Abstract
We measured the ex vivo electrical conductivity of eight human metastatic liver tumours and six normal liver tissue samples from six patients using the four electrode method over the frequency range 10 Hz to 1 MHz. In addition, in a single patient we measured the electrical conductivity before and after the thermal ablation of normal and tumour tissue. The average conductivity of tumour tissue was significantly higher than normal tissue over the entire frequency range (from 4.11 versus 0.75 mS cm−1 at 10 Hz, to 5.33 versus 2.88 mS cm−1 at 1 MHz). We found no significant correlation between tumour size and measured electrical conductivity. While before ablation tumour tissue had considerably higher conductivity than normal tissue, the two had similar conductivity throughout the frequency range after ablation. Tumour tissue conductivity changed by +25% and −7% at 10 Hz and 1 MHz after ablation (0.23−0.29 at 10 Hz, and 0.43−0.40 at 1 MHz), while normal tissue conductivity increased by +270% and +10% at 10 Hz and 1 MHz (0.09−0.32 at 10 Hz and 0.37−0.41 at 1 MHz). These data can potentially be used to differentiate tumour from normal tissue diagnostically.
Keywords: cancer, tumor, electrical conductivity, dielectric tissue properties
1. Introduction
The liver is a common site for both primary and secondary (metastatic) cancer. The standard curative treatment for both of these malignancies is liver resection. However, the majority of patients with liver cancer (∼80%) are ineligible for resection (Jamison et al 1997), typically due to the presence of multiple diffuse tumours or other concomitant liver disorders. Radiofrequency (RF) ablation has become a common treatment modality for unresectable liver tumours (Gillams 2005). In a typical ablation, an electrode is introduced into a target tumour and RF current is applied for ∼12−35 min, resulting in tissue heating near the electrode due to resistive heating. The resulting heat propagates into the surrounding target tissue over the course of the ablation, leading to coagulation necrosis in regions that exceed approximately 50 °C. The pattern of RF current deposition near the electrode depends on the relationship between the electrical conductivities of the different tissue types (normal and tumour) at that location. It has been demonstrated in prior studies that accurate knowledge regarding these different conductivities is essential in creating accurate computer and ex vivo models of RF ablation (Solazzo et al 2005).
It is well known that biological tissues have higher electrical conductivity at higher frequencies. This change in conductivity in the radiofrequency range is due to formation of an ion double layer around cell membranes (Foster and Schwan 1989). Researchers have also known for nearly 100 years that malignant tissue has higher electrical conductivity than healthy surrounding tissue, and that this difference could be used in the detection of tumours (Fricke and Morse 1926). Several studies have further demonstrated the difference in conductivity between normal and neoplastic tissues at audio and radiofrequencies in various tumour models (Haemmerich et al 2003, Smith et al 1986, Surowiec et al 1988, Swarup et al 1991). Additionally, two studies have recently shown that malignant tissue has higher electrical conductivity than normal tissue in the human liver at microwave frequencies (915 MHz to 2.2 GHz) (O'Rourke et al 2007, Stauffer et al 2003). However, there are few data available regarding the electrical properties of malignant hepatic tissue in the literature at audio and radiofrequencies. Haemmerich et al (2003) showed that the differences in electrical conductivity between normal hepatic and tumour tissue in a rat model are more pronounced at lower frequencies.
Since these data are unavailable for human tissue, we measured the electrical conductivity of normal and tumour tissue in ex vivo human liver samples over the frequency range of 10 Hz to 1 MHz in this study. In addition, we measured the electrical conductivity in one excised sample before and after performing an RF ablation in both tissue types.
2. Materials and methods
2.1. Measurement system
We used the four-electrode method to measure electrical conductivity in this study. This method has been commonly used in prior ex vivo and in vivo tissue studies (Haemmerich et al 2002, 2003, Rush et al 1963, Steendijk et al 1993, Tang et al 2008, Tsai et al 2002b) because it reduces errors due to polarization impedance and stray capacitance (Tsai et al 2002a). We constructed a custom plunge probe (figure 1) using epoxy and 0.38 mm diameter silver wire electrodes, which were coated with silver chloride prior to the experiments to further reduce interface impedance. The four collinear electrodes were spaced 1.5 mm apart and were 6 mm in length, with the 2 mm nearest the bottom surface of the probe insulated to prevent conduction between the electrodes due to any residual fluid presence on the bottom surface of the probe. This plunge probe design has a measurement volume of ∼6 × 2 mm, and has been used successfully in previous tissue studies (Haemmerich et al 2002, 2003, Tsai et al 2000, 2002b).
Figure 1.
The four-electrode plunge probe design (reproduced with permission from Haemmerich et al 2003).
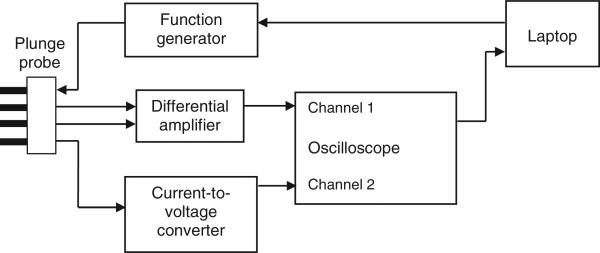
The conductivity measurement system used in this study is described in detail in Tsai et al (2000). We measured conductivity over the frequency range of 10 Hz to 1 MHz, including the standard RF ablation frequency (460 kHz). This frequency range was selected since this measurement system has been well characterized within this range (Tsai et al 2002a), and because the electrical conductivity of tumour and normal tissue at these frequencies is of current research interest (Haemmerich and Wood 2006, Schutt and Haemmerich 2008). Figure 2 shows a block diagram of the measurement system. A custom program (Microsoft Visual Basic) running on a laptop PC controlled the generation of sinusoidal waveforms (Hewlett Packard 33120 A function generator, Palo Alto, CA) over the frequency range. The program received the voltage, current and phase measurements from a digital oscilloscope (HP54600B, Hewlett Packard), and automatically calculated and stored the measured conductivity at each frequency. Each measurement scan over the seven frequencies took approximately 10 s. The estimated maximum error of this electrode and measurement system over this frequency range is ∼2% (Tsai et al 2002a), not including any possible errors due to tissue temperature variation.
Figure 2.
Block diagram of the conductivity measurement system.
2.2. Tissue source and handling
This study was approved by the Institutional Review Board for Human Research at the University of Wisconsin-Madison. Six patients (2 f/4 m) scheduled for surgical removal of colorectal liver metastases were enrolled in this study. Patients were 55 to 76 years old (average 65 yr), and tumour size was between 1 and 9 cm (average 4.5 cm). In three patients, two distinct tumours were present in the excised sample and were both measured, so a total of eight tumour tissue and six normal tissue measurements were obtained. After each surgery, the excised tissue sample including tumour and surrounding normal tissue was transported to the pathology lab where the tumour was centrally sliced to expose the tumour core. For the measurements of tumour tissue, the plunge probe was inserted into the centre of the tumour, while for the measurements of normal liver tissue, the plunge probe was inserted into a non-malignant region of the same sample distant from the tumour. All measurements were performed within ∼30−60 min of tissue removal. We assume that both normal and tumor tissue are sufficiently isotropic to allow the measurement as described above with sufficient accuracy. This is likely true, as previous studies have not shown any anisotropy of liver tissue, and tumour structure is typically irregular such that no directional anisotropy is likely to exist.
In a single tissue sample, we performed an RF ablation with an internally cooled needle electrode for 12 min at the centre of a 5.4 cm diameter tumour to create an ∼2.5 cm diameter coagulation zone. In addition, we performed a similar RF ablation in a section of normal liver tissue for 12 min. Electrical conductivity was measured at the centre of the ablation zone in both tumour and normal tissue before and after ablation.
2.3. Data analysis
Multiple conductivity measurements (minimum 2, average 2.9) were made at each location and frequency during each experiment. The average values at each location and frequency were then corrected based on a calibration of the measurement system in 0.9% saline at room temperature after each experiment. We compared the corrected average values for normal and tumour tissue at each frequency using Student's t-test. Statistical significance was designated as p < 0.05. Additionally, we investigated whether there was a significant relationship between tumour size and electrical conductivity using Pearson's correlation coefficient.
3. Results
Figure 3 and table 1 show the average and standard deviation of the electrical conductivity measurements (n = 8 tumour tissue, n = 6 normal tissue) made without subsequent RF ablation. The average conductivity was significantly lower in normal tissue than in tumour tissue at all seven measurement frequencies (p < 0.01).
Figure 3.
Average electrical conductivity from six normal and eight tumour tissue conductivity measurements without RF ablation. Bars denote the standard deviation (SD) for each measurement series.
Table 1.
Results of six normal and eight tumour tissue conductivity measurements (including statistical significance) without RF ablation.
| Frequency | σ, Normal (mS cm−1) | σ, Tumour (mS cm−1) | p-value |
|---|---|---|---|
| 10 Hz | 0.75 ± 0.28 | 4.11 ± 2.56 | 0.008 |
| 100 Hz | 0.74 ± 0.24 | 4.25 ± 2.58 | 0.006 |
| 1 kHz | 0.74 ± 0.20 | 4.19 ± 2.35 | 0.004 |
| 10 kHz | 0.92 ± 0.23 | 4.30 ± 2.28 | 0.004 |
| 100 kHz | 1.79 ± 0.41 | 4.61 ± 2.02 | 0.005 |
| 460 kHz | 2.60 ± 0.62 | 5.04 ± 1.91 | 0.008 |
| 1 MHz | 2.88 ± 0.82 | 5.33 ± 1.88 | 0.008 |
In the single trial where we measured the electrical conductivity of both tumour and normal tissue before and after RF ablation, we found that both types of ablated tissue had higher conductivity than either tissue type before ablation at lower frequencies (figure 4). The conductivity of ablated normal liver tissue was relatively independent of frequency compared to measurements before ablation.
Figure 4.
Electrical conductivity before and after ablation of normal and tumour tissue samples from a single patient.
Additionally, we found no significant correlation between tumour size and measured electrical conductivity at any frequency (R2 = 0.04 to 0.10, p = 0.43 to 0.64).
4. Discussion
Previous studies have demonstrated in various tumour models that malignant tissue has higher electrical conductivity than normal tissues at radiofrequencies (Haemmerich et al 2003, Smith et al 1986, Surowiec et al 1988, Swarup et al 1991). This effect is likely due to regions of cell necrosis commonly found inside tumour tissue, since the cellular membrane of living cells acts as an electrical insulator, especially at lower frequencies. In the current study, we found a similar relationship between the electrical conductivities of malignant and normal human liver tissue (figure 3) as seen in a previous animal study involving hepatic tumours (Haemmerich et al 2003). The conductivity of tumour tissue was significantly higher at all measured frequencies, with increasing disparity in electrical conductivity at lower frequencies (figure 3, table 1). In addition, the variation in electrical conductivity was considerably higher for tumour tissue, likely due to the high variation (e.g. cell differentiation, necrosis, blood supply, etc) between tumours. We found no significant correlation between tumour size and electrical conductivity.
As mentioned previously, there are no published measurements of the electrical conductivity of human liver tumours in the literature available for comparison. However, we found that our measured values for the conductivity of normal liver tissue were lower (∼25−45%) than those presented in a previous ex vivo study (Gabriel et al 1996). This difference can likely be attributed to the difference in post-excision measurement time between the two studies (30−60 min in our study versus 24−48 h in Gabriel et al) since it has been demonstrated that the electrical conductivity of liver tissue initially decreases by as much as 33% at 500 kHz and 85% at 1 kHz in the first 2 h post excision, before gradually increasing (from 2 to 12 h) to values similar to those measured in vivo (Haemmerich et al 2002).
We further measured the change in electrical tissue conductivity after RF ablation and found both normal and tumour tissue change considerably in conductivity due to tissue coagulation after RF heating (figure 4). Since this measurement was only performed on a single sample, additional studies are required to confirm this difference between patients. A few prior studies have investigated the effect of heating on electrical tissue properties (Pop et al 2003, Chin and Sherar 2001). Pop et al measured electrical conductivity at 460 kHz while heating ex vivo kidney tissue up to ∼80 °C and separated reversible effects directly related to temperature change and irreversible effects (i.e. changes that persist after heating). Both the reversible and irreversible components were related to an increase in electrical conductivity at 460 kHz. In our studies we did not measure temperature, but rather the irreversible component of change in electrical conductivity after thermal ablation. Our results are similar to the data published by Pop et al in that we also generally observed an increase in electrical conductivity due to irreversible changes after ablation. However, there was a marked difference in this change depending on frequency as well as on tissue type (i.e. normal versus tumour). Tumour tissue electrical conductivity increased by 25% at 10 Hz, and decreased by 7% at 1 MHz (figure 4). In contrast, normal tissue electrical conductivity increased by 270% at 10 Hz, and by 10% at 1 MHz. Thus, while normal tissue was considerably less conductive than tumour before ablation—a difference particularly pronounced at frequencies below 100 kHz—after ablation the difference between tumour and normal tissue was considerably reduced (figure 4). It should be noted that we measured in the centre of the ablation zone, where very high temperatures (∼80−100 °C) are obtained. The changes are likely smaller when tissue is heated to lower temperatures as in the study by Pop et al.
The observed differences between normal and tumour tissue may allow for diagnostic detection of tumour via measurement of electrical tissue conductivity, particularly at lower frequencies (figure 3). These measurements could potentially be performed non-invasively, via either MRI-based conductivity imaging (Lee et al 2006) or electrical impedance tomography (EIT) employing skin electrodes (Brown 2003). Alternatively, invasive measurements via needle electrodes inserted into the tissue could also be performed to aid in the guidance of biopsy or ablation procedures; the measurement electrodes could even be located directly on the biopsy or ablation needles. The observed changes in conductivity during ablation (figure 4) may allow real-time monitoring of ablation procedures. In addition, the data on electrical conductivity measured in this study may be valuable in mathematical models of cancer treatment protocols that utilize tissue heating, such as RF ablation or RF hyperthermia. Furthermore, the data presented here support recent studies that have suggested that ablating hepatic tumours at lower frequencies (∼20 kHz) than currently used (∼460 kHz) could preferentially heat tumour tissue and preserve surrounding normal tissue, due to the increased difference in electrical conductivity between normal and malignant tissues at those frequencies (figure 3) (Haemmerich and Wood 2006, Schutt and Haemmerich 2008).
It should again be noted that tissue electrical conductivity changes significantly after tissue is removed from the body (Haemmerich et al 2002). Therefore, the in vivo electrical conductivity of normal and tumour liver tissue may differ from the data presented here. In addition, normal liver tissue was only measured in patients with liver cancer, so any changes in normal liver tissue due to this disease may alter the electrical conductivity in comparison to non-diseased liver tissue.
5. Conclusion
We measured the ex vivo electrical conductivity of normal human liver and metastatic tumour tissue using the four electrode method, and found that the conductivity of tumour tissue was significantly higher over the entire frequency range (10 Hz to 1 MHz), with more pronounced differences at lower frequencies. Thermal ablation results in considerable change of electrical conductivity, particularly for normal tissue. Conductivity changes due to tissue ablation are greater in magnitude at lower frequencies. These data may be useful for tumour detection based on electrical property measurement, as well as for use in mathematical models of tissue heating during RF ablation and RF hyperthermia.
Acknowledgments
This project was supported by NIH Grant Numbers R01 DK58839 and R21 CA135519.
References
- Brown BH. Electrical impedance tomography (EIT): a review. J. Med. Eng. Technol. 2003;27:97–108. doi: 10.1080/0309190021000059687. [DOI] [PubMed] [Google Scholar]
- Chin L, Sherar M. Changes in dielectric properties of ex vivo bovine liver at 915 MHz during heating. Phys. Med. Biol. 2001;46:197–211. doi: 10.1088/0031-9155/46/1/314. [DOI] [PubMed] [Google Scholar]
- Foster KR, Schwan HP. Dielectric properties of tissues and biological materials: a critical review. Crit. Rev. Biomed. Eng. 1989;17:25–104. [PubMed] [Google Scholar]
- Fricke H, Morse S. The electric capacity of tumours of the breast. J. Cancer Res. 1926;10:340–76. [Google Scholar]
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996;41:2251–69. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- Gillams AR. The use of radiofrequency in cancer. Br.J.Cancer. 2005;92:1825–9. doi: 10.1038/sj.bjc.6602582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerich D, Wood BJ. Hepatic radiofrequency ablation at low frequencies preferentially heats tumour tissue. Int. J. Hyperthermia. 2006;22:563–74. doi: 10.1080/02656730601024727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerich D, Ozkan R, Tungjitkusolmun S, Tsai JZ, Mahvi DM, Staelin ST, Webster JG. Changes in electrical resistivity of swine liver after occlusion and postmortem. Med. Biol. Eng. Comput. 2002;40:29–33. doi: 10.1007/BF02347692. [DOI] [PubMed] [Google Scholar]
- Haemmerich D, Staelin ST, Tsai JZ, Tungjitkusolmun S, Mahvi DM, Webster JG. In vivo electrical conductivity of hepatic tumours. Physiol. Meas. 2003;24:251–60. doi: 10.1088/0967-3334/24/2/302. [DOI] [PubMed] [Google Scholar]
- Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch. Surg. 1997;132:505–10. doi: 10.1001/archsurg.1997.01430290051008. (discussion 11) [DOI] [PubMed] [Google Scholar]
- Lee SH, Seo JK, Park C, Lee BI, Woo EJ, Lee SY, Kwon O, Hahn J. Conductivity image reconstruction from defective data in MREIT: numerical simulation and animal experiment. IEEE Trans. Med. Imaging. 2006;25:168–76. doi: 10.1109/TMI.2005.862150. [DOI] [PubMed] [Google Scholar]
- O'Rourke AP, Lazebnik M, Bertram JM, Converse MC, Hagness SC, Webster JG, Mahvi DM. Dielectric properties of human normal, malignant and cirrhotic liver tissue: in vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007;52:4707–19. doi: 10.1088/0031-9155/52/15/022. [DOI] [PubMed] [Google Scholar]
- Pop M, Molckovsky A, Chin L, Kolios MC, Jewett MA, Sherar MD. Changes in dielectric properties at 460 kHz of kidney and fat during heating: importance for radio-frequency thermal therapy. Phys. Med. Biol. 2003;48:2509–25. doi: 10.1088/0031-9155/48/15/317. [DOI] [PubMed] [Google Scholar]
- Rush S, Abildskov JA, McFee R. Resistivity of body tissues at low frequencies. Circ. Res. 1963;12:40–50. doi: 10.1161/01.res.12.1.40. [DOI] [PubMed] [Google Scholar]
- Schutt DJ, Haemmerich D. Tumor ablation at low frequencies for preferential heating of tumor: initial ex-vivo studies. IEEE EMBC 2008 (Vancouver) 2008 doi: 10.1109/IEMBS.2008.4649134. [DOI] [PubMed] [Google Scholar]
- Smith SR, Foster KR, Wolf GL. Dielectric properties of VX-2 carcinoma versus normal liver tissue. IEEE Trans. Biomed. Eng. 1986;33:522–4. doi: 10.1109/TBME.1986.325740. [DOI] [PubMed] [Google Scholar]
- Solazzo SA, Liu Z, Lobo SM, Ahmed M, Hines-Peralta AU, Lenkinski RE, Goldberg SN. Radiofrequency ablation: importance of background tissue electrical conductivity—an agar phantom and computer modeling study. Radiology. 2005;236:495–502. doi: 10.1148/radiol.2362040965. [DOI] [PubMed] [Google Scholar]
- Stauffer PR, Rossetto F, Prakash M, Neuman DG, Lee T. Phantom and animal tissues for modelling the electrical properties of human liver. Int. J. Hyperthermia. 2003;19:89–101. doi: 10.1080/0265673021000017064. [DOI] [PubMed] [Google Scholar]
- Steendijk P, Mur G, Van Der Velde ET, Baan J. The four-electrode resistivity technique in anisotropic media: theoretical analysis and application on myocardial tissue in vivo. IEEE Trans. Biomed Eng. 1993;40:1138–48. doi: 10.1109/10.245632. [DOI] [PubMed] [Google Scholar]
- Surowiec AJ, Stuchly SS, Barr JB, Swarup A. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans. Biomed. Eng. 1988;35:257–63. doi: 10.1109/10.1374. [DOI] [PubMed] [Google Scholar]
- Swarup A, Stuchly SS, Surowiec A. Dielectric properties of mouse MCA1 fibrosarcoma at different stages of development. Bioelectromagnetics. 1991;12:1–8. doi: 10.1002/bem.2250120102. [DOI] [PubMed] [Google Scholar]
- Tang C, You F, Cheng G, Gao D, Fu F, Yang G, Dong X. Correlation between structure and resistivity variations of the live human skull. IEEE Trans. Biomed. Eng. 2008;55:2286–92. doi: 10.1109/TBME.2008.923919. [DOI] [PubMed] [Google Scholar]
- Tsai JZ, Cao H, Tungjitkusolmun S, Woo EJ, Vorperian VR, Webster JG. Dependence of apparent resistance of four-electrode probes on insertion depth. IEEE Trans. Biomed. Eng. 2000;47:41–8. doi: 10.1109/10.817618. [DOI] [PubMed] [Google Scholar]
- Tsai JZ, Will JA, Hubbard-Van Stelle S, Cao H, Tungjitkusolmun S, Choy YB, Haemmerich D, Vorperian VR, Webster JG. Error analysis of tissue resistivity measurement. IEEE Trans. Biomed. Eng. 2002a;49:484–94. doi: 10.1109/10.995687. [DOI] [PubMed] [Google Scholar]
- Tsai JZ, Will JA, Hubbard-Van Stelle S, Cao H, Tungjitkusolmun S, Choy YB, Haemmerich D, Vorperian VR, Webster JG. In-vivo measurement of swine myocardial resistivity. IEEE Trans. Biomed. Eng. 2002b;49:472–83. doi: 10.1109/10.995686. [DOI] [PubMed] [Google Scholar]