Abstract
We have investigated the role of foxd3 activity in conjunction with signaling by the kit tyrosine kinase receptor in zebrafish black pigment cell (melanophore) development. As loss-of-function of these molecules individually has distinct effects on melanophore number, we have examined the phenotype of double mutants. Individuals with a null mutation in kit have fewer melanophores than wildtype, with cells lost through death. When kit mutants are injected with foxd3 antisense morpholino oligonucleotides or crossed with a foxd3 zebrafish mutant, they have more melanophores than their uninjected or foxd3+ counterparts. Examination of foxd3 loss-of-function in two additional kit mutants that differentially alter kit-dependent migration and survival indicates a change in melanophore number in survival mutants only. Consistently, TUNEL analysis confirms a partial rescue of melanophores from cell death. Ectopic expression of foxd3 indicates that foxd3 promotes early melanophore death only when kit is inactive. Taken together, these data suggest a kit-dependent role for foxd3 in the regulation of melanophore survival.
Keywords: melanophore, survival, morphology, kit signaling, foxd3, neural crest, zebrafish
INTRODUCTION
Early during vertebrate development, cells localized to the lateral neural plate boundary respond to extrinsic signals inducing them to become a transient, migratory population of cells, the neural crest. Upon their induction, neural crest cells respond to extrinsic and intrinsic cues to promote their terminal differentiation into several distinct cells types, including pigment cells, glia, neurons of the dorsal root ganglia and cartilage of the facial bony structures (for a recent review see Sauka-Spengler and Bronner-Fraser, 2006). Neural crest and resulting derivatives have not only become an intriguing model for understanding mechanisms controlling cell fate decisions and migration, but also for examining the events important for lineage survival once fate decisions are made.
Neural crest derived melanocytes (melanophores in fish and frogs) are providing extensive insight into mechanisms promoting lineage survival (Cooper and Raible, 2008). Several genes, including Kit tyrosine kinase receptor (Kit), Bcl-2, MAPK, ADAMTS20 and Mitf have been implicated in melanocyte survival (Geissler et al., 1988; Hodgkinson et al., 1993; Opdecamp et al., 1997; Dorsky et al., 1998; Lister et al., 1999; Parichy et al., 1999; Takeda et al., 2000; McGill et al., 2002; Widlund et al., 2002; Dunn et al., 2005; Silver et al., 2008). The latter four genes function downstream of kit signaling (Geissler et al., 1988; Hodgkinson et al., 1993; Opdecamp et al., 1997; Dorsky et al., 1998; Lister et al., 1999; Parichy et al., 1999; Takeda et al., 2000; McGill et al., 2002; Widlund et al., 2002; Dunn et al., 2005; Silver et al., 2008). Originally identified as a key factor in mouse melanogenesis, hematopoeisis and primordial germ cell development (Geissler et al., 1988; Copeland et al., 1990; Bernex et al., 1996), Kit signaling is also essential for embryonic melanophore survival in zebrafish (Parichy et al., 1999). kitb5 mutant zebrafish have a reduction in kita activity. A second kit orthologue, kitb, is not expressed in pigment cells (Mellgren and Johnson, 2005); to avoid confusion with previous studies, hereafter we will refer to the kita gene as kit. In kit mutants, melanophores are initially specified and differentiate, but are reduced in number as compared to wildtype zebrafish. Furthermore, the majority of melanophores fail to leave sites of origin and undergo apoptosis, most evident between 5 and 6 days post-fertilization (dpf;Parichy et al., 1999). Zebrafish mutagenesis screens have allowed the identification of novel melanophore survival genes, including touchtone and TRPM7, which function in distinct pathways to regulate maintenance of the melanophore lineage (Cornell et al., 2004; McNeill et al., 2007). Additionally, loss of kit function in zebrafish is not lethal (this study; Parichy et al., 1999). Thus, zebrafish melanophores are a resource for discovering novel survival genes as well as genes functioning to modulate kit signaling.
Both loss- and gain-of-function studies in chick and zebrafish have uncovered functions for foxd3 in black pigment cell development. Specifically, chick melanocytes are strongly affected by foxd3 loss-of-function, as this promotes an increase in the percentage of melanocytes developing in neural crest explant culture. Overexpression of foxd3 leads to inhibition of chick melanogenesis (Kos et al., 2001). Intriguingly, loss of zebrafish foxd3 activity leads to distinct effects on the onset of melanogenesis. Delays in mitfa expression and melanoblast migration are observed in the foxd3 zebrafish mutants, mother superior (mos188) and foxd3zdf10, yet the number of melanophores is similar to wildtype by day 3 and 5, respectively (Montero-Balaguer et al., 2006; Stewart et al., 2006). Examination of morpholino oligonucleotide (MO) mediated loss-of-function in zebrafish shows more subtle changes in expression patterns of melanophore markers (Lister et al., 2006; Ignatius et al., 2008). These seemingly conflicting results may suggest subtle differences in the foxd3 protein level, activity or temporal requirements necessary to mediate Foxd3 function. Consistently, other forkhead transcription factor family members, such as FoxO1 and FoxO3, show a high degree of post-translational modification in response to kit signaling, critical to their intracellular localization and subsequent effects on regulating cell survival (Engstrom et al., 2003). Whether foxd3 is similarly regulated or regulated via alternative genetic mechanisms remains poorly understood.
As loss of foxd3 or kit activity have opposing effects on zebrafish melanophore location and numbers, we hypothesized that foxd3 may modulate kit function. To test the relationship between foxd3 and kit signaling in melanophore development, we investigated the effect of foxd3 loss-of-function on melanophores in multiple zebrafish kit mutants. Our evidence suggests foxd3 loss-of-function modifies melanophore morphology and numbers when the survival function of the kit receptor is defective. Consistently, TUNEL analysis indicates a rescue of kit negative melanophores when foxd3 activity is reduced. Last, melanophore specification from neural crest and cell differentiation are largely unaffected by foxd3 MO injection. Our data suggest foxd3 and kit signaling oppose each other in the survival of embryonic melanophores and may cooperate to establish proper melanophore patterning and number.
RESULTS
kitw34 has defects in kit dependent melanophore migration and survival
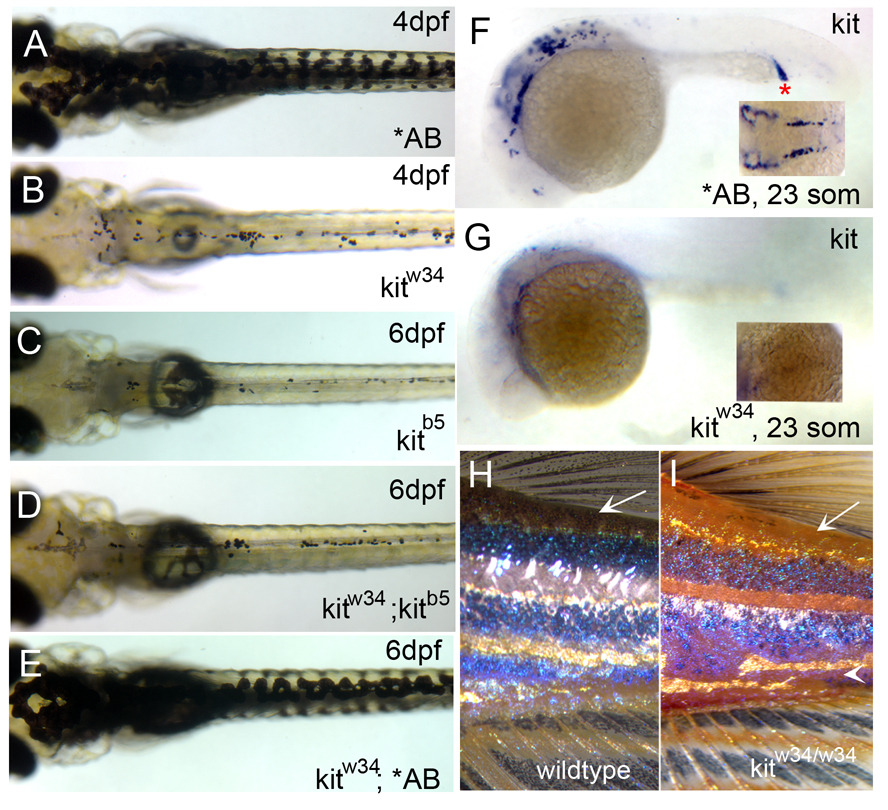
Zebrafish melanophores are sensitive to kit loss-of-function as characterized with the kit null mutant, kitb5 (previously known as sparse; Johnson et al., 1995; Parichy et al., 1999). kitb5 mutants display defects in melanophore migration and survival, and also show fewer melanophores than wildtype zebrafish. We have isolated a mutant, kitw34 that has defects in melanophore migration and survival, similar to kitb5. Homozygous kitw34 larvae show substantial cell loss by 4 dpf, and migration away from sites of origin to peripheral locations in the anterior region of the head, lateral stripe and ventral stripe are all defective (Fig. 1A and 1B). Analysis of 6dpf progeny resulting from a kitw34/kitb5 cross indicate similar defects in melanophore migration, morphology and survival as observed in each homozygous allele alone (Fig. 1C–1E). Lack of kit message at 23 somite stage (Fig. 1F and 1G) and exclusion of kit exon 10 in kitw34 genomic DNA (data not shown) further support the notion that kitw34 harbors a null mutation in the kit tyrosine kinase receptor gene.
Fig. 1.
Characterization of kitw34 mutant larvae. (A) AB zebrafish at 4dpf, illustrating wildtype melanophore morphology and patterning. (B) kitw34 zebrafish at 4dpf, have small, rounded melanophores. Lateral and ventral stripe melanophores are largely missing by this stage. (C–E) kitw34/kitb5 complementation analysis. (C) In kitb5, 6dpf zebrafish, the majority of melanophores have died. (D) While progeny from a homozygous kitb5 and kitw34 cross show defects in melanophore morphology and survival, progeny from a kitw34 and AB cross are rescued and show wildtype melanophore patterning (E). (F, G) In situ analysis for kit mRNA during early melanogenesis. (F) Wildtype zebrafish express kit in the head, anterior trunk and intermediate cell mass (red asterisk) at 23 somites. (G) kitw34 embryos lack kit expression. H, I) kitw34 adults have a reduction in melanophores. (H) Wildtype pigment pattern showing three distinct dark stripes on the flank near the dorsal and caudal fins. (I) kitw34 adult showing reduced overall pigmentation, especially in dorsal areas (arrow) and throughout the dark stripes. The darks stripes are disorganized and the ventral stripe is lost with kit loss-of-function (arrowhead).
We also examined the effects of kitw34 loss-of-function on adult pigment pattern. Similar to homozygous kitb5 mutants (Parichy et al., 1999), we see a reduction in overall pigmentation in kitw34 adults (Fig. 1H and 1I). In fish with wildtype pigmentation, dark stripes consist of melanophores, intermingled with shiny silver iridophores and occasional xanthophores (Parichy and Turner, 2003). The lighter, interstripes consist of iridophores and yellow xanthophores, with melanophores diffusely dispersed throughout the scales. In kitw34 mutant adults, the dark stripes are present, but lighter and less organized than those found in wildtype adults (Fig. 1I, arrowhead). In addition, dorsally positioned melanophores are largely missing in the skin, scales and dorsal fin (Fig.1H and I, arrow). Thus, similar to kitb5 mutants, kitw34 mutant zebrafish illustrate the requirement for kit signaling in melanophore maintenance and adult pigment pattern.
Inhibition of foxd3 activity partially rescues melanogenesis in kit null mutants
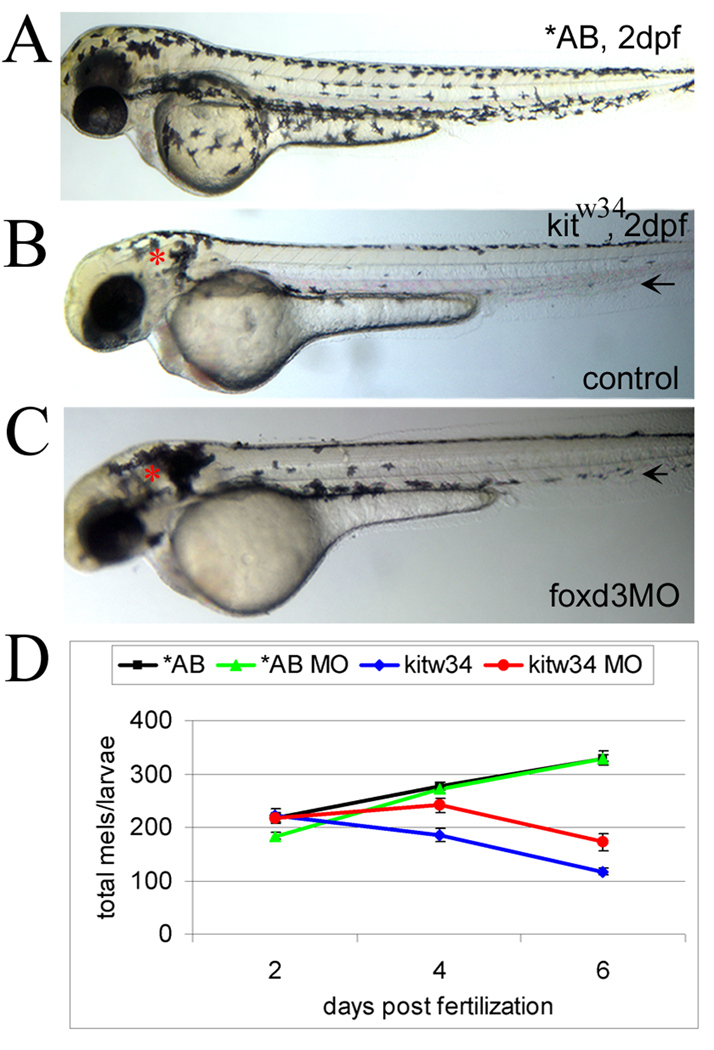
Little is known regarding the factors involved in generating specificity with kit signaling. Using kitw34 mutants, we looked for genetic modulators of kit function, with the goal of identifying molecules involved in transducing or blocking kit signals. Studies in chick and zebrafish suggest a role for forkhead transcription factor foxd3 in inhibition of melanogenesis (Kos et al., 2001; Montero-Balaguer et al., 2006; Stewart et al., 2006). Furthermore, additional evidence indicates changes in melanophore distribution in 5 dpf foxd3 null zebrafish mutants (foxd3zdf10; Stewart et al., 2006), suggesting a role for foxd3 in embryonic melanophore migration, a function regulated by kit signaling. To test the interaction between foxd3 and kit during melanogenesis, we reduced foxd3 protein levels using translation blocking foxd3 MO in kitw34 embryos. Following injection of foxd3 MO at 1–2 cell stage, uninjected (control) and injected (foxd3MO) embryos were grown to 2dpf and images were taken. At 2dpf, kitw34 mutant melanophores are largely absent from the lateral and ventral stripes (compare AB (wildtype) to kitw34 in Fig. 2A and 2B). In kitw34/foxd3 morphants (Fig. 2C), more melanophores are present in the ventral stripe at 2dpf, as compared to controls (Fig. 2B, black arrow). Additional areas (near the otic vesicle, for example, red asterisks) appear to have more melanophores than uninjected controls.
Fig. 2.
foxd3 loss-of-function reduces loss of melanophores in kitw34 zebrafish (A) In 2 dpf wildtype zebrafish (AB), melanophores have migrated extensively, anteriorly to the head and ventrally over the yolk. (B) In 2 dpf kitw34 zebrafish, melanophores are specified, but largely fail to migrate from sites of origin: the dorsal trunk and area caudal to the otic vesicle (red asterisk). (C) kitw34 zebrafish injected with foxd3 MO have more melanophores, especially apparent near the otic vesicle (foxd3MO, red asterisk). Both the ventral and lateral stripes show an increase in melanophores (black arrows). (D) Line graph showing counts of total melanophores in 2, 4 and 6dpf AB or kitw34 larvae, either uninjected (control) or injected with foxd3 MO. A significant change in the number of melanophores is observed in kitw34 mutants after injection of foxd3 MO (red) as compared to kitw34 uninjected controls (blue; p<0.0001 by 2-way ANOVA; 7–11 fish per time point), whereas AB foxd3 morphants (green) show no significant difference as compared to uninjected AB controls (black; p=0.49 by 2-way ANOVA; 9–13 fish per time point).
To examine the temporal requirement for kit and foxd3 in regulating melanophore numbers, control and foxd3 MO injected AB (wildtype) and kitw34 larvae were collected at 2, 4 and 6 dpf and total melanophores were counted (Fig. 2D). At 2 dpf, the number of melanophores is similar under all conditions, despite the differences in distribution noted above. While melanophores continue to be added to wildtype animals over the next four days, melanophores are lost in kitw34mutants, consistent with previous reports (Parichy et al., 1999). This loss is significantly reduced in kitw34 mutants injected with foxd3 MO as compared to uninjected controls. Uninjected and MO injected wildtype larvae have similar numbers of melanophores throughout this time course. Although foxd3 MO injection strongly reduces foxd3 protein production (Lister et al., 2006), it is conceivable that kit foxd3 morphants retain some foxd3 activity or MO efficacy is reduced at the times phenotypes are observed. To better characterize the importance of Foxd3 protein levels on melanophore development, we compared cell numbers in larvae mutant for both kit and foxd3. Consistent with our MO experiments, kitw34/w34;foxd3zdf10/zdf10 zebrafish show significant rescue in total melanophore numbers at 4dpf, as compared to kitw34/w34 and kitw34/w34; foxd3zdf10/+ siblings (Table I). This data further supports a role for foxd3 in the regulation of melanophore numbers in zebrafish.
TABLE 1.
Effect of kitw34 and foxd3zdf10 loss-of-function on total melanophores
| GENOTYPE | AVERAGE # TOTAL MELANOPHORES* |
|---|---|
| kitw34/+ | 307.8 ± 42.6 (6) |
| kitw34/w34 | 118.7 ± 41.8 (7) |
| kitw34/w34; foxd3zdf10/+ | 122.2 ± 47.6 (10) |
| kitw34/w34; foxd3zdf10/zdf10 | 176.0 ± 41.9 (10) |
Larvae were fixed at 4dpf for counting.
Melanophore averages for each genotype ± standard deviation are shown. The number of fish counted is in parentheses. There is a significant difference in the number of melanophores between kit/foxd3 double mutants and kit single mutants (p,0.05 by Tukey’s posthoc test after 1-way ANOVA).
foxd3 loss-of-function rescues melanophore localization in kitw34 mutants
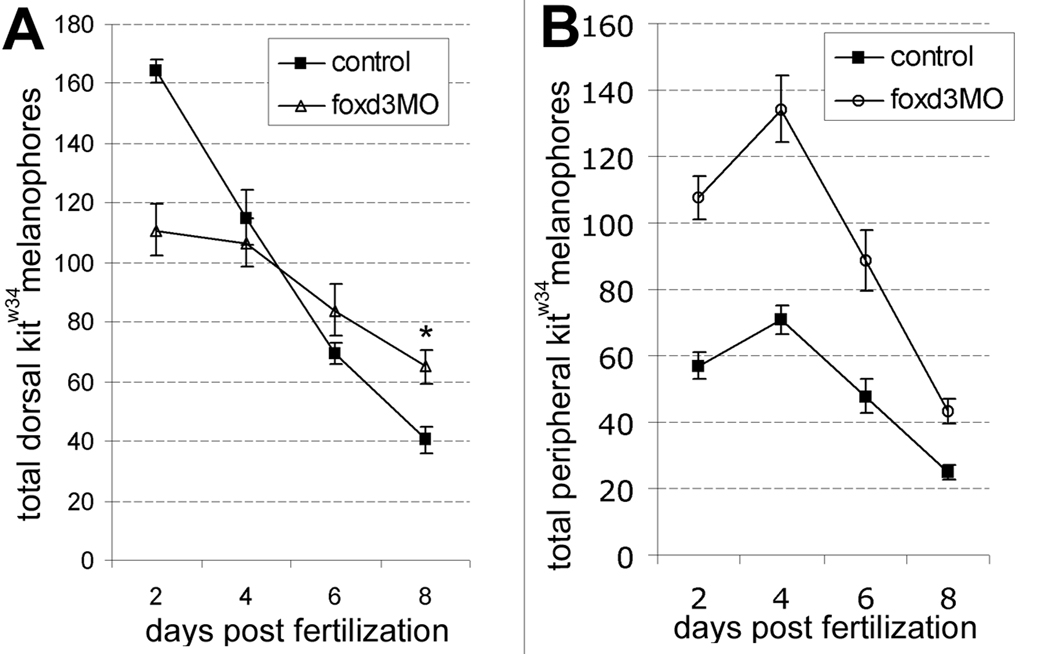
Along with changes in melanophore numbers, we examined melanophore localization in kitw34 mutants injected with foxd3 MO. During quantification of total melanophores, cell location at 2, 4, 6 and 8dpf was noted for each condition: melanophores localized to the lateral and ventral stripes were designated as migrated or peripheral melanophores, and those localized to the head and dorsal trunk areas were designated unmigrated or dorsal melanophores. Dorsal trunk melanophores, initially present in higher numbers in controls, persist longer in animals injected with foxd3 MO, resulting in a significant increase at 8dpf (Fig. 3A). Peripherally localized melanophores are significantly increased with foxd3 loss-of-function as indicated by quantification (Fig. 3B). Taken together, these data suggest that along with influencing cell number, foxd3 activity inhibits embryonic melanophore migration in kit zebrafish mutants.
Fig. 3.
foxd3 loss-of-function alters the number of melanophores localized to the dorsal stripe and periphery. A) In the dorsal stripe, kitw34 control embryos (solid squares) lose melanophores quickly, showing a dramatic reduction by 8 dpf. Conversely, melanophores persist in kitw34 foxd3 morphants at higher numbers (*, p<0.05 by Bonferroni posttest at 8 dpf). Note that dorsal melanophores are initially present at lower numbers in kitw34 animals injected with foxd3 MO than in controls, but are present at higher numbers at 2 dpf in the periphery. B) The few melanophores detected in kitw34 lateral and ventral stripes (solid squares) at 2 dpf initially increase, and then steadily decline with each subsequent time point. Peripheral melanophores are detected at significantly higher numbers in 2 dpf in kitw34 foxd3 morphants as compared to uninjected kitw34 controls at all time periods (p<0.05 a by Bonferroni posttest).
Melanophore specification is unaltered in kitw34/foxd3 morphants
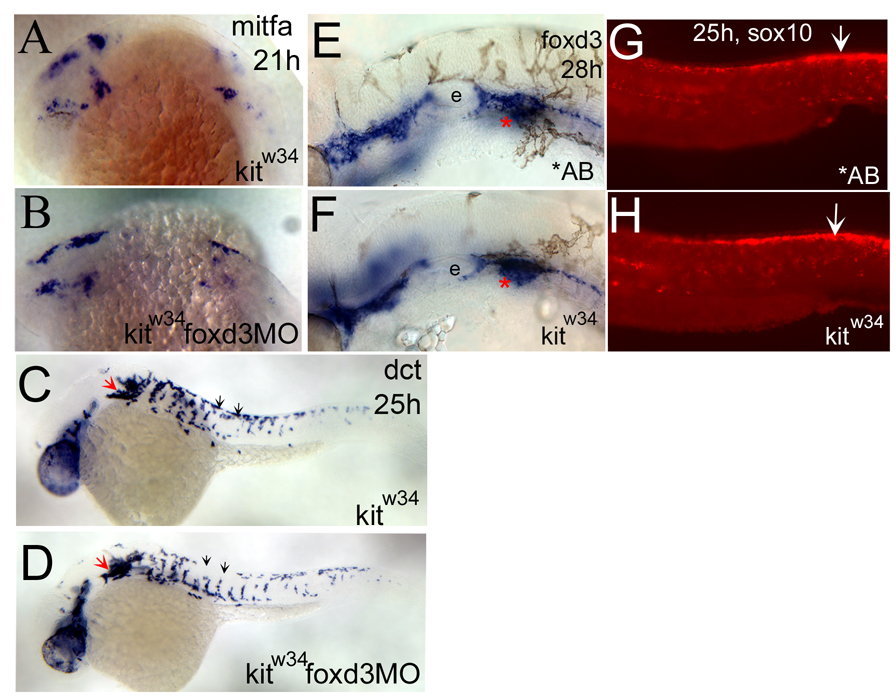
To test whether foxd3 interacts with kit signaling to regulate melanophore specification from neural crest cells, kitw34 embryos were injected with foxd3 MO and examined by in situ hybridization. Melanophore precursors were visualized using RNA antisense probes for the mitfa and dopachrome tautomerase (dct) genes, which are markers for melanophore specification and differentiation, respectively (Lister et al., 1999; Kelsh et al., 2000). At 21 hours post fertilization (hpf), mitfa is detected in several distinct patches caudal to the eye and in the anterior trunk. Comparison of mitfa expression in uninjected and MO injected kitw34 embryos indicates similar levels of transcript at this stage (Figure 4A and 4B). Later at 25 hpf, some differentiating melanoblasts marked by dct expression are migrating ventrally from the dorsal trunk (black arrows, Fig 4C), while some cells remain in clumps caudal to the otic vesicle (red arrow, Fig 4C). Although a slight decrease in dorsal stripe melanoblasts is apparent with foxd3 loss-of-function (compare Fig. 4C and 4D, black arrows), kitw34 embryos injected with foxd3 MO show similar melanophore characteristics and dct expression levels to uninjected controls at 25 hpf. Thus, foxd3 MO injection does not appear to affect melanoblast specification or differentiation from neural crest in kitw34 mutants, consistent with previous results in wildtype embryos (Lister et al., 2006).
Fig. 4.
foxd3 loss-of-function does not affect the number of melanoblasts specified from kitw34 neural crest. A,B) mitfa expression is detected in the head and anterior trunk of kitw34 21hpf embryos. This pattern is not dramatically altered in kitw34 foxd3 morphants. C, D) In 25 hpf kitw34 embryos, dopachrome tautomerase (dct) positive cells are found caudal to the eye and ear (red arrow), and in streams migrating ventrally between somites from the dorsal trunk (black arrows). kitw34 foxd3 morphants show some differences in migration (compare C and D, black arrows) but the overall number of cells appears similar. E, F) 28hpf AB and kitw34 embryos, processed by in situ hybridization for foxd3 message. foxd3 expression appears unchanged by kit loss-of-function. e; developing ear or otic vesicle G, H) 25hpf AB and kitw34 embryos, processed by fluorescent in situ hybridization for sox10 message. sox10 expression in premigratory neural crest cells is mostly unchanged (white arrows), although there is some reduction in peripheral expression, consistent with the reduction in melanoblast migration observed in kitw34 mutants.
To confirm that kit loss-of-function primarily affects melanophore survival and not other neural crest derivatives, we analyzed expression patterns of foxd3 and sox10 in kitw34 and wildtype embryos. sox10 is expressed by all neural crest cells and critical for their differentiation into pigment and glia cells (Dutton et al., 2001). foxd3, expressed primarily by neural crest derived glia at 25hpf (Kelsh et al., 2000), is not affected by kit loss-of-function, as compared to wildtype (AB) embryos (Fig. 4E and 4F). Consistently, sox10 expression in trunk premigratory neural crest cells (Fig. 4G and 4H, white arrows) is also largely unaffected by kit loss-of-function. There is some reduction in dorsal and peripheral sox10 expression, consistent with the decrease in melanoblast numbers and migration observed in kitw34 mutants. Taken together, these data suggest that kit and foxd3 do not interact to increase the number of neural crest cells that give rise to melanoblasts. Furthermore, foxd3 expression is not regulated by kit function, suggesting that the observed genetic interaction between these two genes is by some other mechanism.
foxd3 loss-of-function rescues melanophore morphology and survival in distinct kit alleles
Following specification, melanophores begin to process migratory and survival signals. As previously described, melanophore migration and survival both depend on kit function (Parichy et al., 1999). We hypothesized that foxd3 inhibits melanophore survival, and thus foxd3 loss-of-function rescues some kit-dependent melanophores from death. An alternative explanation is that increases in melanophores result from other neural crest cells switching fates to the melanophore lineage when the function of both genes is inhibited. To distinguish between these two possibilities, we utilized previously characterized kit zebrafish mutants, kitj1e60 and kitj1e78 (Rawls and Johnson, 2003). Unlike the kitb5 and kitw34 mutations, the kitj1e60 and kitj1e78 mutations have partial, distinct defects in kit signaling: 1) the kitj1e60 allele encodes a single amino acid substitution in the second IgG repeat of the extracellular domain (C146R), a portion of the receptor thought to participate in ligand binding (Blechman et al., 1993; Lev et al., 1993; Rawls and Johnson, 2003). This mutation renders melanophores deficient in migration to peripheral locations. However, the number of kitj1e60 melanophores remains fairly constant throughout embryonic development. 2) The kitj1e78 allele encodes a single amino acid substitution in the intracellular domain of the receptor (A779T). kitj1e78 melanophores are deficient in processing survival signals, yet migration patterns are similar to that of wildtype fish (Rawls and Johnson, 2003). We reasoned that if blocking foxd3 function was promoting survival, then we should see increases in melanophores after foxd3 MO injection in kitj1e78 but not kitj1e60 mutants. If additional melanophores were coming from another source, then we predicted to see increases in both mutants.
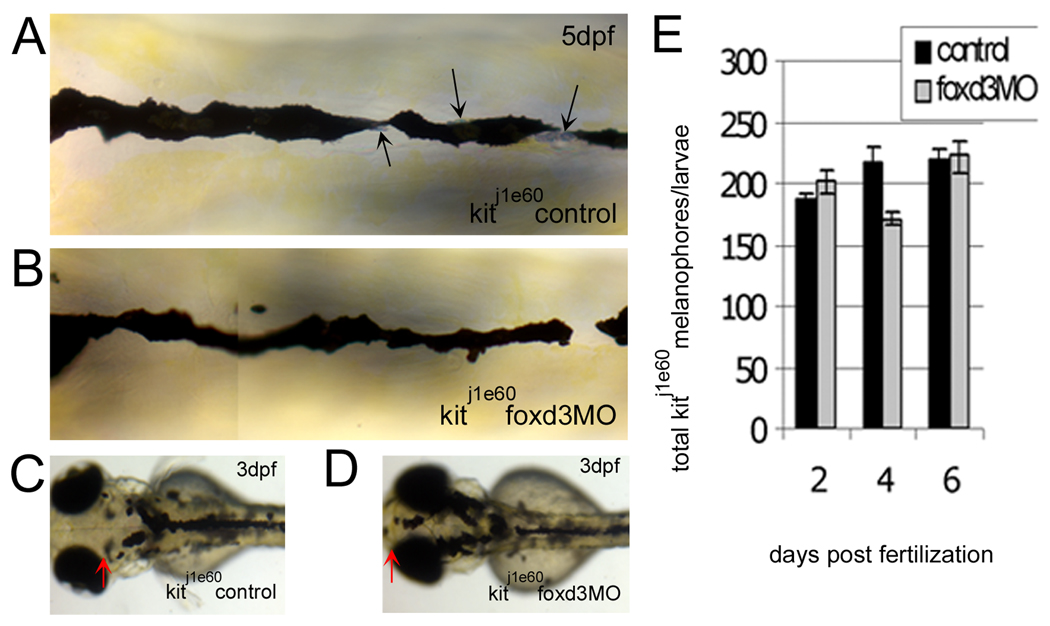
We injected kitj1e60 melanophore migration mutants with foxd3 MO and examined their melanophores. Melanophore morphology (Fig. 5A – 5D) in kitj1e60 foxd3 morphants is similar to uninjected controls. Furthermore, we do not detect significant increases in total melanophores at any time point examined (Fig. 5E). To confirm that our foxd3 MO worked as expected in these experiments, we tallied trunk and tail iridophores at 4 dpf, which are known to be reduced by foxd3 loss-of-function (Lister et al., 2006; Stewart et al., 2006). Indeed, iridophores are reduced in kitj1e60/foxd3 morphants (18 ± 14 iridophores) as compared to uninjected controls (52 ± 3 iridophores; Fig. 5A and 5B, black arrows). Consistent with results in kitw34 embryos, foxd3 loss-of-function in kitj1e60 mutants increases melanophore localization to areas away from sites of origin (compare 5C and 5D, red arrows). Although the total number of melanophores does not increase in kitj1e60 mutants after foxd3 MO injection, the extent of their migration over the head is restored closer to wildtype. These results further suggest that foxd3 and kit play opposing roles in regulating melanophore migration.
Fig. 5.
The increase in kit mutant melanophores with foxd3 loss-of-function is not due to transfating of other foxd3 dependent cell types. A-D) kitj1e60 melanophore migration mutants were injected (or left uninjected; control) with foxd3 MO (foxd3MO), imaged at 3 (C, D) and 5dpf (A, B) and fixed at 2, 4 and 6 dpf for melanophore quantification. A) Melanophores localized to the anterior dorsal trunk display relatively normal, non-apoptotic morphology. Black arrows indicate silver pigment cells, iridophores. B) In kitj1e60 foxd3 morphants, foxd3 dependent iridophores are gone indicating MO function (average control and morphant iridophores ± standard deviation at 4dpf are 52±3 and 18±14, respectively), yet melanophore morphology and number remain similar to uninjected controls. C) kitj1e60 control melanophores do not localize to the anterior most portion of the head, indicating a migration defect. D) The presence of melanophores over the head is partially rescued with foxd3 loss-of-function (red arrows indicate anterior extent of melanophores). E) kitj1e60 melanophores are not significantly increased with foxd3 loss-of-function at 2, 4 and 6 dpf (p=0.31 by 2-way ANOVA; 8–15 fish per time point).
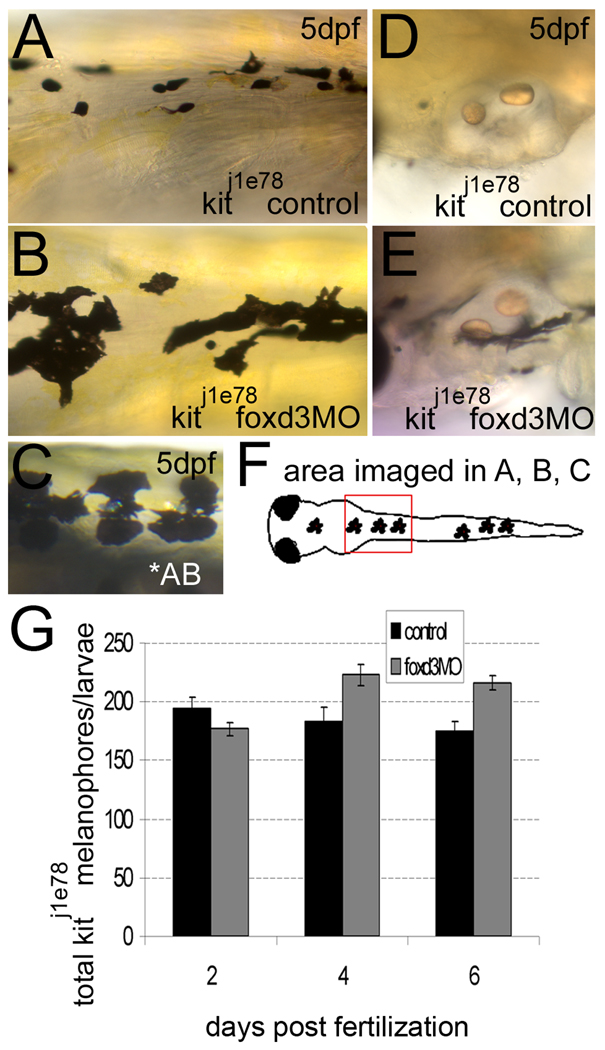
We next examined the effects of foxd3 loss-of-function in kitj1e78 melanophore survival mutants. We observe rounded, small melanophores, a phenotype indicative of apoptosis (Parichy et al., 1999), as early as 5dpf in the dorsal anterior trunk in control animals (Fig. 6A), and considerable changes in melanophore morphology with foxd3 MO injection. kitj1e78/foxd3 morphants have flattened melanophores, a phenotype closer to that observed in wildtype larvae (Fig. 6A–C). Additionally, areas lacking melanophores in 5 dpf kitj1e78 embryos, presumably due to cell death, are partially rescued after MO injection (compare Fig. 6D and 6E). Quantification of melanophores at 2, 4 and 6 dpf confirms a significant increase in melanophores beginning at 4dpf (Fig. 6G). Iridophore counts confirm foxd3 MO function (average control and morphant iridophores ± standard deviation is 35 ± 12 and 4 ± 3, respectively).
Fig. 6.
foxd3 loss-of-function improves the number and morphology of kit j1e78 survival mutant melanophores. A, B, D, E) kitj1e78 survival mutants were injected with foxd3 MO (foxd3MO) or uninjected embryos were examined as controls. A) Control kitj1e78 melanophores are small and rounded, characteristic of apoptosing melanophores. B) loss of foxd3 activity restores some wildtype morphology to kitj1e78 melanophores, and increases the number of melanophores (compare 6B to AB wildtype melanophores in 6C). D) kitj1e78 controls lack ventral stripe melanophores near the otic vesicle, which have apoptosed and been removed from the fish. E) kitj1e78 foxd3 morphants show increases in melanophores at this location, suggesting increased survival. F) Cartoon illustrating the location of images taken in A–C. G) Quantification at 2, 4 and 6 dpf of kitj1e78 and kitj1e78 foxd3 morphant melanophores. There is a statistically significant increase in kitj1e78 animals after foxd3 MO injection (p<0.005 by 2-way ANOVA; 10–19 fish per time point).
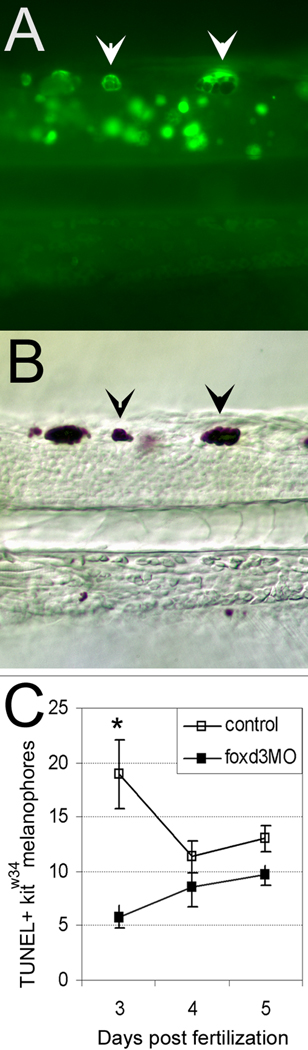
To directly test the role of foxd3 in melanophore survival, kitw34 larvae were injected with foxd3 MO and examined by TUNEL assay at 3, 4 and 5dpf. Since previous studies suggest the number of TUNEL positive cells begins to increase in kitb5 mutants at 3dpf and peaks at 5.6dpf (Parichy et al., 1999), we decided to examine these same time points in our kitw34 mutants. TUNEL positive melanophore carcasses are most abundant in control 3dpf kitw34 larvae and often observed in apparent clusters (Fig. 7A–C, arrowheads). A significant decrease in melanophore carcasses is detected at 3dpf in kitw34 foxd3 morphants (Fig. 7C). Although the number of TUNEL positive carcasses in kitw34 larvae is highest at 3dpf, they are visible at all time points examined. Taken together, these data suggest that foxd3 regulates melanophore number by antagonizing kit-dependent melanophore survival, between 2 and 3dpf.
Fig. 7.
foxd3 loss-of-function partially rescues melanophores from apoptosis in kitw34 larvae. A) 3dpf kitw34 larvae analyzed by TUNEL assay. Examples of posterior trunk melanophores positive for TUNEL signal are marked with white arrowheads. B) Brightfield image of larvae shown in A. TUNEL positive melanophores (black arrowheads) are distinct in size and shape. C) Line graph indicating the total number of TUNEL positive melanophores found in the dorsal stripe of uninjected or foxd3 MO injected kitw34 larvae quantified at 3, 4 and 5 dpf. There is a significant decrease in TUNEL positive melanophores at 3 dpf with foxd3 loss-of-function. (control n=45 fish, 649 TUNEL+ cells; foxd3MO n=64 fish, 493 TUNEL+ cells; *, p<0.001 by Bonferroni posttest at 3dpf).
Ectopic expression of foxd3 leads to precocious melanoblast death in kitw34 mutants
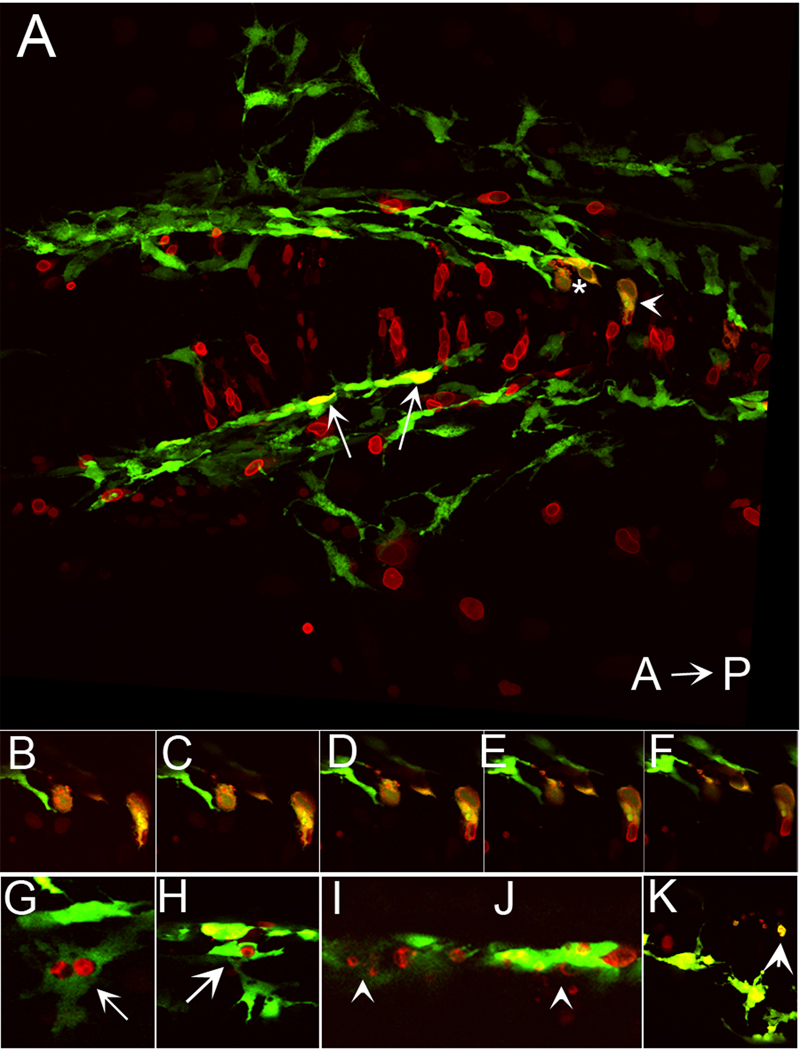
Given that foxd3 loss-of-function rescues melanophore survival in kitw34 zebrafish larvae, we predicted that its ectopic expression would induce melanophore death. To test this possibility, we expressed myc epitope tagged-foxd3 under the control of a heatshock promoter (hs:mycfoxd3), to bypass the early requirement for foxd3 during zebrafish gastrulation and mesoderm induction (Steiner et al., 2006). Following injection of hs:mycfoxd3 into embryos resulting from a kitw34/+ intercross, kitw34 and wildtype embryos were grown to 19hpf and heat shocked at 37°C for 1 hour. We looked for foxd3 expressing cells that showed fragmentation and blebbing, indicators of apoptosis, at 28hpf, when kit signaling is not yet required for melanophore survival (Rawls and Johnson, 2003). kit mutant animals were identified by their characteristic migration pattern. kitw34 larvae transgenic for green fluorescent protein (gfp) driven by a previously characterized 836bp portion of the mitfa promoter (Tg(mitfa:gfp)w47 Lister et al., in preparation; Dorsky et al., 2000) were used for identification of melanoblasts. Once embryos with fragmenting cells were identified, confocal images were taken of each embryo and the number of gfp/foxd3+ cells was counted (Table 2). Although we observe gfp/foxd3+ cells with wildtype morphology in both wildtype and kitw34 embryos (representative examples shown in Fig. 8A, 8G–H, white arrows), we observe fragmenting gfp/foxd3+ cells only in kitw34 embryos (representative examples shown in Fig. 8A–F, 8I–K, white asterisk and arrowheads; see Table 2 for quantification and statistical analysis). These results demonstrate that foxd3 expression promotes melanophore death in the absence of kit function.
TABLE 2.
Effect of ectopic foxd3 expression on melanophore morphology. Data are the numbers of cells with indicated morphology and genotype.
| Wildtype or kitw34/+ | kitw34/w34 | |
|---|---|---|
| Fragmented | 0 | 7 |
| Normal | 13 | 11 |
Larvae were fixed at ~28hpf and antibody stained for Foxd3 (anti-Myc) and GFP. Fish were screened for the presence of foxd3 fragmented cells and GFP expression. Positive fish were imaged and the number of cells with the indicated morphologies were counted in the different genetic backgrounds. Distributions are significantly different (p=0.025, Fisher’s exact test).
Fig. 8.
Ectopic expression of foxd3 promotes cell death in kit mutants. A) Confocal stack image showing a dorsal view of posterior head of 28 hpf kitw34/w34 embryo transgenic for mi::gfp and also expressing myc-foxd3 (red) under the control of a heat shock promoter. Expression of myc-foxd3 was induced at 19 hpf. Both wildtype (arrows) and fragmenting (asterisk and arrowhead) melanophores are observed. B- F) Confocal slices showing fragmenting cells indicated by asterisk and arrowhead in A. Note the presence of Foxd3 positive fragments within the GFP+ melanophores. G–H) Confocal slices showing additional examples of GFP/Foxd3+ cells designated as normal melanophores. I–J) Confocal slices showing examples of GFP/Foxd3+ cells designated as fragmenting melanophores (arrowheads).
DISCUSSION
Our results indicate a genetic interaction between foxd3 and kit during embryonic melanophore development that functions to regulate melanophore migration and survival. foxd3 loss-of-function increases the number of melanophores in peripheral locations and significantly increases the total number of melanophores in three distinct kit melanophore survival alleles: kitw34 (null), kitb5 (null; data not shown) and kitj1e78 (melanophore survival mutant). In situ hybridization analysis of kitw34 melanophore precursors after foxd3 MO injection suggests that melanophore specification is unaltered, although additional cell counts at early stages might more definitively resolve this issue. Quantification of total melanophores in kitj1e60 (melanophore migration) mutants following foxd3 MO injection, suggests that foxd3 dependent derivatives are not being redirected from other neural crest lineages to the melanophore lineage. Total melanophore numbers are similar between uninjected control and MO injected larvae at 2dpf in kitw34 and kit j1e78 foxd3 MO experiments, providing additional support for foxd3 function at a later stage of kit-dependent embryonic melanophore development. Last, examination of indicators of apoptosis (TUNEL and cell fragmentation) in foxd3 loss and gain of function conditions, respectively, suggest that foxd3 promotes melanophore death, probably between 2 and 3dpf. These data suggest that foxd3 modulates kit migration and survival function during zebrafish melanogenesis.
Function of foxd3 and kit interaction during melanophore development
Our data suggests a genetic interaction between kit and foxd3 activity, that initially regulates migration (prior to 2dpf), then later, melanophore survival (post 2dpf). foxd3 loss-of-function significantly decreases apoptosis in kitw34 melanophores at 3dpf, a process known to be regulated by kit signaling. Overexpression of foxd3 via heatshock at 19hpf, a time when kit is not required for promoting melanophore survival (Rawls and Johnson, 2003), induced precocious cell death (as indicated by cell fragmentation) in kit null larvae, only. These results suggest that foxd3 and kit act antagonistically in the regulation of cell survival. Regulation of migration is somewhat more complicated. Loss of either kit or foxd3 function results in a reduction of ventrally migrating melanophores, which might suggest that the wildtype function both promote migration, while the combined loss of both somewhat restores migration. However, the combined loss-of-function reveals antagonistic interactions. Future studies using time-lapse analysis may help resolve whether alterations in migration are due to changes in motility.
There are several mechanisms that may explain the genetic interaction observed between kit and foxd3. The two genes might function in the same pathway. foxd3 could act upstream of kit signaling to regulate melanophore survival. This mechanism supposes that some kit activity remains in mutants, and this activity is enhanced by loss of foxd3. However, both kit null alleles show enhanced melanophore survival after foxd3 loss of function. Since the kitw34 mutant is a deletion resulting in a frameshift, we think it unlikely that it has any residual activity. Alternatively, foxd3 might act downstream of kit, with kit signaling functioning to downregulate foxd3 activity. In the absence of kit function, an increase in foxd3 activity would disrupt melanophore migration and survival; and conversely loss of foxd3 activity would therefore partially rescue the kit mutant phenotype. Consistently, kit signaling has been found to deactivate other members of the forkhead transcription factor family, FoxO1 and FoxO3, in multipotent progenitor cell lines, by controlling trafficking of these forkhead proteins out of the nucleus (Engstrom et al., 2003).
It is also conceivable that kit regulates another factor that is parallel or downstream to foxd3 activity that functions in melanophore migration and/or survival, such as mitfa. As one function of mouse Mitf is to transactivate bcl-2, which in turn would inhibit cell death (McGill et al., 2002), the balance of Mitf activation and repression by kit and foxd3 might explain their epistatic interaction. Activation of kit signaling results in increased Mitf transcriptional activity (Hemesath et al., 1998). Recent studies demonstrate that foxd3 directly represses mitfa activity (Curran et al., submitted). Consistently, histone deacetylase 1 dependent reduction of foxd3 expression is necessary to increase mitfa expression and subsequent specification of melanoblasts from neural crest cells (Ignatius et al., 2008). We presume that activity of mitfa is not a limiting factor for cell survival in wildtype animals, since foxd3 loss-of-function (resulting in increased mitfa activity) has no effect on melanophore numbers. We hypothesize that mitfa activity becomes limiting after its reduction with kit loss-of-function, and melanophore survival becomes sensitive to additional changes in mitfa activity resulting from manipulation of foxd3. Further experiments on the roles of zebrafish foxd3 on mitfa expression and the temporal requirements for these distinct activities are needed to address this model.
kit signaling, foxd3 and disease
Activating mutations in the kit receptor have been correlated with several types of cancer, including gastrointestinal stromal tumors (Hirota et al., 1998) and melanoma (Lefevre et al., 2004). Loss-of-function in mice has been associated with sterility and anemia, as kit plays a critical role in promoting the survival of primordial germ cells and hematopoetic precursor cells in mammals. One disorder linked to defects in both kit and foxd3 expression is the melanocyte specific disorder, Vitiligo, which results from a gradual loss of pigment cells and is characterized by light patches of skin and hair (reviewed in Imokawa, 2004; Goding, 2007). The exact mechanisms underlying the progression of Vitiligo are not entirely understood, but one mechanism being explored involves the loss of expression and/or activity of key melanocyte survival genes, including kit and its downstream targets, Mitf and bcl-2. Conversely, the link between Vitiligo and foxd3 involves overexpression. An uncommon variant sequence in the foxd3 promoter, - 639G>T, was linked to patients with Vitiligo (Alkhateeb et al., 2005). Additional characterization of the variant promoter in luciferase assays suggests it has higher transcriptional activity than the wildtype promoter in cells expressing foxd3 endogenously. The authors suggest a model where melanoblast precursors containing the variant promoter sequence, may have enhanced foxd3 expression, leading to alterations in melanocyte development (Alkhateeb et al., 2005). At what stage of Vitiligo progression foxd3 may exert its effect is unclear, however, it is plausible that the relationship between kit and foxd3 plays a role in promoting normal melanocyte number, function and homeostasis.
EXPERIMENTAL PROCEDURES
Fish rearing and crosses
Wildtype fish were of the AB (ZDB-GENO-960809-7) strain. Adult zebrafish were maintained on a 14-h/10-h light/dark cycle at 28.5°C. Embryos were acquired from natural crosses, grown at 28.5°C in embryo media until analysis. Embryos were staged according to characterized morphological criteria (Kimmel et al., 1995). The following alleles were used: kitab5, (Parichy et al., 1999); kitaj1e60, kitaj1e78 (Rawls and Johnson, 2003); foxd3zdf10, (Stewart et al., 2006); Tg(mitfa:gfp)w47, (Lister et al., in prep).
Isolation, characterization and sequencing of kitw34
For mutagenesis, adult AB males were treated with N-ethyl N-nitrosourea according to published methods (Solnica-Krezel et al., 1994) and outcrossed to wild-type females. These F1 progeny were intercrossed and the kitw34 mutation was identified by intercrossing adults from one of the resulting F2 families. Complementation analysis was done by crossing homozygous kitw34 females with homozygous kitb5 males. Live embryos were examined for rescue of melanophore migration and survival.
For sequencing the kitw34 allele, total RNA was isolated from 24hpf AB and kitw34 embryos using Trizol (Invitrogen, Carlsbad CA) according to manufacturer's recommendations, followed by cDNA preparation (Superscript First Strand Kit; Invitrogen, Carlsbad CA). PCR primers (sequences available on request), designed to amplify 6 overlapping regions of kita cDNA, and subsequent gel analysis revealed a difference in migration for one specific PCR product, as compared to wildtype. The variant amplicon was sequenced, revealing a large gap in kita cDNA. PCR primers designed to amplify area adjacent to the gap were used to amplify kitw34 genomic DNA. Subsequent gel analysis confirmed kitw34 embryos were lacking exon 10 (along with some adjacent intron DNA), resulting in a frameshift and early stop codon.
MO injection and live imaging
Embryos obtained from natural matings were injected at the 1–2 cell stage with foxd3 MO oligonucleotide designed to bind the 5’ untranslated region of foxd3 message (Lister et al., 2006). Volumes and concentrations ranged from 0.5–1nL per embryo of 1–2mg/mL working stocks. A 10mg/mL MO stock was diluted in 1X Danieu Buffer (58 mM NaCl, 0.7mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES pH 7.6) to generate working stocks.
For imaging, live fish were anesthetized in MESAB (MS-222; Sigma, St Louis, MO) and immobilized in 3% methyl cellulose. Fish were visualized using Nikon SMZ1500 dissecting or Zeiss Axioplan 2 microscopes. Brightfield images were taken using a Spot Camera and Spot advanced software program. Images were processed for color balance and brightness/contrast using Adobe Photoshop Elements 4.0.
Melanophore and iridophore counts
Fish were grown to the appropriate stage at 28.5°C, then soaked in 5mg/mL epinephrine for 5–10 minutes (in embryo media) to aggregate melanosomes around the cell body and improve visibility of individual cells. Larvae were fixed in 4% paraformaldehyde at room temperature for 1–2h or 14–18h at 4°C. Melanophores and iridophores were quantified using dissecting microscopes and intact fixed fish (not images). To assist with iridophore visualization and counting, epi-illumination from a fiber optic light source was used. Tail and trunk iridophores were quantified. “Total” melanophores include cells found in the dorsal and lateral stripes, as well as ventral stripe cells found posterior to the cloaca. In kitw34 foxd3 MO experiments, the complete ventral stripe was counted. Statistical tests were performed using Prism 5.0 (Graphpad Software, San Diego, CA)
In situ hybridization
Embryos were fixed in 4% paraformaldehyde (in phosphate buffered saline, pH 7.2) and processed using standard protocols. Digoxygenin-labeled probes for mitfa (Lister et al., 1999), dopachrome tautamerase (Kelsh et al., 2000), sox10 (ZDB-GENE-011207-1; Dutton et al., 2001), foxd3 (ZDB-GENE-980526-143; Odenthal and Nusslein-Volhard, 1998) and kita genes (Parichy et al., 1999) have been previously described. Fluorescent in situ hybridization was done using a protocol developed in Scott Holley’s laboratory (Julich et al., 2005).
Melanophore TUNEL analysis
TUNEL analysis was done using In Situ Cell Death Detection Kits (Roche, Indianapolis, IN) using the below protocol adapted from C. Moens, R. Kelsh and S. Holley laboratory protocols. Fish were fixed in 4% paraformaldehyde for 1–2 hours at RT or 12–16 hours at 4°C. Larvae were dehydrated, then rehydrated in 25:75, 50:50, 75:25 MEOH/Tris buffered saline (1M Tris, pH 7.5, 1.5M NaCl) containing 2% Triton-X 100 and 5% Tween-20 (TBSTT), 5–10 minutes each. Larvae were incubated in Proteinase K (1ug/ml) diluted in TBSTT for 30 minutes at RT, with continuous rocking. Proteinase K activity was quenched in 2mg/ml glycine, larvae were fixed for 20 minutes in 4% paraformaldehyde and washed in TBSTT. Larvae were washed in Roche TUNEL dilution buffer, incubated in TUNEL enzyme/label mix (Roche In Situ Cell Death Detection kit) for 1 hour on ice, followed by 1 hour incubation at 37°C, protected from light. Larvae were washed in TBSTT, blocked in 150mM maleic acid, 100mM NaCl, (pH 7.5) plus 2% Western Blocking Reagent (Roche, Indianapolis, IN) and incubated in anti-fluor- POD antibody (Roche, Indiananapolis, IN; 1:1000 dilution) for 12–16 hours at 4°C. Next, larvae were washed in maleic acid/NaCl buffer and incubated in TSA plus fluorescein solution (Invitrogen, Carlsbad, CA) for 45–60 minutes. Larvae were dehydrated in MEOH/PBS series, incubated in 1% H2O2 in 100% MEOH, followed by rehydration. Larvae were visualized and imaged using Nikon SMZ1500 dissecting or Zeiss Axioplan 2 microscopes.
Ectopic expression of foxd3 and confocal imaging
Intercrossed kitw34/+ fish, transgenic for mitfa:gfp (Tg(mitfa:gfp)w47; Lister et al., in preparation), were injected with foxd3 under the control of the zebrafish hsp70 heatshock promoter (Halloran et al., 2000) at the 1–2 cell stage. At approximately 19hpf, fish were transferred from 28°C to 37°C for 1 hour, and returned to 28°C until fixation at 28hpf. Following fixation, fish were blocked in antibody block (1xPBS, 0.2% Triton X-100, 2mg/ml BSA, 1%DMSO, 0.02% Na Azide) supplemented with 5% goat serum for at least 1 hour. Fish were stained overnight in mouse anti-Myc (Cell Signaling Technology, Danvers MA) and rabbit anti-GFP (Invitrogen/Molecular Probes, Carlsbad CA) antibodies at 4°C. Fish were washed in PBT (PBS + 0.1% Triton X-100) then stained overnight in anti-mouse Alexa 568 and anti-rabbit Alexa-488 secondary antibodies (Invitrogen/Molecular Probes, Carlsbad CA). Fish were washed in PBT, deyolked and mounted for confocal imaging, using a Zeiss LSM Pascal confocal microscope. Images were processed for color balance and contrast/brightness using Adobe Photoshop Elements 4.0.
ACKNOWLEDGEMENTS
The authors would like to thank Dave White and all current and previous members of the UW Fish Facility for excellent fish care. David Parichy, James Lister and Kevin Curran for critical evaluation of the manuscript. Keith Hultman and Steve Johnson for providing kitj1e78 and kitj1e60 zebrafish mutants. John Kanki and A. Thomas Look for providing foxd3zdf10 /foxd3 zebrafish mutants. This work was supported by a NIH National Research Service Award to C.D.C and a NIH RO1 grant to D.W.R.
Grant sponsor: National Institutes of Health
Grant number: 1F32 HD047108-01 to CDC, 1R01-NS45246 to DWR
REFERENCES
- Alkhateeb A, Fain PR, Spritz RA. Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. Journal of Investigative Dermatology. 2005;125:388–391. doi: 10.1111/j.0022-202X.2005.23822.x. [DOI] [PubMed] [Google Scholar]
- Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122:3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- Blechman JM, Lev S, Brizzi MF, Leitner O, Pegoraro L, Givol D, Yarden Y. Soluble c-kit proteins and antireceptor monoclonal antibodies confine the binding site of the stem cell factor. Journal of Biological Chemistry. 1993;268:4399–4406. [PubMed] [Google Scholar]
- Cooper CD, Raible DW. Mechanisms for reaching the differentiated state: Insights from neural crest derived melanocytes. Sem Cell Dev Biol 2008. 2008 Sep 30; doi: 10.1016/j.semcdb.2008.09.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, Cosman D, Anderson D, Lyman SD, Williams DE. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Yemm E, Bonde G, Li W, d'Alencon C, Wegman L, Eisen J, Zahs A. Touchtone promotes survival of embryonic melanophores in zebrafish. Mech Dev. 2004;121:1365–1376. doi: 10.1016/j.mod.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article] [PubMed] [Google Scholar]
- Dunn KJ, Brady M, Ochsenbauer-Jambor C, Snyder S, Incao A, Pavan WJ. WNT1 and WNT3a promote expansion of melanocytes through distinct modes of action. Pigment Cell Res. 2005;18:167–180. doi: 10.1111/j.1600-0749.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Engstrom M, Karlsson R, Jonsson JI. Inactivation of the forkhead transcription factor FoxO3 is essential for PKB-mediated survival of hematopoietic progenitor cells by kit ligand. Experimental Hematology. 2003;31:316–323. doi: 10.1016/s0301-472x(03)00002-x. [DOI] [PubMed] [Google Scholar]
- Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Goding CR. Melanocytes: The new Black. The International Journal of Biochemistry and Cell Biology. 2007;39:275–279. doi: 10.1016/j.biocel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Ignatius MS, Moose HE, El-Hodiri HM, Henion PD. colgate/hdac1 Repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev Biol. 2008;313:568–583. doi: 10.1016/j.ydbio.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Research. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Africa D, Walker C, Weston JA. Genetic control of adult pigment stripe development in zebrafish. Developmental Biology. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- Julich D, Hwee Lim C, Round J, Nicolaije C, Schroeder J, Davies A, Geisler R, Lewis J, Jiang YJ, Holley SA. beamter/deltaC and the role of Notch ligands in the zebrafish somite segmentation, hindbrain neurogenesis and hypochord differentiation. Dev Biol. 2005;286:391–404. doi: 10.1016/j.ydbio.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Schmid B, Eisen JS. Genetic analysis of melanophore development in zebrafish embryos. Developmental Biology. 2000;225:277–293. doi: 10.1006/dbio.2000.9840. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- Lefevre G, Glotin AL, Calipel A, Mouriaux F, Tran T, Kherrouche Z, Maurage CA, Auclair C, Mascarelli F. Roles of stem cell factor/c-Kit and effects of Glivec/STI571 in human uveal melanoma cell tumorigenesis. Journal of Biological Chemistry. 2004;279:31769–31779. doi: 10.1074/jbc.M403907200. [DOI] [PubMed] [Google Scholar]
- Lev S, Blechman J, Nishikawa S, Givol D, Yarden Y. Interspecies molecular chimeras of kit help define the binding site of the stem cell factor. Molecular & Cellular Biology. 1993;13:2224–2234. doi: 10.1128/mcb.13.4.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JA, Cooper C, Nguyen K, Modrell M, Grant K, Raible DW. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Developmental Biology. 2006;290:92–104. doi: 10.1016/j.ydbio.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- McNeill MS, Paulsen J, Bonde G, Burnight E, Hsu MY, Cornell RA. Cell death of melanophores in zebrafish trpm7 mutant embryos depends on melanin synthesis. J Invest Dermatol. 2007;127:2020–2030. doi: 10.1038/sj.jid.5700710. [DOI] [PubMed] [Google Scholar]
- Mellgren EM, Johnson SL. kitb, a second zebrafish ortholog of mouse Kit. Development Genes & Evolution. 2005;215:470–477. doi: 10.1007/s00427-005-0001-3. [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M, Lang MR, Sachdev SW, Knappmeyer C, Stewart RA, De La Guardia A, Hatzopoulos AK, Knapik EW. The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Dev Dyn. 2006;235:3199–3212. doi: 10.1002/dvdy.20959. [DOI] [PubMed] [Google Scholar]
- Opdecamp K, Nakayama A, Nguyen MT, Hodgkinson CA, Pavan WJ, Arnheiter H. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development. 1997;124:2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM. Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Dev Biol. 2003;256:242–257. doi: 10.1016/s0012-1606(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Johnson SL. Temporal and molecular separation of the kit receptor tyrosine kinase's roles in zebrafish melanocyte migration and survival. Developmental Biology. 2003;262:152–161. doi: 10.1016/s0012-1606(03)00386-5. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Current Opinion in Genetics & Development. 2006;16:360–366. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Silver DL, Hou L, Somerville R, Young ME, Apte SS, Pavan WJ. The secreted metalloprotease ADAMTS20 is required for melanoblast survival. PLoS Genet. 2008;4:e1000003. doi: 10.1371/journal.pgen.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, Schier AF, Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AB, Engleka MJ, Lu Q, Piwarzyk EC, Yaklichkin S, Lefebvre JL, Walters JW, Pineda-Salgado L, Labosky PA, Kessler DS. FoxD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development. 2006;133:4827–4838. doi: 10.1242/dev.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Developmental Biology. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, He X, Fisher DE. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]