Abstract
To evaluate the efficacy of dexamethasone (DEX) in anti-SSA/Ro–exposed fetuses newly diagnosed with congenital heart block (CHB). Previous use of DEX has been anecdotal with varying reports of therapeutic benefit. Multicenter open label study non-randomized involving 30 pregnancies treated with DEX (22-3rd degree; 6-2nd degree; 2-1st degree) and 10 untreated (9-3rd degree;1-1st degree). Initial median ventricular rates, age at diagnosis, and degree of cardiac dysfunction were similar between groups. Six deaths occurred in the DEX group. There was no reversal of 3rd degree block, with therapy or spontaneously. In fetuses treated with DEX, 1/6 with 2nd degree progressed to 3rd degree and three remained in 2nd degree (postnatally-- one paced, two progressed to 3rd); two reverted to normal sinus rhythm (NSR) (postnatally--one progressed to 2nd). DEX reversed both fetuses with 1st degree to NSR by seven days with no regression upon discontinuation. Absent DEX, the one 1st degree detected at 38 wks had NSR at birth (overall stability or improvement 4/8 DEX vs. 1/1 non-DEX). Median gestational birth age was 37 weeks vs. 38 weeks, DEX vs. non-DEX, p=0.019. Prematurity and small for gestational age were restricted to the DEX group. Pacemaker use and growth parameters at birth and one year were similar between groups. In conclusion, these data confirm the irreversibility of 3rd degree block and progression of 2nd to 3rd degree despite DEX. A potential benefit of DEX in reversing 1st or 2nd degree was supported in rare cases, but should be weighed against potential steroid side effects such as growth restriction.
Keywords: congenital heart block, dexamethasone, anti-SSA/Ro antibodies, anti-SSB/La antibodies, neonatal lupus
Introduction
Although evidence is limited and uncontrolled, maternal dexamethasone (DEX) has been utilized to treat fetal CHB in the anticipation that it may reduce the degree of block and/or prevent or ameliorate an associated cardiomyopathy (1). Accordingly, a multicenter study was initiated to prospectively evaluate the efficacy of DEX in anti-SSA/Ro exposed fetuses newly diagnosed with varying degrees of CHB. This study was part of the PR Interval and Dexamethasone Evaluation (PRIDE) study, in which the unaffected fetuses were reported separately (2). Randomization of the treatment was not feasible given the rapidity with which Institutional Review Board (IRB) approval was required. Thus, therapeutic decisions were made by the physicians and patients.
Methods
Patients entered this prospective, multicenter, observational study between December 2000 and April 2006. A total of 40 pregnant women with anti-SSA/Ro antibodies (+/− anti-SSB/La antibodies) were enrolled from 33 centers across the U.S. by participating clinicians who included rheumatologists, pediatric cardiologists, and obstetricians. The study was approved by the IRB of NYU School of Medicine. Written informed consent was obtained from all subjects who agreed to have medical records and echocardiographic tapes sent to the principal.
Although the initial study was designed to be a randomized controlled trial of DEX 4 mg per day compared to placebo, this was not feasible for two reasons: 1) it proved impossible to obtain IRB approval from the many sites within a week of identification of an affected fetus; and 2) the first several women refused randomization. Therefore, the decision to treat with DEX was made by the managing physicians.
At study entry, all patients fulfilled the following criteria: 1) presence of anti-SSA/Ro and/or anti-SSB/La antibodies documented by a commercial laboratory or in the research laboratory of JPB; and 2) presence of any degree of fetal heart block, diagnosed echocardiographically. Exclusion criteria included the presence of structural heart disease associated with heart block (e.g., heterotaxia, complete atrioventricular septal defect), or the absence of acceptable quality imaging as determined in the core laboratory.
Data were obtained on maternal health status as well as previous and current pregnancies. Mothers could be clinically asymptomatic or have a rheumatic disease. Rheumatologic diagnoses were assigned based on case report forms filled out by the participating obstetricians and cardiologists performing the echocardiograms and verified by telephone interviews and review of medical records when available. The following categories were assigned in the majority of cases: 1) asymptomatic if a patient denied any clinical symptoms which would be consistent with systemic lupus erythematosus (SLE) or Sjögren's syndrome (SS); 2) undifferentiated autoimmune disease if insufficient criteria for SLE of SS; 3) SLE if four American College of Rheumatology criteria were satisfied (3); and 4) possible, probable, or definite SS if patients had at least dry eyes and/or dry mouth plus evidence of objective criteria in addition to autoantibodies as per the European Classification (4); 5) SLE and SS.
Fetal echocardiographic protocols were established focusing on the evaluation of structural heart disease, the presence of hydrops fetalis or abnormal fluid collections, the assessment of systolic ventricular function by M-mode or two-dimensional echocardiography, the presence of valvular regurgitation by Doppler, and the qualitative tissue characteristics on two-dimensional echocardiography (5).
Heart rate and rhythm were determined by Doppler or M-mode echocardiography, allowing for the detection of 2nd or 3rd degree CHB. To detect 1st degree block, the fetal mechanical Doppler PR interval was determined during 1 to 1 conduction, according to a method previously developed and validated (5, 6, 7). Briefly, from an apical 5 chamber or left ventricular long axis view, the pulsed Doppler sample volume was placed in the left ventricular outflow tract between the anterior leaflet of the mitral valve and the aortic valve. The pulsed Doppler signal thus obtained showed simultaneously the mitral valve inflow and the aortic outflow signals. A time interval was then measured from the onset of the mitral “a” wave (mechanical atrial systole) to the beginning of aortic flow (mechanical ventricular systole). A PR interval of ≥150 msec (3 S.D. above the mean) was considered 1st degree block.
All submitted fetal and postnatal echocardiograms and electrocardiograms were over read in the core lab. Any disputed results were adjudicated by consensus opinion. Electrocardiograms and echocardiograms were requested at birth and at one year of age. Growth parameters at birth and one year were evaluated.
All sera were initially evaluated in the clinical immunology laboratory of the NYU-Hospital for Joint Diseases. Specifically, screening evaluation for the presence of antibodies to anti-SSA/Ro and/or anti-SSB/La was done by ELISA (Diamedix Corporation, Miami, FL). In this commercial test, the cutoff for normal has been established at 19 E.U. for both SSA/Ro and SSB/La. However, patients with anti-SSA/Ro titers lower than 35 E.U. were not included in the study. In addition to evaluation by commercial ELISA, sera were also evaluated by ELISA using the recombinant proteins 48kD SSB/La, 52kD SSA/Ro, and 60kD SSA/Ro which were synthesized and purified as previously described (2, 8).
Continuous variables such as gestational age and ventricular rate at diagnosis were compared between treatment groups using the Mann-Whitney test (Graph Pad, Instat3 software). Categorical variables were compared using the Fisher's Exact Test (Graph Pad, Instat3 software). P-value < 0.05 was considered statistically significant.
Results
Forty mothers identified with a CHB-fetus were enrolled. The maternal health status was as follows: 11 women were asymptomatic, 10 had SS, six had SLE, one had SLE/SS, one had discoid lupus, 10 had UAS, and one had rheumatoid arthritis. Recurrent fetal disease was common: four women (10%) had a prior offspring with CHB, and five women (13%) had a previous infant with the characteristic rash of neonatal lupus. All mothers with a previously affected child were in the DEX group.
Thirty of these mothers elected to take DEX and were thus assigned to the DEX group and 10 did not and were assigned to the non-DEX group. DEX was given at 4 mg/day with total cumulative doses ranging from 28–532 mg (mean = 257 mg +/- 185 S.D.). Five fetuses in the DEX group had been previously enrolled in the PRIDE study (3 3rd degree block, 2 1st degree block) (2).
The median titers of anti-SSA/Ro antibodies were high in both groups, 17,280 E.U. vs. 18,048 E.U., p=0.40. Twenty of the thirty mothers in the DEX group had anti-SSB/La antibodies compared to seven of the ten mothers who did not take DEX, median 218 E.U. vs. 173.5 E.U., p=0.91.
As summarized in Table 1, the gestational age at diagnosis was slightly but not significantly lower in the DEX group (median = 21.5 weeks) versus the non-DEX group (median = 23.5 weeks), p=0.09. Combining the groups and evaluating gestational age at diagnosis the median was 22 weeks, range 18-38 weeks. Ninety percent were diagnosed in the gestation age range of 19-28.5 weeks. Fifty percent were diagnosed in the gestational age range of 20-24.5 weeks.
Table 1. Clinical parameters for the DEX and non-DEX groups.
| DEX (N=30) | No DEX (N=10) | p value | |
|---|---|---|---|
| Age at Diagnosis (gestational weeks) | |||
| N | 30 | 10 | |
| Mean | 22.1 | 24.8 | |
| Median | 21.5 | 23.5 | p=0.09 |
| range | 18–30 | 20–38 | |
| Ventricular Rate (bpm) | |||
| N | 28* | 9* | |
| Mean | 69 | 62 | |
| Median | 60 | 60 | p=0.9 |
| range | 48–120 | 50–70 | |
| Cardiac manifestations other than purely conduction system disease | |||
| N | 30 | 10 | |
| 7 (23%)** | 2 (20%)*** | p=1.0 | |
| Age at birth (gestational weeks) | |||
| N | 26**** | 10 | |
| Mean | 36.3 | 38.2 | |
| Median | 37 | 38 | p= 0.019 |
| Range | 28.5–40 | 36.5–40 | |
| Pacemaker | |||
| N**** | 26 | 10 | |
| 11 (42%) | 5 (50%) | p=0.72 | |
Excludes first-degree block (two for DEX, one for no-DEX).
3 pericardial effusions; 1 cardiomegaly; 3 atrioventricular valve regurgitation.
1 scalp edema; 1 atrioventricular valve regurgitation.
Excludes four deaths.
Median fetal ventricular rates at diagnosis of 2nd or 3rd degree blocks were equivalent between the groups (60 bpm DEX vs. 60 bpm non DEX, p=0.9). It was not likely that the decision to treat with dexamethasone was based on heart rate at the time of diagnosis since median heart rates were not statistically different between the fetuses with third degree in either group, (p = 0.40). As expected, median heart rates were significantly faster in the fetuses with 2nd degree compared to third degree in the DEX group. Cardiac manifestations other than purely conduction system disease were defined as any abnormal fluid collection (effusions, ascites, hydrops), poor myocardial function which may be manifest as tricuspid regurgitation, poor systolic function of the myocardium, or cardiomegaly out of proportion to bradycardia, as well as noncardiac effects or steroid (drug) side effects, such as IUGR, oligohydramnios, or fetal distress, are excluded. These findings suggestive of an associated carditis, were common, but no different between groups (p=1.0). There were three pericardial effusions, one cardiomegaly, and three AV valve regurgitation cases in the DEX group (7/30 = 23%) compared to one scalp edema and one AV regurgitation in the non-DEX group (2/10 = 20%, p=1.0). In the DEX group, there were 16 females and 13 males (one demise of unknown sex). In the non-DEX group, there were nine females and one male (p=0.064).
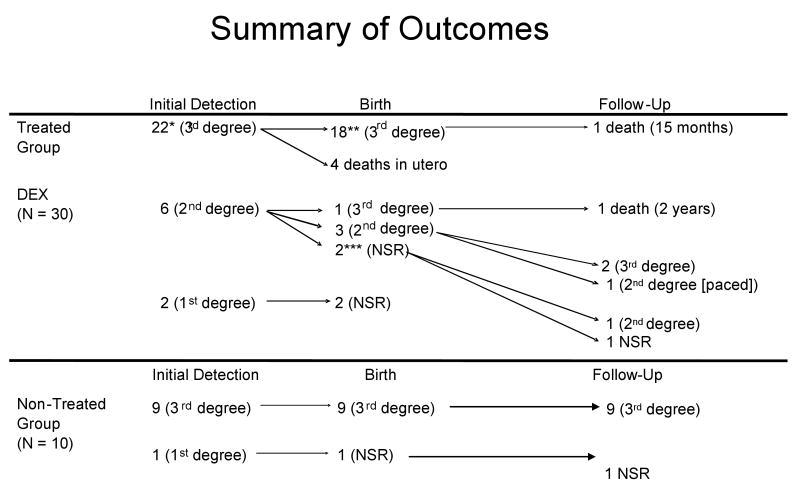
As summarized in Figure 1, 22 fetuses in the DEX group presented in utero with 3rd degree block, none recovered any conduction, and five of these patients died. There were six fetuses who presented with 2nd degree block, one of whom progressed to 3rd degree at birth and subsequently died. Three of these fetuses stabilized at 2nd degree block at birth, but two progressed to complete block and the third required a pacemaker although remaining in 2nd degree block. Two patients reverted to normal sinus rhythm (NSR) in utero, but one of these progressed postnatally back to 2nd degree block. The other remains in NSR at two years. Two fetuses presented with 1st degree block (one had a previous child with CHB and the other not) and in each, the PR interval decreased to a normal range within seven days of DEX and remained in NSR up to two years of follow up (2).
Figure 1.
Summary of outcomes from detection to birth through follow up, separated into treated and untreated groups. NSR = normal sinus rhythm.
*In one non-fatal case, maternal terbutaline was administered.
**In two non-fatal cases, postnatal steroids were administered.
***In one non-fatal case, postnatal steroids were administered, however, there was progression to 2nd degree.
There were six (20%) deaths in this group of 30 fetuses exposed to DEX. Four deaths occurred in utero. One presented at 20.5 weeks with a dilated right ventricle (RV) and endocardial fibroelastosis. The second presented at 20 weeks with hydrops fetalis. A third had diminished amniotic fluid at 27 weeks. Finally, a fetus at 24 weeks had hydrops. Autopsies were done in three cases and revealed necrosis, fibrosis, and calcification in the conduction system. The two deaths before 25 weeks revealed an extensive mononuclear cell infiltrate. Two of the deaths occurred after birth. One was born with 3rd degree block but was not paced, required a heart transplant by one year of age and died at 15 months from transplant rejection (9). Another death occurred in a child diagnosed with 2nd degree block in utero who progressed to 3rd degree at birth despite DEX. Although the heart rate was 54 bpm, no pacemaker was placed and the child died at home at two years of age during a febrile viral illness with seizure.
As summarized in Figure 1, nine fetuses presented with 3rd degree block. One fetus was noted to have an isolated prolonged PR interval during maternal labor at term, with a birth PR interval of 154 msec, and a completely normal electrocardiogram by one month of age. There were no deaths in this group.
As shown in Table 1, the median gestational age at birth was significantly less in the DEX group (37 weeks) compared to the non-DEX group (38 weeks), p=0.019. Prematurity was present in 12 (40%) of the DEX group but in one of the neonates in the non-DEX group. Small size for gestational age only occurred in the DEX group. Pacemaker use occured in 43% of the cohort by two years of age, with no significant differences observed between the groups, DEX (11/26 = 42%) and non-DEX groups (5/10 = 50%), p=0.72. Similar results were obtained when outcomes were compared between treatment groups in only those who presented in utero with 3rd degree block. Three fetuses in the DEX group were also given corticosteroid therapy postnatally while none in the non-DEX group received postnatal DEX. Failure to thrive and congestive heart failure were common.
Growth data are presented in Table 2, with parameters presented by treatment group and cumulative exposure to DEX. Weight, height, and head circumference were derived exclusively from prematurity-specific growth curves. Several DEX-treated newborns were premature and/or small for gestational age (40%) with some persisting in failing to thrive at one year of age.
Table 2. Growth and safety data for DEX and NON-DEX groups.
| Conduction Abnormalities |
Birth | One Year Survival | DEX | |||||
|---|---|---|---|---|---|---|---|---|
| Weight percentile |
Length percentile |
Head Circumference percentile |
Weight percentile |
Length percentile |
Head Circumference percentile |
(mg) | ||
| 3rd degree (N=22) |
mean | 30 | 36 | 37 | 20.2 | 33 | 50 | |
| median | 25 | 25 | 25 | 10 | 25 | 50 | ||
| range | 3-75 | 3-90 | 3-90 | 3-75 | 3-90 | 3-97 | 28-476 | |
| 2nd degree (N=6) |
mean | 36 | 56 | 39 | 21 | 44 | 38.3 | |
| median | 38 | 75 | 25 | 3 | 50 | 38 | ||
| range | 3-75 | 15-90 | 3-75 | 3-75 | 25-50 | 3-75 | 84-448 | |
| 1st degree (N=2) |
mean | 43 | 58 | 25 | 86 | 97 | 74 | |
| median | 43 | 58 | 25 | 86 | 97 | 74 | ||
| range | 10-75 | 25-90 | 25 | 75-97 | 97-97 | 50-97 | 140-532 | |
| No DEX | ||||||||
| 3rd degree (N=9) |
mean | 25 | 47 | 31 | 24 | 57 | 58 | |
| median | 25 | 50 | 25 | 25 | 75 | 50 | ||
| range | 5-50 | 10-90 | 10-90 | 10-50 | 10-75 | 50-75 | N/A | |
Discussion
This component of the PRIDE study (2) comprises prospective observational data obtained with the use of a structured protocol including serological confirmation and echocardiographic oversight from 40 fetuses diagnosed with CHB. Confirming and extending published studies, once fetal 3rd degree block was detected, it was irreversible, regardless of treatment with DEX (1). Moreover, 2nd degree block progressed to 3rd degree block despite DEX as previously reported (10). This study also reaffirms prior findings that anti-SSA/Ro–associated CHB is usually diagnosed before 27 weeks of gestation, has a high morbidity (43% paced by two years of age) and a high mortality (20% in the DEX group) (11). That 50% of cases are diagnosed between 20 and 24.5% weeks of gestation should guide clinicians in the timing of echocardiograms. Thus, echocardiographic surveillance in advance of 20 weeks might be important to identify potentially treatable abnormalities.
The rationale for DEX as a potential treatment for CHB is based on the presumed contribution of inflammation to the pathologic cascade eventuating in fibrosis of the conducting system. Specifically, autopsy studies of fetuses dying with CHB have demonstrated a macrophage infiltrate (12) and in vitro studies demonstrate macrophage release of TNFα when cocultured with apoptotic human fetal cardiocytes bound by anti-SSA/Ro and SSB/La antibodies (13). Thus, treatment with corticosteroids is reasonable but only fluorinated corticosteroids are available in active form in the fetal circulation when given to the mother (14). The potential efficacy of maternal DEX in cases of hydrops or incomplete block has been reported (1). Based on the one year survival of 90% of 22 DEX treated cases of 3rd degree block compared to a 46% survival in untreated cases in a single center, Jaeggi et al. concluded that DEX was an effective strategy (15). This same group also used beta adrenergic agents. In contrast, based on a single anti-SSA/Ro pregnancy in which DEX did not prevent CHB, Breur et al. performed a meta-analysis of 15 previous studies comprising 93 fetal cases of CHB. The authors concluded that maternal DEX was neither safe nor effective (16).
Based on a relatively limited number of cases, there may be a potential benefit for the gestational use of fluorinated corticosteroids in reversing or stabilizing 1st or 2nd degree CHB, but these events are rare, and decisions to use DEX must be tempered by the potential adverse effects of prenatal steroids. Prenatal exposure to steroids has raised concerns of growth restriction, including head growth, and long-term neurodevelopmental impairments, as well as oligohydramnios. Leung et al. (17) concluded, in the circumstance of threatened premature labor, in which antenatal corticosteroids are used to promote fetal lung maturation, that even brief fetal exposure may not be justified to be repeated more than once. However, in two large randomized control trials there were no differences in birth weight or head circumference between repeat and single course antenatal corticosteroids (both only analyzed intramuscular betamethasone) (18). In a small and underpowered study, Brucato et al. (19) reported no neurodevelopmental problems in anti-SSA/Ro-SSB/La–exposed children whose mothers had taken high dose DEX in a context similar to the study herein.
Prematurity and growth restriction are common findings in fetuses and infants affected by CHB, as seen in this cohort. The role of DEX–induced adverse effects versus the effects of this serious disease itself cannot be determined in this unblinded, non-randomized study in which it is likely that the sickest fetuses were those chosen to receive the drug. Similar to our experience, Skog et al. (20) reported on the growth of a series of 32 pregnancies in 30 anti-SSA/Ro 52–positive mothers in which seven fetuses had 2nd or 3rd degree block, eight developed 1st degree block, and 17 had normal cardiac conduction. Three of six surviving fetuses with >1st degree block received fluorinated steroids. Fetuses with 2nd or 3rd degree block were smaller by −0.98 +/–0.77 S.D. (Z scores) in weight at birth and did not show any catch-up during infancy. In contrast, fetuses with 1st degree block or normal conduction had a weight reduction of − 0.51 +/–1.01 S.D. (Z scores) with a catch-up during early infancy.
Several limitations are acknowledged. The cohort was relatively small albeit the largest prospectively evaluated within a six year time span and thus was not confounded by major changes in obstetric care. Although initially designed as a randomized and blinded study, this was not feasible. The logistics of patient identification throughout the country and obtaining local IRB approval within a few days of enrollment proved to be an insurmountable challenge. Mothers and their physicians were often reluctant to agree to randomization to placebo. This was because they were either convinced that DEX was the standard of care and should be provided, or that the risks of DEX were too high in the absence of efficacy data. The multicenter nature of the study led to individual local practitioners often having differing approaches to diagnosis and treatment of the disease, making uniformity of data difficult and opening the strong possibility of bias in terms of offering DEX to the sickest fetuses. Finally, this study was not designed to assess the safety of DEX; therefore any observations should be considered anecdotal.
Acknowledgments
We thank the following investigators for contributing to the PRIDE study: Matthew Allsede, Ahmed Baschat, Tara Becker, TM Biancaniello, Morton Borg, Patrick Callahan, Eric J. Carlson, Edward Chien, Kathryn Collins, Bettina F. Cuneo, William Cusick, Curt DeGroff, Mary Donofrio, Robin Doroshow, James FX Egan, Mohammed Elkousy, Llyod Feit, Carlen Gomez, Nina Gotteiner, Julie Glickstein, David Hirsch, Eby Michael, Michelle Miller, Alan Peaceman, Howard Rosenfeld, Frederick S. Sherman, Russell Shiff, Ewing Stanford, John R. Stanley, Janet Stein, Amy Sullivane, Kern Sylvestre, Mary VanDerVelde, Sharon Weil-Chalker, Luciana Young. We also thank the NIH Advisory Board (DSMB: Kathryn Reed, Ralph Kauffman, Daniel Skupski) for valuable guidance. We appreciate the help of Amy Lawless in the preparation of the manuscript.
This work was funded by NIH-NIAMS Grant No. RO1 AR42455-01 (Maternal Autoantibodies: Pathogenesis of Neonatal Lupus) to Dr. Buyon, NIH, Contract NO1-AR-4-2220 (Research Registry for Neonatal Lupus) to Dr. Buyon, NIH-NIAMS grant 1-R01-AR46265 (the PRIDE study), and a generous gift from the Lee family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody associated congenital heart block. Arthritis Rheum. 1999;42:2335–2345. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CKL, Glickstein JS, Buyon JP. Utility of cardiac monitoring in fetuses at risk for congenital heart block: The PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's Syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glickstein JS, Buyon JP, Friedman D. The fetal PR interval: pulsed Doppler echocardiographic assessment. Am J Cardiol. 2000;86:236–239. doi: 10.1016/s0002-9149(00)00867-5. [DOI] [PubMed] [Google Scholar]
- 6.Glickstein J, Buyon J, Kim M, Friedman D, PRIDE investigators The fetal Doppler mechanical PR interval: A validation study. Fetal Diagn Ther. 2004;19:31–34. doi: 10.1159/000074256. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal D, Friedman DM, Buyon J, Dubin A. Validation of the Doppler PR interval in the fetus. J Am Soc Echocardiogr. 2002;15:1029–1030. doi: 10.1067/mje.2002.121438. [DOI] [PubMed] [Google Scholar]
- 8.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, Chan EK. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 9.Chan JB, Levi DS, Lai CK, Alejos JC, Fishbein MC. Cellular rejection of conduction system after orthotopic heart transplantation for congenital atrioventricular block. J Heart Lung Transplant. 2006;25(11):1371–5. doi: 10.1016/j.healun.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Askanase AD, Friedman DM, Copel J, Dische MR, Dubin A, Starc TJ, Katholi MC, Buyon JP. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-Ro/La antibodies. Lupus. 2002:145–151. doi: 10.1191/0961203302lu173oa. [DOI] [PubMed] [Google Scholar]
- 11.Buyon JP, Clancy RM. Neonatal lupus. Wallace DJ, Hahn BH, editors. Dubois' Lupus Erythematosus. (7th) 2006:1058–1080. [Google Scholar]
- 12.Clancy RM, Kapur RP, Molad RP, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 13.Miranda-Carús ME, Dinu Askanase A, Clancy RM, DiDonato F, Chou TM, Libera MR, Chan EKL, Buyon JP. Anti-SSA/Ro and -SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of α by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 14.Blanford AT, Pearson Murphy BE. In vitro metabolism of prednisolone, dexamethasone, betamethasone, and cortisol by the human placenta. Am J Obstet Gynecol. 1977;127:264–267. doi: 10.1016/0002-9378(77)90466-5. [DOI] [PubMed] [Google Scholar]
- 15.Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 16.Breur JM, Visser GH, Kruize AA, Stoutenbeek P, Meijboom EJ. Treatment of fetal heart block with maternal steroid therapy: case report and review of the literature. Ultrasound Obstet Gynecol. 2004;24:467–472. doi: 10.1002/uog.1713. [DOI] [PubMed] [Google Scholar]
- 17.Leung TN, Lam PM, Ng PC, Lau TK. Repeated courses of antenatal corticosteroids: is it justified? Acta Obstet Gynecol Scand. 2003;82:589–596. doi: 10.1034/j.1600-0412.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1248–50. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 19.Brucato A, Astori MG, Cimaz R, Villa P, Li Destri M, Chimini L, Vaccari R, Muscarà M, Motta M, Tincani A, Neri F, Martinelli S. Normal neuropsychological development in children with congenital complete heart block who may or may not be exposed to high-dose dexamethasone in utero. Ann Rheum Dis. 2006;65:1422–1426. doi: 10.1136/ard.2005.049866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skog A, Wahren-Herlenius M, Sundström B, Bremme K, Sonesson SE. Outcome and growth of infants fetally exposed to heart block–associated maternal anti-Ro 52/SSA autoantibodies. Pediatrics. 2008:e803–809. doi: 10.1542/peds.2007-1659. [DOI] [PubMed] [Google Scholar]