 21 | 8 (38.1) | 1.8 (0.65–4.8) | … | ||
| No. of lifetime sex partners | .04 | … | ||||
| ≤1 | 103 | 25 (24.3) | 1.0 | … | ||
| >1 | 16 | 8 (50.0) | 3.1 (1.05–9.3) | … | ||
| History of tooth extraction | .37 | … | ||||
| No | 83 | 21 (25.3) | 1.0 | … | ||
| Yes | 36 | 12 (33.3) | 1.5 (0.62–3.4) | … | ||
| History of acupuncture | .16 | … | ||||
| No | 111 | 29 (26.1) | 1.0 | … | ||
| Yes | 8 | 4 (50.0) | 2.8 (0.63–12.7) | … | ||
| History of surgery | .18 | … | ||||
| No | 108 | 28 (25.9) | 1.0 | … | ||
| Yes | 11 | 5 (45.5) | 2.4 (0.64–8.5) | … | ||
| History of blood transfusion | .049 | … | ||||
| No | 113 | 29 (25.7) | 1.0 | … | ||
| Yes | 6 | 4 (66.7) | 5.8 (1.07–43.4) | … | ||
| History of medical injectionb | .064 | … | ||||
| No | 33 | 5 (15.2) | 1.0 | … | ||
| Yes | 86 | 28 (32.6) | 2.7 (0.94–7.8) | … |
NOTE. CI, confidence interval; OR, odds ratio.
Multivariate logistic regression analysis using variables significant at the P ≤ .05 level in the univariate analysis.
Intravenous or intramuscular use of medicines for treating diseases.
In those seropositive for HCV antibodies, the odds of having donated plasma were 7.6 times higher than the odds of having donated blood (table 2). When we excluded the 28 subjects who said that they had donated plasma, the significant risk factors did not differ (data not shown), but the OR for being HCV seropositive was 8.0 (95% CI, 3.6–18.6), compared with an OR of 14.4 when both plasma and blood donors were included in the model.
Discussion
This community-based cross-sectional study found high HCV seroprevalence rates in subjects in rural Shanxi Province, where there had been an illegal commercial plasma/blood-collection center. Of subjects 18–59 years old, 8.2% were infected with HCV. In former plasma/blood donors, the HCV seroprevalence rate was 27.7%, whereas, in nondonors, the HCV seroprevalence rate was 2.6%, which is similar to that in the general population of China [2, 14]. Selling blood/plasma was the strongest predictor of being HCV seropositive; the risk in former plasma/blood donors was 14.4 times higher than that in nondonors, after adjustment for other confounding factors. In former donors, those who had donated plasma had 7.6 times the risk of being HCV seropositive than that in those who had donated whole blood only. Caution should be used when interpreting any association between HCV seropositivity and whole-blood donation, however. In the study villages, plasma donation is more stigmatized than whole-blood donation, because villagers associate plasma donation, during which erythrocytes are reinfused, with the risk of HIV infection. Therefore, some former plasma donors may have reported themselves as being former blood donors, thereby exhibiting the so-called social desirability bias [15]. It might be interesting to investigate how the 11 nondonors acquired HCV infection, because misclassification due to either inaccurate recall or the social desirability bias may play a role. Because only a small number of HCV infections were present in spouses, the statistical power of the study was inadequate to assess the association between donation and spouses' HCV status or other reasons associated with HCV seropositivity in the 11 nondonors.
Unlike the association between illegal private blood-collection centers and HIV infection that was found in a study published elsewhere [16], selling plasma and/or blood in official collection centers was significantly associated with HCV seropositivity in our univariate analysis but did not remain significant in our multivariate analysis. A possible explanation is that the unadjusted association is confounded by selling plasma; our subjects commonly sold plasma at both official and illegal commercial plasma-collection centers. Moreover, whether this reflects poor memory of the type of donation center that was visited nearly 10 years previously or whether there were also hygiene problems in government hospitals and official blood-collection centers is not known.
Exposure to contaminated blood or blood products is the major risk factor for HCV seropositivity; sexual transmission of HCV is far less efficient [17]. A prospective study reported that persons in long-term monogamous partnerships had a risk of 0%–0.6%/year for HCV seroconversion, whereas persons with multiple partners or those at risk of acquiring sexually transmitted diseases had a risk of 0.4%–1.8%/year [18]. The present study has shown that subjects with a history of blood transfusion had ∼8 times the risk of HCV seropositivity than that of those without a history of blood transfusion; this suggests the vital need for local blood banks to screen all blood products for HCV. Subjects with >1 lifetime sex partner also had a higher risk of HCV seropositivity than did those with ≤1 lifetime sex partner (P = .058). In the study villages, neither commercial sex work nor having multiple lifetime sex partners is common; only 5 subjects reported a history of commercial sex work, and only 30 reported having extramarital sex. Having multiple sequential spouses may increase the risk of acquiring sexually transmitted diseases (e.g., HIV or HCV infection) in this group, because some subjects reported that they remarried after their former spouses had died of blood donation–related diseases, including AIDS. The present study had low statistical power to test the associations between HCV seropositivity and commercial sex work or multiple lifetime sex partners, so we categorized the number of lifetime sex partners as >1 (as is relevant for this low-risk population). Much larger sample sizes would be needed to confirm any independent association between multiple lifetime sex partners and HCV seropositivity and to clarify whether commercial sex work or spouse's HCV status is associated with HCV seropositivity in these communities.
Older age and lower level of education were associated with HCV seropositivity in the univariate analysis, but these associations were not significant in the multivariate analysis. These observations may be related to a cohort effect: former plasma/blood donors who sold plasma/blood in the early 1990s were >35 years old at the time of our 2003 survey, and they likely received less education than younger villagers; furthermore, they had a higher risk of HCV seropositivity because they had sold plasma/blood. It is likely that selling plasma or receiving a blood transfusion is the risk factor of importance, with age and education being associated with the exposure rather than the outcome per se. In those who reported selling plasma/blood, women had a lower risk of HCV seropositivity than did men. No biological mechanism has been reported to suggest that men are more vulnerable to HCV infection; this difference in the HCV seroprevalence rate between the sexes might imply that there are unmeasured risks for male former plasma/blood donors—for example, more frequent donations among men. Residual confounding is still possible, even though we included a variable to dichotomize the frequency of donation (≥ 10 or <10 times), because the original questionnaire did not include donation frequency as a continuous variable. Another explanation for the sex difference is that women may be more likely to recover from HCV infection and subsequently lose anti-HCV reactivity, because of female sex hormones [19, 20].
Our community-based survey had a number of methodological strengths. We conducted a community census from which a random sample was drawn. The study was thoroughly explained to the subjects before the study and when individual informed consent was obtained, and the participation rate was high, even though subjects consented totally voluntarily. The majority of those who did not participate had temporarily out-migrated, and only 6.1% of villagers who were contacted refused to participate. Given China's extensive health-care networks that reach the village level, our experience suggests that good epidemiological studies can be undertaken even in rural China in collaboration with local public-health departments and community leaders.
The present study also had limitations. Because we did not perform HCV RNA testing or recombinant immunoblot testing, some samples that gave positive results for HCV antibodies by EIA may have actually given false-positive results. A complete virological and clinical work-up was not feasible. We did not successfully identify communities with high HIV seroprevalence rates (only 7 [1.3%] of 538 subjects were HIV seropositive), which made a study of risk factors for HIV-HCV coinfection impossible. We conducted our study ∼10 years after the possible HCV exposures had occurred, so death and out-migration may have altered our findings. HIV-related deaths may lead to an underestimation of HCV seroprevalence rates, because HCV shares a blood transmission route with HIV. Non-participants were younger than participants and were less likely to have been infected with HCV though plasma/blood donation; a majority of nonparticipants (81/120) were temporary out-migrants and may have been more likely than participants to engage in behaviors that put them at risk of HCV infection, such as illicit drug (e.g., heroin or marijuana) use or commercial sex work. Therefore, a lack of data regarding nonparticipants may have biased the estimates of HCV seroprevalence rates in either direction.
Unhygienic practices during plasma donation and receipt of blood transfusion are strong risk factors for HCV seropositivity in rural central China. Improved blood-collection and blood-banking practices are an urgent health priority. Technical support and drugs are needed to assist these central Chinese provinces in the treatment of patients with HCV infection.
Acknowledgments
We thank the entire China Integrated Programs for Research on AIDS team and participating staff at the Shanxi Province Center for Disease Control and the Wenxi County Center for Disease Control, for their contributions to data collection, data management, and laboratory testing; the Provincial and County Department of Health, for cooperation; Rodney Hoff (National Institute of Allergy and Infectious Diseases, Bethesda, Maryland), for his generous help in many areas; Roger Detels (University of California, Los Angeles), for his critical review of the manuscript; and the subjects, for their participation in our study.
Financial support: National Institute of Allergy and Infectious Diseases (grant U19 AI51915-03 to Y.S.); Fogarty International Center (grant D43 TW010035-06 to S.H.V.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Koff RS, Hepatitis C. In: Infectious diseases. 2nd. Gorbach SL, Bartlett JG, Blacklow NR, editors. Philadelphia: W. B. Saunders; 1998. pp. 864–71. [Google Scholar]
- 2.Liu CB. Epidemic and risk factors of viral hepatitis in general population in China. Zhongguo Gan Bing Za Zhi. 1998;6:67–70. [Google Scholar]
- 3.Yuan YH, Hong KX, Liu SZ, et al. Community-based survey of HCV and HIV coinfection in injection drug abusers in Sichuan Province of China. World J Gastroenterol. 2004;10:1589–93. doi: 10.3748/wjg.v10.i11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garten RJ, Lai S, Zhang J, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in southern China. Int J Epidemiol. 2004;33:182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention. National Hepatitis C prevention strategy. [1 May 2005]; Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/HCV_infection.htm.
- 6.Chandra M, Khaja MN, Farees N, et al. Prevalence, risk factors and genotype distribution of HCV and HBV infection in the tribal population: a community based study in south India. Trop Gastroenterol. 2003;24:193–5. [PubMed] [Google Scholar]
- 7.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–9. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 8.Meng DA. Serological study on hepatitis C infection in plasma donors. Zhonghua Yu Fang Yi Xue Za Zhi. 1990;24:193–5. [PubMed] [Google Scholar]
- 9.Chen X, He JM, Wu HQ, et al. A seroepidemiological and behavioral factors study of HIV, HCV, HBV and syphilis infection among commercial blood donors. Shi Yong Yu Fang Yi Xue. 1999;6:174–6. [Google Scholar]
- 10.Liu SZ, Fu JH, Cui YH, et al. A study on prevalence and subtypes of hepatitis C virus among blood donors in some areas of Shandong Province. Zhongguo Gong Gong Wei Sheng. 2000;16:527–8. [Google Scholar]
- 11.Zhang SY. Conditional logistic regression analysis of the influential factors of HCV infection in one blood donator aggregated village. Yu Fang Yi Xue Wen Xiang Xin Xi. 2000;6:3–4. [Google Scholar]
- 12.Yin N, Mei S, Li L, et al. Study on the epidemiology and distribution of human immunodeficiency virus-1 and hepatitis C virus infection among intravenous drug users and illegal blood donors in China. Zhong-hua Liu Xing Bing Xue Za Zhi. 2003;24:962–5. [PubMed] [Google Scholar]
- 13.Qiao XC, Nie XY, Guo XL. Analysis of the epidemic of HIV/AIDS in Shanxi Province and the strategy for HIV/AIDS prevention and control. Zhongguo Xing Bing Ai Zi Bing Fang Zhi. 2004;10:190–2. [Google Scholar]
- 14.Ding X, Gu H, Zhong ZH, Zilong X, et al. Molecular epidemiology of hepatitis viruses and genotypic distribution of hepatitis B and C viruses in Harbin, China. Jpn J Infect Dis. 2003;56:19–22. [PubMed] [Google Scholar]
- 15.Lau JT, Tsui HY, Wang QS. Effects of two telephone survey methods on the level of reported risk behaviours. Sex Transm Infect. 2003;79:325–31. doi: 10.1136/sti.79.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Qian X, Cao GH, et al. Study on the seropositive prevalence of human immunodeficiency virus in village residents living in rural region of central China. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:317–21. [PubMed] [Google Scholar]
- 17.Brook MG. Sexually acquired hepatitis. Sex Transm Infect. 2002;78:235–40. doi: 10.1136/sti.78.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36(Suppl 1):S99–S105. doi: 10.1053/jhep.2002.36797. [DOI] [PubMed] [Google Scholar]
- 19.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–33. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 20.Spada E, Mele A, Berton A, et al. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673–81. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]


