Abstract
Background
Infection with intestinal helminths may stimulate dysfunctional immune responses in human immunodeficiency virus (HIV)–infected persons. Studies have yielded conflicting results regarding the impact of antihelminthic treatment on plasma concentrations of HIV-1 RNA.
Methods
We conducted a prospective study of 54 HIV-1– and helminth-coinfected and 57 HIV-1–infected, helminth-uninfected asymptomatic adults living in Lusaka, Zambia, to assess the impact of antihelminthic treatment on plasma concentrations of HIV-1 RNA.
Results
Median baseline viral load was 0.33 log10 copies/mL lower in the helminth-infected group than in the uninfected group. Mean viral load between pretreatment and posttreatment visits increased in the helminth-infected (mean, 4.23 vs. 4.29 log10 copies/mL; P = .6) and helminth-uninfected (mean, 4.39 vs. 4.52 log10 copies/mL; P = .2) groups. Helminth-infected participants with high pretreatment viral loads had a mean 0.25-log10 copies/mL decrease after treatment (P = .3), and helminth-uninfected participants had a mean 0.02-log10 copies/mL decrease (P = .8).
Conclusions
We did not find an overall association between treatment of intestinal helminth infections and reduction in viral load in coinfected adults. Future studies may need to focus on adults with intense helminth infections who live in rural areas or on adults or children who harbor higher helminth burdens and plasma concentrations of HIV-1 RNA.
Recent observations suggest an interaction in intestinal helminth and HIV-1 coinfection. Ecological data suggest an overlap in the geographic distribution of these infections [1–4]. Immunologic evidence yields several mechanisms by which chronic helminth infection may facilitate HIV replication within a coinfected host [5, 6]. It is well established that HIV thrives in an immune system in which the cellular compartment is activated, and, although nonspecific activation of host immunity can be elicited by HIV alone, it is enhanced by coinfection with other pathogens. Helminth infections also shift the dominant profile of T helper lymphocyte subsets from a type 1 to a type 2 (TH2) response. Investigations of Ethiopian immigrants in Israel have revealed cytokine imbalances that reflect this shift [7, 8]. Additional studies have demonstrated increased apoptotic and anergic activity of lymphocyte subsets in helminth-infected adults [9–11]. The net effect is a pattern of immune dysregulation that might be expected to diminish the host cell’s capacity to contain retroviral replication.
These findings have prompted the hypothesis that treatment of helminth coinfections may be of direct clinical benefit to HIV-infected individuals, especially if it lowers viral load. The few human studies that have addressed this hypothesis have been inconsistent in their findings. Although one study demonstrated a mean decrease of 0.36 log10 copies/mL in plasma concentrations of HIV-1 RNA after treatment of intestinal helminth infections, others failed to find a similar effect [12–15]. The inconsistency of these results may be due to study limitations as a result of small sample size, the absence of comparison groups, and/or a cross-sectional analytic design. To address these issues, we conducted a prospective study to assess the impact that antihelminthic treatment has on plasma concentrations of HIV-1 RNA in asymptomatic adults living in Lusaka, Zambia, and coinfected with HIV-1 and intestinal helminths.
SUBJECTS, MATERIALS, AND METHODS
Study population and design
From March to December 2003, we screened 428 adults for HIV serostatus and the presence of helminth infection or Schistosoma mansoni on examination of stool samples. Participants were recruited from 3 citywide voluntary, confidential HIV testing and counseling centers. Participants were eligible for enrollment if they provided informed consent, were serologically confirmed as being infected with HIV-1, were 19–45 years old, had a total lymphocyte count of ≥1000 cells/μL, and provided at least 1 stool sample for examination for the presence of helminth ova and larvae. Individuals were excluded if they had been treated for active tuberculosis in the previous year, were pregnant (as determined by rapid human chorionic gonadotropin ELISA; Biotec Laboratories), were smear positive for malaria, or had received antihelminthic treatment or antiretroviral therapy in the preceding 4 weeks or 3 months, respectively. We also excluded individuals who presented with advanced HIV disease, defined as stage 3 or 4 of the revised World Health Organization (WHO) Kigali HIV staging system [16].
Eligible participants were recruited into 2 study arms on the basis of helminth status; helminth-infected participants were assigned to an experimental treatment group, whereas helminth-uninfected participants were followed to compare viral load and CD4+ and CD8+ cell count trends over a contemporaneous period. The participants in the treatment arm were coinfected with HIV-1 and helminths and were individually matched to HIV-1–infected, helminth-uninfected participants on the basis of sex and age (±4 years). Both groups were followed over the course of 5 study visits, which occurred at baseline and 1 week before the initiation of treatment and at 4 weeks, 10 weeks, and 16 weeks after the initiation of treatment. Participants in the experimental arm were treated at both the 1-week and 4-week visits with a 3-day course of albendazole (400 mg on the initial day and 200 mg on the 2 subsequent days; Albenza; GlaxoSmith-Kline) and a 1-day regimen of praziquantel (40 mg/kg spread over 2 doses separated by 4–6 h; Biltricide; Bayer). Receipt of the first doses was directly observed, and adherence to the regimen was assessed by participant interview. All participants were clinically evaluated at each scheduled visit and were offered primary medical care as indicated.
Data collection and laboratory procedures
During the screening process, we obtained sociodemographic, behavioral, and medical data via a structured interview. At follow-up visits, we evaluated participants for disease progression in accordance with basic clinical and laboratory parameters. Helminth status was determined before enrollment and again at the 10-week posttreatment visit. At enrollment, eligible participants were asked to provide 1 fresh and 2 preserved stool samples. Of the enrolled participants, 1 had 1 stool sample analyzed, 8 had 2 analyzed, 104 had 3 analyzed, and 13 had ≥4 analyzed (mean, 3.04 stool samples). A statistically equivalent number of stool samples was analyzed at the completion of the study (mean, 2.96 stool samples; P = .4). The mean number of stool samples analyzed per participant did not differ between the helminth-infected and helminth-uninfected groups at enrollment (3.11 vs. 2.97 stool samples; P = .3) or at the completion of the study (2.92 vs. 3.00 stool samples; P = .3).
Fresh stool samples were collected in sterilized plastic containers, and preserved stool samples were collected in 10% formalin ParaPak vials (Meridian Diagnostics). The fresh and preserved stool samples were examined within 24 and 72 h of collection, respectively, by formol-ether concentration techniques [17, 18]. For participants whose fresh stool samples contained helminth ova or larvae, we estimated the intensity of infection by quantitative analysis of the egg burden, in accordance with the Kato-Katz thick-smear technique [19]. For quality-control purposes, 10% of the stool samples were examined twice, by formol-ether concentration and the Kato-Katz thick-smear technique. Important discrepancies in ova identification or quantification were found in 2 (5.3%) of 38 stool samples and were resolved by the examination of an additional stool sample.
Participants were tested and counseled for HIV infection in accordance with a locally validated dual rapid test algorithm and quality-control procedure (Determine HIV-1/2 by Abbott Laboratories and Capillus HIV-1/HIV-2 by Trinity Biotech) [20]. Whole blood for immunologic and virologic analysis was obtained from enrolled participants at the baseline and 1-week pretreatment visits and at the 10- and 16-week posttreatment visits. Automated complete blood cell counts were quantified by a Coulter Counter, and CD4+ and CD8+ cell subsets were quantified by a FACSCount (both from Becton Dickinson). Plasma was stored at − 80°C and was shipped to the National Institute of Allergy and Infectious Diseases at the National Institutes of Health for HIV-1 RNA quantification (Chiron Quantiplex HIV-1 for bDNA).
Statistical analysis
We analyzed participants who completed follow-up in accordance with the study arm to which they were originally assigned. Helminth-uninfected participants who became newly infected with intestinal helminths during follow-up were still considered to be, in the primary analysis, in the helminth-uninfected comparison group. This was done so as not to introduce bias by selectively moving subjects from one study arm to another. Only 2 helminth-uninfected participants were newly infected during follow-up. When we analyzed all data, first by considering these 2 participants to be uninfected, then by considering them to be infected, and finally by excluding them altogether, the results from all 3 analyses differed only marginally. Hence, we present the findings of the primary analysis only.
Data were entered on site, and the analysis was performed using SAS (version 9.0; SAS Institute). Data were double entered with a discrepancy rate of <2%. Differences in proportions of sociodemographic characteristics by helminth status were assessed by Fisher’s exact test. Mean plasma concentrations of HIV-1 RNA and CD4+ and CD8+ cell counts for baseline and 1-week pretreatment visits were compared with averaged values for the 10- and 16-week posttreatment visits by use of the Wilcoxon rank sum test. Univariate comparisons of pretreatment and posttreatment log10 viral load between the 2 study arms were also evaluated by the Wilcoxon rank sum test. Changes in viral load across all 4 time points were examined using longitudinal data analytic methods. Specifically, we estimated changes in log10 viral load within participants and between experimental and comparison groups from a linear model using the SAS MIXED procedure for repeated measures. Model-selection strategies were used to adjust for potentially important covariates, such as age, sex, CD4+ cell count, and intensity of helminth infection. Variance-covariance structure and model-fit diagnostics were assessed for each model.
Ethics
Study procedures were approved by the University of Alabama, Birmingham (UAB), Institutional Review Board; the University of Zambia Research Ethics Committee for the University Teaching Hospital (UTH) of Lusaka; and the Fogarty International Center of the National Institutes of Health. Written, informed consent was obtained from all study participants, and human-experimentation guidelines of the UAB Institutional Review Board and the UTH Research Ethics Committee were followed.
RESULTS
Baseline characteristics
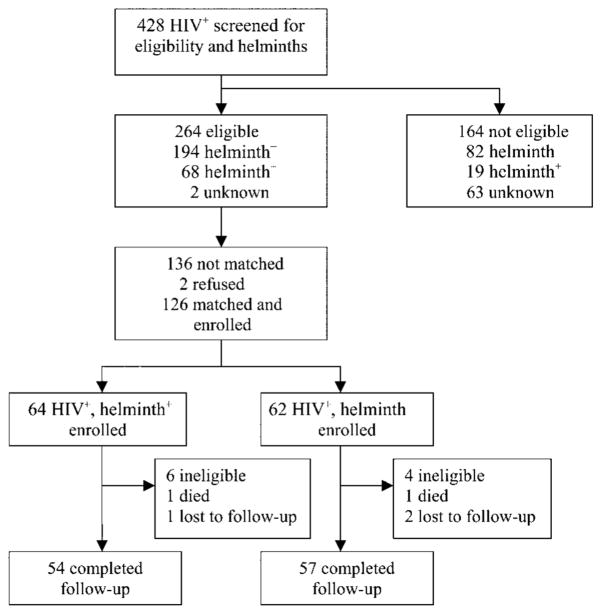
Of 428 individuals screened for the study, 264 were eligible to participate; of the 264 participants, 26% were infected with at least 1 type of intestinal helminth (figure 1). Of the 264 eligible participants, 126 were individually matched and enrolled into the study. Ten participants were later deemed to be ineligible when they were confirmed as being HIV seronegative; 2 participants died because of causes unrelated to the study (cerebral malaria and bacterial meningitis); and 3 were lost to follow-up, leaving 54 participants in the experimental arm and 57 participants in the comparison arm who completed the 5 study visits. Of the 54 helminth-infected participants, 26 were infected with Ascaris lumbricoides, 18 were infected with hookworm, 5 were infected with S. mansoni, 4 were infected with Strongyloides stercoralis, and 1 was infected with Hymenolepsis nana.
Figure 1.
Trial profile. +, positive; −, negative.
The majority of participants were female (61.3%), reported an income of <US$1/day (62.2%), and lived in the eastern portion of Lusaka (76.6%). We observed no differences in baseline characteristics between the helminth-infected group and the helminth-uninfected group (table 1). The mean age of the participants in the 2 study arms was similar (30.2 vs. 30.5 years; P = .6), as were unemployment rates (50.0% vs. 49.1%; P = .6). The median total lymphocyte count was 1786 cells/μL in the helminth-infected group and 1625 cells/μL in the helminth-uninfected group (P = .4). Except for hematocrit (median, 35.8%; SD, 6.8%), for which the reference range is 37%–54%, hematologic values were within locally established reference ranges.
Table 1.
Baseline characteristics of helminth-infected and helminth-uninfected HIV-1–infected adults.
| Variable | Helminth-infected group (n = 54) | Helminth-uninfected group (n = 57) | P |
|---|---|---|---|
| Age, mean (SD), years | 30.2 (6.1) | 30.5 (5.7) | .6 |
| Sociodemographic characteristic, no. (%) | |||
| Sex, male | 21 (38.9) | 22 (38.6) | .9 |
| Primary education or less | 29 (53.7) | 21 (38.6) | .1 |
| Illiterate | 8 (14.8) | 15 (26.3) | .2 |
| Employment | |||
| Unemployed | 27 (50.0) | 28 (49.1) | .6 |
| Self-employed | 15 (27.8) | 20 (35.1) | |
| Formally employed | 12 (22.2) | 9 (15.8) | |
| Income <US$1/day | 33 (61.1) | 36 (63.2) | .8 |
| Location of residence | |||
| West Lusaka | 12 (22.2) | 14 (24.5) | .8 |
| East Lusaka | 42 (77.8) | 43 (75.4) | |
| Baseline laboratory measures, median (IQR) | |||
| Erythrocytes, ×106 cells/μL | 4.2 (3.9–4.7) | 4.2 (3.9–4.7) | .8 |
| Leukocytes, ×103 cells/μL | 5.0 (4.2–5.8) | 4.6 (4.0–5.5) | .4 |
| Leukocyte differential, % | |||
| Lymphocytes | 35.8 (31.4–41.4) | 7.0 (29.6–42.5) | .8 |
| Monocytes | 8.4 (6.0–10.0) | 8.4 (6.8–10.9) | .4 |
| Granulocytes | 55.2 (48.6–59.6) | 3.3 (45.2–58.7) | .5 |
| Total lymphocyte count, ×103 cells/μL | 1.8 (1.3–2.1) | 1.6 (1.4–2.1) | .4 |
| Platelets, ×103 cells/μL | 226 (187–274) | 224 (192–270) | .8 |
| Hemoglobin, g/dL | 12.1 (10.8–13.3) | 2.2 (10.8–13.6) | .8 |
| Hematocrit, % | 35.4 (32.2–39.8) | 6.6 (32.8–40.6) | .6 |
NOTE. IQR, interquartile range.
Differences in plasma concentrations of HIV-1 RNA and CD4+ and CD8+ cell counts
The median pretreatment plasma concentration of HIV-1 RNA was 0.33 log10 copies/mL higher in the helminth-uninfected group (4.63 log10 copies/mL [interquartile range {IQR}, 4.19–4.83 log10 copies/mL]) than in the helminth-infected group (4.30 log10 copies/mL [IQR, 3.86–4.80 log10 copies/mL]) (P = .09). The magnitude of this difference remained close to 0.30 log10 copies/mL after treatment in the helminth-infected group (4.71 log10 copies/mL [IQR, 4.22–5.02 log10 copies/mL] vs. 4.43 log10 copies/mL [IQR, 3.88–4.87 log10 copies/mL]; P = .03). The mean baseline plasma concentration of HIV-1 RNA was 4.21 log10 copies/mL (SD, 0.83; n = 48) in participants with high- or moderate-intensity helminth infections, compared with 4.14 log10 copies/mL (SD, 0.48; P = .6) in participants with low-intensity helminth infections. The helminth-infected group exhibited consistently higher median CD4+ cell counts across all study visits (pre-treatment, 329 cells/μL [IQR, 208–434 cells/μL] vs. 194 cells/μL [IQR, 152–338 cells/μL]; P = .02; posttreatment, 276 cells/μL [IQR, 171–375 cells/μL] vs. 178 cells/μL [IQR, 112–314 cells/μL]; P = .03). The median CD8+ cell count was statistically equivalent between the treatment and comparison groups at the pretreatment (782 vs. 845 cells/μL; P = .7) and posttreatment (743 vs. 706 cells/μL; P = 1.0) visits.
Impact of antihelminthic treatment
Comparisons between pretreatment and posttreatment visits within each study arm demonstrated a slight increase in mean plasma concentration of HIV-1 RNA and a decline in mean CD4+ cell count within both the helminth-infected (pretreatment, 4.23 vs. 4.29 log10 copies/mL; P = .6; posttreatment, 349 vs. 301 cells/μL; P = .2) and the helminth-uninfected (pretreatment, 4.39 vs. 4.52 log10 copies/mL; P = .2; posttreatment, 280 vs. 230 cells/μL; P = .1) groups (tables 2 and 3). CD8+ cell counts decreased during the same period within each group (P > .05). Plasma concentrations of HIV-1 RNA increased by 0.06 log10 copies/mL (95% confidence interval [CI], .02–.10 log10 copies/mL) in the helminth-infected group and by 0.13 log10 copies/mL (95% CI, −.03 to .29 log10 copies/mL) in the helminth-uninfected group. The interaction between visit and treatment arm yielded a statistically nonsignificant difference of .07 log10 copies/mL (95% CI, −.04 to .20 log10 copies/mL; P = .2) between groups in the change from mean pretreatment to posttreatment plasma concentrations of HIV-1 RNA. There was no significant interaction between visit and treatment group when change over the 4 separate visits, instead of the average of the 2 pretreatment and 2 posttreatment visits, was assessed (P = .2). Similarly, the change in CD4+ cell count differed by only 2 cells/μL (95% CI, − 45 to 49 cells/μL) between the 2 groups (P = .9).
Table 2.
Plasma concentrations of HIV-1 RNA in helminth-infected and helminth-uninfected HIV-1–infected adults before and after antihelminthic treatment.
| Pretreatment |
Posttreatment |
Mean |
||||||
|---|---|---|---|---|---|---|---|---|
| Group, parameter | Baseline | Week 1 | Week 10 | Week 16 | P | Pretreatment | Posttreatment | P |
| Helminth infected (n = 54) | ||||||||
| Log10 HIV-1 RNA copies/mL | 4.20 (0.80) | 4.25 (0.82) | 4.30 (0.82) | 4.26 (0.80) | .4 | 4.23 (0.79) | 4.29 (0.77) | .6 |
| HIV-1 RNA, ×103 copies/mL | 48.4 (75.2) | 97.7 (396) | 60.0 (86.5) | 56.8 (83.9) | .5 | 73.1 (222) | 57.8 (73.1) | .5 |
| Helminth uninfected (n = 57) | ||||||||
| Log10 HIV-1 RNA copies/mL | 4.38 (0.88) | 4.40 (0.92) | 4.52 (0.97) | 4.54 (1.0) | <.001 | 4.39 (0.88) | 4.52 (0.96) | .2 |
| HIV-1 RNA, ×103 copies/mL | 61.0 (82.1) | 81.0 (164) | 114 (217) | 157 (398) | .03 | 71.0 (106) | 132 (282) | .2 |
NOTE. Data are mean (SD), unless otherwise indicated.
Table 3.
CD4+ and CD8+ cell counts in helminth-infected and helminth-uninfected HIV-1–infected adults before and after antihelminthic treatment.
| Pretreatment |
Posttreatment |
Mean |
||||||
|---|---|---|---|---|---|---|---|---|
| Group, parameter | Baseline | Week 1 | Week 10 | Week 16 | P | Pretreatment | Posttreatment | P |
| Helminth infected (n = 54) | ||||||||
| CD4+ | 359 (238) | 338 (206) | 345 (223) | 257 (220) | <.001 | 349 (204) | 301 (200) | .2 |
| CD8+ | 912 (428) | 886 (505) | 930 (475) | 700 (526) | <.001 | 899 (407) | 815 (447) | .2 |
| Helminth uninfected (n = 57) | ||||||||
| CD4+ | 287 (219) | 272 (204) | 247 (218) | 214 (186) | .001 | 280 (206) | 230 (178) | .1 |
| CD8+ | 966 (515) | 874 (427) | 826 (458) | 801 (576) | .007 | 920 (443) | 814 (475) | .1 |
NOTE. Data are mean (SD) cells per μL, unless otherwise indicated.
Plasma concentrations of HIV-1 RNA changed only trivially when the data were adjusted for CD4+ cell count. Thirteen helminth-infected and 26 helminth-uninfected participants had baseline CD4+ cell counts <200 cells/μL. Overall, these participants had higher plasma concentrations of HIV-1 RNA than did those with CD4+ cell counts ≥200 cells/μL at both pretreatment (4.63 vs. 4.13 log10 copies/mL) and posttreatment (4.83 vs. 4.18 log10 copies/mL) visits. Regardless of the CD4+ cell count, plasma concentrations of HIV-1 RNA in the helminth-infected and helminth-uninfected groups increased by a magnitude similar to that of the estimated increase for the whole study population. When we evaluated participants with high (≥5.0 log10 copies/mL; n = 17) baseline plasma concentrations of HIV-1 RNA, we found that mean viral load declined by 0.25 log10 copies/mL after antihelminthic treatment in the helminth-infected group (5.24 vs. 4.99 log10 copies/mL; P = .3) and by 0.02 log10 copies/mL at the same time point in the helminth-uninfected group (5.18 vs. 5.16 log10 copies/mL; P = .8). Adjusting for age, sex, and CD4+ cell count in a mixed model did not alter the primary outcome in the overall or any subset analysis. Treatment of helminth infection was associated with a decrease of 0.12 log10 copies/mL in plasma concentrations of HIV-1 RNA in participants with moderate- or high-intensity infections with A. lumbricoides or hookworm, as classified by the WHO [19]; however, this observation was based on data from 6 participants and was not reflective of any prior hypothesis related specifically to hookworm infection or ascariasis.
DISCUSSION
In the present study, we failed to find an association between treatment of intestinal helminth infection and decreases in plasma concentrations of HIV-1 RNA or changes in CD4+ cell counts in Zambian adults coinfected with HIV-1 and helminths. These results are similar to the findings of previous studies in Kenya and Uganda [13–15], but they conflict with data from a study in Ethiopia that demonstrated a reduction in viral load (0.36 log10 copies/mL) 6 months after intestinal helminth infections in coinfected adults were eliminated [12].
The hypothesis that helminth infections may play a central role in the pathogenesis of HIV-1 infection, especially in developing countries, and contribute to faster progression of HIV-1 disease was originally made on the basis of observations that a TH2 immunologic profile and a pattern of chronic immune activation predominated in patients with helminth infections [21–26]. HIV pathogenesis research also revealed that increased expression of HIV coreceptors was associated with chronic immune activation [27, 28], that peripheral blood mononuclear cells extracted from chronically immune-activated individuals exhibited increased susceptibility to HIV-1 infection [29, 30], and that immune activation during the course of HIV-1 infection was an important factor in driving disease progression to AIDS [31]. It also became clear that chronic immune activation, such as that caused by helminth infections, could result in the converse effect—that is, anergy or a suppression of the immune response [32]. Taken together, the data suggest that the role of the immune dysregulation caused by helminth infection may not be unidirectional but instead may be more complex, having polar effects that depend on a number of still unidentified factors.
In the pivotal study that inspired the present one, Wolday et al. [12] found that 13 Ethiopians living in Addis Ababa and coinfected with HIV-1 and either intestinal nematodes or S. mansoni experienced a mean decrease of 0.36 log10 copies/mL in plasma concentrations of HIV-1 RNA after having their helminth infections cleared with antihelminthic medications (albendazole and/or praziquantel). To date, Wolday et al. [12] are the only investigators to have published such a finding. Before the Ethiopian study, Lawn et al. [13] found that treatment of 30 Kenyan adults living in a rural setting who were coinfected with HIV-1 and S. mansoni was correlated with a mean increase in viral load of 0.33 log10 copies/mL 1–15 months after treatment (mean follow-up, 5.6 months). This increase was unlikely to be related to the elimination of schistosomes, because adults who were evaluated ≤5 months after antihelminthic treatment exhibited an increase in viral load of 0.08 log10 copies/mL, whereas participants whose follow-up visits occurred ≥6 months after treatment had a mean viral load that was 0.56 log10 copies/mL higher than baseline levels. It is more likely that an increase in viral load reflects HIV-1 disease progression in antiretroviral-naive adults.
In a cross-sectional analysis of HIV-1–infected Ugandan adults, Elliott et al. [14] observed that persons coinfected with HIV-1 and helminths had a viral load 0.21 log10 copies/mL lower and a median CD4+ cell count 199 cells/μL higher than those in HIV-1–infected, helminth-uninfected persons. These relative differences were still apparent at 5 weeks and 4 months after treatment of helminth infections. However, one-third of the treated participants still harbored intestinal helminths at the 4-month follow-up visit, which makes it difficult to remove the confounding effects of persistent helminth infection from observed null associations. The same group of investigators recently reported the results of a prospective study in which they followed a much larger cohort of Ugandan adults coinfected with HIV-1 and helminths [15]. Despite the increase in sample size, the findings were similar. After adjusting for potential confounding variables, the investigators found no overall impact of antihelminthic treatment on plasma concentrations of HIV-1 RNA.
We believe the present study improves on previous studies with regard to sample size, study design, and/or analytic approach. We enrolled into the study and assessed 54 participants coinfected with HIV-1 and helminths, and only 2 had helminth infections at the end of follow-up. The present study’s sample size of successfully treated helminth-infected participants is nearly double that in most previous studies, except for the Ugandan cohort followed by Brown et al. [15]. Our study and the 2 Ugandan studies [14, 15] improve on the study designs of Wolday et al. [12] and Lawn et al. [13] by including an HIV-1–infected, helminth-uninfected cohort to assess secular trends in viral load and CD4+ cell count over a concurrent period. Despite the use of a longitudinal study design with true comparison groups, neither Ugandan study [14, 15] accounted for the time-dependent variability of primary outcome measurements, such as CD4+ cell count or viral load, in their analyses. Brown et al. [15] assessed the mortality associated with helminth infection using linear regression techniques but, like Elliott et al. [14], did not employ longitudinal analytic methods when evaluating the impact of antihelminthic treatment on CD4+ cell count or HIV-1 viral load. In fact, only Lawn et al. [13] analyzed their data longitudinally; however, that study may not be comparable to others, because it did not include a comparison group and focused on only S. mansoni infection, which may exhibit kinetics of infection and clearance that differ from those characteristics of intestinal nematodes. Furthermore, the present study is the only one to account for potential regression-to-the-mean effects on outcome measurements by obtaining and analyzing average, rather than individual, HIV-1 load and CD4+ cell count values before and after the administration of antihelminthic treatment.
Despite improving on the design of previous studies, the present study has certain limitations. Similar to other investigators, we screened participants for prevalent, not incident, helminth infections. This limits the interpretation of our data, because the duration of helminth infection may play an important role in the activation of host immunity. Furthermore, we could not ascertain when HIV-1 infection occurred. We do not believe this limitation to be a major source of bias, because we used clinical and laboratory surrogates to screen for stage of infection. We may have failed to adjust for potential unknown confounding variables by not conducting a randomized cohort study; we could not, however, ethically justify the withholding of antihelminthic treatment from helminth-infected participants. By restricting the population to asymptomatic antiretroviral-naive adults with a total lymphocyte count ≥1000 cells/μL and matching by age and sex, we nonetheless minimized the potential confounding influence of these factors. Unlike the Ugandan studies [14, 15], which employed serologic assays for helminth detection, we used only fecal methods to detect helminth ova and larvae and therefore had limited sensitivity to identify helminths such as S. mansoni and S. stercoralis, which excrete low, variable numbers of eggs in stool. However, our study used 2 fecal methods to analyze a median of 3 stool samples from each participant, whereas other studies [12–15] analyzed only 1 sample from each participant.
It is possible that the high sensitivity of our parasitologic methods and the low intensity of infection in this urban setting biased our results to a null outcome. More than 85% of participants in the experimental arm harbored low-intensity infections. The intensity of helminth infection (as measured by ova count) in the population in the present study and in the study by Elliott et al. [14] was an order of magnitude lower than that in the population followed by Wolday et al. [12]. Thus, the difference in intensity of infection possibly explains some of the discrepancy in results. Coinfections may exert more influence on the dynamics of HIV-1 replication in persons with high-intensity helminth infections and high plasma concentrations of HIV-1 RNA. Despite the trend that clearance of intestinal helminth coinfections may not influence plasma concentrations of HIV-1 RNA, the continued investigation of this question is warranted in coinfected children, who are well known to have both high-intensity helminth infections [33, 34] and high plasma concentrations of HIV-1 RNA [35–37], as well as in rural inhabitants, who may also have high-intensity helminth infections. It is also prudent to recognize the inherent advantage of antihelminthic treatment for HIV-infected persons, although we cannot confirm that viral load reduction will be among its benefits.
Acknowledgments
We thank Kara Counseling, Susan Allen, Brad Fuller, and the staff of the Zambia University of Alabama, Birmingham, HIV Research Project, for their assistance in recruiting participants for the study; Julie Metcalf and Robin Dewar of the National Institute of Allergy and Infectious Diseases and Paddy Mukando, Joshua Kashitala, Joshua Siame, Alison Taylor, Patrick Chipaila, Francis Kasolo, Sandi Sianongo, and the rest of the clinical and laboratory staff of the Fogarty International Research Collaborative Award Project, for their hard work and dedication to the study; and the participants in the study, for their cooperation.
Financial support: National Institutes of Health (grant R03 TW05929); University of Alabama, Birmingham, Medical Scientist Training Program (grant T32 GM008361); John J. Sparkman Center for International Public Health Education; US Department of Defense National Security Education Program; “1917” Clinic of the University of Alabama, Birmingham; World Health Organization; GlaxoSmith-Kline; Bayer.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Borkow G, Bentwich Z. Eradication of helminthic infections may be essential for successful vaccination against HIV and tuberculosis. Bull World Health Organ. 2000;78:1368–9. [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Joint Report on HIV/AIDS. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: UNAIDS and World Health Organization; 1997. [Google Scholar]
- 3.Bentwich Z, Maartens G, Torten D, Lal AA, Lal RB. Concurrent infections and HIV pathogenesis. AIDS. 2000;14:2071–81. doi: 10.1097/00002030-200009290-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bentwich Z. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:312. doi: 10.1016/s0169-4758(00)01693-8. [DOI] [PubMed] [Google Scholar]
- 5.Kalinkovich A, Weisman Z, Bentwich Z. Role of TH1 and TH2 in the pathogenesis of AIDS and various other diseases. Harefuah. 1995;128:228–33. [PubMed] [Google Scholar]
- 6.Fincham J. Helminths, HIV/AIDS and tuberculosis. AIDS Bulletin. 2001;10:3. [Google Scholar]
- 7.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 8.Bentwich Z, Weisman Z, Moroz C, Bar-Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinkovich A, Weisman Z, Bentwich Z. Chemokines and chemokine receptors: role in HIV infection. Immunol Lett. 1999;68:281–7. doi: 10.1016/s0165-2478(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 10.Borkow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–60. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messele T, Abdulkadir M, Fontanet AL, et al. Reduced naive and increased activated CD4+ and CD8+ cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–50. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolday D, Mayaan S, Mariam ZG, et al. Treatment of intestinal worms is associated with decreased HIV plasma viral load. J Acquir Immune Defic Syndr. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Karanja DM, Mwinzia P, et al. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000;14:2437–43. doi: 10.1097/00002030-200011100-00004. [DOI] [PubMed] [Google Scholar]
- 14.Elliott AM, Mawa PA, Joseph S, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. 2003;97:103–8. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Kizza M, Watera C, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis. 2004;190:1869–79. doi: 10.1086/425042. [DOI] [PubMed] [Google Scholar]
- 16.Lifson AR, Allen S, Wolf W, et al. Classification of HIV infection and disease in women from Rwanda: evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med. 1995;122:262–70. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Melvin MD. Human Health Services publication (CDC) 3. Atlanta, GA: Centers for Disease Control and Prevention; 1982. Laboratory procedures for the diagnosing of intestinal parasites; pp. 80–82. [Google Scholar]
- 18.World Health Organization. Basic laboratory methods in medical pathology. 1. London: World Health Organization; 1991. [Google Scholar]
- 19.World Health Organization. Bench aids for the diagnosis of intestinal parasites. London: World Health Organization; 1994. [Google Scholar]
- 20.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11 (Suppl 1):S103–10. [PubMed] [Google Scholar]
- 21.Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–52. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–8. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 23.Clerici M, Shearer GM. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 24.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S, Del Prete G, Manetti R, et al. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 26.Romagnani S, Maggi E. Th1 versus Th2 responses in AIDS. Curr Opin Immunol. 1994;6:616–22. doi: 10.1016/0952-7915(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 27.Kalinkovich A, Weisman Z, Leng Q, et al. Increased CCR5 expression with decreased beta chemokine secretion in Ethiopians: relevance to AIDS in Africa. J Hum Virol. 1999;2:283–9. [PubMed] [Google Scholar]
- 28.Kalinkovich A, Borkow G, Weisman Z, Tsimanis A, Stein M, Bentwich Z. Increased CCR5 and CXCR4 expression in Ethiopians living in Israel: environmental and constitutive factors. Clin Immunol. 2001;100:107–17. doi: 10.1006/clim.2001.5040. [DOI] [PubMed] [Google Scholar]
- 29.Shapira-Nahor O, Kalinkovich A, Weisman Z, et al. Increased susceptibility to HIV-1 infection of peripheral blood mononuclear cells from chronically immune activated individuals. AIDS. 1998;12:1731–3. [PubMed] [Google Scholar]
- 30.Gopinath R, Ostrowski M, Justement SJ, Fauci AS, Nutman TB. Filarial infections increase susceptibility to human immunodeficiency virus infection in peripheral blood mononuclear cells in vitro. J Infect Dis. 2000;182:1804–8. doi: 10.1086/317623. [DOI] [PubMed] [Google Scholar]
- 31.Hazenberg MD, Otto SA, van Benthem BHB, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 32.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–30. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bundy DA, de Silva NR. Can we deworm this wormy world? Br Med Bull. 1998;54:421–32. doi: 10.1093/oxfordjournals.bmb.a011698. [DOI] [PubMed] [Google Scholar]
- 34.Chan MS. The global burden of intestinal nematode infections—fifty years on. Parasitol Today. 1997;13:438–43. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 35.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997;336:1337–42. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 36.De Rossi A, Masiero S, Giaquinto C, et al. Dynamics of viral replication in infants with vertically acquired human immunodeficiency virus type 1 infection. J Clin Invest. 1996;97:323–30. doi: 10.1172/JCI118419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–61. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]