Abstract
Objective:
This large, prospective, community-based study characterized neuropsychological functioning and academic achievement at the time of the first recognized seizure and identified risk factors for cognitive deficits.
Methods:
We compared 282 children (ages 6–14 years, IQ ≥70) with a first recognized seizure to 147 healthy siblings on a battery of well-standardized and widely used neuropsychological and academic achievement tests and examined relationships with demographic and clinical variables.
Results:
In this intellectually normal cohort, 27% with just one seizure and up to 40% of those with risk factors exhibited neuropsychological deficits at or near onset. Risk factors associated with neuropsychological deficits included multiple seizures (i.e., second unprovoked seizure; odds ratio [OR] = 1.96), use of antiepileptic drugs (OR = 2.27), symptomatic/cryptogenic etiology (OR = 2.15), and epileptiform activity on the initial EEG (OR = 1.90); a child with all 4 risks is 3.00 times more likely than healthy siblings to experience neuropsychological deficits by the first clinic visit. Absence epilepsy carried increased odds for neuropsychological impairment (OR = 2.00).
Conclusions:
A subgroup of intellectually normal children with seizures showed neuropsychological deficits at onset. Academic achievement was unaffected, suggesting that there is a window early in the disorder for intervention to ameliorate the impact on school performance. Therefore, the risk factors identified here (especially if multiple risks are present) warrant swift referral for neuropsychological evaluation early in the course of the condition.
GLOSSARY
- AED
= antiepileptic drug;
- ANOVA
= analysis of variance;
- CELF
= Clinical Evaluation of Language Fundamentals;
- CI
= confidence interval;
- CTOPP
= Comprehensive Test of Phonological Processing;
- OR
= odds ratio;
- PURS
= prior unrecognized seizure;
- WCST
= Wisconsin Card Sorting Test;
- WRAML
= Wide Range Assessment of Memory and Learning.
Cognitive challenges are well established in children with epilepsy1–4 and could appear at or before seizure onset based on the transient cognitive impairment model.5 Supporting studies have been limited by small sample sizes (n = 12–72 for most),6–13 exclusion of seizure types/etiologies,6–10 long latencies between seizure onset and cognitive assessment,7,8,12,14 using gross outcome measures11,12,14 or testing limited functions,6 no control group,12,14 using retrospective report,14 or ignoring prior unrecognized seizures, observed in one third of children.15,16 Understanding risk factors for early neuropsychological deficiencies is critical for early intervention for common cognitive and academic comorbidities.2,17
We conducted a prospective study with a large, representative cohort of children identified within 3 months of their first identified seizure, together with healthy siblings, using standardized, individually administered tests. We hypothesized that the seizure group would score lower than controls on these tests and lower scores would be associated with prior unrecognized seizures, antiepileptic drug (AED) use, symptomatic/cryptogenic etiology, and EEG abnormality. Differences by epileptic syndrome were also explored.
METHODS
Participants.
Seizure group.
Children were recruited from 2000 to 2004 at 2 academic medical centers (Indianapolis, Cincinnati) and through private practice physicians and school nurses throughout Indiana. Inclusion criteria were age 6–14 years, a first-recognized unprovoked seizure within 3 months of enrollment, and IQ of at least 70. Children were excluded if seizures were provoked (i.e., associated with toxin, infection, trauma, or mass lesion) or if they had a chronic condition limiting daily activities. Children were not excluded for having prior unrecognized seizures. Seizure type and epileptic syndrome were classified by board-certified child neurologists using International League Against Epilepsy criteria18,19 following review of all relevant information available at the evaluation of the first recognized seizure. Institutional review boards at both participating institutions approved the study. Parents provided written consent and children gave assent.
Of the 349 children who agreed to participate in the study, 23 scored below 70 on screening, 11 did not provide any data, and 33 were unable to complete testing (e.g., typically scheduling/travel), for a final sample of 282. There were no differences between those who completed neuropsychological testing and those who did not on age, sex, race, or socioeconomic status (p > 0.10).
Control group.
For each child in the seizure group, we attempted to recruit a healthy sibling age 2–18 years (preferring ages 6+ for cognitive testing). Of the 232 eligible siblings, 50 were too young (<6 years) to complete neuropsychological testing, and 35 others could not travel to the medical center for testing, resulting in 147 sibling controls.
Demographic characteristics.
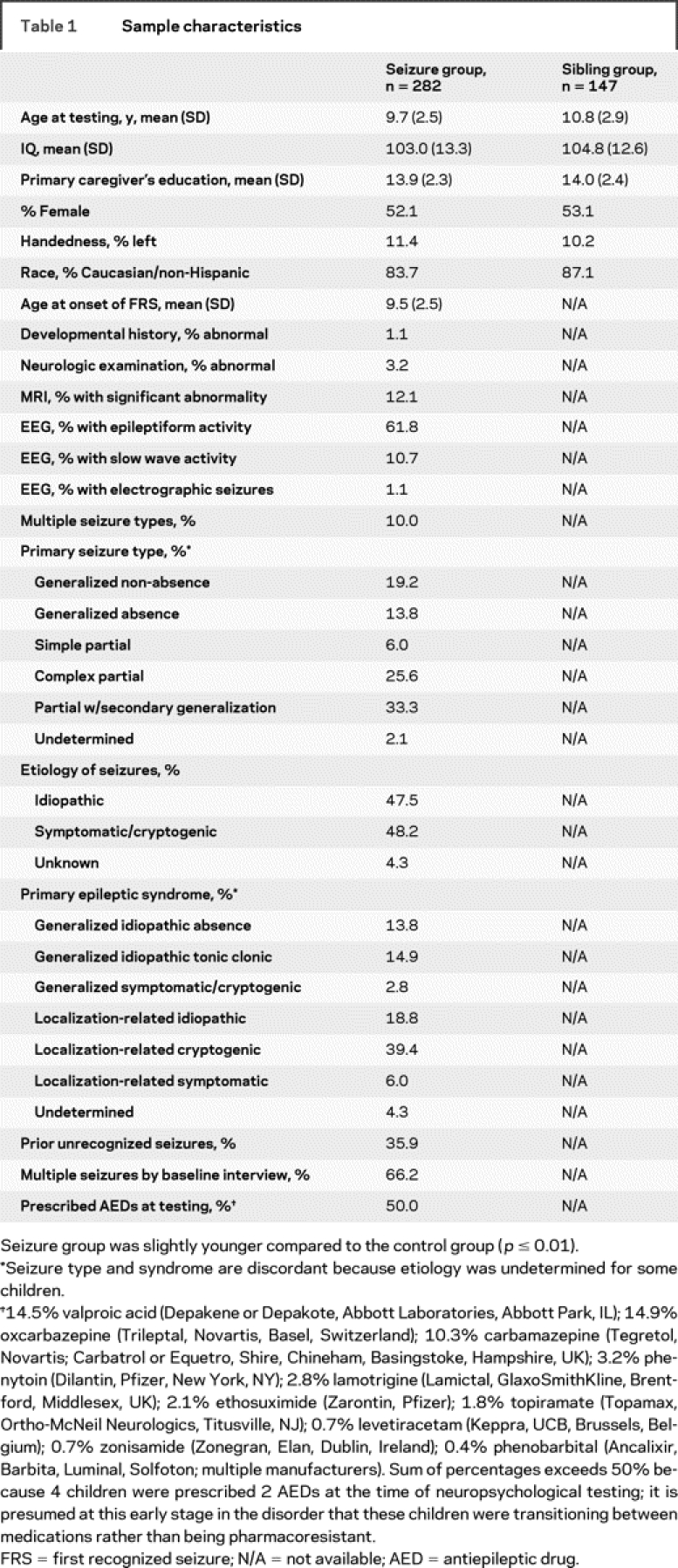
Seizure and sibling groups were comparable demographically (table 1). Estimated IQ spanned from borderline to superior; the mean and median IQs were typical of other studies.9,10
Table 1 Sample characteristics
Clinical characteristics and representativeness of the population.
Most children had one seizure type; partial seizures were more frequent than generalized, characteristic of other studies, especially after age 5.20–22 The proportion with symptomatic/cryptogenic etiology was lower than past studies22 but not unexpected for onset after age 5.
Detailed descriptions of prior events were obtained by structured interviews and evaluated by a board-certified child neurologist (D.W.D.) for likelihood of being unprovoked epileptic seizures. The rate of prior seizures was similar to previous prospective cohorts.15,16
Details, including antiepileptic drugs prescribed, are provided in table 1. This sample has been the subject of other reports.23–28 There is no overlap in analyses or results.
Measures.
EEG.
EEG reports were reviewed by board-certified child neurologists (D.W.D., T.J.D.) and coded for presence of electrographic seizures, epileptiform discharges, and slow wave activity.
Neuropsychological and academic achievement testing.
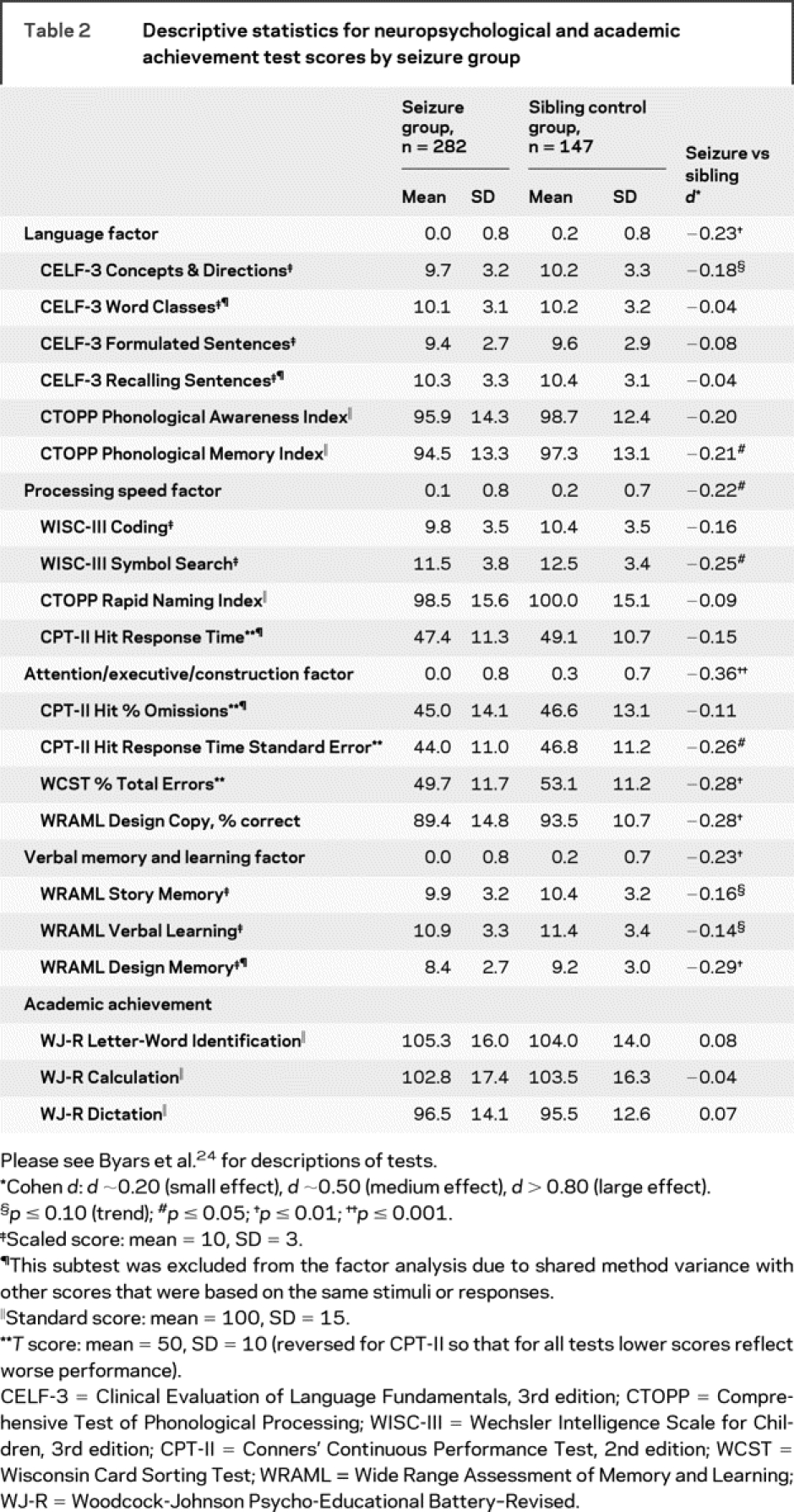
Within 6 months (mean = 2.8, SD = 1.3) of their first recognized seizure, children were tested using standardized individually administered tests. Factor analysis reduced the neuropsychological test variables to 4 factors: language, processing speed, verbal memory and learning, and attention/executive/construction24 (table 2). Neuropsychological deficit was defined as 1.3 SD below the sibling norm on any of the 4 factors; this cutoff corresponds with the 10th percentile, which defines the upper boundary of “borderline intellectual deficiency” on intellectual tests.
Table 2 Descriptive statistics for neuropsychological and academic achievement test scores by seizure group
Statistical analyses.
A linear mixed model was used to compare children with seizures and their siblings on neuropsychological and academic achievement scores. Family was a random effect, and group was a fixed effect (one-tailed tests for directional hypotheses). This analysis was conducted first on the 4 neuropsychological factors and then on the individual test variables. For individual tests, p values were adjusted for multiple comparisons within each domain using Hochberg’s step-up Bonferroni method.
Next, to test for the hypothesized role of a priori risk factors, multivariate logistic regression was performed to assess the associations of AED use (yes/no), multiple seizures by baseline interview including prior unrecognized seizures (yes/no), etiology (idiopathic vs symptomatic/cryptogenic), and epileptiform activity on initial EEG (yes/no) with neuropsychological deficit (present/absent) in children with seizures.
Further in-depth analyses using continuous outcome variables were conducted to examine specific risk factors and interactions. Two-sample t tests were used to test for differences by prior unrecognized seizure (PURS), etiology (symptomatic/cryptogenic vs idiopathic), use of AEDs at neuropsychological testing, epileptiform activity, EEG slowing, and electrographic seizures; all tests were one-tailed (directional hypotheses) except for use of AEDs, which was two-tailed. Analysis of variance (ANOVA) was conducted to test for differences among epileptic syndromes and medications (none, valproic acid, oxcarbazepine, or carbamazepine). These analyses were conducted on the 4 neuropsychological factors and the 3 academic achievement scores. When a significant main effect was found for syndrome or medication, Tukey’s adjustment for multiple comparisons was used. When multiple post hoc tests were performed to investigate the possibility of interactions, Hochberg’s step-up Bonferroni method was used to adjust for multiple comparisons.
Finally, to characterize the degree of risk associated with each risk factor that emerged in the prior analyses, univariate logistic regression analyses were conducted to generate odds ratios (OR) comparing the seizure group children who possessed the risk factor to the sibling group and another OR comparing the seizure group children who possessed the risk factor to the seizure group children without the risk factor. In addition, we identified the seizure group children who possessed all 4 risk factors of interest and computed the same ORs to characterize cumulative risk.
RESULTS
Comparisons between children with seizures vs siblings.
Children with seizures scored lower than siblings on all neuropsychological factors, especially attention/executive/construction (table 2). Groups did not differ in academic achievement. The case-control differences in the seizure group children who had sibling controls distributed relatively normally for each neuropsychological factor; there was clustering in the lower range, probably representing a subgroup with multiple risks.
Multivariate analysis of risk factors.
The proportion of children in the seizure group exhibiting a neuropsychological deficit was 27.4% vs 18.2% of siblings (χ2 p = 0.04, table 3). Compared to siblings, almost twice as many children with seizures exhibited a deficit in attention/executive/construction, verbal memory and learning, and language (13.9 vs 7.7 for each factor); the margin was slightly narrower for processing speed (13.5 vs 8.4).
Table 3 Odds ratios for neuropsychological deficit in at least one neuropsychological domain, by clinical risk factor
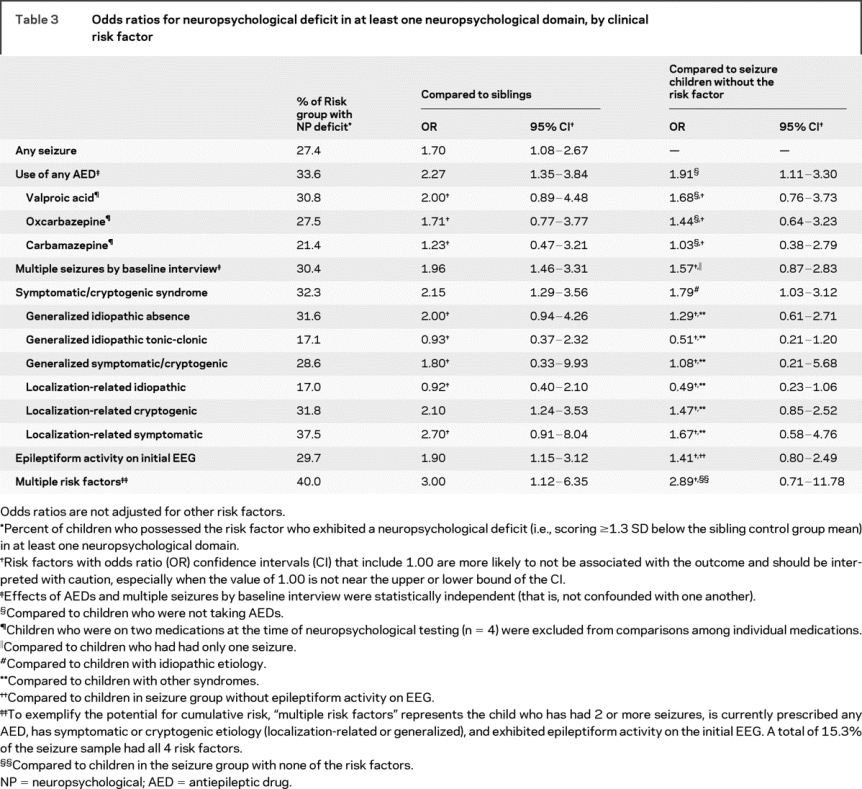
On multivariate logistic regression analysis, only symptomatic/cryptogenic etiology was a risk factor for neuropsychological deficit (p = 0.02). When conducted separately by etiology, no predictors were significant for either the symptomatic/cryptogenic (42 of 88 impaired) or idiopathic (28 of 104 impaired) subgroups. However, within the idiopathic subgroup, OR estimates were larger than 2.0 for recurrence (OR = 2.1, 95% confidence interval [CI] 0.69, 6.7) and epileptiform activity (OR = 2.7, 95% CI 0.74, 10.0); within the symptomatic/cryptogenic subgroup, AED use carried an elevated risk (OR = 2.5, 95% CI 0.97, 6.2). Therefore, small sample sizes could have limited the detection of significant and clinically meaningful effects after subdividing the sample by etiology. Because inclusion of interaction terms into a global multivariate model further reduces power, hypothesized main effects and clinically meaningful interactions among risk factors were examined further using continuous outcome variables and theoretically relevant variables and interactions (described below).
Comparisons between prior seizure subgroups.
Children with prior seizures did not differ from those without prior seizures on any neuropsychological factor or academic achievement score (p > 0.05).
Comparisons by presumed etiology and epileptic syndrome.
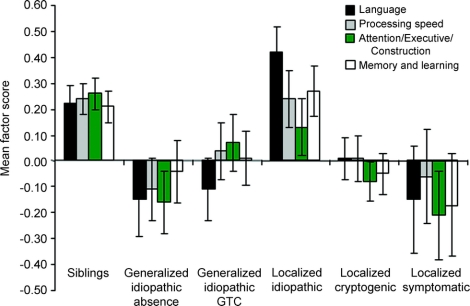
Children with symptomatic/cryptogenic etiology (n = 130) performed worse than those with idiopathic etiology (n = 133) on verbal memory and learning only (p = 0.06, trend). Analysis by specific syndrome groups, however, revealed differences within syndromes on 3 of 4 neuropsychological factors (figure 1). On language, all other syndrome groups scored lower than the localization-related idiopathic group (absence, tonic-clonic, partial cryptogenic, p ≤ 0.02; localization-related symptomatic, p = 0.10, trend). For attention/executive/construction, both localization-related cryptogenic (p = 0.009) and generalized idiopathic absence groups (p = 0.02) scored lower than siblings. For verbal memory and learning, the localization-related cryptogenic group scored lower than the siblings (p = 0.06, trend). Also, for academic achievement in writing, generalized idiopathic tonic-clonic (p = 0.01) and localization-related cryptogenic groups (p = 0.05) scored lower than the localization-related idiopathic group. Epileptic syndrome was not related to processing speed or to academic achievement in reading or math (p > 0.10).
Figure 1 Neuropsychological factor scores by epileptic syndrome
On language, all other syndrome groups scored lower than the localization-related idiopathic group. For attention/executive/construction, both the generalized idiopathic absence and the localization-related cryptogenic groups scored lower than siblings. For verbal memory and learning, the localization-related cryptogenic group scored lower than the siblings. Sibling controls, n = 147; generalized idiopathic/absence, n = 39; generalized idiopathic/tonic-clonic, n = 42; localization-related/idiopathic, n = 53; localization-related/cryptogenic, n = 111; localization-related/symptomatic, n = 17. Although the generalized symptomatic/cryptogenic group (n = 8) was included in the comparisons between etiologies, it was excluded from the analysis comparing specific syndromes because of small sample size.
Comparisons among medication subgroups.
For processing speed, there was a main effect for AED (p = 0.009); children on valproic acid scored lower than the no-AED group. For language, verbal memory and learning, and attention/executive/construction, overall models were nonsignificant. The relationship with processing speed remained for children with symptomatic/cryptogenic etiology (p = 0.01) but not for children with idiopathic etiology. There were no differences on academic achievement.
Comparisons among medication and multiple-seizure subgroups.
Among children with seizures, those on AEDs performed worse than those not on AEDs on all neuropsychological factors (processing speed, p = 0.002; others, p ≤ 0.05, two-tailed) but not on academic achievement (p > 0.10). To control for the potential confound between multiple seizures and AED use, post hoc 2 × 2 (multiple seizures × AED use) ANOVAs compared 4 subgroups (single seizure/no AED, n = 77; single seizure/on AED, n = 18; multiple seizures/no AED, n = 63; multiple seizures/on AED, n = 123).
There was a main effect for AED use on processing speed (p = 0.001), language (p = 0.04), and verbal memory and learning (p = 0.05); children on AEDs scored lower irrespective of whether they had had a single seizure or multiple seizures. When examined separately by etiology, this pattern was observed only for symptomatic/cryptogenic etiology and only for processing speed (p = 0.001) and language (p = 0.02). These relationships remained unchanged after controlling for EEG epileptiform activity.
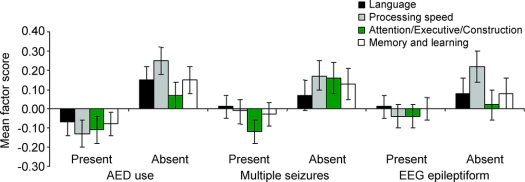
There was a main effect of multiple seizures on attention/executive/construction (p = 0.03); children with multiple seizures scored lower irrespective of AED use (figure 2). Although the largest differences were in construction and attention, executive functioning was also significantly compromised in this subgroup. When examined separately by etiology, the effect of multiple seizures did not reach significance for the smaller subgroups.
Figure 2 Neuropsychological factor scores by antiepileptic drug (AED) use, multiple seizures, and epileptiform activity on initial EEG
Among children who had a seizure, children who were prescribed AEDs (n = 141) scored lower than those who were unmedicated (n = 140) on processing speed (p = 0.001), language (p = 0.04), and verbal memory and learning (p = 0.05), even in children who had had only one seizure and in children without epileptiform activity on EEG. Children who had 2 or more seizures before baseline interview (n = 187) had lower scores compared to children with only one seizure (n = 95) on attention/executive/construction (p = 0.03), even in children who were unmedicated. Children who exhibited epileptiform activity on the initial EEG (present group, n = 173) scored lower than those without epileptiform activity (absent group, n = 107) on the processing speed factor (p = 0.004, d = 0.31), even in children who were unmedicated.
Relationships with EEG variables.
Epileptiform activity was associated with slower processing speed (p = 0.004, d = 0.31) (figure 2). Because children with epileptiform activity on EEG are more likely to be medicated, we conducted a post hoc 2 × 2 (presence of epileptiform activity × AED use) ANOVA to determine if the association between epileptiform activity and neuropsychological function was related to AED use. The relationship between epileptiform activity and processing speed was attenuated after controlling for AED use but still apparent at the level of a statistical trend (p = 0.07). Epileptiform activity was unrelated to academic achievement. Results were the same within symptomatic/cryptogenic etiology. There were no associations with epileptiform activity within idiopathic etiology (p > 0.10).
Children with slowing on EEG (n = 30) did not differ from those without slowing (n = 250) on any neuropsychological domain or academic achievement variable (p > 0.10). When examined separately by etiology, slowing on EEG did not significantly affect any neuropsychological domain for children with either symptomatic/cryptogenic or idiopathic etiology. Although only 3 children exhibited electrographic seizures on their initial EEG, they scored far lower than other children with seizures on attention/executive/construction (d = 1.83), verbal memory and learning (d = 1.47), language (d = 1.24) processing speed (d = 1.07), letter-word identification (d = 0.99), calculation (d = 0.36), and dictation (d = 0.73).
Odds ratios for neuropsychological deficits.
ORs were computed to quantify the degree of risk associated with each risk factor suggested to be important in any of the above analyses (table 3).
DISCUSSION
In this prospective design with a large, community-based cohort of children with school-age onset of seizures and sibling controls, 27% of children with one seizure and up to 40% of those with risk factors exhibited neuropsychological deficits at or near onset. Several risk factors were identified.
A second unprovoked seizure was associated with deficiencies in attention, construction, and executive functioning, even in unmedicated, drug-naïve children. This is consistent with past studies of children diagnosed with epilepsy6–8,10,13 and adds to the conceptualization of deleterious effects of seizures, showing that they are not limited to children with generalized symptomatic epilepsies, frequent seizures, high AED use, or very early onset, as suggested elsewhere.29
Multivariate modeling identified symptomatic/cryptogenic syndromes as being associated with neuropsychological difficulties, even in this intellectually normal cohort. This finding is consonant with a previous report by our team correlating MRI abnormalities with neuropsychological deficits at onset.24 However, the two etiology subgroups appeared to have unique risk profiles, and there were children within the symptomatic/cryptogenic subgroup who did well and children outside that group who did not do so well (e.g., absence), thereby warranting further exploration of complex interactions that can go undetected in a single multifactorial model.
Use of AEDs was associated with deficiencies in all neuropsychological domains; for processing speed, language, and verbal memory and learning, this relationship was evident even in children who had had only one seizure and in children without epileptiform activity on EEG. Many articles report minimal cognitive deficits with most AEDs when used in monotherapy,30,31 but studies of cognitive effects of AEDs in children have been lacking in number and in quality.32,33 The present study was not designed to examine adverse cognitive effects of individual AEDs but addressed methodologic limitations in this area32 by using sensitive tests, recruiting a large sample, controlling for some critical interacting factors, and controlling for multiple statistical tests. Our results support the conclusion of Kwan and Brodie32 that children on AEDs should be individually monitored for neuropsychological effects of AEDs. As for specific AEDs, valproate was associated with slower processing speed; however, because this study was not a randomized trial, extraneous factors might have contributed to this relationship.
The large sample permitted comparisons across syndrome groups. Not surprisingly, the localization-related idiopathic group was relatively unaffected neuropsychologically.34 All other syndromes were equally compromised compared to siblings. This adds to increasing evidence suggesting that absence seizures might not be as “benign” as once thought.34–36 Attempts to examine unique risk factors by etiology were unsuccessful, perhaps because of heterogeneity or reduction in power (i.e., cutting the sample in half for those analyses).
Epileptiform activity on the initial EEG was associated with slower psychomotor speed, and this relationship was not an artifact of AED use. Past studies have documented relationships between epileptiform discharges and various neuropsychological functions.37 Taken together with the present investigation, initial EEG provides a biomarker for processing speed deficits, which can facilitate rapid identification of a neuropsychological function that is critical to cognitive development in childhood.38 Thus, in addition to predicting surgical outcome, epileptiform activity on the EEG can predict neuropsychological deficits, further supporting the assertion that EEG provides a “window into the anatomy, severity, and functional implications of epilepsy.”39,40
Several factors individually double the odds of neuropsychological deficits compared to a healthy sibling: a single seizure (and slightly higher for a second seizure), symptomatic/cryptogenic etiology, childhood absence epilepsy syndrome, epileptiform activity on initial EEG, and use of AEDs. Although symptomatic/cryptogenic etiology explained the most variance in multivariate logistic models, this does not negate risk associated with other factors. Compared to other children in the seizure group with no risk factors, those with symptomatic/cryptogenic etiology have an OR of 1.79; however, children with multiple risk factors (which might or might not include symptomatic/cryptogenic etiology) are almost 3 times more likely to have neuropsychological impairment (OR = 2.89) compared to children who have only one seizure and no other risk factors.
The absence of academic achievement differences runs contrary to other reports, but there are possible explanations. One study included very early onset and, thus, catastrophic epilepsies associated with developmental delays.14 Another tested children an average of 10 months after onset7; their results might represent changes across the first year. Finally, one study reported high rates of special educational placements,10 but attention deficits and behavior problems might have accounted for the classroom placements because academic achievement test scores were nonsignificant. Taken together with the present findings, neuropsychological deficits might emerge concomitantly with seizure onset but exert their influence on academic achievement cumulatively over time. Given the relationship between neuropsychological functioning and academic achievement3 and the high rates of learning disability in children with chronic seizures,2 these findings suggest that there might be a window during which educational interventions could prevent or minimize the long-term impact on educational attainment, and presumably upon vocational success as adults.
The lower age limit of this study excluded children with more severe syndromes of infancy and early childhood; this tradeoff was deemed appropriate for the goals of the present study because earlier onset is associated with broader intellectual disability. In this study, neuropsychological assessment was not conducted as close to “onset” as it had been in other studies9,10; however, age at onset in those studies was defined as a diagnosis of epilepsy following the second unprovoked seizure, so our studies might not be very different in this regard. Effect sizes were generally small, which suggests that the degree of neuropsychological impairment is subtle; however, these indices are attenuated to some degree by the restriction of range in cognitive dysfunction due to exclusion of children with early onset and IQ <70. Finally, although our sample size would permit examination of AED × syndrome interactions, results would remain difficult to interpret because our study design did not control for the choice of treatment, dosing, or timing relative to neuropsychological evaluation.
This study has important clinical implications for the neurologist, pediatrician, neuropsychologist, and educational specialist. Children with multiple seizures (i.e., a diagnosis of epilepsy), with symptomatic/cryptogenic etiology, on AEDs, or with epileptiform discharges on the initial EEG—especially if they possess multiple risks—should undergo a prompt neuropsychological evaluation to characterize neuropsychological deficits and to develop interventions to prevent or minimize their impact on long-term outcomes.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by C.S. Johnson and S.M. Perkins.
ACKNOWLEDGMENT
The authors thank Angela McNelis, DNS, for coordinating interviews; Beverly Musick for coordinating data management; and Carolanne Benson and Paul Buelow for technical and administrative support. They also thank Patricia A. Taylor-Cooke, Jennifer I. Koop, Brenna C. LeJeune, Stacey E. Woodrome, Sherry Mullinix Pryor, Tiffany J. McCall Neal, Jennifer M. Katzenstein, Natalie C. Cunningham, Janet L. Kain, Joy M. Fairbanks, Deborah Doescher, Jennifer Frey, Wendi Lopez, Chrisha Wayt, Catherine McNutt, and Erin Cooper for coordinating and collecting neuropsychological data. Finally, they thank the children and their families who participated in this project.
DISCLOSURE
Dr. Fastenau receives royalties from Western Psychological Services for publication of the Extended Complex Figure Test, a neuropsychological test and manual; has received speaking honoraria and travel subsidies from National Institutes of Health for reviewing grant applications and from American Epilepsy Society, International Neuropsychological Society, National Academy of Neuropsychology, and University of California–San Diego for speaking; and has received research support from the NIH (grant #NS22416, coinvestigator). His wife is an employee of Eli Lilly & Co., in which she holds stock options and participates in a stock-based pension. C.S. Johnson and her husband hold stock in Genentech; she received research support from the NIH (grant #NS22416, coinvestigator). Her husband is employed by Eli Lilly & Co. and he holds stock in that company. Dr. Perkins and her husband hold stock options in Biosante Pharmaceutical, Cortex Pharmaceutical, Crucell N.V., Isis Pharmaceuticals, Oracle, and Vical Incorporated; Dr. Perkins has received honoraria for statistical workshops given in the Southwestern Ohio Area Research Network Faculty Development Series; has received honoraria from National Institute of Allergy and Infectious Diseases and the Veterans Administration for reviewing grant applications; and has received research support from the NIH (grant #NS22416, coinvestigator). Dr. Byars (coinvestigator), Dr. DeGrauw (coinvestigator), and Dr. Austin (principal investigator) received research support from the NIH (grant #NS22416). Dr. Dunn received research support from the NIH (grant #NS22416, coinvestigator), Eli Lilly, and GlaxoSmithKline, and has received speaker honorarium from McNeil.
Address correspondence and reprint requests to Dr. Philip S. Fastenau, Department of Neurology, University Hospitals Case Medical Center, 11100 Euclid Avenue, HAN 5040, Cleveland, OH 44106-5040 Philip.Fastenau@uhhospitals.org
Editorial, page 496
e-Pub ahead of print on August 12, 2009, at www.neurology.org.
Supported by the NIH/National Institute of Neurological Disorders and Stroke (NS22416, J.K. Austin, PI).
Disclosure: Author disclosures are provided at the end of the article.
Received October 3, 2008. Accepted in final form May 11, 2009.
REFERENCES
- 1.Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia 1985;26:395–400. [DOI] [PubMed] [Google Scholar]
- 2.Fastenau PS, Shen J, Dunn DW, Austin JK. Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. J Learn Disabil 2008;41:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fastenau PS, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK. Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia 2004;45:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidenberg M, Beck N, Geisser M, et al. Academic achievement of children with epilepsy. Epilepsia 1986;27:753–759. [DOI] [PubMed] [Google Scholar]
- 5.Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav 2004;5 suppl 1:S25–S34. [DOI] [PubMed] [Google Scholar]
- 6.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia 2000;41:426–431. [DOI] [PubMed] [Google Scholar]
- 7.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 2006;129:2609–2619. [DOI] [PubMed] [Google Scholar]
- 8.Kolk A, Beilmann A, Tomberg T, Napa A, Talvik T. Neurocognitive development of children with congenital unilateral brain lesion and epilepsy. Brain Dev 2001;23:88–96. [DOI] [PubMed] [Google Scholar]
- 9.Williams J, Bates S, Griebel ML, et al. Does short-term antiepileptic drug treatment in children result in cognitive or behavioral changes? Epilepsia 1998;39:1064–1069. [DOI] [PubMed] [Google Scholar]
- 10.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A, Dutch Study Group of Epilepsy in C. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”: a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 2003;112:1338–1344. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois BF, Prensky AL, Palkes HS, Talent BK, Busch SG. Intelligence in epilepsy: a prospective study in children. Ann Neurol 1983;14:438–444. [DOI] [PubMed] [Google Scholar]
- 12.McNelis AM, Johnson CS, Huberty TJ, Austin JK. Factors associated with academic achievement in children with recent-onset seizures. Seizure 2005;14:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stores G, Williams PL, Styles E, Zaiwalla Z. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child 1992;67:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol 2005;47:749–753. [DOI] [PubMed] [Google Scholar]
- 15.Shinnar S, Berg AT, Moshe SL, et al. Risk of seizure recurrence following a first unprovoked seizure in childhood: a prospective study. Pediatrics 1990;85:1076–1085. [PubMed] [Google Scholar]
- 16.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics 2001;107:115–122. [DOI] [PubMed] [Google Scholar]
- 17.Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol 2003;45:50–54. [PubMed] [Google Scholar]
- 18.CCT-ILAE. Proposal for revised clinical and electroencephalographic classification of epileptic seizures: from the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501. [DOI] [PubMed] [Google Scholar]
- 19.CCT-ILAE. Proposal for revised classification of epilepsies and epileptic syndromes: Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 20.Berg AT, Levy SR, Testa FM, Shinnar S. Classification of childhood epilepsy syndromes in newly diagnosed epilepsy: interrater agreement and reasons for disagreement. Epilepsia 1999;40:439–444. [DOI] [PubMed] [Google Scholar]
- 21.Hauser WA. Epidemiology of epilepsy in children. In: Pellock JM, Dodson WE, Bourgeois BFD, eds. Pediatric Epilepsy: Diagnosis and Therapy (2nd Ed.). New York, NY: Demos Medical Publishing; 2001:81–96. [Google Scholar]
- 22.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia 1999;40:445–452. [DOI] [PubMed] [Google Scholar]
- 23.Baum KT, Byars AW, deGrauw TJ, et al. Temperament, family environment, and behavior problems in children with new-onset seizures. Epilepsy Behav 2007;10:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byars AW, deGrauw TJ, Johnson CS, et al. The association of MRI findings and neuropsychological functioning after the first recognized seizure. Epilepsia 2007;48:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doescher JS, deGrauw TJ, Musick BS, et al. Magnetic resonance imaging (MRI) and electroencephalographic (EEG) findings in a cohort of normal children with newly diagnosed seizures. J Child Neurol 2006;21:491–495. [PMC free article] [PubMed] [Google Scholar]
- 26.Arthur TM, deGrauw TJ, Johnson CS, et al. Seizure recurrence risk following a first seizure in neurologically normal children. Epilepsia 2008;49:1950–1954. [DOI] [PubMed] [Google Scholar]
- 27.Byars AW, Byars KC, Johnson CS, et al. The relationship between sleep problems and neuropsychological functioning in children with new-onset seizures. Epilepsy Behav 2008;13:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalnin AJ, Fastenau PS, deGrauw TJ, et al. MR imaging findings in children with first recognized seizure. Pediatr Neurol 2008;39:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vingerhoets G. Cognitive effects of seizures. Seizure 2006;15:221–226. [DOI] [PubMed] [Google Scholar]
- 30.Bourgeois BF. Antiepileptic drugs, learning, and behavior in childhood epilepsy [see comment]. Epilepsia 1998;39:913–921. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen J, Aldenkamp AP. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res 1995;22:65–95. [DOI] [PubMed] [Google Scholar]
- 32.Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet 2001;357:216–222. [DOI] [PubMed] [Google Scholar]
- 33.Loring DW, Meador KJ. Cognitive side effects of antiepileptic drugs in children. Neurology 2004;62:872–877. [DOI] [PubMed] [Google Scholar]
- 34.Mandelbaum DE, Burack GD. The effect of seizure type and medication on cognitive and behavioral functioning in children with idiopathic epilepsy. Dev Med Child Neurol 1997;39:731–735. [DOI] [PubMed] [Google Scholar]
- 35.Poblano A, Ibarra J, Muniz A, Garza S. Absence seizures effects on reading revealed by video-electroencephalography. Rev Invest Clin 2001;53:136–140. [PubMed] [Google Scholar]
- 36.Sengoku A, Kanazawa O, Kawai I, Yamaguchi T. Cognitive function during absence seizures. Japanese J Psychiatry Neurol 1988;42:558–559. [DOI] [PubMed] [Google Scholar]
- 37.Koop JI, Fastenau PS, Dunn DW, Austin JK. Neuropsychological correlates of electroencephalograms in children with epilepsy. Epilepsy Res 2005;64:49–62. [DOI] [PubMed] [Google Scholar]
- 38.Kail R, Hall LK. Processing speed, naming speed, and reading. Dev Psychol 1994;30:949–954. [Google Scholar]
- 39.Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology 2008;71:413–418. [DOI] [PubMed] [Google Scholar]
- 40.Miller JW, Gotman J. The meaning of interictal spikes in temporal lobe epilepsy: should we count them? Neurology 2008;71:392–393. [DOI] [PubMed] [Google Scholar]